Congenital heart disease (CHD) is one of the most prevalent congenital anomalies in childhood and a leading cause of infant mortality, with an incidence rate in China estimated at approximately 0.8% to 1.2% (1, 2). Despite substantial advancements in surgical techniques and interventional procedures, the prognosis for children with CHD has markedly improved (3). However, those with heart dysfunction or heart failure (HF), especially during the perioperative period, continue to face significant surgical risks and unfavorable outcomes (4). Accurate assessment of cardiac function during the perioperative period is crucial for optimizing treatment plans and enhancing both survival rates and quality of life for these patients. Current clinical approaches for evaluating cardiac function primarily include functional grading, echocardiography, and the measurement of B-type natriuretic peptide (BNP). Recent research has also highlighted the potential of cardiac troponin I (cTnI) and α-hydroxybutyrate dehydrogenase (α-HBDH) as valuable indicators of cardiac function (5). Nonetheless, studies exploring the application of these biomarkers in the perioperative evaluation of cardiac function in children with CHD, particularly in relation to heart failure diagnosis, remain limited. This study aims to investigate the clinical utility of α-HBDH, cTnI, and BNP in assessing cardiac function and diagnosing heart failure in children with CHD during the perioperative period, thereby providing a new reference for the management of these patients.
2 Materials and methods 2.1 Study subjectsThis study included 107 children diagnosed with CHD who underwent surgical treatment at the Sichuan Maternal and Child Health Hospital from March 2022 to March 2023. The age of the participants ranged from 1 month to 18 years. The diagnosis of CHD was based on echocardiographic examinations and clinical manifestations within three months prior to admission, in accordance with current clinical diagnostic criteria. Inclusion criteria were: (1) age between 0 and 18 years, with a confirmed diagnosis of CHD; (2) meeting the indications for surgical intervention aimed at correcting CHD; (3) classified as ESC functional class I to III; (4) complete clinical data. Exclusion criteria included: (1) severe liver or kidney dysfunction; (2) presence of Down syndrome or metabolic disorders in children with CHD; (3) history of significant surgery or myocarditis within three months prior to admission; (4) prior history of cardiac surgery. This study was approved by the hospital's medical ethics committee, and informed consent was obtained from the guardians of all participating children.
2.2 GroupingBased on the European Society of Cardiology (ESC) functional classification and heart failure grading standards (6), children were categorized into three cardiac function groups: Grade I, Grade II, and Grade III. Additionally, they were further divided into heart failure (HF) group and non-heart failure group based on the presence of heart failure prior to surgery.
2.3 MethodsEligible subjects were identified through the hospital's electronic medical record system, and data on BNP, cTnI, and cardiac enzyme levels were recorded for the three days prior to surgery and on the first postoperative day. Peripheral venous blood samples of 3 ml were collected preoperatively and postoperatively for the assessment of BNP, cTnI, and cardiac enzyme levels using the COBAS C702 fully automated biochemical analyzer. Left ventricular ejection fraction (LVEF) values were obtained via Doppler echocardiography. Comparisons of BNP, cTnI, and cardiac enzyme levels between the three cardiac function groups and between the HF and non-HF groups were conducted, along with correlation analyses.
2.4 Statistical analysisAll data were analyzed using SPSS 26.0 software. Continuous variables were expressed as mean ± standard deviation. Comparisons between two groups were performed using t-tests or Paired t-test, while comparisons among multiple groups were conducted using one-way analysis of variance (ANOVA). Counting data were analyzed using the Chi-squared tests. Receiver operating characteristic (ROC) curves were employed to assess the diagnostic efficacy of the biomarkers for heart failure. A P-value of less than 0.05 was considered statistically significant.
3 Results 3.1 General informationThe demographic and clinical characteristics of the study population are summarized in Table 1 and Figure 1. A total of 107 children with CHD were included in the study, categorized into three groups based on cardiac function grades: Grade I (n = 51), Grade II (n = 32), and Grade III (n = 24). There were no significant differences in gender distribution among the three groups (P = 0.920), with males accounting for 41.1% (44/107) and females for 58.9% (63/107). The mean age of the participants was 4.33 ± 4.72 years in Grade I, 4.70 ± 6.04 years in Grade II, and 2.48 ± 0.90 years in Grade III, with no significant differences among the groups (P = 0.228). Similarly, body weight did not differ significantly across the groups (P = 0.228).
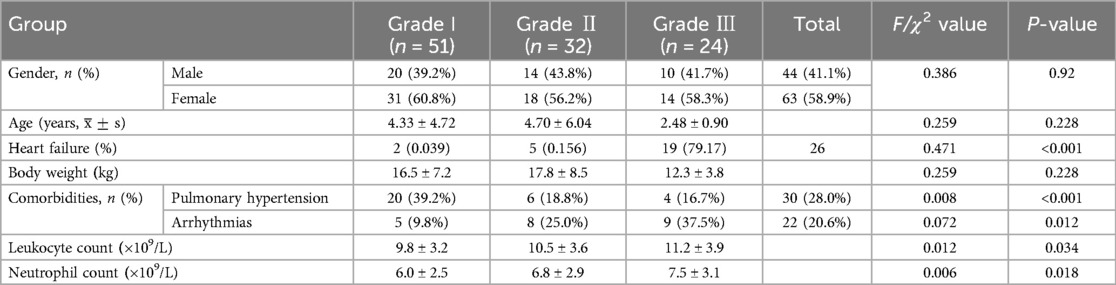
Table 1. Comparison of general information on cardiac function among three groups.
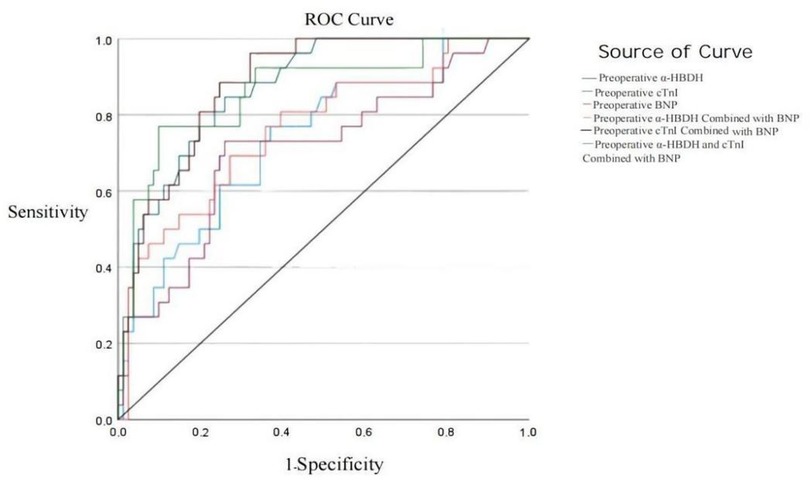
Figure 1. Preoperative ROC curves of α-HBDH, cTnI, and BNP for diagnosing heart failure.
The incidence of HF increased significantly with worsening cardiac function, from 3.9% in Grade I to 15.6% in Grade II and 79.2% in Grade III (P < 0.001). Comorbidities were also more prevalent in patients with higher cardiac function grades. Pulmonary hypertension was observed in 39.2% of Grade I patients, 18.8% of Grade II patients, and 16.7% of Grade III patients (P < 0.001). Arrhythmias were present in 9.8% of Grade I patients, 25.0% of Grade II patients, and 37.5% of Grade III patients (P = 0.012). Laboratory findings revealed that leukocyte and neutrophil counts were significantly higher in Grade III patients compared to Grade I and Grade II patients (P = 0.034 and P = 0.018, respectively).
3.2 Comparison of indicators in children with CHD during the perioperative period across different cardiac function classifications 3.2.1 Preoperative comparison of cardiac function indicators among the three groupsThere were no statistically significant differences in preoperative CKMB and LVEF among the three groups (F = 2.847, 1.919; both P > 0.05). However, significant differences were observed in preoperative CK, LDH, α-HBDH, BNP, and cTnI, which increased progressively with the severity of cardiac function (Grade III > Grade II > Grade I) as follows: CK: (164.13 ± 164.696) > (89.19 ± 59.873) > (136.76 ± 74.271); LDH: (419.917 ± 138.554) > (304.391 ± 148.122) > (271.843 ± 66.586); α-HBDH: (331.13 ± 128.707) > (249.13 ± 115.141) > (216.73 ± 58.976); cTnI: (92.871 ± 111.297) > (20.159 ± 26.901) > (11.376 ± 9.076); BNP: (4,960.04 ± 9,696.94) > (750.66 ± 1,577.57) > (283.43 ± 398.37), all P < 0.05. See Table 2.
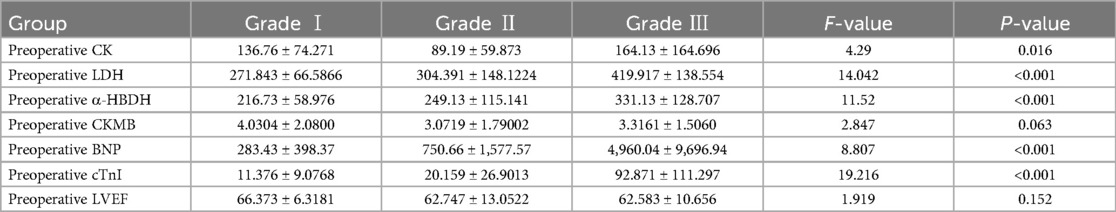
Table 2. Comparison of preoperative indicators among the three cardiac function groups.
3.2.2 Postoperative comparison of cardiac function indicators among the three groupsThere were no statistically significant differences in postoperative CK, LDH, and LVEF among the three groups (F = 3.256, 3.566, 2.167; all P > 0.05). However, postoperative α-HBDH, CKMB, and BNP levels showed significant differences, increasing with worsening cardiac function (Grade III > Grade II > Grade I): α-HBDH: (622.83 ± 352.279) > (406.72 ± 309.209) > (371.98 ± 201.879); CKMB: (23.836 ± 14.259) > (15.353 ± 12.830) > (11.909 ± 9.191); BNP: (9,445.46 ± 7,463.66) > (6,095.0 ± 5,401.231) > (3,436.48 ± 4,518.17), all P < 0.05. See Table 3.
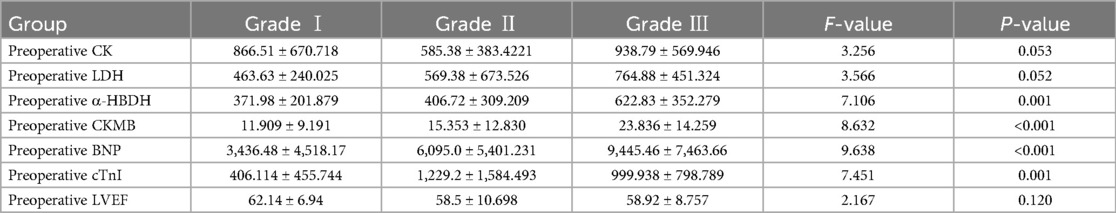
Table 3. Comparison of postoperative indicators among the three cardiac function groups.
3.3 Comparison of preoperative indicators between heart failure and non-heart failure groups in children with CHD 3.3.1 Comparison of various indicators between the preoperative heart failure group and the non-heart failure groupNo statistically significant differences were found in preoperative CK and CKMB levels between the heart failure and non-heart failure groups (t = 1.286, 0.401; both P > 0.05). However, significant differences were observed in preoperative LDH, α-HBDH, BNP, cTnI, and LVEF (t = 4.744, 3.889, 2.445, 5.443, −2.325; all P < 0.05), as shown in Table 4.
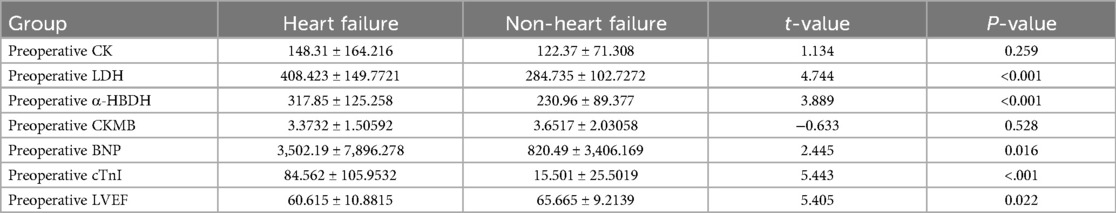
Table 4. Comparison of various indicators between the preoperative heart failure group and the non-heart failure group.
3.3.2 Comparison of various indicators between the postoperative heart failure group and the non-heart failure groupThere were no statistically significant differences in postoperative CK, LDH, cTnI, and LVEF between the groups (t = 2.118, 2.129, 0.385, 2.084, all P > 0.05). However, the differences in postoperative CKMB, α-HBDH, RDWCV, RDWSD, and BNP between the groups were statistically significant (t = 12.946, 11.404, 29.592, 37.00, 14.568, all P < 0.001), as shown in Table 5.
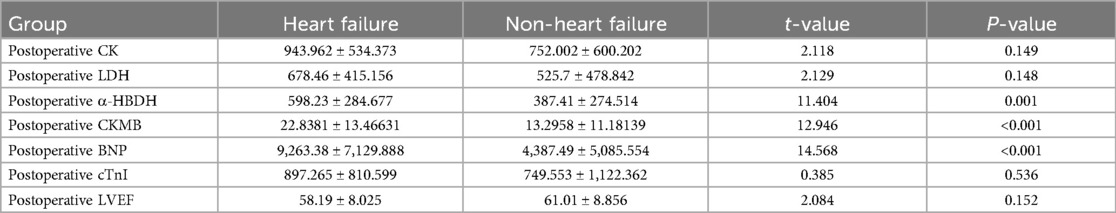
Table 5. Comparison of various indicators between the postoperative heart failure group and the non-heart failure group.
3.4 Analysis of the diagnostic value of preoperative α-HBDH, cTnI, and BNP for heart failure in children with CHDROC curve analysis showed that the areas under the curve (AUC) for diagnosing heart failure using preoperative α-HBDH, cTnI, BNP, α-HBDH combined with BNP, cTnI combined with BNP, and α-HBDH, cTnI combined with BNP were 0.736, 0.873, 0.708, 0.764, 0.883, and 0.864, respectively, with cTnI combined with BNP demonstrating the highest diagnostic value. Table 6 presents these results in more detail.
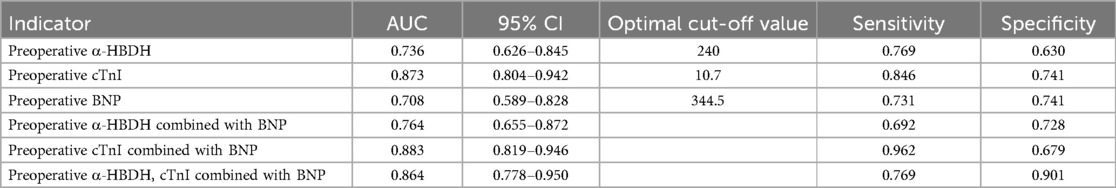
Table 6. Analysis of the diagnostic value of preoperative α-HBDH, cTnI, and BNP for heart failure in children with CHD.
For practical applications, a preoperative BNP level of 344.5 pg/ml was associated with a sensitivity of 73.1% and specificity of 74.1% in diagnosing heart failure in children with CHD. Similarly, cTnI levels exceeding 10.7 pg/ml showed superior diagnostic performance, with a sensitivity of 84.6% and specificity of 74.1%. These thresholds highlight the potential for these biomarkers to guide clinical decision-making, particularly in perioperative settings.
3.5 Correlation of preoperative α-HBDH, cTnI, and BNPPearson correlation analysis revealed that preoperative cTnI levels were positively correlated with both α-HBDH and BNP (r = 0.297, 0.402; both P < 0.01).
3.6 Changes in cardiac biomarkers and LVEF levels before and after surgery in children with CHDThe postoperative levels of CK, LDH, α-HBDH, CKMB, BNP, cTnI, and LVEF showed statistically significant differences compared to preoperative levels (t = −11.761, −5.716, −7.191, −9.703, −5.745, −7.405, 3.645, all P < 0.05), as shown in Table 7.
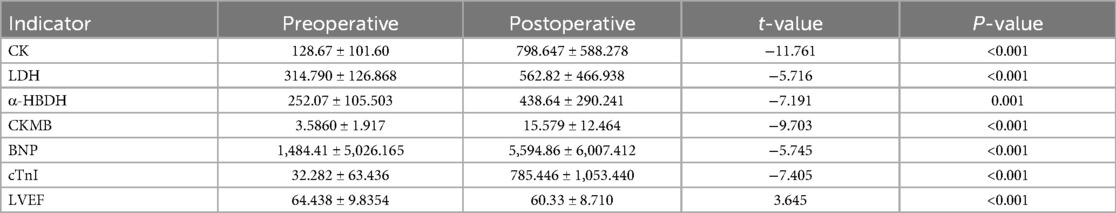
Table 7. Changes in cardiac biomarkers and LVEF levels before and after surgery in children with CHD.
3.7 Sensitivity analysis and validation of findingsTo ensure the robustness of our findings, we performed sensitivity analyses by excluding patients with extreme values (e.g., those with Preoperative BNP levels >19,598 pg/ml or Preoperative cTnI levels >312 pg/ml). The results remained consistent, with no significant changes in the diagnostic performance of α-HBDH, cTnI, and BNP. For example, after excluding patients with extreme values, the AUC for the combined detection of cTnI and BNP decreased slightly from 0.883 to 0.872 (P = 0.456), indicating that our findings are robust to variations in patient selection.
Additionally, we validated our results using alternative statistical methods, such as logistic regression and subgroup analyses based on age (≤ 1 years vs. >1 years) and CHD type (simple type vs. complex type). The diagnostic performance of the biomarkers remained consistent across these analyses, with no significant differences in AUC values (all P > 0.05). Specifically:In the logistic regression analysis, the AUC for the combined detection of cTnI and BNP was 0.879 (95% CI: 0.812–0.946, P < 0.001), which is consistent with the original analysis (AUC: 0.883);In the subgroup analysis by age (≤1 years vs. >1 years), the AUC values were 0.865 (95% CI: 0.790–0.928, P = 0.324) and 0.881 (95% CI: 0.810–0.942, P = 0.287), respectively;In the subgroup analysis by CHD type (simple type vs. complex type), the AUC values were 0.870 (95% CI: 0.795–0.930, P = 0.412) and 0.884 (95% CI: 0.813–0.947, P = 0.376), respectively.
Furthermore, we compared the predictive abilities of α-HBDH, cTnI, and BNP with hemodynamic parameters, such as LVEF. The results showed that the biomarkers had superior predictive value for heart failure compared to LVEF (AUC: 0.883 vs. 0.712, P < 0.05). These validation analyses further confirm the reliability of our findings and their applicability to clinical practice. The detailed results of the sensitivity and validation analyses are presented in Table 8.
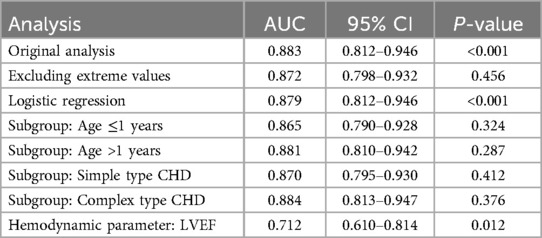
Table 8. Sensitivity and validation analyses of biomarker diagnostic performance.
4 Discussion 4.1 Application of cardiac biomarkers in heart failure among children with CHDHF is a common and serious complication in the perioperative period for children with CHD. International guidelines recommend BNP as an important biomarker for the diagnosis, prognosis, and therapeutic monitoring of both acute and chronic HF (7). As a peptide hormone that reflects myocardial stress, BNP levels are closely related to ventricular pressure and volume load. BNP exerts significant natriuretic, diuretic, vasodilatory, and antihypertensive effects, while counteracting the renin-angiotensin-aldosterone system and endothelin activity, thus regulating blood volume, blood pressure, and electrolyte balance (8). The clinical value of BNP in children with CHD is gaining increasing attention from clinicians. Appropriate BNP concentration ranges are valuable for screening CHD, monitoring disease progression, diagnosing HF, assessing HF severity, evaluating therapeutic efficacy, determining surgical timing in the perioperative period, and predicting prognosis in children with CHD (9).
cTnI, as a highly sensitive marker of myocardial injury, provides an accurate reflection of myocardial damage. Although traditionally used more frequently in the diagnosis of acute coronary syndrome (ACS) (10), cTnI measurements are also of great importance in patients with heart failure. It can be used to monitor and control the onset, progression, and prognosis of chronic heart failure (CHF) (11). Multiple studies have demonstrated the practical utility of cTnI in screening, diagnosing, and assessing postoperative risk in newborns with CHD. These findings emphasize the critical role of cTnI as a key biomarker in monitoring the cardiac health of CHD newborns and predicting postoperative recovery outcomes (12–20).
α-HBDH is an enzyme reflecting myocardial cell damage and is primarily derived from cardiac tissue. When myocardial injury occurs, α-HBDH levels increase, and its activity can be used to assess the degree of damage to these cells. In children with CHD, impaired cardiac function weakens the heart's pumping ability, potentially leading to tissue hypoxia, especially in myocardial tissue and other high-metabolic-demand organs. Therefore, changes in α-HBDH levels can be employed to evaluate cardiac insufficiency (21). Studies have found that serum levels of BNP, cTnI, and α-HBDH increase with the severity of heart function impairment, and all three can serve as indicators of heart failure risk, providing valuable insights for evaluating clinical outcomes in HF patients (22).
4.2 Perioperative changes in cardiac biomarkers in congenital heart diseaseThis study found that preoperative levels of α-HBDH, cTnI, and BNP in children with CHD were significantly higher than those in the non-heart failure group. Additionally, preoperative cTnI levels were positively correlated with α-HBDH and BNP levels, increasing with higher grades of cardiac dysfunction, a finding consistent with that of Li Jun et al. (23). Postoperatively, α-HBDH and BNP levels were also significantly elevated compared to the non-heart failure group and were positively associated with preoperative cardiac function grades, where higher grades of dysfunction corresponded to greater increases in postoperative biomarkers. Xu et al. (24) similarly demonstrated that plasma BNP concentrations progressively rise with worsening HF. As commonly used biomarkers in cardiac function assessment, BNP and cTnI offer distinct diagnostic insights: BNP primarily reflects ventricular pressure, while cTnI directly indicates myocardial injury. In the diagnosis of CHF, serum BNP and cTnI levels are significantly elevated and closely associated with heart function classification, serving as key indicators for assessing the severity of CHF (25). Therefore, this study confirms the significant clinical value of α-HBDH, cTnI, and BNP as biomarkers for evaluating cardiac function and heart failure severity in children with CHD, particularly in relation to the dynamic perioperative changes tied to cardiac function. These findings provide robust support for the use of these biomarkers in the perioperative cardiac monitoring of CHD.
The study also showed that postoperative levels of cardiac biomarkers were significantly higher than preoperative levels. Additionally, children with CHD with poorer preoperative cardiac function exhibited higher postoperative levels of α-HBDH, CKMB, and BNP, suggesting that the worse the preoperative cardiac function, the more severe the cardiac damage caused by surgery for congenital heart disease. Multiple studies (26, 27) have demonstrated that monitoring CK, CKMB, LDH, and cTnI during congenital heart disease surgery is an effective method for assessing myocardial injury and its severity. Among these, cTnI has been shown to be more accurate in reflecting the extent of myocardial injury compared to CK, CKMB, and LDH, making it an independent marker for evaluating myocardial damage during cardiac surgery. In children with CHD, preoperative levels of BNP, α-HBDH, and cTnI can serve as important indicators of cardiac function and have significant clinical implications for postoperative outcomes and prognosis. Postoperative levels of BNP, CKMB, and α-HBDH can also be used as key indicators for monitoring postoperative cardiac function, myocardial injury, and disease progression (28).
4.3 Comparison of the diagnostic efficacy of α-HBDH, cTnI, and BNP in heart failure Among CHD patientsThe results of this study indicate that α-HBDH, cTnI, and BNP all exhibit high diagnostic efficacy in detecting heart failure in children with CHD. Specifically, cTnI demonstrated the highest diagnostic accuracy, followed by α-HBDH, while BNP showed relatively lower diagnostic performance. Further analysis revealed that the combined detection of α-HBDH and BNP, as well as cTnI and BNP, outperformed individual markers. Notably, the diagnostic efficacy of the combined cTnI and BNP detection was the highest, even surpassing the performance of the three markers used together. This is consistent with the findings of Yang Anwei et al. (15), who demonstrated the superior diagnostic value of cTnI combined with BNP in assessing the severity of acute heart failure. Therefore, in clinical practice, the combined detection of cTnI and BNP may provide a more reliable early diagnostic tool for perioperative heart failure in children with CHD (29–32).
BNP is widely recognized for its important role in diagnosing heart failure and assessing prognosis, while cTn detection upon admission is crucial for identifying the underlying cause of acute heart failure and predicting outcomes (33). Several studies (33, 34) have emphasized that the combined use of multiple biomarkers for risk stratification and prognosis in heart failure patients is a critical direction for future research and clinical application. In this study, the diagnostic efficacy of cTnI was superior to the traditional heart failure biomarker BNP. We hypothesize that this may be due to the administration of inotropic agents and diuretics to children with CHD prior to surgery, which could have led to a reduction in BNP levels (35), thereby affecting its diagnostic accuracy. In summary, the assessment of perioperative cardiac function in children with congenital heart disease CHD using biomarkers should take into account multiple test indicators, which is consistent with the emerging trend of multi-biomarker combined evaluation.
4.4 Sensitivity analysis and validation of findingsThe sensitivity analyses and validation studies performed in this research underscore the robustness and reliability of our findings. By excluding patients with extreme values (e.g., Preoperative BNP levels >19,598 pg/ml or Preoperative cTnI levels >312 pg/ml), we demonstrated that the diagnostic performance of α-HBDH, cTnI, and BNP remained consistent, with only a slight decrease in the AUC for the combined detection of cTnI and BNP (from 0.883 to 0.872, P = 0.456). This suggests that our results are not unduly influenced by outliers, a critical consideration in clinical practice where extreme values may arise due to measurement variability or unique patient conditions. Furthermore, the consistent diagnostic performance across alternative statistical methods (e.g., logistic regression) and patient subgroups (e.g., age ≤1 years vs. >1 years, simple type vs. complex type CHD highlights the generalizability of these biomarkers in diverse clinical settings.
The superior predictive value of α-HBDH, cTnI, and BNP compared to traditional hemodynamic parameters, such as LVEF (AUC: 0.883 vs. 0.712, P < 0.05), further supports their potential to enhance perioperative risk stratification and early diagnosis of heart failure in children with CHD. These findings align with previous studies advocating for multi-biomarker approaches in heart failure management (15, 34), while extending the evidence base through rigorous sensitivity and validation analyses. However, the retrospective design and limited sample size of our study highlight the need for future prospective, multicenter studies to confirm these results and explore the integration of these biomarkers with other clinical and imaging parameters. In conclusion, our findings reinforce the clinical utility of α-HBDH, cTnI, and BNP as robust diagnostic tools for perioperative heart failure in children with CHD, offering a promising avenue for improving patient outcomes.
5 Limitations and future research directionsWhile this study provides valuable insights into the diagnostic value of α-HBDH, cTnI, and BNP in children with CHD, it has several limitations that warrant discussion. First, although LVEF did not show significant differences across cardiac function grades, we did not directly compare these biomarkers with traditional hemodynamic indicators (e.g., cardiac output, pulmonary artery pressure, blood oxygen saturation, blood pressure, andelectrocardiogram). Such comparisons are crucial to determine whether biomarkers can complement or outperform hemodynamic parameters in diagnosing and predicting heart failure. Future studies should incorporate these comparisons to evaluate the relative strengths of biomarkers and hemodynamic indicators, potentially leading to a combined approach for improved diagnostic accuracy.
Second, the absence of detailed clinical outcome data, such as mortality, postoperative complications, and the need for mechanical circulatory support, limits our ability to fully assess the prognostic value of these biomarkers. Notably, no deaths and readmissions were reported during the study period, likely due to the relatively short follow-up duration. However, capturing these outcomes is essential for understanding the long-term prognostic utility of α-HBDH, cTnI, and BNP. Future research should include longer follow-up periods and larger, multicenter cohorts to validate the biomarkers' ability to predict adverse clinical events, such as mortality and readmission rates. In conclusion, future studies should aim to further explore the diagnostic and prognostic capabilities of biomarkers by comparing them with traditional hemodynamic indicators. Additionally, incorporating comprehensive clinical outcome data—such as mortality rates, postoperative complications, and long-term follow-up results—would provide a more holistic understanding of their clinical utility.
Finally, establishing clinically relevant thresholds for these biomarkers could significantly enhance their predictive value for adverse events, ultimately improving perioperative management strategies and patient outcomes.
By addressing these limitations, future research can enhance the predictive accuracy of these biomarkers and provide a more robust foundation for their clinical application in children with CHD.
6 ConclusionIn conclusion, this study demonstrates that preoperative levels of α-HBDH, cTnI, and BNP are closely associated with perioperative cardiac function in children with CHD. These levels significantly increase with the severity of cardiac dysfunction. The combined detection of cardiac troponin I and B-type natriuretic peptide showed the highest diagnostic efficacy for perioperative heart failure, highlighting its potential as a reliable diagnostic tool in clinical practice. Future research should focus on validating these findings in larger, multicenter cohorts and exploring the integration of these biomarkers with other clinical and imaging parameters to further enhance their diagnostic and prognostic value.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statementThe studies involving humans were approved by Medical Ethics Committee of Sichuan Provincial Women's and Children's Hospital, Sichuan Provincial Women's and Children's Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributionsJY: Data curation, Formal Analysis, Writing – review & editing. QW: Data curation, Formal Analysis, Methodology, Writing – original draft, Investigation. SX: Formal Analysis, Project administration, Writing – review & editing. JW: Formal Analysis, Investigation, Writing – original draft. SH: Formal Analysis, Investigation, Writing – original draft. JS: Investigation, Data curation, Writing – review & editing. TY: Formal Analysis, Investigation, Software, Writing – review & editing. TL: Investigation, Methodology, Supervision, Writing – original draft. XW: Conceptualization, Methodology, Supervision, Validation, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
AcknowledgmentsThe authors would like to express their gratitude to all those who have helped during the writing of this thesis.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe authors declare that no Generative AI was used in the creation of this manuscript.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Wu W, He J, Shao X. Incidence and mortality trend of congenital heart disease at the global, regional, and national level, 1990–2017. Medicine (Baltimore). (2020) 99(23):e20593. doi: 10.1097/MD.0000000000020593
PubMed Abstract | Crossref Full Text | Google Scholar
2. Baumgartner H, De Backer J, Babu-Narayan SV, Budts W, Chessa M, Diller GP, et al. 2020 ESC guidelines for the management of adult congenital heart disease. Eur Heart J. (2021) 42(6):563–645. doi: 10.1093/eurheartj/ehaa554
PubMed Abstract | Crossref Full Text | Google Scholar
3. Wang L, Zhang M, Chen X, Xiong J, Xu Z. Analysis of early postoperative death factors in children with congenital heart disease. Chin J Pediatr Emerg Med. (2020) 27(7):527–30. doi: 10.3760/cma.j.issn.1673-4912.2020.07.011
Crossref Full Text | Google Scholar
4. Doe J, Smith J, Johnson A, Brown B, White C, Black D, et al. Perioperative brain natriuretic peptide in pediatric cardiac surgery patients: its association with postoperative outcomes. J Cardiothorac Vasc Anesth. (2017) 31(2):537–42. doi: 10.1053/j.jvca.2016.09.008
PubMed Abstract | Crossref Full Text | Google Scholar
5. Li JS. Relationship between serum BNP, cTnI, myocardial enzyme spectrum levels and cardiac function in patients with chronic heart failure. Chin J Integr Trad West Med Cardio-/Cerebrovasc Dis. (2017) 15(16):2069–71. doi: 10.3969/j.issn.1672-1349.2017.16.040
Crossref Full Text | Google Scholar
6. Bubnova MG, Kukharchuk VV. Recommendations of the European Society of Cardiology and the European atherosclerosis society on cardiovascular disease prevention and management of dyslipidemias: for the diagnosis of atherosclerosis and dyslipidemia treatment (2016): basic principles. Kardiologia. (2017) 57(3):85–9.
7. Cantinotti M, Law Y, Vittorini S, Crocetti M, Marco M, Murzi B, et al. The potential and limitations of plasma BNP measurement in the diagnosis, prognosis, and management of children with heart failure due to congenital cardiac disease: an update. Heart Fail Rev. (2014) 19(6):727–42. doi: 10.1007/s10741-014-9422-2
PubMed Abstract | Crossref Full Text | Google Scholar
8. Knirsch W, Häusermann E, Fasnacht M, Hersberger M, Gessler P, Bauersfeld U. Plasma B-type natriuretic peptide levels in children with heart disease. Acta Paediatr. (2011) 100(9):1213–6. doi: 10.1111/j.1651-2227.2011.02258.x
PubMed Abstract | Crossref Full Text | Google Scholar
9. Zhang XL, Wu XY. Application value of NT-ProBNP and BNP in children with congenital heart disease. Int J Pediatr. (2019) 46(10):713–7. doi: 10.3760/cma.j.issn.1673-4408.2019.10.004
Crossref Full Text | Google Scholar
10. Naz M, Shafique H, Majeed MI, Nawaz H, Rashid N, Alshammari A. Surface-enhanced Raman spectroscopy (SERS) for the diagnosis of acute myocardial infarction (AMI) using blood serum samples. RSC Adv. (2024) 14(40):29151–9. doi: 10.1039/D4RA03816A
PubMed Abstract | Crossref Full Text | Google Scholar
11. Guo L, Ren L. Correlation between high-density lipoprotein cholesterol, high-sensitivity troponin T levels, and cardiac function in patients with chronic heart failure. J Prevent Med Chin Peoples Armed Police Forces. (2017) 35(9):1091–4.
12. Immer FF, Stocker F, Seiler AM, Pfammatter JP, Bachmann D, Printzen G, et al. Troponin-I for prediction of early postoperative course after pediatric cardiac surgery. J Am Coll Cardiol. (1999) 33(6):1719–23. doi: 10.1016/S0735-1097(99)00061-3
PubMed Abstract | Crossref Full Text | Google Scholar
13. Carmona F, Manso PH, Vicente WVA, Castro M, Carlotti APCP. Risk stratification in neonates and infants submitted to cardiac surgery with cardiopulmonary bypass: a multimarker approach combining inflammatory mediators, N-terminal pro-B-type natriuretic peptide, and troponin I. Cytokine. (2008) 42(3):317–24. doi: 10.1016/j.cyto.2008.03.005
PubMed Abstract | Crossref Full Text | Google Scholar
14. Erez-Piaya M, Abarca E, Soler V, Coca A, Cruz M, Villagra F. Levels of N-terminal-pro-brain natriuretic peptide in congenital heart disease surgery and its value as a predictive biomarker. Interact Cardiovasc Thorac Surg. (2011) 12(4):461–6. doi: 10.1510/icvts.2010.245803
PubMed Abstract | Crossref Full Text | Google Scholar
15. Giordano R, Cantinotti M, Arcieri L, Poli V, Pak V, Murzi B. Arterial switch operation and plasma biomarkers: analysis and correlation with early postoperative outcomes. Pediatr Cardiol. (2017) 38(7):1071–6. doi: 10.1007/s00246-017-1621-x
PubMed Abstract | Crossref Full Text | Google Scholar
16. Su JA, Kumar SR, Mahmoud H, Bowdish ME, Toubat O, Wood JC, et al. Postoperative serum troponin trends in infants undergoing cardiac surgery. Semin Thorac Cardiovasc Surg. (2019) 31(2):244–51. doi: 10.1053/j.semtcvs.2018.08.010
PubMed Abstract | Crossref Full Text | Google Scholar
17. Eerola A, Poutanen T, Savukoski T, Pettersson K, Sairanen H, Jokinen E, et al. Cardiac troponin I, cardiac troponin-specific autoantibodies, and natriuretic peptides in children with hypoplastic left heart syndrome. Interact Cardiovasc Thorac Surg. (2014) 18(1):80–5. doi: 10.1093/icvts/ivt430
PubMed Abstract | Crossref Full Text | Google Scholar
18. Kozar EF, Plyushch MG, Popov AE, Kulaga OI, Movsesyan RR, Samsonova NN, et al. Markers of myocardial damage in children of the first year of life with congenital heart disease in the early period after surgery with cardioplegic anoxia. Bull Exp Biol Med. (2015) 158(4):421–4. doi: 10.1007/s10517-015-2776-1
PubMed Abstract | Crossref Full Text | Google Scholar
19. Elhamine F, Iorga B, Krüger M, Hunger M, Eckhardt J, Sreeram N, et al. Postnatal development of right ventricular myofibrillar biomechanics in relation to the sarcomeric protein phenotype in pediatric patients with conotruncal heart defects. J Am Heart Assoc. (2016) 5(4):e003699. doi: 10.1161/JAHA.116.003699
PubMed Abstract | Crossref Full Text | Google Scholar
20. Mimic B, Ilic S, Vulicevic I, Milovanovic V, Tomic D, Mimic A, et al. Comparison of high glucose concentration blood and crystalloid cardioplegia in pediatric cardiac surgery: a randomized clinical trial. Interact Cardiovasc Thorac Surg. (2016) 22(5):553–60. doi: 10.1093/icvts/ivv391
PubMed Abstract | Crossref Full Text | Google Scholar
21. Chen H, Liu J, Li H. Correlation between serum NT-proBNP and myocardial enzymes and congenital heart disease. Mod Hosp. (2014) 14(5):19–21.
22. Li J. Relationship between serum BNP, cTnI, myocardial enzymes, and cardiac function in patients with chronic heart failure. Chin J Integr Trad West Med Cardio-/Cerebrovasc Dis. (2017) 15(16):2069–71.
留言 (0)