Worldwide between 8% and 10% of all inflammatory bowel disease (IBD) are diagnosed in childhood (<18 years) and classified as pediatric IBD (pIBD) (1, 2). A subset of pIBD with disease onset younger than 6 years of age is further classified as VEO-IBD (3). In the last 2 decades, IBD has emerged as a global disease. Whilst the incidence of pIBD is somewhat stable in regions with a high baseline prevalence, a rapid rise in the prevalence of pIBD has been reported in many newly industrialized countries (4). The data regarding VEO-IBD is conflicting. A Canadian study on childhood and adolescent IBD showed a rapid increase in the incidence of VEO-IBD (5) but another study from Israel showed a stable incidence of VEO-IBD (6).
Currently, data on VEO-IBD are mainly from North America and Europe (7, 8). VEO-IBD represents approximately 15%–19% of all children with IBD (9, 10). The highest incidence was observed in Wisconsin (United States; 3.7/100,000 persons/year), with lower rates in Saudi Arabia (0.19/100,000 persons/year) (11). Nearly 30% of all VEO-IBD are a monogenic variant of IBD (12, 13).
There is a paucity of data on the incidence of VEO-IBD in developing and recently developed countries from Asia (11). The published studies from Asia on VEO-IBD are mainly on monogenic IBD (13–16). We recently published data from our multicentre Asian pIBD registry, demonstrating that 30% of all pIBD in Asian children were VEO-IBD (17). The aim of this current study is to detail the disease phenotype and 5-year outcome of these children VEO-IBD compared with the LO-pIBD cohort.
Materials and methods Multicentre Asian PIBD registry networkThe establishment of the Asian pIBD Multicentre Registry Network has been described previously (17). Established in 2017, the network includes 7 Asian pediatric gastroenterology centres from 5 South Asian and Southeast Asian countries (the Philippines Malaysia, Singapore, Sri Lanka and Thailand). The aim was to investigate the natural history and outcome of pIBD among children residing in South Asia and Southeast Asia. Standardized, anonymized data on children < 18 years of age diagnosed with IBD from the participating centres were collected retrospectively and prospectively between 1 January 1995 to 30 June 2023. Patients admitted before 31 December 2011 were collected retrospectively while data entered after that were collected prospectively.
Ethics approvalStudy data were collected and managed using REDCap electronic data capture tools (18) hosted at the Singapore Clinical Research Institute. We obtained ethics approval from the National Healthcare Group (NHG) Domain Specific Review Board (NUH/2019-00060, approved 23 January 2020) of Singapore as well as from the respective centres' ethics review boards.
Data collectionEpidemiological parameters (demographics, clinical features/biochemistry/disease severity scores), disease phenotype and behaviour at diagnosis were extracted. Clinical remission rates, medication utilization patterns, surgery and mortality were analysed for both 1YFU and 5-year follow up (5YFU).
Inclusion and exclusion criteriaInclusion criteria for the current study were: (a) gastroenterologist-confirmed diagnosis of pIBD at the participating centre; (b) age below 18 years at the point of diagnosis; and (c) resident (citizens, permanent citizens or long-term visitors) of the country of the participating centre. Excluded were medical tourists who originated from outside the 5 countries as well as children with a confirmed diagnosis of monogenic IBD. We excluded monogenic disease as genetic service was only available in Singapore and Malaysia.
DefinitionsVEO-IBD was defined by an age of onset less than 6 years and LO-pIBD by an age of onset of between 6 and 18 years of age. Diagnosis of pIBD, disease phenotype and behaviour was classified according to Paris modification of Montreal classification (1). We used the pediatric CD activity index (PCDAI) for disease activity assessment of CD, and the pediatric UC activity index (PUCAI) for the assessment of UC and IBD-U (19, 20). The disease activity of CD, UC and IBD-U were classified into inactive (PCDAI ≤ 10 or PUCAI < 10); mild (PCDAI > 10–30 or PUCAI =10−34) and moderate severe activity (PCDAI > 30 or PUCAI ≥ 35) (19, 20).
For CD, classification of L4 disease was based on findings on gastric and duodenal involvement from upper GI endoscopy. Jejunal and proximal ileal disease were diagnosed based on findings from small bowel imaging such as intestinal ultrasound, computerised tomography of abdomen, or magnetic resonance enterography. Video capsule endoscopy was not performed.
Perianal disease was defined as the presence of fistula (including fistula diagnosed with imaging studies), anal canal ulcers or abscess (1). Extraintestinal manifestation was defined as the presence of the following symptoms and signs: fever above 38.5 degree Celsius for three days over the previous week, arthritis, uveitis, erythema nodosum or pyoderma gangrenosum (13). Bowel surgery was defined as bowel resection and/or creation of a stoma, excluding excision of perianal abscess or seton placement.
Definition of ethnicities was similar to that described by Huang et al (17). South Asians included people of Indians, Bangladeshi, Pakistani, Sri Lankan (Sinhalese/Tamil), or Maldivian origin. Chinese included descendants of immigrants from China.
GrowthAnthropometric z-scores for children aged 0−5 years and 5-18 years were based on the World Health Organization (WHO) Child Growth Standards and the WHO Growth reference 2007, respectively. We defined growth faltering as height z-score for age and gender of less than or equal to −2 at diagnosis or at any time during disease course.
Therapeutic optionsDuring the study period, enteral nutrition for CD, corticosteroids, 5-aminosalicylate acid (5-ASA), and immunomodulators were available in all participating centres while biologics were readily available only in centers from Malaysia and Singapore.
Statistical methodsStatistical analysis was performed using STATA/MP Version 17.0. (StataCorp 2021). We compared disease phenotype and outcome between the key phenotypic subgroups (CD and UC). Continuous variables were compared using the student's t-test while categorical variables were compared using the chi-square test. A p-value of < 0.05 was deemed significant.
ResultsA total of 112 (25.5%) of the 440 children in the Asian pIBD Multicentre Registry were diagnosed to have VEO-IBD. Two children with monogenic disease, one each for XIAP deficiency and trichoenterohepatic syndrome (mutation in SKIV2l gene), were excluded. The mean age at diagnosis was 3.57 (SD = 1.39) years (Table 1). There was a slight male preponderance in both VEO-IBD (55.4%) and LO-pIBD (55.8%). A positive family history of IBD was uncommon in both age groups (1.79% for VEO-IBD vs. 3.96% for LO-pIBD; p = 0.322). The duration of follow-up measured as years from diagnosis to latest follow up was significantly longer for VEO-IBD than LO-pIBD [5.19 (±4.12) vs. 4.10 (± 3.47) years (p = 0.0131)]. Approximately 1 in 8 children with pIBD had growth faltering at diagnosis (11.6% for VEO-IBD vs. 11.0% for pIBD; p = 0.534) (Table 2).
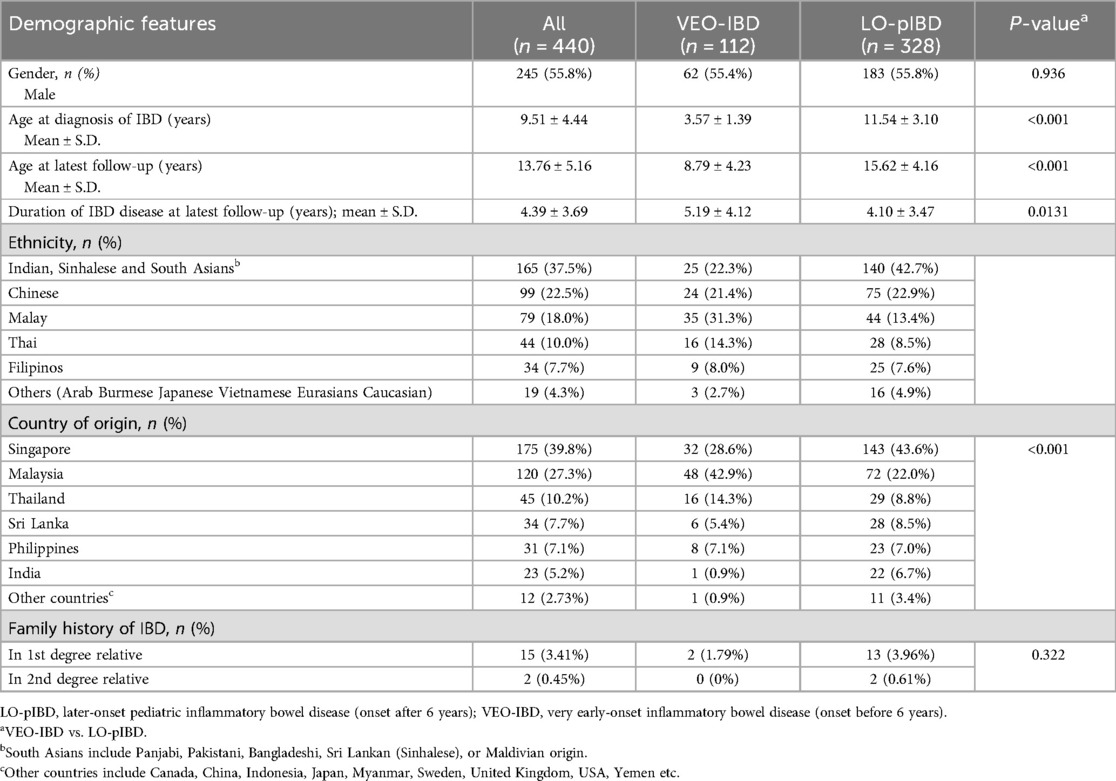
Table 1. Demographic features in 440 Asian children with pediatric inflammatory bowel disease.
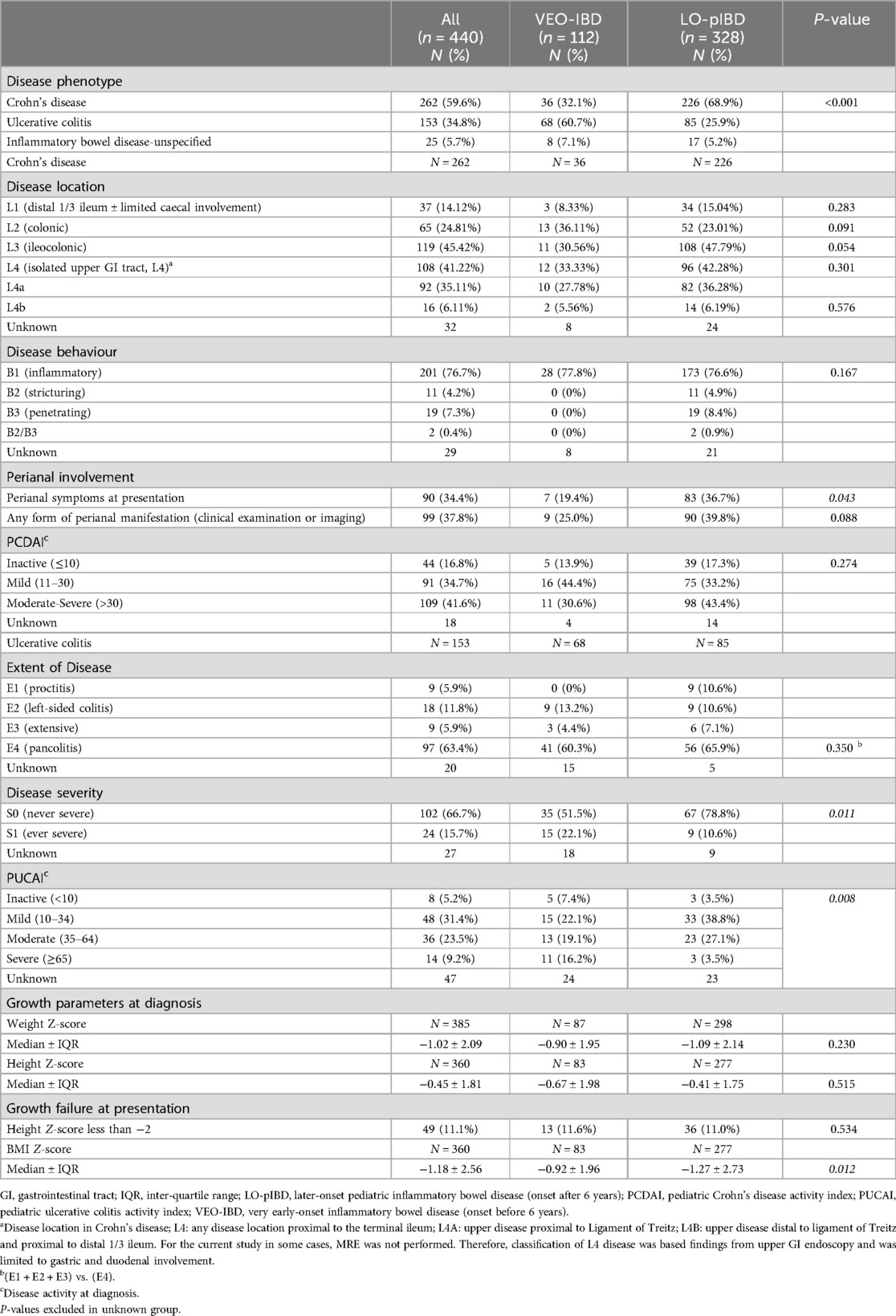
Table 2. Disease phenotype and behaviour, disease activity and growth parameters at diagnosis.
Co-existing immune-mediated disorders and extraintestinal manifestationFor extra-intestinal manifestations, oral ulcers were significantly more common in LO-pIBD (18.9% vs. 5.4%, p = 0.001) whilst immune-mediated liver disease (primary sclerosing cholangitis and autoimmune hepatitis) was more common in VEO-IBD (15.2% vs. 6.1%, p = 0.003) (Table 3).
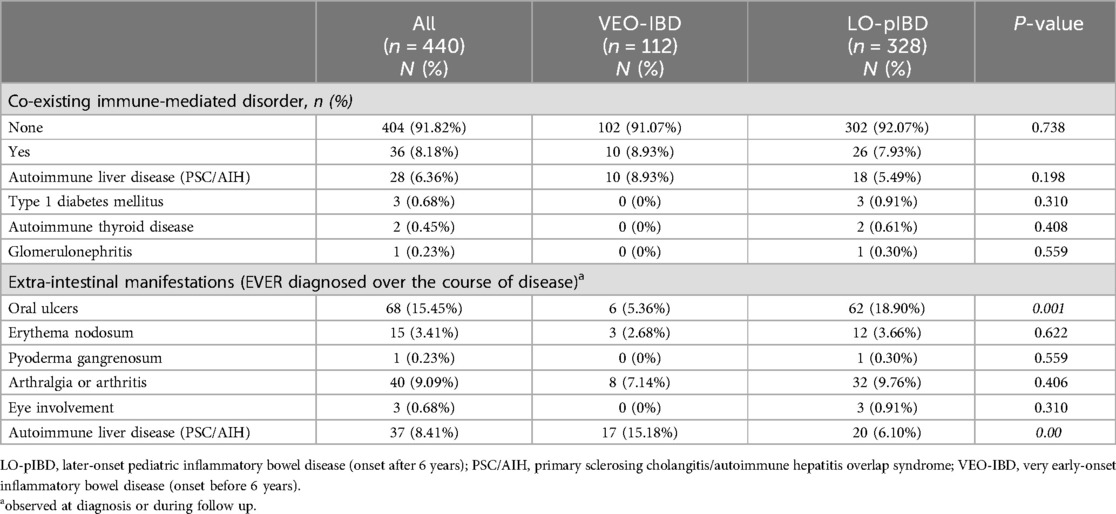
Table 3. Co-existing immune-mediated disorders and extra-intestinal manifestations.
Disease phenotype and disease activity at diagnosisUC was the more common disease phenotype in VEO-IBD while CD was more commonly seen in LO-pIBD (p < 0.001; Table 2). IBD-U was seen in 5.7% (n = 25) of the overall cohort (Table 2). Pancolitis was equally common between VEO-pUC and LO-pediatric UC (LO-pUC) (60.3% vs. 65.9%; p = 0.350). Children with VEO-UC were more likely to have a severe disease activity (PUCAI ≥ 65) at diagnosis compared to LO-pUC (16.2% vs. 3.5%; p = 0.008) (Table 2).
Colonic disease was equally common between VEO-CD (23%) and LO-pediatric CD (36.1%) (p = 0.091) (Table 2). No difference in disease behavior in CD was observed between both age groups. Inflammatory phenotype predominated in both (77.8% vs. 76.6%; p = 0.167). Stricturing and penetrating disease were not observed in VEO-CD, unlike in LO-pCD which was noted at 4.9% and 8.4%, respectively. No significant difference in perianal disease (25.0% vs. 39.8%; p = 0.09) or upper gastrointestinal involvement (L4, Paris classification) (33.3% vs. 42.3%; p = 0.301) was observed between both age groups. At diagnosis, similar number of patients with VEO-CD (30.6%) and LO-pCD (43.4%) presented with moderate-to-severe disease activity scores (PCDAI ≥ 30) (p = 0.273) (Table 2).
TherapiesUse of corticosteroid for induction was significantly higher in children with VEO-IBD than LO-pIBD (73.2% vs. 47.9%; p < 0.01; Table 4), even after adjusting for co-existing immune mediated liver disease (p < 0.001). Similarly, as compared to LO-pIBD, use of azathioprine (27.7% vs. 15.9%; p = 0.006) and biologics (11.6% vs. 5.2%; p = 0.020) were significantly higher in VEO-IBD in the course of the disease. No difference in the use of 5-aminosalicylates, methotrexate, antibiotics, or exclusive enteral nutrition (as induction for CD) was observed in both age groups (Table 4). Use of biologics was more common in VEO-IBD (13/80) than LO-pIBD (17/215; p = 0,035; Table 4) amongst centres from Malaysia and Singapore (n = 295).
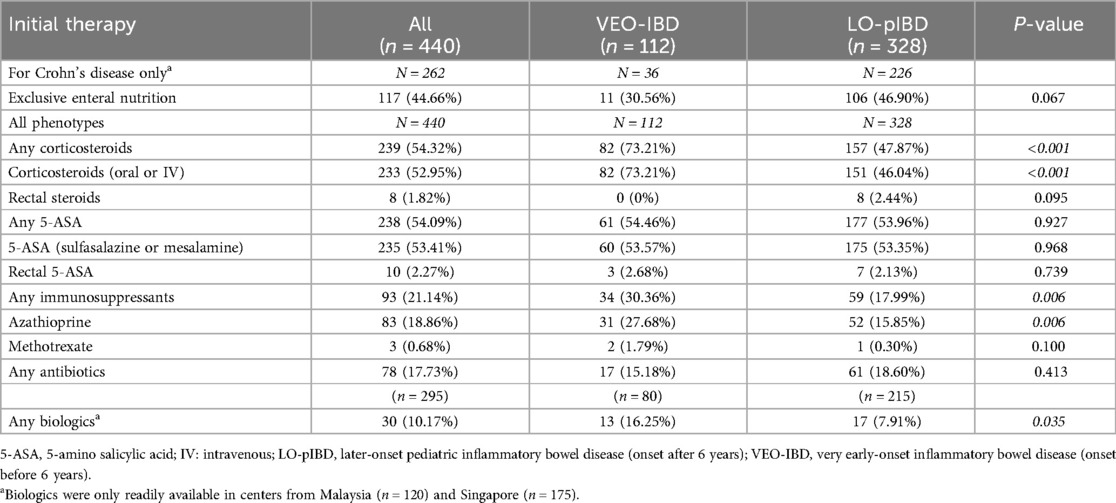
Table 4. Medical therapy received at diagnosis.
Disease activity at 1 and 5 year after diagnosisData on disease activity were available in 273 patients at 1YFU and 130 patients 5YFU (Table 5). The proportion of children with an inactive or mild disease activity for VEO-CD and LO-pCD was 96.8%% vs. 97.3 (p = 0.85) at 1YFU, and 100% vs. 98.3 (p = 0.82) at 5YFU, respectively. For VEO-UC and LO-pUC, the proportion of inactive and mild disease activity was 89.2% vs. 94.5% (p = 494) at 1YFU and 95.7% vs. 97/0% (p = 0.528), respectively.
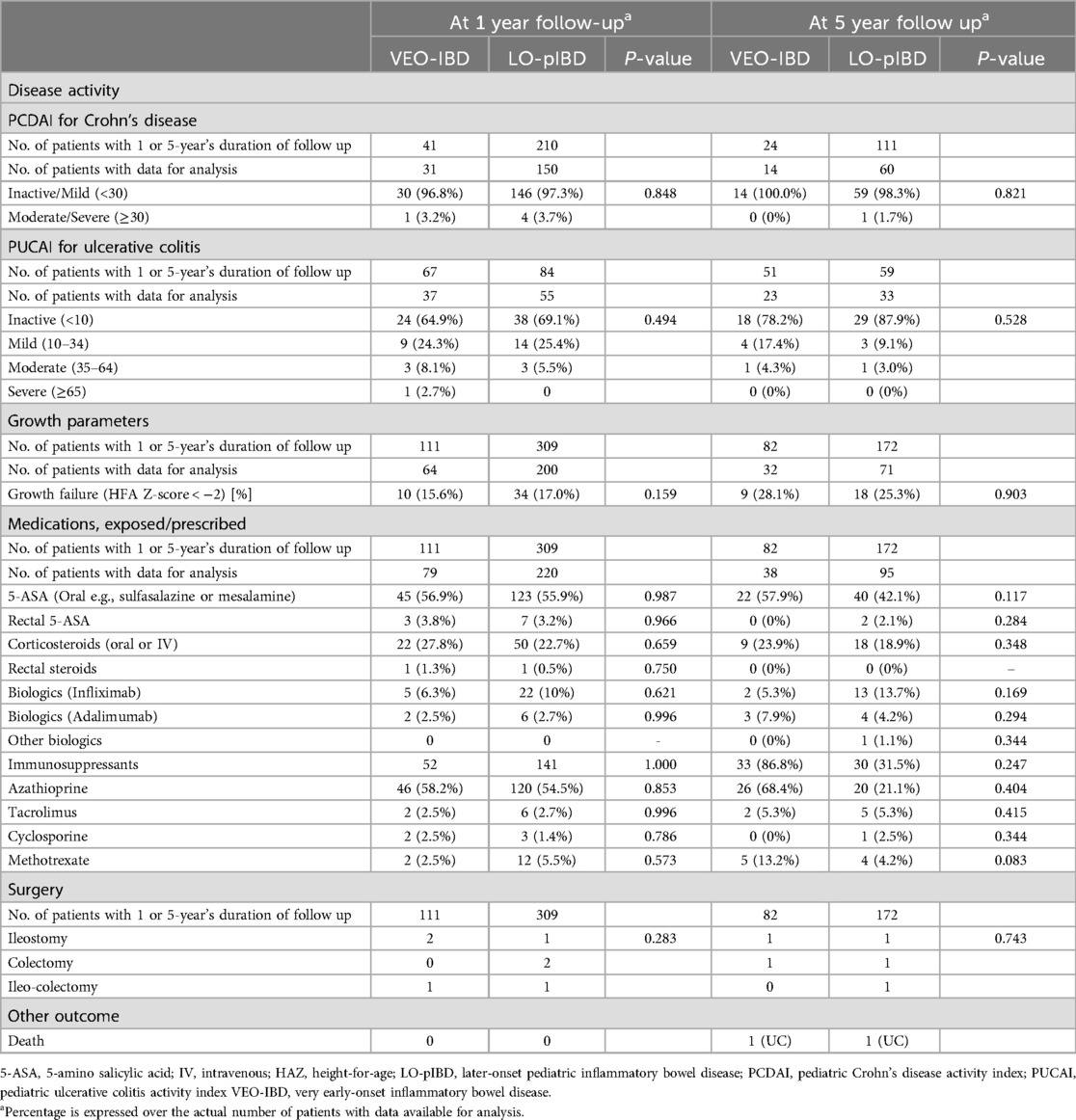
Table 5. Outcome of Asian children with inflammatory bowel disease at 1 and 5 years after diagnosis.
Bowel surgeryBowel surgery rate was 2.4% and 1.7% for VEO- and LO-IBD at 5 years, respectively.
MortalityTwo deaths (1.8% of 112), both had UC, were observed (Table 5). One with VEO-UC succumbed to disseminated venous thromboembolism died 18 months after diagnosis after his parents abandoned further treatment and sought traditional and spiritual therapy. The second child with severe UC died of disseminated tuberculosis 12 months after biologic was started. At initial diagnosis, screening for latent tuberculosis was negative.
DiscussionIn the current study, 25.5% of children in our registry with pIBD had VEO-IBD, which is comparable to other referral centre-based studies from Asia, such as 19.2% from India (10) 26.1% from China (21). With the exception of a few differences, phenotypes and outcome of VEO- and LO-pIBD in this study were largely similar. The major difference was UC was the more common disease phenotype in VEO-IBD whilst CD was more common in LO-pIBD. On the other hand, no difference in the disease location and behaviour were observed between both age groups. In addition, perianal disease was equally common in both age groups, similar to other studies (13, 22).
For disease location, we observed a very similar disease involvement for both VEO-CD and VEO-UC compared to a North American cohort (22). For VEO-UC, pancolitis was seen in two-thirds of patients, similar to a large North American cohort by Kerur et al. (8). For VEO-CD, isolated colitis was the most common disease location, also similar to the another North American study by Olivia-Hammer et al. (22).
Although disease activity at diagnosis was less severe in VEO-CD than LO-CD, overall clinical remission rates at 5 years was comparable between both age groups. In both age groups, approximately 3–4 in 10 children had moderate-to-severe disease at diagnosis with the vast majority noted to have inactive-to-mild disease at 1YFU, and remaining so at 5 years.
Studies on VEO-IBD in Caucasian children have reported a more severe disease course 5 years after diagnosis (23). They were more likely to need steroids during the course of disease compared with LO-IBD (22, 24). Failure of biologics therapy at 1-year post-diagnosis was also higher in VEO-IBD compared to the LO-pIBD (25). A North American study noted the risk of colectomy for VEO-IBD group was 0%–3% by 1 year, 3%–12% by 3 years, 14%–15% by 5 years after diagnosis (8).
In our study, use of biologics was not common. In centres where biologics were readily available, 10% of all patients in both age group needed biologics, being more common in VEO-IBD than in LO-pIBD. Limited healthcare resources in the other centers were reason for the limited access to biologics (26).
However, despite the relatively low use of biologics (at ∼10%), the outcome of our patients at 1 year and 5 years appears to be good. At 5 year follow up, 100% of VEO-CD and 95% of VEO-UC had clinically inactive disease. In contrast, a Japanese study reported that of 52% of 44 patients with VEO-IBD required biologics (27). Another study from North America on VEO-IBD reported that 85.7% of patients achieved steroid-free clinical remission at 56 months follow up (28). Shim et al. from Korea also reported that children with VEO-IBD have higher rates of affected first degree relatives, a more severe disease course, and a higher rate of resistance to immunosuppressive treatment (29).
Despite an initial difference in disease severity, VEO-IBD in this cohort was not more resistant to treatment compared to LO-pIBD. More than 9 in 10 children with VEO-CD and VEO-UC had an inactive or mild disease 1 year after diagnosis, and moderate to severe disease remained under control 5 years after disease onset. The great majority of both VEO-CD and VEO-UC had either inactive or mild disease activity 5 years after diagnosis. The risk of surgery at 5 years after diagnosis was approximately 2%–3% much lower than the 14%–15% at 5 years observed by Kerur et al. in North America (8).
Our study excluded children with monogenic disease because genetic service for pIBD was not available in centres outside Singapore and Malaysia because of high cost. It is noteworthy that studies on VEO-IBD from Asia where monogenic disease were included, signficant percentage of monogenic disease have been reported; 31% in India (13), 16.7% in China (30), and 11.6% in Japan (15). It is therefore possible that some patients with undiagnosed monogenetic disease were inadvertently included in our cohort.
In addition, by excluding monogenic disease, there is a possibility of excluding late-onset monogenic disease by excluding and thus possibly influencing the results of current study (31). Thus, we readily acknowledge the importance of genetic studies in pIBD in view of rapid advances in this field and a wider availability of genetic analysis. Future studies from our Network should include analysis of monogenic disease in Asian children with pIBD.
Recent studies on IBD and pIBD from Asia have shown evidence of rapidly rising incidence of pIBD in Asia (17, 23). The changing epidemiology have been attributed to evolving environmental factors such as rapid urbanization and a shift to a westernised diet observed in developed society (such as Singapore) and recently developed countries (such as Malaysia and Thailand) (17).
In the current study, we are unable to estimate the population incidence of VEO-IBD among countries taking in this network. However, authors from France (25) and Korea (29) have observed the relative stability of population incidence of VEO-IBD over time, implying that this disease is less likely to be influenced by any shift in environmental factors as compared IBD in other age groups.
The strength of the current study is it is the largest cohort of VEO-IBD in Asian children on clinical characteristics and disease outcome in children with VEO-IBD. The findings on disease phenotype and outcome of predominantly non-monogenic VEO-IBD would be useful in providing guidance in anticipatory care, prognosis and treatment in this emerging patient population.
Limitations of the study include its retrospective nature and the issue of missing data. Anthropometric data were not available in nearly 40% of the cohort both at 1 and 5 years after diagnosis. Similarly, disease activity scores were not complete at follow up. Secondly, limited access to magnetic resonance enterography in some centres and unavailability of video capsule endoscopy have limited our ability to diagnose jejunal and ileal involvement. Therefore classification of L4 disease for CD may not be complete. Thirdly, although we did not include monogenic IBD in this cohort, children with a monogenic disease were likely to be included since monogenic testing is unavailable in most participating centres. Finally, we are unable to eliminate referral bias entirely since each of the participating centre in the current study is a referral or academic centre in their respective country. Thus, we were unable to estimate the prevalence of VEO-IBD in our respective populations.
ConclusionsIn summary, in this first multicentre, multi-ethnic Asian cohort with pIBD, VEO-IBD represents 25.2% of all pIBD. Despite difference in phenotype, similarities in disease severity at diagnosis as well as the varying use of corticosteroids, immunosuppressants, and biologic therapy, overall clinical outcomes at 5 years was good and comparable between VEO-IBD and LO-IBD.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementWe obtained ethics approval from the National Healthcare Group (NHG) Domain Specific Review Board (NUH/2019-00060, approved 23 January 2020) of Singapore as well as from the respective centers' ethics review boards. All study participants or their legal guardian provided informed written consent about personal and medical data collection prior to study enrolment.
Author contributionsWSL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. KSC: Data curation, Methodology, Writing – original draft, Writing – review & editing. JGH: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. PT: Data curation, Writing – original draft, Writing – review & editing. KM: Data curation, Writing – original draft, Writing – review & editing. AR: Data curation, Writing – original draft, Writing – review & editing. VL: Data curation, Writing – original draft, Writing – review & editing. KH: Data curation, Writing – original draft, Writing – review & editing. SR: Data curation, Writing – original draft, Writing – review & editing. YW: Data curation, Formal Analysis, Funding acquisition, Project administration, Validation, Writing – original draft, Writing – review & editing. ST: Data curation, Writing – original draft, Writing – review & editing. MA: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. The current study received an unrestricted support from Singapore Clinical Research Institute, Singapore, for hosting the database.
AcknowledgmentsThe authors thank Dr. Lim QY for helping with formatting of the manuscript.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Crocetti E, Bergamaschi W, Russo AG. Population-based incidence and prevalence of inflammatory bowel diseases in Milan (Northern Italy), and estimates for Italy. Eur J Gastroenterol Hepatol. (2021) 33(1S):e383–9. doi: 10.1097/MEG.000000000002107
PubMed Abstract | Crossref Full Text | Google Scholar
2. Hammer T, Nielsen KR, Munkholm P, Burisch J, Lynge E. The Faroese IBD study: incidence of inflmmatory bowel disease acrosee 54 years of population-based data. J Crohn’s Colitis. (2016) 10:934–42. doi: 10.1093/ecco-jcc/jjw050
Crossref Full Text | Google Scholar
3. Ouahed J, Spencer E, Kotlarz D, Shouval DS, Kowalik M, Peng K, et al. Very early onset inflammatory bowel disease: a clinical approach with a focus on the role of genetics and underlying immune deficiencies. Inflamm Bowel Dis. (2019) 26(6):820–42. doi: 10.1093/ibd/izz259
PubMed Abstract | Crossref Full Text | Google Scholar
4. Sykora J, Pomahacova R, Kreslova M, Cvalinova D, Stych P, Schwarz J. Current global trends in the incidence of pediatric-onset inflammatory bowel disease. World J Gastroenterol. (2018) 24(25):2741–63. doi: 10.3748/wjg.v24.i25.2741
PubMed Abstract | Crossref Full Text | Google Scholar
5. Carroll MW, Kuenzig ME, Mack DR, Otley AR, Griffiths AM, Kaplan GG, et al. The impact of inflammatory bowel disease in Canada 2018: children and adolescents with IBD. J Can Assoc Gastroenterol. (2019) 2(Suppl 1):S49–67. doi: 10.1093/jcag/gwy056
PubMed Abstract | Crossref Full Text | Google Scholar
6. Atia O, Benchimol EI, Ledderman N, Greenfeld S, Kariv R, Weisband YL, et al. Incidence, management, and outcomes of very early onset inflammatory bowel diseases and infantile-onset disease: an epi-IIRN study. Clin Gastroenterol Hepatol. (2023) 21(10):2639–48.e6. doi: 10.1016/j.cgh.2022.10.026
PubMed Abstract | Crossref Full Text | Google Scholar
7. Bramuzzo M, Arrigo S, Romano C, Filardi MC, Lionetti P, Agrusti A, et al. Efficacy and safety of infliximab in very early onset inflammatory bowel disease: a national comparative retrospective study. United European Gastroenterol J. (2019) 7(6):759–66. doi: 10.1177/2050640619847592
PubMed Abstract | Crossref Full Text | Google Scholar
8. Kerur B, Benchimol EI, Fiedler K, Stahl M, Hyams J, Stephens M, et al. Natural history of very early onset inflammatory bowel disease in North America: a retrospective cohort study. Inflamm Bowel Dis. (2021) 27(3):295–302. doi: 10.1093/ibd/izaa080
PubMed Abstract | Crossref Full Text | Google Scholar
9. Heyman MB, Kirschner BS, Gold BD, Ferry G, Baldassano R, Cohen SA, et al. Children with early-onset inflammatory bowel disease (IBD): analysis of a pediatric IBD consortium registry. J Pediatr. (2005) 146(1):35–40. doi: 10.1016/j.jpeds.2004.08.043
PubMed Abstract | Crossref Full Text | Google Scholar
10. Srivastava A, Sathiyasekharan M, Jagadisan B, Bolia R, Peethambaran M, Mammayil G, et al. Paediatric inflammatory bowel disease in India: a prospective multicentre study. Eur J Gastroenterol Hepatol. (2020) 32(10):1305–11. doi: 10.1097/MEG.0000000000001859
PubMed Abstract | Crossref Full Text | Google Scholar
11. Kuenzig ME, Fung SG, Marderfeld L, Mak JWY, Kaplan GG, Ng SC, et al. Twenty-first century trends in the global epidemiology of pediatric-onset inflammatory bowel disease: systematic review. Gastroenterology. (2022) 162(4):1147–59 e4. doi: 10.1053/j.gastro.2021.12.282
PubMed Abstract | Crossref Full Text | Google Scholar
12. Charbit-Henrion F, Parlato M, Hanein S, Duclaux-Loras R, Nowak J, Begue B, et al. Diagnostic yield of next-generation sequencing in very early-onset inflammatory bowel diseases: a multicentre study. J Crohns Colitis. (2018) 12(9):1104–12. doi: 10.1093/ecco-jcc/jjy068
PubMed Abstract | Crossref Full Text | Google Scholar
13. Poddar U, Aggarwal A, Jayalakshmi K, Sarma MS, Srivastava A, Rawat A, et al. Higher prevalence of monogenic cause among very early onset inflammatory bowel disease in children: experience from a tertiary care center from northern India. Inflamm Bowel Dis. (2023) 29(10):1572–8. doi: 10.1093/ibd/izac254
PubMed Abstract | Crossref Full Text | Google Scholar
14. Banerjee R, Pal P, Nabi Z, Shava U, Ganesh G, Reddy DN. Very early onset inflammatory bowel disease in a south Asian country where inflammatory bowel disease is emerging: a distinct clinical phenotype from later onset disease. Intest Res. (2021) 19(4):398–407. doi: 10.5217/ir.2020.00107
PubMed Abstract | Crossref Full Text | Google Scholar
15. Kudo T, Arai K, Uchida K, Tajiri H, Hokari R, Suzuki Y, et al. Very early-onset inflammatory bowel disease in Japan: a nationwide survey. J Gastroenterol Hepatol. (2021) 36(1):151–5. doi: 10.1111/jgh.15146
PubMed Abstract | Crossref Full Text | Google Scholar
16. Kudo T, Fujii T, Maisawa SI, Sasaki M, Uchida K, Ida S, et al. Multicenter prospective survey on early-onset inflammatory bowel disease in Japan. Digestion. (2021) 102(3):368–76. doi: 10.1159/000507570
PubMed Abstract | Crossref Full Text | Google Scholar
17. Huang JG, Wong YKY, Chew KS, Tanpowpong P, Calixto Mercado KS, Reodica A, et al. Epidemiological characteristics of Asian children with inflammatory bowel disease at diagnosis: insights from an Asian-pacific multi-centre registry network. World J Gastroenterol. (2022) 28(17):1830–44. doi: 10.3748/wjg.v28.i17.1830
PubMed Abstract | Crossref Full Text | Google Scholar
18. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. (2009) 42(2):377–81. doi: 10.1016/j.jbi.2008.08.010
PubMed Abstract | Crossref Full Text | Google Scholar
19. Turner D, Otley AR, Mack D, Hyams J, de Bruijne J, Uusoue K, et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology. (2007) 133(2):423–32. doi: 10.1053/j.gastro.2007.05.029
PubMed Abstract | Crossref Full Text | Google Scholar
20. Hyams J, Markowitz J, Otley A, Rosh J, Mack D, Bousvaros A, et al. Evaluation of the pediatric crohn disease activity index: a prospective multicenter experience. J Pediatr Gastroenterol Nutr. (2005) 41(4):416–21. doi: 10.1097/01.mpg.0000183350.46795.42
PubMed Abstract | Crossref Full Text | Google Scholar
21. Wang XQ, Zhang Y, Xu CD, Jiang LR, Huang Y, Du HM, et al. Inflammatory bowel disease in Chinese children: a multicenter analysis over a decade from Shanghai. Inflamm Bowel Dis. (2013) 19(2):423–8. doi: 10.1097/MIB.0b013e318286f9f2
PubMed Abstract | Crossref Full Text | Google Scholar
22. Oliva-Hemker M, Hutfless S, Al Kazzi ES, Lerer T, Mack D, LeLeiko N, et al. Clinical presentation and five-year therapeutic management of very early-onset inflammatory bowel disease in a large North American cohort. J Pediatr. (2015) 167(3):527–32.e1-3. doi: 10.1016/j.jpeds.2015.04.045
PubMed Abstract | Crossref Full Text | Google Scholar
23. Ledder O, Catto-Smith AG, Oliver MR, Alex G, Cameron DJ, Hardikar W. Clinical patterns and outcome of early-onset inflammatory bowel disease. J Pediatr Gastroenterol Nutr. (2014) 59(5):562–4. doi: 10.1097/MPG.0000000000000465
PubMed Abstract | Crossref Full Text | Google Scholar
24. Aloi M, Lionetti P, Barabino A, Guariso G, Costa S, Fontana M, et al. Phenotype and disease course of early-onset pediatric inflammatory bowel disease. Inflamm Bowel Dis. (2014) 20(4):597–605. doi: 10.1097/01.MIB.0000442921.77945.09
PubMed Abstract | Crossref Full Text | Google Scholar
25. Bequet E, Sarter H, Fumery M, Vasseur F, Armengol-Debeir L, Pariente B, et al. Incidence and phenotype at diagnosis of very-early-onset compared with later-onset paediatric inflammatory bowel disease: a population-based study [1988–2011]. J Crohns Colitis. (2017) 11(5):519–26. doi: 10.1093/ecco-jcc/jjw194
PubMed Abstract | Crossref Full Text | Google Scholar
26. Lee WS, Arai K, Alex G, Treepongkaruna S, Kim KM, Choong CL, et al. Medical management of pediatric inflammatory bowel disease (PIBD) in the Asia pacific region: a position paper by the Asian pan-pacific society for pediatric gastroenterology, hepatology, and nutrition (APPSPGHAN) PIBD working group. J Gastroenterol Hepatol. (2022) 38:510–38. doi: 10.1111/jgh.16097
Crossref Full Text | Google Scholar
27. Usami M, Takeuchi I, Kyodo R, Hirano Y, Kashiwagi K, Fujikawa H, et al. Clinical features of very early-onset inflammatory bowel disease in Japan: a retrospective single-center study. Intest Res. (2022) 20:475–81. doi: 10.5217/ir.2021.00142
PubMed Abstract | Crossref Full Text | Google Scholar
28. Chapuy L, Leduc B, Godin D, Damphousse A, Patey N, Dal Soglio D, et al. Phenotype and outcomes of very early onset and early onset inflammatory bowel disease in a Montreal pediatric cohort. Front Pediatr. (2023) 11:1157025. doi: 10.3389/fped.2023.1157025
PubMed Abstract | Crossref Full Text | Google Scholar
29. Shim JO. Recent advances in very early onset inflammatory bowel disease. Pediatr Gastroenterol Hepatol Nutri. (2022) 1:41–9. doi: 10.5223/pghn.2019.22.1.41
Crossref Full Text | Google Scholar
30. Fang YH, Luo YY, Yu JD, Lou JG, Chen J. Phenotypic and genotypic characterization of inflammatory bowel disease in children under six years of age in China. World J Gastroenterol. (2018) 24(9):1035–45. doi: 10.3748/wjg.v24.i9.1035
PubMed Abstract | Crossref Full Text | Google Scholar
31. Crowley E, Warner N, Pan J, Khalouei S, Elkadri A, Fiedler K, et al. Prevalence and clinical features of inflammatory bowel disease with monogenic variants, identified by whole exome sequencing in 1000 children at a single center. Gastroenterology. (2020) 158(8):2208–20. doi: 10.1053/j.gastro.2020.02.023
留言 (0)