Electrolyte imbalances are known to occur in patients with cancer and hospitalized patients receiving palliative care.[1] Hospitalisation in patients with advanced cancer indicates poor symptom control or worsening disease-related morbidity. Electrolyte imbalance is a significant issue in palliative care due to its association with prognosis and other factors such as hospitalisation duration and costs.[1-5] While studies explore these issues in the context of cancer patients on active treatment, very few studies document the pattern of electrolyte imbalance noted in hospitalised patients with advanced cancer. Albumin is a well-known prognostic marker in oncology, and literature is evolving in palliative oncology.[6,7] Patients admitted to the emergency department undergo various baseline blood investigations. The characteristics of patients admitted and the association of these investigations with patient outcomes in palliative oncology was an area of interest for us. Our study aimed to generate data and contribute to research in this field to plan and prognosticate and possibly improve the quality of life for cancer patients receiving palliative care.
MATERIALS AND METHODSAfter institutional review board approval (ref # 22-483), a retrospective, observational study was carried out in the Department of Pain and Palliative Care at the Bahrain Oncology Centre. Electronic records from January 1, 2019, 12:00 am to October 1, 2021, 12:00 am were obtained. We measured the following:
Patient characteristics including age, sex, and type of cancer: solid/haematological
Symptom pattern at the time of presentation and clinical disposition following arrival: emergency or ward
Measurement of electrolyte imbalance and blood parameters: we documented the levels of cations (Sodium: Na, Potassium: K, Calcium: Ca, Magnesium: Mg), along with renal function and serum albumin levels
Comorbid medical issues such as major systemic illnesses, drugs that may cause electrolyte imbalance, presence of stomas, feeding tubes, and a history or previous electrolyte imbalance.
Electronic records accessed and analysed with a pre-approved pro forma.
Data anonymised by assigning a number e.g. 1,2,3, ... For the same patient with repeated admission, alphabets added to extend the admission number, e.g. 1a, 1b. and so on for the same patient. NO Patient IDENTIFIERS recorded anywhere. Data handling by study team, maintained on secure password protected hospital electronic devices. Data analysed.
Inclusion criteria:
Patients presenting to the emergency room, with advanced cancer and receiving supportive care.
All patients admitted to oncology ward/ Palliative Ward/ ICU
Admission through emergency
Admissions from 1st January 2019 12:00 am to 1st 5 October 2021 12:00 am
Exclusion criteria*:
Elective admissions.
*Note: Owing to COVID-19, for a brief period, most admissions were triaged through Emergency department as per hospital protocol, including elective admissions
The methodology is described below:
Abnormal values of blood reports were defined using the institution’s laboratory values in standard international units as a reference. The files were screened for comorbid histories, such as renal or cardiac disease, urostomas, gastrointestinal stomas, nasogastric or enteral feeding, and possible drugs that could cause electrolyte imbalance.
Descriptive statistics were used in terms of percentages, absolute numbers, or proportions. Analytical statistics were used in terms of regression to measure the association between the variables of interest using SPSS v 25.0.
RESULTSThere were 157 emergency admissions during the study period. A total of 153 patients were admitted to the ward, and four were transferred to the intensive care unit (ICU) from the emergency department. Most of the admissions were for patients with solid tumours (92.4%). The patient characteristics and outcomes are described in Table 1. Symptoms at the time of presentation were clustered into the common symptoms encountered in palliative oncology. We encountered the following symptom patterns in emergency presentations:
Pain
Reduced oral intake
Altered mental status
Fever
Fatigue
Breathlessness
Vomiting and/or constipation
Other symptoms, e.g., bleeding from stoma sites/haematuria, etc.
Table 1: Patient characteristics.
Age 67 (IQR: 56–71) Duration of hospitalisation 12 (IQR: 6–21) Sex, n(%) FemalePain was the most common cardinal symptom at the time of presentation (n = 68); close to half of the patients presented with pain as an isolated symptom (n = 33), whereas the rest reported it as a part of a symptom cluster. The next common symptoms reported either in isolation or with other symptoms were reduced oral intake (n = 32), followed by altered mental status (n = 24), fever (21), dyspnoea (16), fatigue (15), and vomiting (15).
Symptoms that did not fit into the common symptom clusters were categorised as ‘others,’ for example, patients with haematuria and bleeding from colostomy sites, collectively seen in 15 patients.
Long-term outcomesSixty-six patients died within the hospitalisation period (30 had abnormal Na, 16 had abnormal K, 15 with abnormal Mg, 32 had abnormal Ca, and 47 had abnormal albumin). On long-term follow-up one year after the pre-defined study period (in January 2023), 138 patients died, eight (5.1%) survived, and follow-up was lost for seven patients. The proportion of patients with haematological malignancies who were surviving was more (27%) than the patients with solid tumours (3.7%).
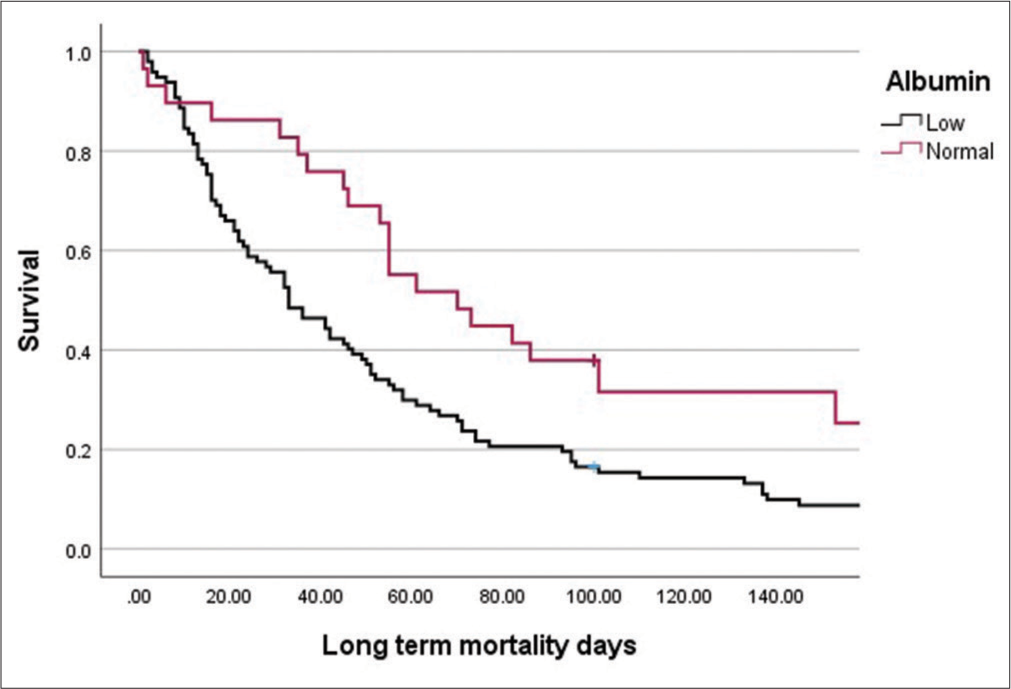
Hyponatraemia was the most common electrolyte imbalance (43%), hypoalbuminaemia was observed in about two-thirds of the patients (66%), and one-third of patients had deranged renal function (33%). On analysis of each subgroup in terms of survival, we observed a weak positive correlation between Na levels and outcome (r = 0.199, P = 0.016) and a strong positive correlation between albumin levels and survival outcomes (r = 0.329, P = 0.000). Patients with normal albumin had a higher chance of survival (odds ratio: 33.1225, 95% confidence interval: 3.415–321.20, P = 0.003). Patients who had long-term survival had normal sodium levels and had no history of electrolyte imbalance. The results are shown in Table 2 (long-term outcome), Table 3 (median days of survival), and Table 4 (bivariate analysis: Levels of electrolytes with outcome: Spearman’s rho), and the survival curve plotted against sodium and albumin levels is shown in Figures 1 and 2, respectively.
Table 2: Outcomes based on patient characteristics and blood investigations.
Outcome P-value Died n(%) Surviving n(%) Type of cancer Solid tumour 130 (96.3) 5 (3.7) 0.015 Haematological cancer 8 (72.7) 3 (27.3) Na level Low 63 (100.0) 0 (0.0) 0.029 Normal 71 (89.9) 8 (10.1) High 3 (100.0) 0 (0.0) K level Low 15 (93.8) 1 (6.3) 0.705 Normal 111 (94.1) 7 (5.9) High 11 (100.0) 0 (0.0) Mg level Low 23 (95.8) 1 (4.2) 0.865 Normal 112 (94.1) 7 (5.9) High 3 (100.0) 0 (0.0) Ca level 0.093 Low 50 (98.0) 1 (2.0) Normal 34 (87.2) 5 (12.8) High 5 (100.0) 0 (0.0) Albumin level 0.002 Low 97 (99.0) 1 (1.0) Normal 23 (82.1) 5 (17.9)Table 3: Median duration of survival in days for Na levels and serum albumin levels.
Median days of survival (IQR) P-value Na level Low 46 (17–76) 0.356 Normal 41 (16–21) High 32 (21–51) Albumin level Low 33 (15–70) 0.004 Normal 55 (41–93.5)Table 4: Bivariate analysis: Levels of electrolytes with outcome (Spearman's rho).
Spearman's rho coefficient P-value Na 0.199 0.016 K −0.050 0.550 Mg 0.013 0.874 Ca 0.167 0.107 Albumin 0.329 0.000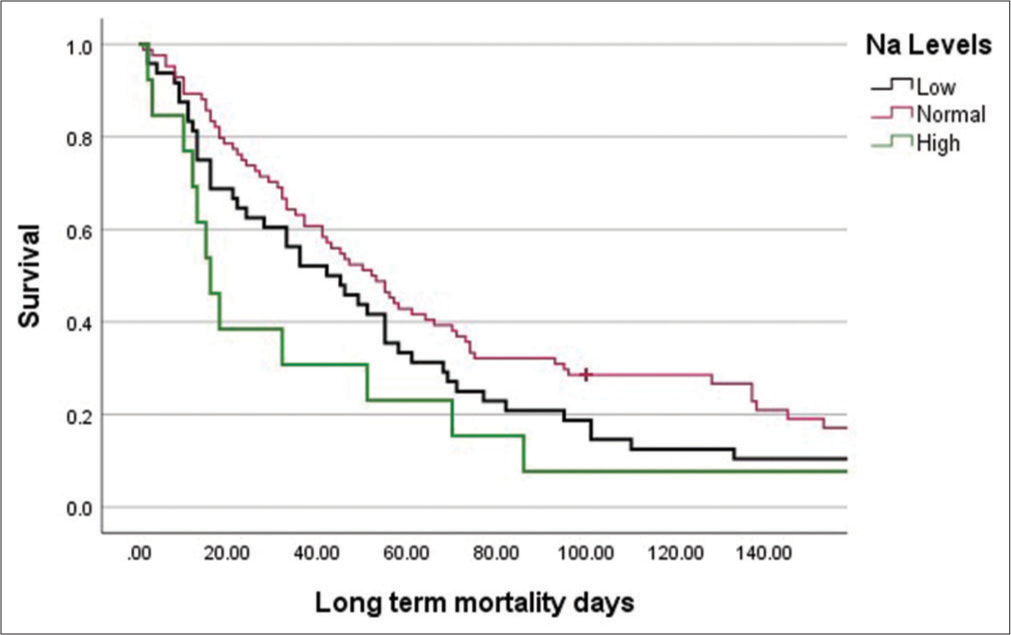
Export to PPT
Export to PPT
DISCUSSIONHospitalisation in patients with advanced cancer on palliative care may indicate poor symptom control or worsening disease-related morbidity. Recognising these issues and addressing these complex clinical states are an important part of managing these patients. We noted that 66 out of 157 patients with advanced cancer receiving palliative care died in the same admission period. There is no local data for the mortality associated with palliative care patients in Bahrain. In a study by El Majzoub et al., the authors observed that the patients admitted through the emergency department made up a significant majority of those who died in the hospital (88%).[8] In addition, they observed significant associations with complaints of respiratory problems, neurologic issues, or fatigue/weakness with in-hospital mortality. The long-term survival for our patients followed up one year after the end of the study period also noted a significant mortality rate, with only 5.1% of the patients surviving. In a descriptive before-and-after Norwegian study including 44 patients with advanced cancer, the authors reported a median survival of 50 days following emergency medicine department (EMD) admissions.[9] Based on our observations, emergency hospitalisation in palliative oncology patients may have prognostic implications. In addition, the symptom clusters identified point to gaps in the continuity of care or provision of palliative care services at the home/community level.
We identified uncontrolled pain as a significant indication for palliative admission, followed by reduced oral intake. Anorexia and reduced food intake in palliative oncology may cause caregiver distress, especially in the social-cultural context. This may be perceived as distress, and it may prompt caregivers to bring their loved ones to the emergency in the absence of distress in the patients themselves. We noted that a large proportion of patients did not have enteral feeding. In addition, our clinical experience suggests that caregivers of patients with other symptoms, including altered sensorium, may later report poor oral intake as one of the prompting symptoms to bring the patient to the hospital. This may further place a strain on resources without significantly improving the quality of life or survival of the patient with advanced cancer. In the absence of formal home care palliative services in the public or private sector, a possible area of research would be to consider the practical role of nasogastric feeding where it is acceptable. This may ensure access to hydration, medications for symptom control, and nutrition, possibly reducing the perceived need to hospitalise patients with this symptom.
We assume emergency hospital admissions in palliative oncology reflect the following:
The gaps in the caregivers’ understanding of the critical nature of the disease lead to unrealistic expectations
The lack of time needed for caregivers and patients to come to terms with the disease status
An unpredictable course of the cancer itself leads to distress and poor symptom control.
Ethically, intensive care admissions in advanced cancer patients pose a dilemma between the principles of distributive justice and beneficence. Real-world scenarios may not offer simple solutions. The issue of resource utilisation and patient harm due to aggressive treatment is often discussed in palliative oncology. Some patients in palliative oncology may not be on active disease-directed treatment, but they may still have a life expectancy in the range of months. Acute, reversible conditions in these patients may be mitigated by brief intensive care stays, returning the patient back to a potentially good quality of life with a positive survival outcome. At the same time, patients need to be treated keeping in mind non-maleficence - ‘do not harm,’ as aggressive and advanced treatment in these patients may worsen the quality of life without improving survival. Such treatment may also deplete limited resources and, in a utilitarian concept, impinge on the principles of distributive justice. In real-world scenarios, patients with advanced cancers will continue to be admitted to intensive care units in resource-rich settings. The challenge would be to initiate conversations in the critical care unit for those who may not have been exposed to palliative care. Ensuring continuity of care in these patients is important, and offering palliative care to patients with advanced cancers must be part of institutional treatment protocols.
Till the time robust home healthcare delivery systems or early palliative care referral may address these issues, our data demonstrate the real-world needs of several communities where healthcare systems operate out of institutions.
While studies explore the issues of electrolyte imbalance in the context of cancer patients on active treatment, very few studies document the pattern of electrolyte imbalance noted in emergency admissions in palliative oncology. Our findings in these patients are discussed below.
HyponatraemiaHyponatraemia is the most common tumour-related electrolyte imbalance.[1] Nair et al. documented the prevalence of hyponatraemia in hospitalised patients after reviewing 2666 inpatient charts and found out that it was significant in palliative care patients.[2] Similar studies document the prevalence of hyponatraemia in patients with cancer receiving palliative care.[10] Castillo et al. reviewed the diagnosis and management of hyponatraemia in cancer patients. They identified hyponatraemia as a negative prognostic factor, usually caused by the syndrome of inappropriate antidiuretic hormone secretion.[11] They observe variable reported rates of the prevalence of hyponatraemia, ranging from 15% in some studies to 25–44% in others. In our study, we observed the prevalence of hyponatraemia to be 43.3%, and it was the most common electrolyte abnormality observed. It was the most common electrolyte abnormality in the past medical history (11% of patients). There was a weak correlation between sodium levels and mortality.
HypernatraemiaHypernatraemia is relatively uncommon in cancer patients, as a study by Salahudeen et al. involving 3446 patients reported a frequency of 2.6%, as opposed to 46% for hyponatraemia.[3] The study went on to point out that most cases were hospital-acquired, with an impact in terms of higher morbidity, longer stay, and higher financial costs. In this context, it may be an anticipated and preventable dyselectrolytemia. In our study, we have observed comparable numbers, with a prevalence of 2.5%. Seo et al. conducted a retrospective cohort study, from which they concluded that hypernatremia was predictive of shorter patient survival and emphasised the need for greater clinical attention to its prognostic utility.[4] We could not draw any conclusion from our observations on hypernatremia in the present study.
HypokalaemiaAlthough reviews mention hypokalaemia as a common electrolyte problem in cancer with multiple possible mechanisms that may cause it, there is a dearth of literature on the prevalence of hypokalaemia in the cancer population.[1] Treatment in these conditions addresses the underlying cause, addresses the symptoms, and is aimed at preventing life-threatening complications that may potentially arise secondary to hypokalaemia.[12,13] In the update by Kardalas et al., they cited the prevalence in hospitalised patients to be 20%, with about 5% being clinically significant.[14] We observed the prevalence of hypokalaemia to be lower in our study (12.1%). In patients with oncology, symptoms of hypokalaemia, such as fatigue and ileus may mimic cancer fatigue or malignant bowel obstruction. More data are needed on the prevalence of this symptom in the cancer palliative care population to plan and guide treatment strategies.
HyperkalaemiaHougen et al. reported a retrospective cohort study of 96,337 patients, concluding that hyperkalaemia was an independent risk factor for all-cause mortality, cardiovascular events, hospitalisations, and ICU admissions.[5] As in hypokalaemia, treatment remains directed towards preventing the accompanying life-threatening complications.[15] Our study observed a prevalence of 7% in palliative oncology admissions. In our clinical practice, we observe that new-onset hyperkalaemia is commonly associated with worsening renal function, and patients with advanced cancers may have obstructive uropathies, urosepsis, or sepsis as a proximal cause. There remains a dearth of literature on the prevalence of hyperkalaemia in hospitalised cancer patients, and more studies may help anticipate and correct this silent symptom. Magnesium: There is a dearth of literature related to the prevalence of hypo- and hypermagnesaemia in hospitalised cancer patients.
HypomagnesaemiaMagnesium is an intracellular cation, and its deficiency frequently occurs (7–12%) in hospitalised patients, and it is correlated to an increased risk of death.[1] Our observations of prevalence are slightly higher (15.9%). Although the symptoms of hypomagnesaemia may be non-specific, we have observed it to occur in our practice in patients with poor oral intake, alongside hypokalaemia. In such cases, clinical correction of magnesium precedes that of potassium. In a study by López-Saca et al., two cases of opioid refractory pain attacks were described, which were corrected with magnesium replacement.[16]
HypermagnesaemiaHypermagnesaemia is a rare electrolyte abnormality.[1] Takahashi and Uchino. reported a cross-sectional study of 533 patients admitted to a palliative care centre, out of whom hypermagnesaemia was observed in 123 patients (23.08%).[17] In this study, predictors of hypermagnesaemia included renal dysfunction, short prognosis prediction, and oral magnesium oxide laxative use, with a plausible incidence of hypermagnesaemia being 20%. However, our observations on hypermagnesaemia are much lower 1.9% than in this study.
HypocalcaemiaHypocalcaemia can arise from multiple causes.[18] In the cancer population, the use of bone-directed treatments such as bisphosphonates and Denosumab are specific examples of causative factors for hypocalcaemia, with a reported incidence of 9.6% in patients treated with Denosumab.[19] In a review of the literature, Kreutle et al. reported that the true incidence of hypocalcaemia was difficult to measure with bisphosphonate therapy.[20] Broadbent et al. have described a case report of severe symptomatic hypocalcaemia following bisphosphonate therapy in a patient with advanced cancer, pointing to the significance of assessment of this ion status in palliative care.[21] Hypocalcaemia can be caused by myriad mechanisms, and there is a dearth of literature about its prevalence in the palliative care cancer population. Our study reported a prevalence of 34.4% of hypocalcaemia.
HypercalcaemiaAsonitis et al. reviewed the literature on hypercalcaemia of malignancy and cited how cancers were the more prevalent cause of hypercalcaemia in patients presenting to the emergency department.[22] This electrolyte disturbance is the most common metabolic life-threatening emergency encountered in advanced cancers and is associated with a poor prognosis. It has been estimated to occur in 20% of all patients with cancer, and despite it being a common electrolyte disorder with emergency implications, there is a need to report the incidence of hypercalcaemia in patients with cancer. In this study, we noted the prevalence to be much lower in our hospitalised palliative care population presenting to the emergency (3.8%). Our findings are in line with studies reported in the literature.[23]
AlbuminIn a systematic review of 59 studies, the authors concluded that pre-treatment serum albumin levels provided prognostic significance in oncology.[6] Hypoalbuminaemia may be classified using common terminology criteria for adverse events criteria:
Grade 1: Lower limit of normal (LLN) - 3 g/dL
Grade 2: LLN < 3–2 g/dL
Grade 3: 2 g/dL
Grade 4: Life-threatening consequences; urgent intervention indicated
Grade 5: Death.[24]
The relationship between albumin, protein levels, malnutrition, and survival is documented in the literature. In a study by Ñamendys-Silva, 164 out of 200 patients with advanced malignancy (82%) had serum albumin levels below 35 g/L, and 55.5% of patients had levels of albumin ≤20 g/L. The latter group had the highest mortality rate (73%).[25] Serum albumin is known for its association with prognosis in oncology and cancer cachexia.[6,7,25-28] In the nested case-control study by Liu et al., serum albumin had an independent negative linear association with 1-year mortality in cachectic patients in oncology.[7] When serum albumin level was divided into quartiles, the authors observed that quartiles 3 and 4 (albumin levels >36.9 g/L) had higher survival than quartile 1 (albumin levels <33.1 g/L). Their observation was free of external influences such as age/cancer type/hospital/sex.
In our study, most patients had low albumin levels (66.2%), and hypoalbuminaemia was associated with a long-term mortality of 99%. Our study demonstrates the strong association of this cheap and easily available test with mortality and reinforces its utility as a practical prognostic marker in palliative oncology.
Our study reports the prevalence of electrolyte imbalance, hypoalbuminaemia, and associations with mortality in hospitalised patients in palliative oncology admitted through the emergency. There are limitations of our study due to its retrospective observational nature. Multiple factors may influence morbidity and mortality in patients with advanced disease. We included all patients in palliative oncology as one group for the purpose of the study; this may not reflect the study population in other centres. We cannot comment on prognosis based on the primary cancer type. The symptom clusters at the time of presentation to the emergency may differ possibly between palliative healthcare delivery systems and may not be generalised to the palliative oncology population. However, the associations noted between albumin levels and the high mortality in these patients seem to be consistent across patients with advanced cancer. With larger data and the increasing use of artificial intelligence, we may presuppose that more robust prognostic and predictive indicators may be developed using these variables.
CONCLUSIONPatients with advanced cancer receiving palliative care tend to be quite sick, and significant associations include hyponatraemia and hypoalbuminaemia.
Hypoalbuminaemia in these patients is an independent predictor of long-term mortality. This may have prognostic utility.
Recognising these issues and addressing these complex clinical states are an important part of management in this group of patients.
While studies explore these issues in the context of cancer patients on active treatment, very few studies document the pattern of electrolyte imbalance noted in patients with advanced cancer.
留言 (0)