For children, diarrhea is a prevalent and serious clinical condition (1). Functional diarrhea (FD), the most common type of persistent or chronic diarrhea, is a functional gastrointestinal disorder (2). The Rome IV definition of FD is the presence of loose (mushy) or watery stools four times a day without pain (3). The prevalence of FD is estimated at around 6.4% in the USA and 1.54% in China (4–6). Children who experience recurrent episodes of diarrhea are at greater risk of developing other health conditions, such as under nutrition, diarrheal infections, and immunodeficiency (7). Consequently, those conditions will have a substantial impact on children's quality of life as well as burden the healthcare system.
However, the specific pathogenesis and mechanism of FD remain unclear. In previous studies, intestinal microbiota dysbiosis and intestinal barrier disruption have been suggested as contributing factors to FD (8, 9). In a healthy human intestine, commensal bacteria coexist with pathogenic bacteria in a dynamic balance. It has been suggested that intestinal microbiota dysbiosis (increased numbers of pathogenic bacteria and decreased numbers of commensal bacteria) might result in the disruption of intestinal barriers (10, 11). Tight junction proteins play a vital role in maintaining the integrity of the intestinal barriers by averting the transport of pathogenic bacteria and toxins. Reduced intestinal tight junction proteins can disrupt intestinal barriers, alter intestinal epithelial permeability, eventually cause diarrhea. Recurrent episodes of diarrhea can disrupt the normal intestinal barriers and substantially impair the development of a healthy intestinal microbiota (12). Thus, intestinal microbiota dysbiosis could simultaneously be the cause and consequence of FD.
There are limited conventional treatments available to mitigate the symptoms of FD, such as fluid replacement, antibiotics, and probiotics (13). Children with chronic conditions also have a poor medication compliance rate. Therefore, non-pharmacological and complementary treatments are urgently needed. The abdominal massage, which is a regular and circular movement around the belly button, is widely used for children with diarrhea in China as a simple, safe, and cost-effective method, practiced both in clinics and at home (14, 15). Furthermore, children readily accept abdominal massage as opposed to other types of treatment, such as medication. Many clinical studies have also confirmed that abdominal massage is beneficial for treating common gastrointestinal disorders, such as diarrhea (16), constipation (17), and gastrointestinal dysfunction (18). Consequently, abdominal massage could be an effective complementary therapy for children suffering from FD.
However, preceding evidences regarding its efficacy has been insufficient, and its underlying mechanism has yet to be elucidated. Moreover, we have found that in clinical settings, both clockwise and counter-clockwise massage directions were generally used when a parent massaged a child's abdomen. The direction of massage depends on individual habits, the left-handed people usually use the counter-clockwise direction and the right-handed people generally use the clockwise direction. Nonetheless, researches have been focused less on the impact of massage direction than on treatment outcomes. By analyzing 16S sequencing, we identified substantial changes in intestinal microbiota in rats with FD. After that, we investigated the effects of different directions of abdominal massage on the modulation of intestinal microbiota and intestinal tight junctions. These results may provide useful insight into the therapeutic benefits of abdominal massage for FD.
Materials and methods Animal modelGuangzhou University of Chinese Medicine's Animal Experimentation Ethics Committee approved the experiments (protocol #20210317004). The Animal Experiment Center [SCXK (YUE) 2019-0047] at Guangzhou University of Chinese Medicine supplied 50 specific-pathogen-free Sprague-Dawley rats (age: 3 weeks, weight: 50.30 ± 12.33 g, sex: ratio male: female ratio of 1:1). All animals were housed in standard cages at the Animal Experiment Center of Guangzhou University of Chinese Medicine [SYXK (YUE) 2018-0085]. Growing conditions were as follows: 22 ± 2°C, 40–60% humidity, 12 h light/12 h dark cycle, with food and water provided. The body weights of the animals were observed daily throughout the study.
After 1 week of adaptive feeding, fifty SD rats were randomly divided into five groups, including the control group (n = 10), model group (n = 10), sham-massage group (n = 10), CW-massage group (n = 10), and CCW-massage group (n = 10). The rats in the control group were fed with a regular standard diet and drank water freely every day. For the rest of the rats, miscible liquids (3 g/ml) of Folium Sennae and Rhubarb were administered intragastrically of 10 ml/kg for 1 week (19, 20). Both Folium Sennae and Rhubarb are Chinese herbal medicines. They were commonly used as laxatives to induce diarrhea models (21). During experiment procedure, six SD rats with successful modeling in each group were finally selected for further investigation (n = 6 in each group).
Abdominal massageNo treatment was administered to the control and model groups after modeling. The CW-massage group was treated with clockwise abdominal massage, while the CCW-massage group received counter-clockwise abdominal massage (Figures 1A,B). The frequency of the abdominal massage is 120–160 times per minute, with a duration of 10 min once a day for 14 days. In the sham-massage group, irregular touch stimulation was applied with the same frequency. Several points should be considered when massaging: (a) The abdominal massage is applied to the rat's abdomen using the whorl surface of the index and middle fingers, rubbing gently in a circular motion. (b) Should the rats avoid being massaged on their abdomens, the duration of the massage will be extended for 1~2 min to make up for the time loss owing to the rats' avoidance behavior. (c) If the rats struggled violently, the massage would pause and resume when they become calm again. (d) The same researcher would performe all the massage throughout the experiment.
FIGURE 1
Figure 1. (A) Abdominal massage site. (B) Schematic of massage direction. Created with BioRender.com.
Sample collection and preparationOn the last day, after 12 h of starvation, the rats were sacrificed by anesthetic overdose intraperitoneally. Four rats (sex: ratio male: female ratio of 1:1) from each group were randomly selected. Samples of colon content were collected and stored in a freezer storage tube, then instantly kept at −80°C until examined for intestine microbiota. We also collected, processed, and froze colon tissues at −80°C for RT-qPCR analysis. Other colon tissues were kept at room temperature in 4% paraformaldehyde solution overnight.
Histopathology analysis of colonThe samples were washed with BBS distilled water and transferred to 70% ethyl alcohol for long-term storage. Afterwards, the colon tissues were embedded in paraffin and sectioned at five μm thickness (Leica, RM2245). Hematoxylin and 0.5% eosin were used for histopathological staining (Leica, ST5010). Under a light microscope (Leica, DM2000), tissue sections were viewed at a magnifying power of *100. Each group underwent four biological verifications.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)The mRNA levels of ZO-1, Occludin-1, and Claudin-1 were measured by RT-qPCR. TRIzol reagent (Life, 15596-018) was used to extract RNA from snap-frozen colon tissues of each group. We performed RT-qPCR using the qTOWER 2.0 Real-Time PCR System (ABI, step one plus). The relative quantification of mRNA expression for ZO-1, Occludin-1, and Claudin-1 was analyzed via the 2ΔΔCt method following normalization with Gapdh. The specific primers were designed as follows: ZO-1, TGTCAGCCCTTCTGATGGTG (forward), and CTTCTTTGGCTGCAGGGCTA (reverse); Occludin-1, CATGGCGGCCTTTTGCTTCA (forward) and CCCAGGATTGCGCTGACTAT (reverse); Claudin-1, TCGGCTCTATCGTCAGCAC (forward) and GGACATCCACAGTCCCTCTG (reverse).
Intestinal microbiota analysisThe 16S rDNA amplicon sequencing was executed by Novogene Corporation (Beijing, China). The total genome DNA was derived from colon content samples by CTAB. 1% agarose gels were used to measure DNA concentration and purity. The 16S rDNA V3-V4 regions were performed using the primers 341F (5′-CCTAYGGGRBGCASCAG-3′) and 806R (5′-GGACTACNNGGGTATCTAAT-3′). Sequencing libraries were prepared using NEBNext® Ultra™ IIDNA Library Prep Kit (Cat No. E7645). The Agilent Bioanalyzer 2100 system and Qubit@ 2.0 Fluorometer (Thermo Scientific) were used to assess the quality. Lastly, 250 bp paired-end reads were elicited by Illumina on a NovaSeq platform. FLASH (Version 1.2.11) was used for gaining Raw Tags (22) and Fastp (Version 0.20.0) was used for obtaining Clean Tags. By measuring the Clean Tags against the Silva database, the chimera sequences were identified using Vsearch (Version 2.15.0) and removed to winnow out the Effective Tags (23). Then, using the deblur module in the QIIME2 software (Version QIIME2-202006), initial ASVs (default: DADA2) were obtained, and then ASVs with an abundance of <5 were filtered out (24). As a result, the absolute abundance of ASVs was normalized by using the least abundant sample as the standard.
StatisticsSPSS version 22.0 was used for statistical analysis. The values were presented as the mean ± SD. Multiple-group comparisons were assessed through one-way ANOVA and Tukey tests. P < 0.05 was considered statistically significant. Unless otherwise stated, the data were processed with GraphPad Prism 9.3.1. The 16S rDNA data were run on the Omicshare Platform (www.omicshare.com).
Results Effects of abdominal massage on alleviating diarrheaThe effects of abdominal massage on functional diarrhea were investigated by massaging immature rats for 2 weeks. Except for the control group, watery stools or diarrhea developed in all of the groups after the first day of Folium Sennae and Rhubarb ingestion (Figure 2A). After 2 weeks of abdominal massage, the stools of both CW and CCW groups of massage were normalized (Figure 2B).
FIGURE 2
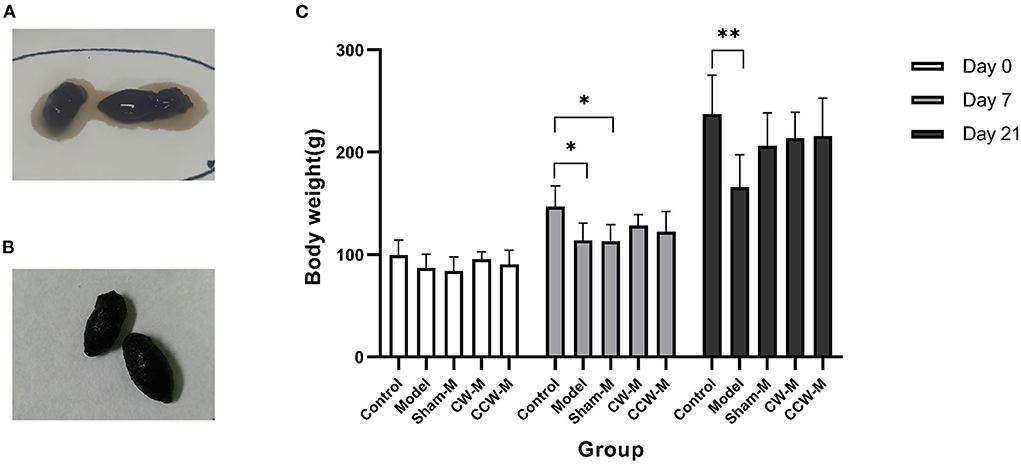
Figure 2. (A) State of stool before abdominal massage. (B) State of stool after abdominal massage. (C) Body weight. Error bars are expressed as means ± SD (n = 6). Statistical significance was determined by one-way ANOVA with Tukey tests for multiple-group comparisons. *P < 0.05; **P < 0.01.
We measured body weight each day. At the beginning of the experiment, all groups of rats had similar weights (P > 0.05). After 7 days of feeding with Folium Sennae and Rhubarb, we observed that the rats in the model had significantly less weight than the rats in the control group (P > 0.05). Compared to the control group, the growth rates of weight in other groups were slower. By the end of the massage (Day 21), no significant differences in weight were found between the sham massage, CW massage, and CCW massage groups. The mean weights in comparison were as follow: control group > CW-massage group > CCW-massage group > sham-massage group > model group (Figure 2C).
Effects of abdominal massage on reversing intestinal barrier disruptionAs demonstrated by histologic analysis and RT-qPCR assay, abdominal massage could restore the damaged intestinal barriers in FD rats. The HE staining of the colon revealed that the epithelial cells in the model group were exfoliated, necrosed, and not arranged neatly as compared to the control group. Also, the glands have partial atrophy as well as mucosal edema. The situation of the sham-massage group was similar to that of the model group. Conversely, two massage groups reversed the above situation partially. The villus structure was relatively intact, and mild submucosa edema was still observed (Figure 3A). Concomitantly, ZO-1, Occludin-1, and Claudin-1 mRNA expression levels were significantly increased in both massage groups (Figures 3B–D). These results indicate that abdominal massage has a significant effect on maintaining intestinal epithelium integrity.
FIGURE 3
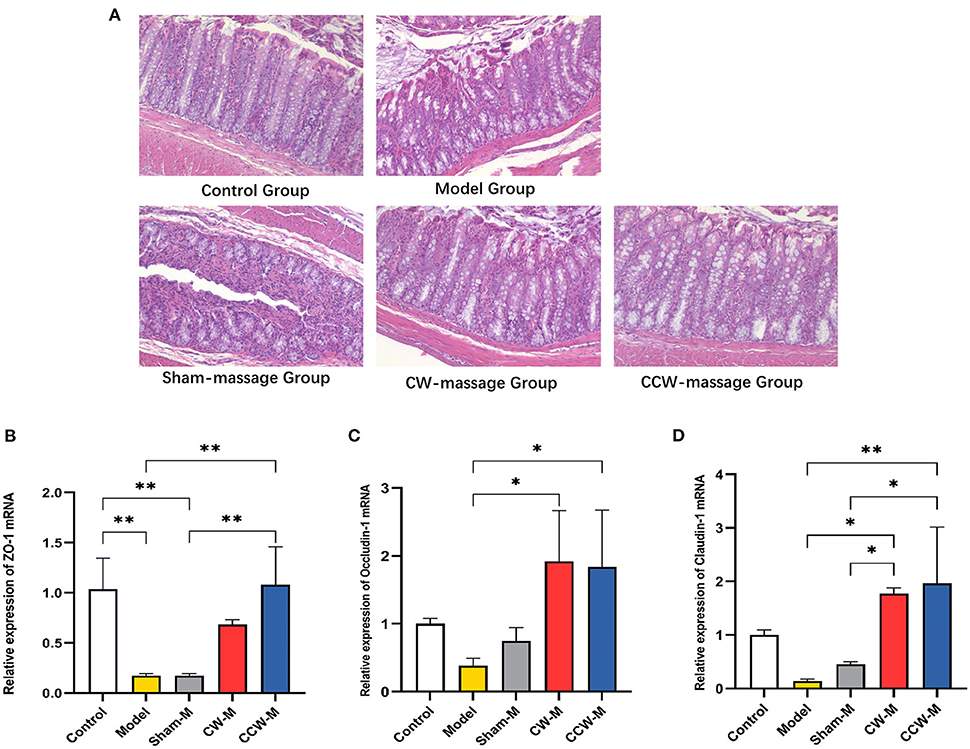
Figure 3. (A) H&E staining of colon tissue sample sections of rats. (B–D) Relative expression of ZO-1, Occludin-1 and Claudin-1 mRNA in colon was assessed using RT-qPCR. Error bars are expressed as means ± SD (n = 3). Statistical significance was determined by one-way ANOVA with Tukey tests for multiple-group comparisons, *P < 0.05; **P < 0.01.
Effects of abdominal massage on eliminating intestinal microbiota dysbiosis caused by functional diarrhea Species annotation and evaluationA total of 110,677 raw reads were generated from each sample in this study. After discarding low-quality sequences, on average 77,611 clean tags were obtained. The average length of the tags was 419. These tags were then subjected to the following analysis and clustered into operational taxonomic units (ASVs).
Alpha analysisAlpha diversity indexes were calculated to assess the richness, diversity, and coverage of the sample microbial community in response to abdominal massage. The Species Accumulation Boxplot has become steady, which indicated that enough sequencing data has been collected and was acceptable (Supplementary Figure 1A). All samples reflected 100% community coverage in their coverage indexes. Clearly, these results were reasonable and reflected the actual microbiological situation. However, richness and diversity did not differ significantly between the groups (P > 0.05, Supplementary Figures 1B–D).
Beta analysisAdditionally, the PCoA and NMDS of Bray-Curtis dissimilarity were used to assess beta diversity within each group. PCoA and NMDS revealed that microbiota structure in the model group was remarkably different from that in the control group. In contrast, the CW-massage and CCW-massage groups were restored to some extent (Figures 4A,B). Based on the analyses of the ANOSIM data, the differences between the five groups in terms of community composition were more apparent than those within each group (ANOSIM R = 0.5421, P = 0.001). These results further indicated that abdominal massage significantly altered the structural diversity of the intestinal microbiota of FD rats.
FIGURE 4
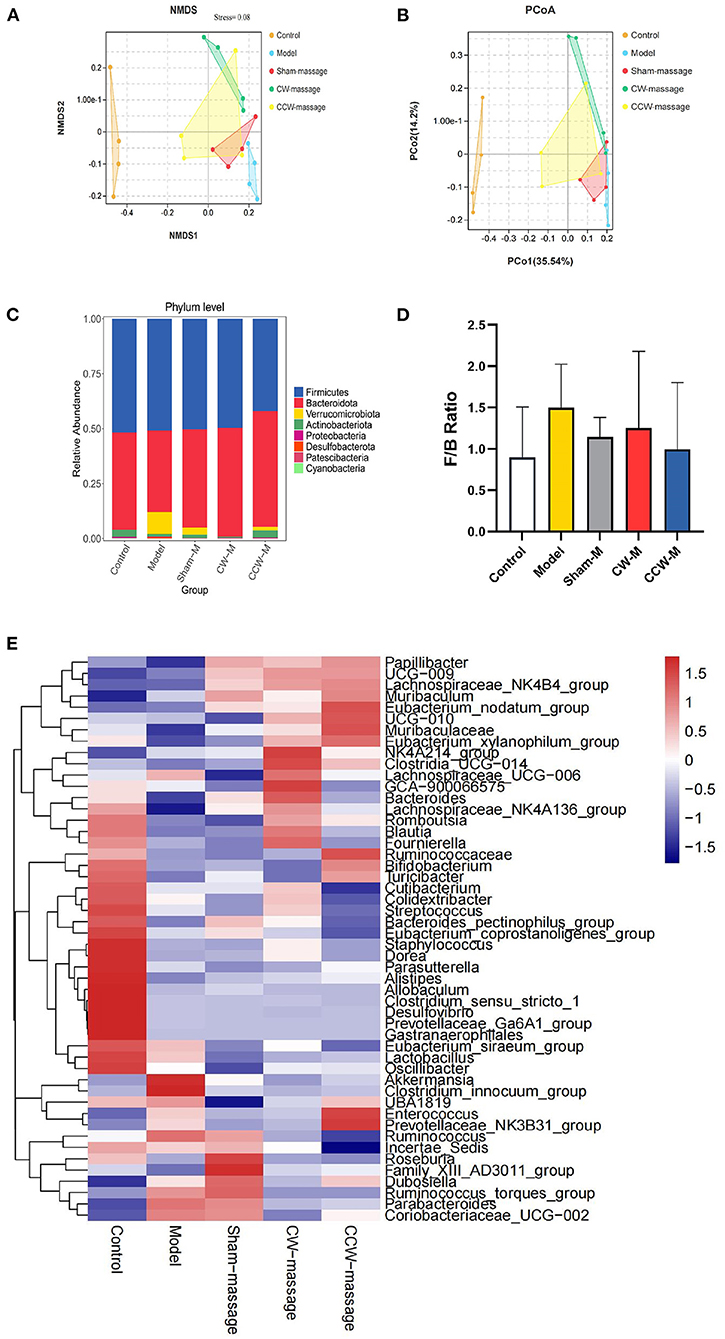
Figure 4. (A) NMDS plot of Bray-Curtis dissimilarity, Stress = 0.08. (B) PCoA plot of Bray-Curtis dissimilarity. (C) Relative abundance of ASVs at phylum level. (D) F/B Ratio in each group. Error bars are expressed as means ± SD (n = 4). Statistical significance was determined by one-way ANOVA with Tukey tests for multiple-group comparisons. (E) Heatmap of relative abundances at the genus level [top 50].
Altered composition of the intestinal microbiotaTo investigate the impact of abdominal massage on the intestinal microbiota in FD rats, the phylum and genus abundance were examined. At the phylum level, Firmicutes, Bacteroidota, Verrucomicrobiota, Actinobacteriota, Proteobacteria, and Desulfobacterota were the dominant microbiota constituents (Figure 4C). In contrast to the control group, the model group exhibited an increment of Firmicutes/Bacteroidetes (F/B) ratio (P > 0.05, Figure 4D). The prevailing microbiota at the genus level were Muribaculaceae, Dubosiella, Lactobacillus, Lachnospiraceae_NK4A136_group, Romboutsia, Bacteroides, Akkermansia, Ruminococcus, and Bifidobacterium (Figure 4E).
Specifically, LEfSe (LDA Effect Size) was used to analyze the differential enrichment of bacterial taxa between the control and model groups or between the sham-massage, CW-massage, and CCW-massage groups. Using a logarithmic cutoff score of 2.0, the following species showed statistically significant differences. In the control group, several genera were overrepresented, such as Bifidobacterium, Faecalibaculum, Turicibacter, and Dorea. While Verrucomicrobiota, Akkermansia, Coriobacteriaceae-UGG-002, Desulfobacterota, Clostridium-innocuum-group, and Ruminococcus_torques_group were gathered in the model group (Figure 5). Moreover, Bifidobacteriaceae were enriched in the CW-massage group, while Cutibacterium, Propionibacteriaceae, and Dorea were raised in the CCW-massage group.
FIGURE 5
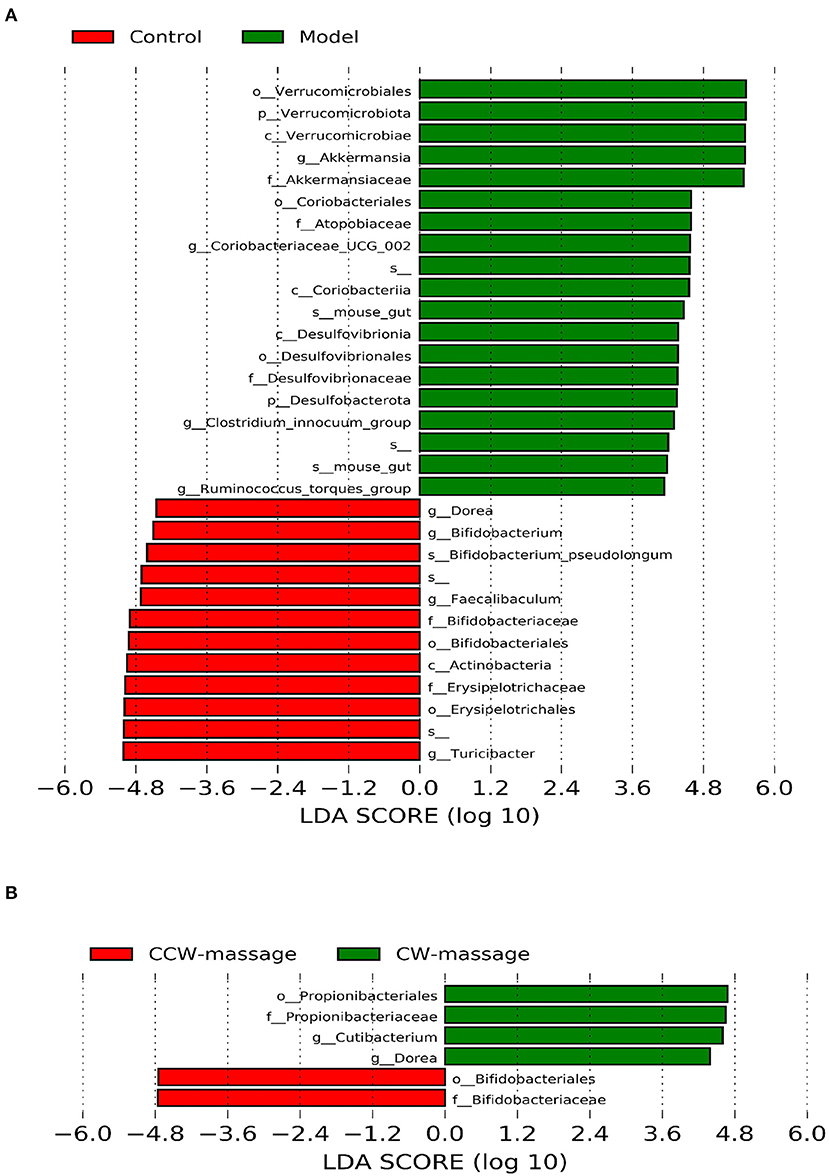
Figure 5. LDA effect size identifies differential abundance of bacteria between the control group and model group (A), or between the sham-massage, CW-massage, and CCW-massage group (B), LDA > 2.0, P < 0.05.
Spearman correlations were performed to further determine the relationship between tight junction proteins in the colon and intestinal microbiota, which have meaningful correlations at the phylum and genus levels. A positive interaction was found between Dorea and ZO-1, while the two bacterial strains (Ruminococcus_torques_group and Clostridium_innocuum_group) were to be negatively interacted with ZO-1, Occludin-1, and Claudin-1 (Figure 6). We concluded that these bacteria were likely to play a significant role in the development of FD and its prevention.
FIGURE 6
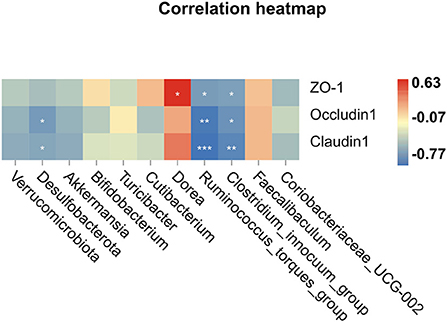
Figure 6. Spearman correlation analysis, *P < 0.05, **P < 0.01, ***P < 0.001.
COG annotation and analysisWe aimed to predict the functional properties of the intestinal microbiota using the PICRUSt software and COG family information to further elucidate the possible role of the intestinal microbiota after FD and abdominal massage (Supplementary File 2). The top 35 functions for each group were chosen to create a heatmap. Also, the heatmaps were clustered according to functional levels. As shown in Figure 6, in the model group, five functional COG categories were decreased, including Transcription[K]; Cell wall/membrane/envelope biogenesis[M]; Signal transduction mechanisms[T]; Replication, recombination, and repair[L] and Defense mechanisms[V]. Then, significant differences in functional COG categories were identified using the LEfSe (Figure 7). We found six significantly different functional COGs between the model and CCW-massage group. The Model group exhibited a high enrichment of three functional COG categories, including Amino acid transport and metabolism[E], Carbohydrate transport and metabolism[G], and Coenzyme transport and metabolism, for General function prediction only[HR]. In contrast, the CCW group was enriched in Transcription[K], Replication, recombination and repair[L], and Defense mechanisms[V].
FIGURE 7
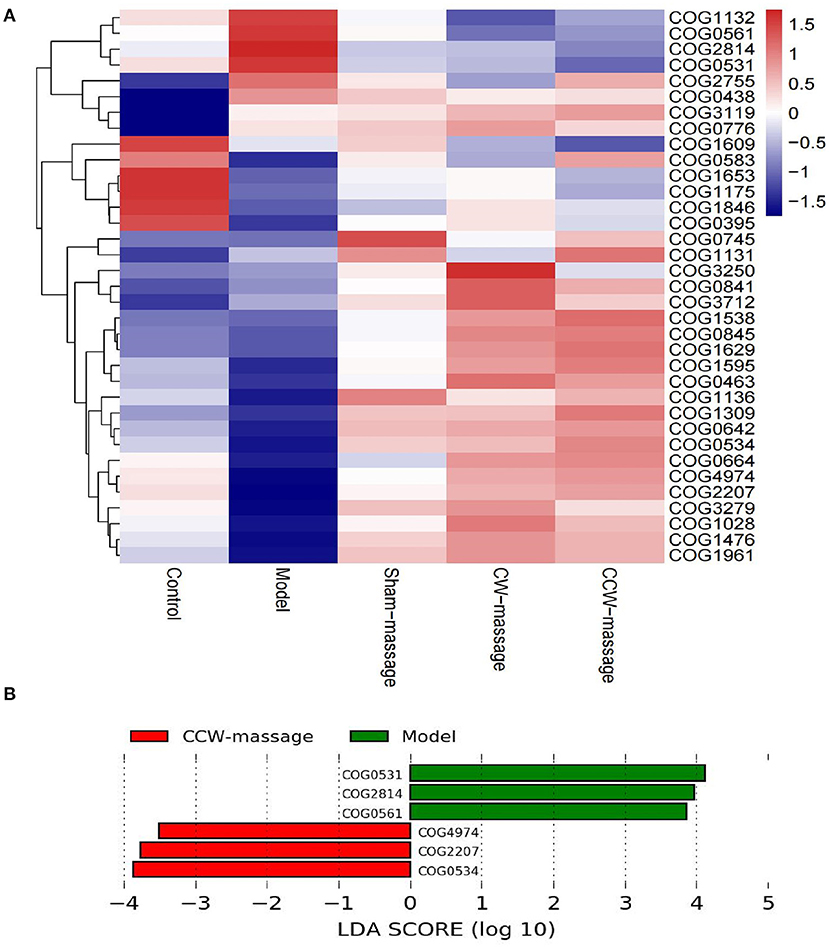
Figure 7. COG Annotation and Analysis. (A) Heatmap of the relative abundances of COG categories between five groups [top 35 functions]. (B) LDA integrated with LEfSe comparison of relative abundances of COG categories between five groups (LDA > 2.0, P < 0.05).
DiscussionIt is well-known that the balance of intestinal microbes plays a crucial role in maintaining the structural integrity of the gastrointestinal mucosal barrier, immunomodulation, and protection against pathogens (25). According to numerous studies, diarrhea may contribute to microbial dysbiosis within the intestines (26). Dysbiosis is comprised of the fall of key taxa, the reduction in taxonomic diversity, metabolic changes, and the increase in pathogenic germs (27). As suggested by previous studies, FD is related to the change of intestinal microbiota (9). Through Beta analysis, we found that community structures were significantly different between the model and control groups. Histological analysis and RT-qPCR assays have demonstrated that FD disrupts the integrity of the intestinal barrier. These observations suggest that FD is probably brought about by intestinal microbiota dysbiosis and disruption of the intestinal barrier. Moreover, In contrast to Irritable Bowel Syndrome or Antibiotic-Associated Diarrhea (28, 29), the FD model rats did not exhibit an overgrowth of colitis-associated bacteria, such as Enterobacter, Salmonella, and Escherichia coli. This finding suggests that FD is not associated with bacteria that cause intestinal infections. It should be noted that according to recent studies, Akkermansia may have a series of protective effects, including the stimulation of mucosal microbial networks and improvements in intestinal barrier function (30). In this study, Akkermansia was enriched in the model group. Rhubarb could be the reason for this phenomena, since Rhubarb has been found to contain ingredients that could increase the content of Akkermansia (31, 32). Therefore, it is not ruled out that Rhubarb could have been interfering with the experimental results.
Abdominal massage has gained more attention in clinics as a complementary therapy. In previous studies, continuous abdominal massage was shown to be able to improve intestinal transit and alleviate visceral hypersensitivity in diarrhea rats (33). In our study, we also discovered that the form of feces and the condition of colonic tissue significantly recovered after abdominal massage.
At the microbiota level, we found that intestinal microbiota was notably changed after abdominal massage. In the Beta analysis (Figures 4A,B), the two massage groups were able to close the distance with the control group in the community structure. These results corroborate that massage can modulate intestinal microbiota to an extent. LEfSe analysis showed that Dorea, Bifidobacterium were enriched to a greater extent, and Clostridium_innocuum_group, Coriobacteriia, Ruminococcus_torques_group were decreased after abdominal massage. To further explore, we investigated the relationship between tight junction protein indexes of the colon and the intestinal microbiota. Epithelial cells were connected through tight junctions to form the mucosal barrier in the intestine. And the integrity of the barrier was heavily dependent on the preservation of tight junction proteins (including ZO-1, Occludin-1, and Claudin-1) (34). In addition, the intestinal microbiota can directly affect epithelial permeability via tight junction protein under normal and pathological conditions (35). It was demonstrated by RT-qPCR that both massage directions could normalize the mRNA expression of ZO-1, Occludin-1, and Claudin-1. Furthermore, Spearman correlation analysis indicated that Ruminococcus_torques_group and Clostridium_innocuum_group were in a strikingly negative relation to tight junction proteins. Observations in this study agreed with those in other studies, which have found a negative correlation between Ruminococcus_torques_group abundance and tight junction protein abundance (36, 37). In part, this could be explained by Ruminococcus_torques_group and Clostridium_innocuum_group participating in the disruption of the intestinal barrier. The toxic metabolite of Clostridium has been previously reported to strengthen oxidative damage in intestinal epithelial cells and compromised the epithelial layer lining the gut (38). Also, the proliferation of Ruminococcus_torques_group can debase the mucin from the mucous layer of epithelial cells on the surface of the intestine, which may be one of the causes of the breakdown of the intestinal barrier (39). These results demonstrate that abdominal massage can partly mitigate FD by reshaping the composition of the intestinal microbiota, decreasing the number of bacteria that damage intestinal mucosal barriers, and restoring the integrity of the intestinal barrier. We speculate that the following mechanism may contribute to its potential effect in altering the intestinal microbiota structure. To begin with, the gastro-intestinal (GI) tract contains numerous mechanosensitive circuits, which are essential to normal gut functioning (40). Secondly, abdominal massage is soft and gentle contact with the skin. The gentle action of this treatment can directly affect the abdomen muscles, stimulate the GI mechanosensitive circuits, adjust the function of the gastrointestinal autonomic nerves, and promote regular peristalsis of the gastrointestinal smooth muscles (41). Furthermore, this beneficial change can alter the composition of vital micronutrients in the gut and ultimately change the microbial composition and structure (42). The mechanism described above may partly explain the positive effects of abdominal massage. Further research is needed to examine the mechanism of action of abdominal massage on intestinal microbiota in the future.
The final step was to use PICRUSt2 to predict functional analysis of the COG database in each group. According to the literature, disturbances in intestinal microbiota function are associated primarily with perturbations in metabolic pathways (43). Our study confirmed this finding. Also, according to COG LEfSe analysis, we can infer that CCW-massage could balance three functions of the rats' intestinal microbiota, including Transcription[K]; Replication, recombination, and repair[L], and Defense mechanisms [V].
This study has certain limitations. It only revealed that abdominal massage improved FD by modulating intestinal microbiota and increasing intestinal tight junction expression. However, it's not clear how abdominal massage affected the microbiota. Therefore, more researches are needed to identify the underlying mechanisms between intestinal microbiota and abdominal massage.
In conclusion, our research demonstrates that FD is not associated with pathogen infection. A dysbiosis of the intestinal microbiota and disruption of the intestinal barrier are most likely to cause FD. Moreover, this study supports that abdominal massage can alleviate FD by altering the intestinal microbiota structure and reducing the number of bacteria detrimental to the intestinal mucosal barrier. Furthermore, the intestinal microbiota and tight junction proteins did not significantly change following treatment with two massage directions. This study confirmed that abdominal massage is an effective complementary therapy for children with FD and provided references for the clinical therapy of FD. Future studies should examine the mechanism of motion of abdominal massage on FD.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statementThe animal study was reviewed and approved by the Animal Experimentation Ethics Committee of Guangzhou University of Chinese medicine.
Author contributionsYH and YS contributed to the concept and design of the study. YH, QM, and XL performed the main experiments. JXH and QXM performed a portion of the statistical analysis. YH wrote the first draft of the manuscript. HL, JYH, and YS have revised this manuscript. All authors have approved the final version before submission.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.922799/full#supplementary-material
References3. Zeevenhooven J, Koppen IJN, Benninga MA. The New Rome IV criteria for functional gastrointestinal disorders in infants and toddlers. Pediatr Gastroenterol Hepatol Nutr. (2017) 20:1–13. doi: 10.5223/pghn.2017.20.1.1
PubMed Abstract | CrossRef Full Text | Google Scholar
4. Zhao Y-F, Guo X-J, Zhang Z-S, Ma X-Q, Wang R, Yan X-Y, et al. Epidemiology of functional diarrhea and comparison with diarrhea-predominant irritable bowel syndrome: a population-based survey in China. PLoS ONE. (2012) 7:e43749. doi: 10.1371/journal.pone.0043749
PubMed Abstract | CrossRef Full Text | Google Scholar
5. Sommers T, Mitsuhashi S, Singh P, Hirsch W, Katon J, Ballou S, et al. Prevalence of chronic constipation and chronic diarrhea in diabetic individuals in the United States. Am J Gastroenterol. (2019) 114:135–42. doi: 10.1038/s41395-018-0418-8
PubMed Abstract | CrossRef Full Text | Google Scholar
6. Huang Y, Tan SY, Parikh P, Buthmanaban V, Rajindrajith S, Benninga MA. Prevalence of functional gastrointestinal disorders in infants and young children in China. BMC Pediatr. (2021) 21:131. doi: 10.1186/s12887-021-02610-6
PubMed Abstract | CrossRef Full Text | Google Scholar
7. Moore SR, Lima NL, Soares AM, Oriá RB, Pinkerton RC, Barrett LJ, et al. Prolonged episodes of acute diarrhea reduce growth and increase risk of persistent diarrhea in children. Gastroenterology. (2010) 139:1156–64. doi: 10.1053/j.gastro.2010.05.076
PubMed Abstract | CrossRef Full Text | Google Scholar
11. Scaldaferri F, Pizzoferrato M, Pecere S, Forte F, Gasbarrini A. Bacterial flora as a cause or treatment of chronic diarrhea. Gastroenterol Clin North Am. (2012) 41:581–602. doi: 10.1016/j.gtc.2012.06.002
PubMed Abstract | CrossRef Full Text | Google Scholar
12. Rouhani S, Griffin NW, Yori PP, Gehrig JL, Olortegui MP, Salas MS, et al. Diarrhea as a potential cause and consequence of reduced gut microbial diversity among undernourished children in Peru. Clin Infect Dis. (2020) 71:989–99. doi: 10.1093/cid/ciz905
PubMed Abstract | CrossRef Full Text | Google Scholar
14. Gao L, Jia C, Huang H. Paediatric massage for treatment of acute diarrhoea in children: a meta-analysis. BMC Complement Altern Med. (2018) 18:257. doi: 10.1186/s12906-018-2324-4
PubMed Abstract | CrossRef Full Text | Google Scholar
15. Karkhaneh M, Zorzela L, Jou H, Funabashi M, Dryden T, Vohra S. Adverse events associated with paediatric massage therapy: a systematic review. BMJ Paediatr Open. (2020) 4:e000584. doi: 10.1136/bmjpo-2019-000584
PubMed Abstract | CrossRef Full Text | Google Scholar
16. Lu T, Yin L, Chen R, Zhang H, Cai J, Li M, et al. Chinese Pediatric Tuina on children with acute diarrhea: a randomized sham-controlled trial. Health Qual Life Outcomes. (2021) 19:4. doi: 10.1186/s12955-020-01636-1
PubMed Abstract | CrossRef Full Text | Google Scholar
17. Birimoglu Okuyan C, Bilgili N. Effect of abdominal massage on constipation and quality of life in older adults: a randomized controlled trial. Complement Ther Med. (2019) 47:102219. doi: 10.1016/j.ctim.2019.102219
PubMed Abstract | CrossRef Full Text | Google Scholar
18. Dehghan M, Malakoutikhah A, Ghaedi Heidari F, Zakeri MA. The effect of abdominal massage on gastrointestinal functions: a systematic review. Complement Ther Med. (2020) 54:102553. doi: 10.1016/j.ctim.2020.102553
PubMed Abstract | CrossRef Full Text | Google Scholar
19. Wang J. Effects of Xiaojianzhong Decoction on Intestinal Microflora and Intestinal Mucosal Immune Function in Young Mouse with Spleen Deficiency Diarrhea [Master's thesis]. Southwest Medical University (2021).
20. Zhiqiang Yan SZ, Yifan Zhang, Chunlin Chen. The composition and diversity of intestinal flora in rats with spleen deficiency diarrhea. J Hunan Agricul University. (2021) 47:580–6.
PubMed Abstract | Google Scholar
21. Lu J, Mao D, Li X, Ma Y, Luan Y, Cao Y, et al. Changes of intestinal microflora diversity in diarrhea model of KM mice and effects of Psidium guajava L. As the treatment agent for diarrhea. J Infect Public Health. (2020) 13:16–26. doi: 10.1016/j.jiph.2019.04.015
PubMed Abstract | CrossRef Full Text | Google Scholar
23. Haas BJ, Gevers D, Earl AM, Feldgarden M, Ward DV, Giannoukos G, et al. Chimeric 16s rRNA sequence formation and detection in sanger and 454-Pyrosequenced PCR Amplicons. Genome Res. (2011) 21:494–504. doi: 10.1101/gr.112730.110
PubMed Abstract | CrossRef Full Text | Google Scholar
24. Li M, Shao D, Zhou J, Gu J, Qin J, Chen W, et al. Signatures within esophageal microbiota with progression of esophageal squamous cell carcinoma. Chin J Cancer Res. (2020) 32:755–67. doi: 10.21147/j.issn.1000-9604.2020.06.09
PubMed Abstract | CrossRef Full Text | Google Scholar
25. Carco C, Young W, Gearry RB, Talley NJ, McNabb WC, Roy NC. Increasing evidence that irritable bowel syndrome and functional gastrointestinal disorders have a microbial pathogenesis. Front Cell Infect Microbiol. (2020) 10:468. doi: 10.3389/fcimb.2020.00468
PubMed Abstract | CrossRef Full Text | Google Scholar
26. Rhoades NS, Hendrickson SM, Prongay K, Haertel A, Gill L, Edwards RA, et al. Growth faltering regardless of chronic diarrhea is associated with mucosal immune dysfunction and microbial dysbiosis in the gut lumen. Mucosal Immunol. (2021) 14:1113–26. doi: 10.1038/s41385-021-00418-2
PubMed Abstract | CrossRef Full Text | Google Scholar
28. Wang L, Alammar N, Singh R, Nanavati J, Song Y, Chaudhary R, et al. Gut microbial dysbiosis in the irritable bowel syndrome: a systematic review and meta-analysis of case-control studies. J Acad Nutr Diet. (2020) 120:565–86. doi: 10.1016/j.jand.2019.05.015
PubMed Abstract | CrossRef Full Text | Google Scholar
29. Zhang W, Zhu B, Xu J, Liu Y, Qiu E, Li Z, et al. Bacteroides Fragilis protects against antibiotic-associated diarrhea in rats by modulating intestinal defenses. Front Immunol. (2018) 9:1040. doi: 10.3389/fimmu.2018.01040
PubMed Abstract | CrossRef Full Text | Google Scholar
30. Macchione IG, Lopetuso LR, Ianiro G, Napoli M, Gibiino G, Rizzatti G, et al. Akkermansia Muciniphila: key player in metabolic and gastrointestinal disorders. Eur Rev Med Pharmacol Sci. (2019) 23:8075–83. doi: 10.26355/eurrev_201909_19024
PubMed Abstract | CrossRef Full Text | Google Scholar
31. Régnier M, Rastelli M, Morissette A, Suriano F, Le Roy T, Pilon G, et al. Rhubarb supplementation prevents diet-induced obesity and diabetes in association with increased Akkermansia Muciniphila in mice. Nutrients. (2020) 12:E2932. doi: 10.3390/nu12102932
PubMed Abstract | CrossRef Full Text | Google Scholar
32. Neyrinck AM, Etxeberria U, Taminiau B, Daube G, Van Hul M, Everard A, et al. Rhubarb extract prevents hepatic inflammation induced by acute alcohol Q17 intake, an effect related to the modulation of the gut microbiota. Mol Nutr Food Res. (2017) 61:1–12. doi: 10.1002/mnfr.201500899
PubMed Abstract | CrossRef Full Text | Google Scholar
33. Li B, Luo X-F, Liu S-W, Zhao N, Li H-N, Zhang W, et al. Abdominal massage reduces visceral hypersensitivity via regulating GDNF and PI3K/AKT signal pathway in a rat model of irritable bowel syndrome. Evid Based Complement Altern Med. (2020) 2020:3912931. doi: 10.1155/2020/3912931
PubMed Abstract | CrossRef Full Text | Google Scholar
34. D L, Xy J, Ls Z. Enriched environment on the intestinal mucosal barrier and brain-gut axis in rats with colorectal cancer. Exp Biol Med. (2018) 243:1185–98. doi: 10.1177/1535370218815437
PubMed Abstract | CrossRef Full Text | Google Scholar
36. Hu R, He Z, Liu M, Tan J, Zhang H, Hou D-X, et al. Dietary protocatechuic acid ameliorates inflammation and up-regulates intestinal tight junction proteins by modulating gut microbiota in LPS-challenged piglets. J Anim Sci Biotechnol. (2020) 11:92. doi: 10.1186/s40104-020-00492-9
PubMed Abstract | CrossRef Full Text | Google Scholar
37. Zhou Y, Zhang M, Zhao X, Feng J. Ammonia exposure induced intestinal inflammation injury mediated by intestinal microbiota in broiler chickens via TLR4/TNF-α signaling pathway. Ecotoxicol Environ Saf. (2021) 226:112832. doi: 10.1016/j.ecoenv.2021.112832
PubMed Abstract | CrossRef Full Text | Google Scholar
38. Adesso S, Ruocco M, Rapa SF, Dal Piaz F, Di Iorio BR, Popolo A, et al. Effect of Indoxyl Sulfate on the repair and intactness of intestinal epithelial cells: role of reactive oxygen species' release. Int J Mol Sci. (2019) 20:2280. doi: 10.3390/ijms20092280
PubMed Abstract | CrossRef Full Text | Google Scholar
39. Zhen Z, Xia L, You H, Jingwei Z, Shasha Y, Xinyi W, et al. An integrated gut microbiota and network pharmacology study on Fuzi-Lizhong Pill for treating diarrhea-predominant irritable bowel syndrome. Front Pharmacol. (2021) 12:746923. doi: 10.3389/fphar.2021.746923
PubMed Abstract | CrossRef Full Text | Google Scholar
41. Chong Guan C, Cheng Z, Xie F, Wang R, Zhang J, Yao F, et al. Efficacy of abdomen-rubbing qigong exercise for chronic insomnia: study protocol for a randomized controlled trial. Trials. (2021) 22:774. doi: 10.1186/s13063-021-05528-7
PubMed Abstract | CrossRef Full Text | Google Scholar
42. Vicentini FA, Keenan CM, Wallace LE, Woods C, Cavin J-B, Flockton AR, et al. Intestinal microbiota shapes gut physiology and regulates enteric neurons and glia. Microbiome. (2021) 9:210. doi: 10.1186/s40168-021-01165-z
PubMed Abstract | CrossRef Full Text | Google Scholar
43. Peng W, Huang J, Yang J, Zhang Z, Yu R, Fayyaz S, et al. Integrated 16s rRNA sequencing, metagenomics, and metabolomics to characterize gut microbial composition, function, and fecal metabolic phenotype in non-obese type 2 diabetic Goto-Kakizaki rats. Front Microbiol. (2019) 10:3141. doi: 10.3389/fmicb.2019.03141
留言 (0)