Dear Editor,
Men with invasive ductal carcinoma [Infiltrative ductal carcinoma (IDC)] of the breast constitute a rare subset of cancer patients, accounting for about 0.2% of total cancers.1 Hormonal, environmental, and genetic factors are implicated in its pathogenesis. The risk factors particularly associated with male breast cancer (MBC) include hyperestrogenism, gynaecomastia, Klinefelter’s syndrome, and hormone therapy for prostate cancer. Many of these patients are also found to have hormone receptor positivity and underlying BRCA2 germline mutations.2,3 Clinical, dermoscopic, and histopathological examination (HPE) can aid in reaching the correct diagnosis of this rare condition.
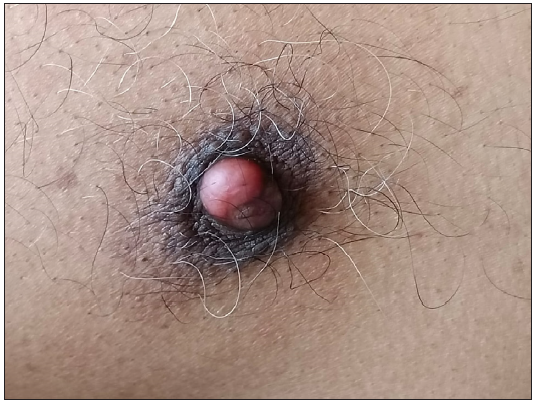
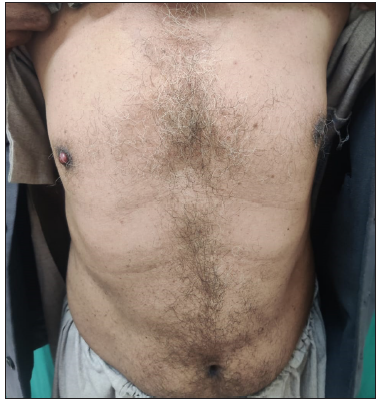
A 60-year-old male with no known comorbidities presented to our outpatient department with a reddish swelling over the right nipple for 2 months. There was no history of preceding trauma, itching, or discharge from the lesion. He also reported a sudden generalised eruption of small brownish skin lesions appearing one month before the aforesaid lesion. These started over the trunk and progressed over two months to involve other body parts. On examination, we noted a solitary, pinkish-red, firm, non-tender nodule, measuring 1.8 × 1.1 × 1.1 cm over the right nipple area [Figure 1]. There was no associated lymphadenopathy. There were multiple small brown papules and plaques with a flat rough surface distributed over the trunk [Figure 2]. Clinical differentials of pyogenic granuloma (PG), eccrine poroma (EP), and apocrine hidrocystoma (AH) were considered for the nipple lesion.
Export to PPT
Export to PPT
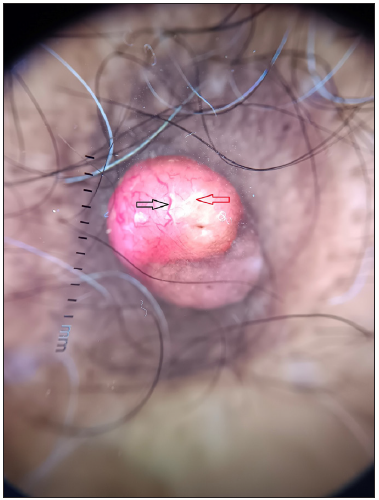
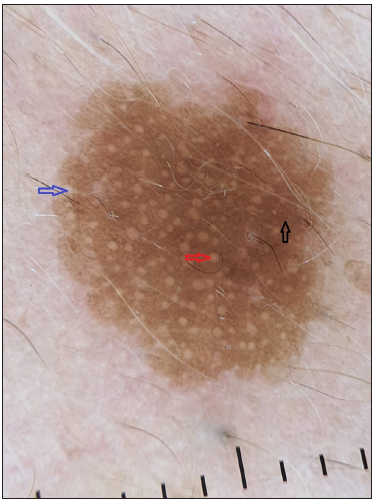
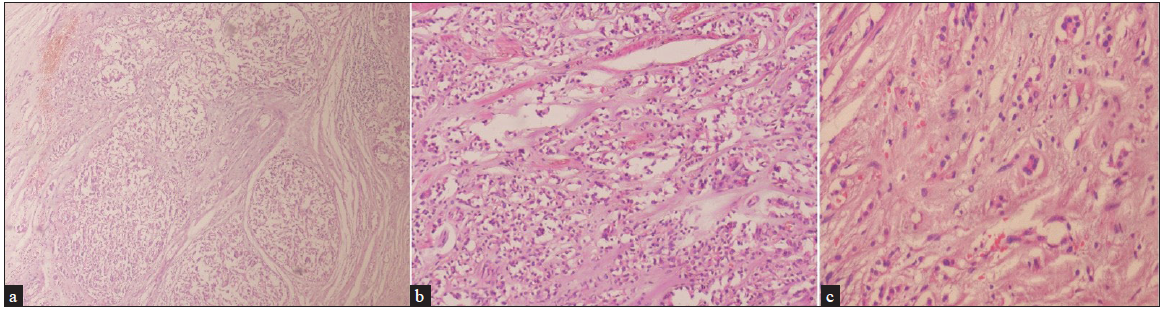
Dermoscopy of the nodule revealed branching vessels of variable diameter against a pink background with white areas interspersed in between [Figure 3]. Dermoscopy of the brown plaques revealed milia-like cysts, moth-eaten borders and pigment network [Figure 4] which were suggestive of seborrheic keratoses (SK) presenting as a sign of ‘Leser-Trelat’, thereby giving a clue to the presence of an underlying malignancy. The patient was subjected to routine laboratory investigations and fine-needle aspiration cytology (FNAC) of the lesion which showed clusters of polymorphous cells forming vague acini, indicative of infiltrative ductal carcinoma (IDC) of the breast. A lesional biopsy revealed tumour cells scattered individually with focal acini formation, moderate pleomorphism, and scanty mitosis [Figure 5]. A thorough radiological examination, including contrast-enhanced computed tomography (CECT) of the chest, abdomen, and pelvis, indicated no abnormalities. The patient underwent a radical mastectomy and axillary lymph node dissection. Histological analysis of the 12 × 9 × 1 cm mastectomy specimen was consistent with Scarff–Bloom–Richardson (SBR) Grade II IDC with no evidence of tumour invasion in level I and level II lymph nodes and American Joint Committee on Cancer (AJCC) stage IIIB (pT4bN0M0). Estrogen, progesterone, Ki-6(MIB-1) receptor positivity, and HER/2 negativity were noted. Subsequently, the patient underwent chemotherapy and later was lost to follow-up.
Export to PPT
Export to PPT
Export to PPT
Male breast cancer (MBC) is an underdiagnosed entity. The age at presentation is often advanced in men as compared to women due to the delay in diagnosis and the low index of suspicion.3,4 It presents as an asymptomatic palpable lump behind the areola and, in many cases, may be associated with spotting or bleeding from the nipple.1 The Leser Trelat sign, a paraneoplastic syndrome has been linked to a variety of cancers, most commonly adenocarcinoma of the colon, breast, and stomach. These lesions are well-defined pigmented lesions that range in colour from skin coloured to tan to brown-black. They have a ‘stuck-on’ appearance and a waxy or velvety texture.5
IDC can mimic other dermatological conditions that can be differentiated on dermoscopy. [Table 1]6 Akay et al.7 in 2019 have reported a unique case of a pigmented primary MBC mimicking melanoma. The dermoscopy revealed features like peppering, collarette of scales, ulceration with haemorrhages, central white structureless areas, white lines, and polymorphic vascular structures.
Table 1: Differential diagnosis & their observed dermoscopic features
S.no. Disease Dermoscopic features observed 1. Pyogenic granuloma Reddish homogenous areas, white collarette at the periphery, white rail lines, and a polymorphic vascular pattern. 2. Eccrine poroma Branched vessels with rounded endings, yellow structureless areas, and milky-red globules. 3. Apocrine hidrocystoma Pink, blue, or yellow homogenous areas with arborising vessels and brown pigment lobules. 4. Our case Arborising branching vessels of variable diameter against a pink background with interspersed white structureless areas.Due to its rarity and close clinical resemblance to various appendageal tumours, diagnosis of MBC becomes challenging. Dermoscopy can serve as a valuable bedside tool in differentiating these conditions with confirmation by histology. This case report describes the dermoscopic features of IDC which have not been reported in the literature so far.
留言 (0)