In recent years, the incidence of thyroid cancer has been gradually increasing. In 2022, the prevalence of thyroid cancer in China was 33.02 per 100,000, ranking third among all malignant tumors in women (1). Thyroidectomy is the primary treatment for thyroid cancer. The accurate identification and preservation of parathyroid gland (PG) during thyroidectomy are critical to prevent postoperative complications such as hypocalcemia (2, 3). It is widely accepted that the incidence of temporary and permanent hypoparathyroidism after surgery ranges from 5.94% to 67.69% and 0% to 20% respectively (4). However, in some center, the incidence of permanent hypoparathyroidism has been reported to exceed 30% (5). Accurate identification and timely autotransplantation can significantly reduce the incidence of hypoparathyroidism and enhance the quality of life for patients (6).
Near-infrared autofluorescence (NIRAF) imaging has emerged as a promising technique for the intraoperative detection of PGs (7, 8). This non-invasive imaging modality provides real-time visualization of PGs, assisting surgeons in accurately locating and preserving these vital structures during surgery. However, NIRAF also has certain limitations. While NIRAF has demonstrated high sensitivity and precision in identifying PGs in certain patients (9, 10), the fluorescence intensity (FI) can vary significantly due to factors such as surrounding anatomical structures and disease pathology of PG (11, 12). False-positive results may also occur when other tissues, such as thyroid or adipose tissues, exhibit similar autofluorescence characteristics, leading to misidentification of PGs (13). Conversely, false-negative results can arise when PGs do not exhibit sufficient fluorescence, especially in cases of pathological changes like parathyroid adenomas or hyperplasia, which can alter the autofluorescence properties of the tissues (14, 15).
Currently, clinical researches on the variations in FI of normal PGs across different thyroid diseases is relatively limited. In this study, we investigated the FI of PGs in patients with various thyroid and parathyroid diseases, aiming to optimize the application of NIRAF for more effective surgical guidance.
2 Methods 2.1 Study designThis is a prospective cohort study conducted at the Shanghai Sixth People’s Hospital, involving patients who underwent thyroidectomy and parathyroidectomy between August 2021 and September 2023. All surgeries were performed by the same treatment team with more than 10 years of surgical experience. This study was approved by the human subjects ethics board of Shanghai Sixth People's Hospital [Approval No: 2022-075-(1)]. The written informed consent statements were obtained from all patients in this study.
2.2 Inclusion and exclusion criteriaInclusion criteria were: (i) age >18 years, (ii) condition fulfilled the surgical indications, (iii) first-time thyroidectomy or parathyroidectomy, (iv) intraoperative use of the NIRAF device. Exclusion criteria were: history of thyroidectomy or parathyroidectomy.
2.3 Surgical procedureThe commercially available NIRAF device (ARGOS 300PT, Microscopic Intelligence Co., China) was used in the study for detecting PGs. The device consists of a NIRAF camera, a computer system, and a monitor. The NIRAF camera, which emits near-infrared (NIR) light at 785 nm and detects the autofluorescence of PGs at 820 nm, was positioned at a fixed height of 15 cm above the surgical field. The angle of illumination was maintained perpendicular to the tissue surface to ensure consistent light exposure, and the autofluorescence signal was captured as a bright grayscale image displayed on the monitor. Before detecting PGs, ambient light was turned off to minimize interference from external light sources. During the procedure, the NIRAF camera was operated by a single trained assistant, who adjusted its position as needed until the PG was clearly imaged on the monitor to reduce inter-observer variability.
Open thyroidectomy with NIRAF was performed in three stages: Stage I (Surgical field assessment after thyroid membrane dissection), Stage II (Surgical field assessment after thyroid gland removal), and Stage III (Assessment of removed specimens). Stages I and III were used during parathyroidectomy. Only Stage III was used during laparoscopic thyroidectomy. The visualization results from all three stages are shown in Figures 1, 2. If PGs were not detected in one stage, further detection was carried out in the subsequent stage.
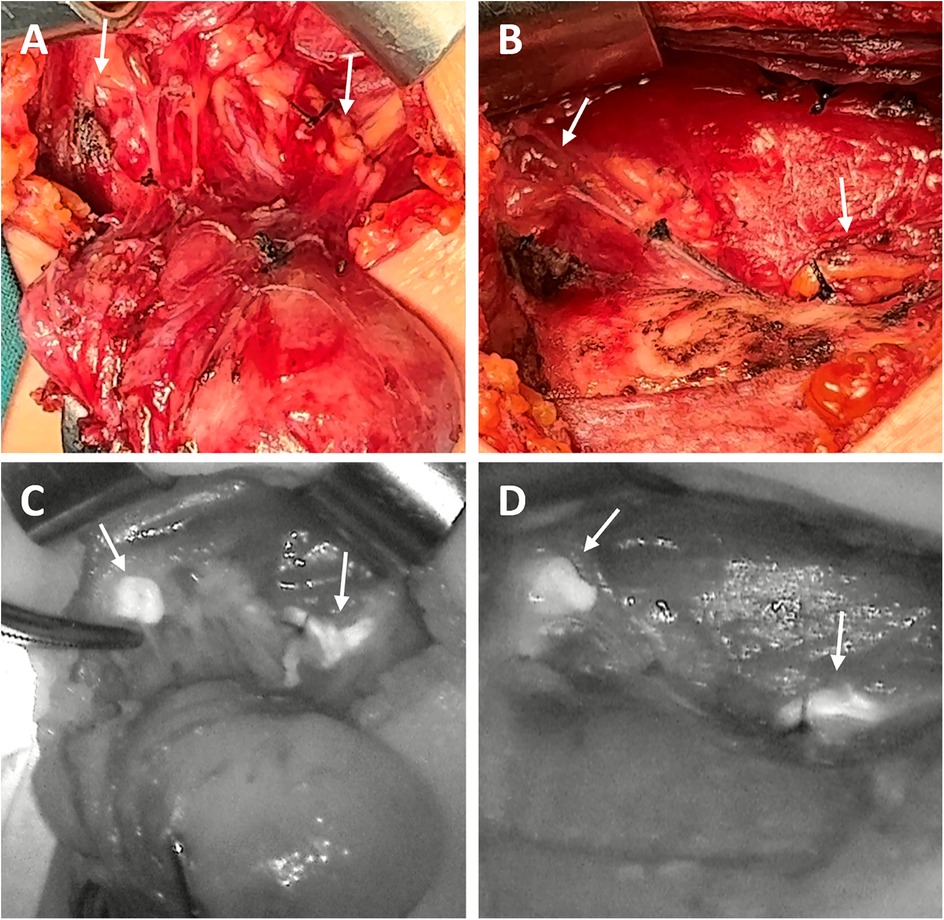
Figure 1. The surgical images (A,B) and corresponding NIRAF images (C,D) of PG detection in one patient. Two normal PGs (indicated by arrows) with intraoperative autofluorescence with NIRAF in Stage I (A,C) and in Stage II (B,D).
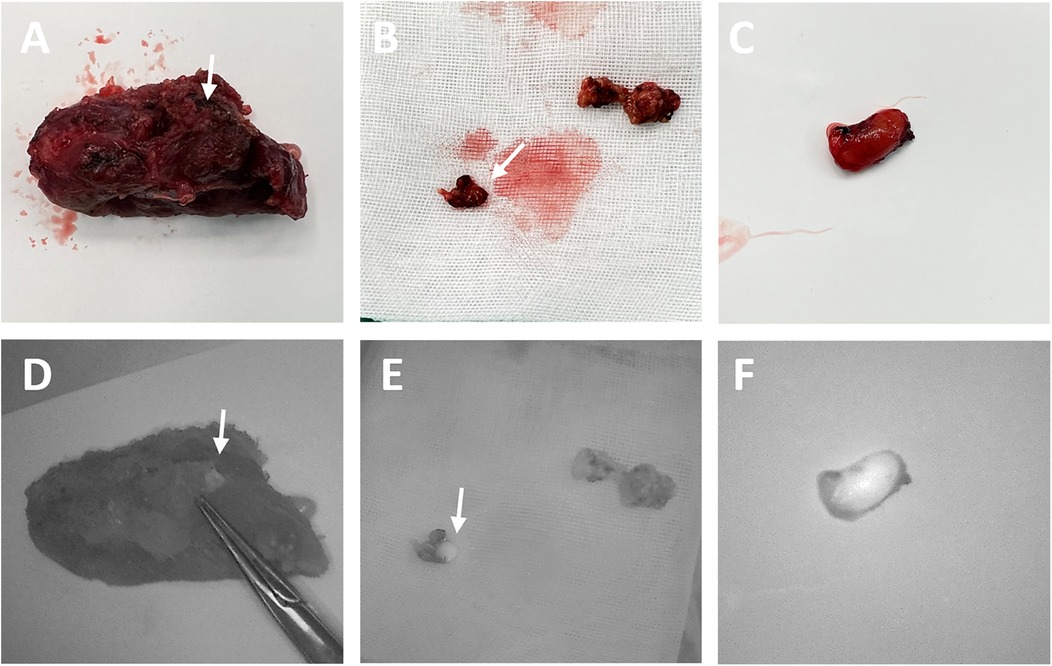
Figure 2. The surgical images (A–C) and corresponding NIRAF images (D–F) of PG detection in stage III. The inadvertently excised PGs (indicated by arrows) were identified using NIRAF in the isolated thyroid gland (D), central lymph nodes (E) and suspected PG tissue (F).
PG validation was performed using the immune colloidal gold technique (ICGT), which measures parathyroid hormone (PTH) levels and has demonstrated superior diagnostic accuracy compared to frozen pathological examination (16). ICGT was applied to all specimens suspected to be PGs based on the surgeon's visual inspection or NIRAF detection, including both PGs preserved in the surgical field and those removed during the procedure, to ensure no potential PGs were overlooked. Each excised specimen, including false positive tissues, was pathologically examined according to the 5th edition of the WHO classification of thyroid neoplasms (17). All acquired NIR image data were systematically archived for further analysis.
2.4 Image processingThe FI values of the target tissues and background were quantified using ImageJ software. The region of interest (ROI) tool was employed to outline the borders of the PG, thyroid gland, central lymph node, false-positive tissue and background, allowing for the measurement of their mean FI values within the range of 0–255 pixels. The FI values for all target tissues were normalized against the FI of the background (target tissue FI/background FI). The background was defined as the same side of the surgical field in Stage I and II, while a similarly sized region overlying the isolated tissues served as the background in Stage III (10).
2.5 NIRAF efficiencyThe identification rate for both surgeon's naked eyes and NIRAF were calculated. The number of true positives(TP), true negatives(TN), false positives(FP) and false negatives(FN) identified by NIRAF were recorded in Stage I–III. To evaluate the performance of NIRAF at different surgical stages, the identification results of PGs in each stage were treated as independent samples. Additionally, the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated.
The number and location of PGs at different stages were recorded, and the false-positive and false-negative results were analyzed. Each normal PG was grouped according to the pathological findings of the ipsilateral thyroid lobe (the side from which the parathyroid glands were examined) to analyze the FI values.
2.6 Statistical analysisStatistical analysis was performed using SPSS software (version 26.0). Data normality was assessed using the Kolmogorov–Smirnov test. Continuous variables were presented as mean ± standard deviation (SD), while categorical variables were presented as frequencies and percentages. Categorical variables were compared using the χ2 test or Fisher's exact test, whereas continuous variables were analyzed using the independent t-test or one-way ANOVA with the posthoc analysis correction using the Bonferroni test for multiple comparisons.
3 ResultsThe clinical characteristics of the 105 patients are presented in Table 1. A total of 239 PGs were identified during surgery, with 81.2% (194/239) identified visually by surgeons and 94.1% (225/239) detected using NIRAF. Postoperative pathological examination confirmed PGs in 8 patients, with 4 detected in thyroid glands and 4 in central lymph nodes. The NIRAF identification rate was significantly higher than visual identification (p < 0.001). Among the detected PGs, 167 were identified in Stage I, 172 in Stage II, and 52 in Stage III. The mis-cut rate of PGs was 7.7% (17/221) and the autotransplantation rate was 16.3% (36/221) in thyroidectomy. A total of 411 true positives, 43 false positives, 148 true negatives, and 20 false negatives were recorded across all stages. Based on these values, the sensitivity, specificity, PPV, and NPV of NIRAF were calculated as 95.4%, 77.5%, 90.5%, and 88.1%, respectively.

Table 1. Clinical characteristics of 105 patients in study.
According to the thyroid and parathyroid pathological findings from 105 patients, the results included 88 cases of papillary thyroid carcinoma (PTC), 1 case of follicular thyroid carcinoma (FTC), 15 cases of nodular thyroid gland, 8 cases of follicular nodules (FND), 2 cases of normal thyroid, 8 cases of hyperthyroidism, 3 cases of thyroid hyperplasia, and 13 cases of primary hyperparathyroidism (PHPT). The distribution of the normalized FI values for PGs in different diseases is shown in Figure 3 and Table 2. The FI of PGs was significantly higher in PTC and follicular nodules compared to PHPT (p < 0.001; p = 0.006) and hyperthyroidism (p = 0.006; p = 0.033). After applying the Bonferroni correction, the comparison between follicular nodules and hyperthyroidism was not statistically significant. Additionally, FI in PGs with nodular thyroid gland was significantly higher compared to hyperthyroidism (p = 0.027). No significant difference in FI was observed between patients with Hashimoto's thyroiditis (HT) and those without HT (p = 0.902).
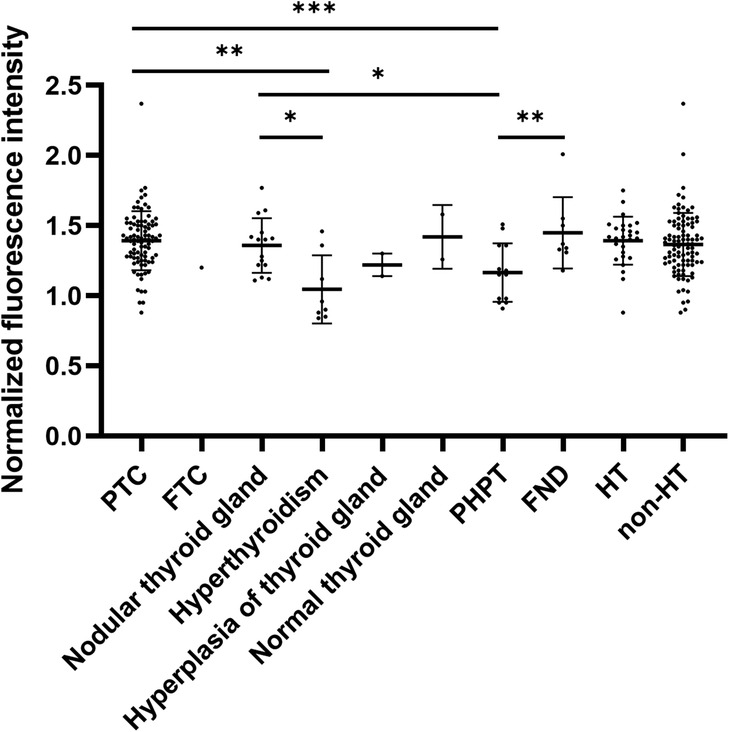
Figure 3. Normalized FI of PGs in ipsilateral thyroid pathology and PHPT. *p < 0.05, **p < 0.01, ***p < 0.001.
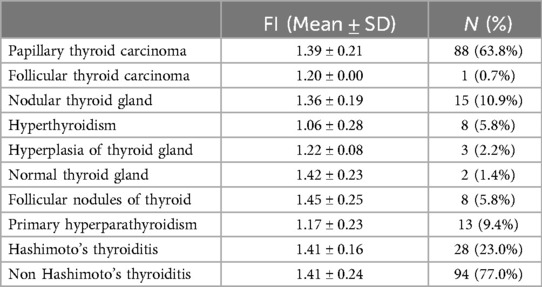
Table 2. Normalized FI of PGs in different diseases of thyroid lobe and PHPT.
In Stage II, inferior PGs exhibited significantly higher FI than superior PGs (p < 0.001). The FI of PGs was also significantly higher in Stage I and Stage II compared to Stage III. The FI of PGs was significantly higher than that of the thyroid gland in Stage I (p < 0.001). The FI values of PGs, thyroid glands, and central lymph nodes across the three stages are detailed in Table 3.
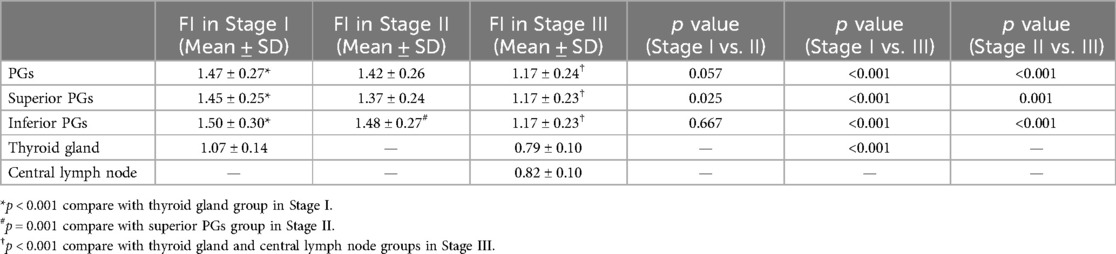
Table 3. Normalized FI of PG, thyroid gland, central lymph node in different stages.
False positives included tissues from PTC, normal thyroid, nodular thyroid glands, scabs, adipose tissue, and central lymph nodes. The FI of scabs and nodular thyroid tissue was significantly higher than that of other tissues (Table 4). False-negative PGs were observed in 7/88 (7.95%) PTC cases, 2/8 (25.0%) hyperthyroidism cases, 1/8 (12.5%) FND cases, and 2/13 (15.38%) PHPT cases.
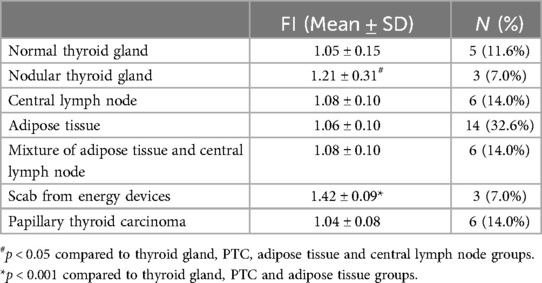
Table 4. Normalized FI of false-positive tissues.
4 DiscussionThe identification and preservation of PGs are crucial in thyroid surgery to prevent postoperative complications such as hypoparathyroidism and hypocalcemia. Due to their delicate structure and similarity to surrounding tissues, PGs are particularly vulnerable to injury during surgery (4, 18). While visual identification remains the primary method for detecting PGs, its accuracy highly depends on the surgeon's experience (5), which reported rates ranging from 61% to 93.6% (19–21). Even experienced surgeons may miss PGs during the procedure. Advanced techniques such as nano-carbon technology have improved PG identification (22, 23), but their accuracy is compromised in cases of lymphatic or tumor obstruction (23). Additionally, the high cost and reliance on visual identification limit their widespread use. Frozen section pathological examination and ICGT have demonstrated high accuracy and are considered reliable adjuncts in PG identification (16, 24). Therefore, we utilize ICGT to validate suspected PG in our study.
NIRAF imaging has emerged as a promising technique for real-time, non-invasive PG identification. Paras et al. demonstrated that PGs exhibit maximum FI at 820–830 nm under 785 nm NIR light excitation (25). This technology enables surgeons to detect PGs at any time, facilitating in situ preservation or timely autotransplantation, thereby reducing complications (26). Indocyanine green (ICG) is the natural dye, which can enhance the FI of PGs when combined with NIRAF (27). However, the low tissue specificity of ICG may cause fluorescence signals in thyroid and surrounding tissues, thereby interfering with the accurate identification of PGs by NIRAF (28). In our study, we did not employ the combination of ICG and NIRAF, focusing instead on the intrinsic fluorescence properties of NIRAF for PG identification.
Our study confirmed that NIRAF significantly improves PG identification rates compared to visual inspection, with a sensitivity of 95.4% and a positive predictive value of 90.5%, consistent with previous studies (19, 29, 30). In this study, the FI of PGs was based on the pathological results of the ipsilateral thyroid lobe (the side from which PGs were examined) and PHPT themselves rather than patients' number. This approach ensures consistency in the analysis and minimizes potential confounding effects from the contralateral thyroid lobe, enabling a systematic investigation into the relationship between the pathology of thyroid and parathyroid diseases and PG autofluorescence. However, false-negative results of PGs were observed, particularly in cases with PTC (7/88, 7.95%), hyperthyroidism (2/8, 25.0%), FND (1/8, 12.5%), and PHPT (2/13, 15.38%). To further investigate, we calculated the normalized FI values of PGs across different thyroid diseases. The blood supply to PGs is primarily provided by the inferior thyroid artery or directly from the thyroid gland itself (31). Therefore, PGs associated with hyperthyroidism, thyroid hyperplasia exhibited lower FI, likely due to hypervasular nature of these tissues. Previous study have demonstrated that blood has a higher absorption coefficient for NIR light than other tissues (11), increased vascularity in these conditions may enhance light absorption, reducing FI of PGs. These findings emphasize the need for cautious interpretation of NIRAF images in hyperthyroidism and thyroid hyperplasia, as lower FI may increase the risk of inadvertent PG excision.
Additionally, NIRAF occasionally identified false-positive tissues, such as thyroid gland, lymph nodes, adipose tissue, and scabs from energy devices. We calculated the FI values of different false-positive tissues separately and found that the scabs from energy devices with the highest FI value. Due to the discrepancy between the morphology and location of scabs and PGs, they are easily distinguishable by surgeons. However, in some patients, false positives may occur where PTC or nodular thyroid gland is adjacent to or protrudes from the thyroid peritoneum. Additionally, false positives can arise from adipose tissues and lymph nodes, introducing challenges in visual identification. Therefore, when autofluorescence is detected on the thyroid, it may be advisable to incise the thyroid surface to determine whether the fluorescence originates from the thyroid tumor or PGs within the gland (32, 33). Furthermore, in cases where the presence of false positive tissues remains uncertain, ICGT technology provides a reliable approach to reduce the risk of misjudgment.
We also observed dynamic FI variations across surgical stages. Superior PGs showed higher FI in Stage I, while inferior PGs exhibited increased FI in Stage II. Currently, there is limited research addressing the FI differences between superior and inferior PGs. We hypothesize that, given the FI of PGs remains consistent post-excision, the in vivo variations may be linked to factors such as adipose tissue exposure or vascularization in the surgical field (11). Superior PGs, being more fixed and less covered, are readily identifiable by NIRAF early in surgery. In contrast, inferior PGs, often obscured by adipose tissue and lymph nodes, exhibit lower FI initially but show increased FI as surgery progresses and these tissues are dissected. This dynamic change likely reflects improved visualization and vascular exposure of inferior PGs, highlighting the importance of meticulous dissection and real-time FI monitoring for PG identification and preservation. We observed the lower FI in Stage III compared to Stages I and II, which we attribute to changes in the reference background. During Stage III, the PGs are typically situated within surrounding tissues, such as lymph nodes, thyroid glands, or adipose tissues. These tissues, which dominate the field of view, exhibit inherent autofluorescence that interferes with the FI of the PGs, complicating their detection. Therefore, careful interpretation of NIRAF signals is essential during Stage III to minimize the risk of missing PGs.
Interestingly, both HT and non-HT patients exhibited higher FI of PGs compared to other diagnoses, although no significant difference was observed between HT and non-HT groups. Given that HT was not the primary surgical indication and both groups encompassed heterogeneous diagnoses of thyroid diseases, we hypothesize that the higher FI may be due to confounding factors. Our preliminary analysis suggests that HT is not the significant determinant of PG autofluorescence. Future studies with larger sample sizes and a specific focus on thyroiditis are warranted to elucidate the relationship between HT and PG autofluorescence.
To our knowledge, this is the first study to classify PG FI values based on the pathological results of the ipsilateral thyroid lobe, providing a comprehensive understanding of autofluorescence variations across different thyroid diseases. However, our study has limitations, including a small sample size, particularly for 1 case of FTC and 2 cases of normal thyroid glands. These cases were excluded from the final analysis, as their inclusion would not provide meaningful statistical power or reliable results. Secondly, this was a single-center study, which may introduce center-specific biases. Future research should aim to include a more diverse range of participants from multiple centers to validate and extend the current results. Additionally, further investigation into the molecular mechanisms underlying the variations in fluorescence intensity could provide valuable insights into the autofluorescence properties of parathyroid tissues.
5 ConclusionsNIRAF has demonstrated high efficiency in identifying PGs at various stages in surgery, surpassing conventional visual identification by surgeons. The FI of superior and inferior PGs exhibits significant variability across different intraoperative stages. Special attention during surgery is required when identifying PGs in patients with PHPT and hyperthyroidism, as the PGs exhibit lower FI compared to those in patients with PTC or nodular thyroid disease.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe studies involving humans were approved by Institutional Ethics Review Board and the Ethics Committee of Shanghai Sixth People's Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributionsFY: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. XY: Formal Analysis, Visualization, Writing – review & editing. ZL: Data curation, Visualization, Writing – review & editing. YW: Data curation, Writing – review & editing. QL: Methodology, Project administration, Supervision, Writing – review & editing. BW: Funding acquisition, Project administration, Validation, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Interdisciplinary Program of Shanghai Jiao Tong University (No. YG2023LC10).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References2. Swift WM, Iorio CB, Hamdi OA, Mallawaarachchi I, Wages NA, Shonka DC Jr. Change in parathyroid hormone levels from baseline predicts hypocalcemia following total or completion thyroidectomy. Head Neck. (2022) 44(7):1588–95. doi: 10.1002/hed.27057
PubMed Abstract | Crossref Full Text | Google Scholar
3. Liu RH, Razavi CR, Chang HY, Tufano RP, Eisele DW, Gourin CG, et al. Association of hypocalcemia and magnesium disorders with thyroidectomy in commercially insured patients. JAMA Otolaryngol Head Neck Surg. (2020) 146(3):237–46. doi: 10.1001/jamaoto.2019.4193
PubMed Abstract | Crossref Full Text | Google Scholar
4. Koimtzis GD, Stefanopoulos L, Giannoulis K, Papavramidis TS. What are the real rates of temporary hypoparathyroidism following thyroidectomy? It is a matter of definition: a systematic review. Endocrine. (2021) 73(1):1–7. doi: 10.1007/s12020-021-02663-8
PubMed Abstract | Crossref Full Text | Google Scholar
5. Abood A, Ovesen T, Rolighed L, Triponez F, Vestergaard P. Hypoparathyroidism following total thyroidectomy: high rates at a low-volume, non-parathyroid institution. Front Endocrinol. (2024) 15:1330524. doi: 10.3389/fendo.2024.1330524
PubMed Abstract | Crossref Full Text | Google Scholar
6. Koimtzis G, Stefanopoulos L, Geropoulos G, Papavramidis T. The outcomes of parathyroid gland autotransplantation during thyroid surgery: a systematic review, meta-analysis and trial sequential analysis. Endocrine. (2025) 87(1):27–38. doi: 10.1007/s12020-024-04011-y
PubMed Abstract | Crossref Full Text | Google Scholar
7. Takeuchi M, Takahashi T, Shodo R, Ota H, Ueki Y, Yamazaki K, et al. Comparison of autofluorescence with near-infrared fluorescence imaging between primary and secondary hyperparathyroidism. Laryngoscope. (2021) 131(6):E2097–104. doi: 10.1002/lary.29310
PubMed Abstract | Crossref Full Text | Google Scholar
8. Domoslawski P, Adamiecki M, Antkowiak L, Pasko K, Chabowski M, Grzegrzolka J, et al. Influence of single experience with intraoperative near-infrared autofluorescence on postoperative parathyroid insufficiency after thyroidectomy—a preliminary clinical study. Int J Med Sci. (2022) 19(8):1334–9. doi: 10.7150/ijms.72886
PubMed Abstract | Crossref Full Text | Google Scholar
9. Dip F, Falco J, Verna S, Prunello M, Loccisano M, Quadri P, et al. Randomized controlled trial comparing white light with near-infrared autofluorescence for parathyroid gland identification during total thyroidectomy. J Am Coll Surg. (2019) 228(5):744–51. doi: 10.1016/j.jamcollsurg.2018.12.044
PubMed Abstract | Crossref Full Text | Google Scholar
10. Kose E, Rudin AV, Kahramangil B, Moore E, Aydin H, Donmez M, et al. Autofluorescence imaging of parathyroid glands: an assessment of potential indications. Surgery. (2020) 167(1):173–9. doi: 10.1016/j.surg.2019.04.072
PubMed Abstract | Crossref Full Text | Google Scholar
11. Han YS, Kim Y, Lee HS, Kim Y, Ahn YC, Lee KD. Detectable depth of unexposed parathyroid glands using near-infrared autofluorescence imaging in thyroid surgery. Front Endocrinol. (2023) 14:1170751. doi: 10.3389/fendo.2023.1170751
PubMed Abstract | Crossref Full Text | Google Scholar
12. Kose E, Kahramangil B, Aydin H, Donmez M, Berber E. Heterogeneous and low-intensity parathyroid autofluorescence: patterns suggesting hyperfunction at parathyroid exploration. Surgery. (2019) 165(2):431–7. doi: 10.1016/j.surg.2018.08.006
PubMed Abstract | Crossref Full Text | Google Scholar
13. De Leeuw F, Breuskin I, Abbaci M, Casiraghi O, Mirghani H, Ben Lakhdar A, et al. Intraoperative near-infrared imaging for parathyroid gland identification by auto-fluorescence: a feasibility study. World J Surg. (2016) 40(9):2131–8. doi: 10.1007/s00268-016-3571-5
PubMed Abstract | Crossref Full Text | Google Scholar
14. Idogawa H, Sakashita T, Homma A. A novel study for fluorescence patterns of the parathyroid glands during surgery using a fluorescence spectroscopy system. Eur Arch Otorhinolaryngol. (2020) 277(5):1525–9. doi: 10.1007/s00405-020-05849-4
PubMed Abstract | Crossref Full Text | Google Scholar
15. DiMarco A, Chotalia R, Bloxham R, McIntyre C, Tolley N, Palazzo FF. Autofluorescence in parathyroidectomy: signal intensity correlates with serum calcium and parathyroid hormone but routine clinical use is not justified. World J Surg. (2019) 43(6):1532–7. doi: 10.1007/s00268-019-04929-9
PubMed Abstract | Crossref Full Text | Google Scholar
16. Xia W, Zhang J, Shen W, Zhu Z, Yang Z, Li X. A rapid intraoperative parathyroid hormone assay based on the immune colloidal gold technique for parathyroid identification in thyroid surgery. Front Endocrinol. (2020) 11:594745. doi: 10.3389/fendo.2020.594745
PubMed Abstract | Crossref Full Text | Google Scholar
17. Baloch ZW, Asa SL, Barletta JA, Ghossein RA, Juhlin CC, Jung CK, et al. Overview of the 2022 WHO classification of thyroid neoplasms. Endocr Pathol. (2022) 33(1):27–63. doi: 10.1007/s12022-022-09707-3
PubMed Abstract | Crossref Full Text | Google Scholar
18. Papavramidis TS, Chorti A, Tzikos G, Anagnostis P, Pantelidis P, Pliakos I, et al. The effect of intraoperative autofluorescence monitoring on unintentional parathyroid gland excision rates and postoperative PTH concentrations-a single-blind randomized-controlled trial. Endocrine. (2021) 72(2):546–52. doi: 10.1007/s12020-020-02599-5
PubMed Abstract | Crossref Full Text | Google Scholar
19. Takahashi T, Yamazaki K, Ota H, Shodo R, Ueki Y, Horii A. Near-infrared fluorescence imaging in the identification of parathyroid glands in thyroidectomy. Laryngoscope. (2021) 131(5):1188–93. doi: 10.1002/lary.29163
PubMed Abstract | Crossref Full Text | Google Scholar
20. Solorzano CC, Thomas G, Baregamian N, Mahadevan-Jansen A. Detecting the near infrared autofluorescence of the human parathyroid: hype or opportunity? Ann Surg. (2020) 272(6):973–85. doi: 10.1097/SLA.0000000000003700
PubMed Abstract | Crossref Full Text | Google Scholar
21. Thomas G, Solorzano CC, Baregamian N, Mannoh EA, Gautam R, Irlmeier RT, et al. Comparing intraoperative parathyroid identification based on surgeon experience versus near infrared autofluorescence detection—a surgeon-blinded multi-centric study. Am J Surg. (2021) 222(5):944–51. doi: 10.1016/j.amjsurg.2021.05.001
PubMed Abstract | Crossref Full Text | Google Scholar
22. Lin DX, Zhuo XB, Lin Y, Lei WD, Chang GJ, Zhang Y, et al. Enhancing parathyroid preservation in papillary thyroid carcinoma surgery using nano-carbon suspension. Sci Rep. (2024) 14(1):24680. doi: 10.1038/s41598-024-76126-1
PubMed Abstract | Crossref Full Text | Google Scholar
23. Liu J, Xu C, Wang R, Han P, Zhao Q, Li H, et al. Do carbon nanoparticles really improve thyroid cancer surgery? A retrospective analysis of real-world data. World J Surg Oncol. (2020) 18(1):84. doi: 10.1186/s12957-020-01852-5
PubMed Abstract | Crossref Full Text | Google Scholar
24. Wang B, Zhu Y, Zhou S, Lu C, Zhang A, Tian J, et al. The effect of carbon nanoparticles vs. immune colloidal gold technique test strips on parathyroid protection in total thyroidectomy: a randomized clinical trial study. Head Neck. (2024) 46(7):1727–36. doi: 10.1002/hed.27685
PubMed Abstract | Crossref Full Text | Google Scholar
25. Paras C, Keller M, White L, Phay J, Mahadevan-Jansen A. Near-infrared autofluorescence for the detection of parathyroid glands. J Biomed Opt. (2011) 16(6):067012. doi: 10.1117/1.3583571
PubMed Abstract | Crossref Full Text | Google Scholar
26. Silver Karcioglu AL, Triponez F, Solorzano CC, Iwata AJ, Abdelhamid Ahmed AH, Almquist M, et al. Emerging imaging technologies for parathyroid gland identification and vascular assessment in thyroid surgery: a review from the American head and neck society endocrine surgery section. JAMA Otolaryngol Head Neck Surg. (2023) 149(3):253–60. doi: 10.1001/jamaoto.2022.4421
PubMed Abstract | Crossref Full Text | Google Scholar
27. Vidal Fortuny J, Sadowski SM, Belfontali V, Guigard S, Poncet A, Ris F, et al. Randomized clinical trial of intraoperative parathyroid gland angiography with indocyanine green fluorescence predicting parathyroid function after thyroid surgery. Br J Surg. (2018) 105(4):350–7. doi: 10.1002/bjs.10783
PubMed Abstract | Crossref Full Text | Google Scholar
28. Alesina PF, Meier B, Hinrichs J, Mohmand W, Walz MK. Enhanced visualization of parathyroid glands during video-assisted neck surgery. Langenbecks Arch Surg. (2018) 403(3):395–401. doi: 10.1007/s00423-018-1665-2
PubMed Abstract | Crossref Full Text | Google Scholar
29. Mohan Kumar R, Pannu A, Metcalfe E, Senbeto M, Balasubramanian SP. Findings of pilot study following the implementation of point of care intraoperative PTH assay using whole blood during surgery for primary hyperparathyroidism. Front Endocrinol. (2023) 14:1198894. doi: 10.3389/fendo.2023.1198894
PubMed Abstract | Crossref Full Text | Google Scholar
30. Kuo TC, Chen KY, Lai CW, Lin MT, Chang CH, Wu MH. Analysis of near-infrared autofluorescence imaging for detection of inadvertently resected parathyroid glands after endoscopic thyroidectomy. Eur J Surg Oncol. (2024) 50(11):108648. doi: 10.1016/j.ejso.2024.108648
PubMed Abstract | Crossref Full Text | Google Scholar
31. Burger F, Fritsch H, Zwierzina M, Prommegger R, Konschake M. Postoperative hypoparathyroidism in thyroid surgery: anatomic-surgical mapping of the parathyroids and implications for thyroid surgery. Sci Rep. (2019) 9(1):15700. doi: 10.1038/s41598-019-52189-3
PubMed Abstract | Crossref Full Text | Google Scholar
32. Ward CJ, Kelly YM, Syed SM, Meier RPH, Ando T, Wisel SA, et al. Procurement of deceased donor parathyroid glands with the aid of near-infrared autofluorescence imaging. Transplant Direct. (2022) 8(4):e1306. doi: 10.1097/TXD.0000000000001306
留言 (0)