Prader-Willi syndrome (PWS) is a complex, rare multisystem genetic disorder with an estimated prevalence of 1/10,000–1/30,000 (1). There are three main classes of chromosomal abnormalities accounting for PWS: paternal 15q11–q13 deletions (60% of cases), maternal uniparental disomy (UPD) of chromosome 15 (36% of cases) or an imprinting defect (4% of cases) (2).
A complex hypothalamic-pituitary dysfunction is currently thought to be responsible for the entire PWS phenotype with implications on the endocrine and neurologic systems (3). Individuals with PWS have an increased risk of metabolic complications, including severe obesity (4). In the Italian PWS registry, morbid obesity was observed in 54.5% of individuals enrolled in 2019 – 2020 period (5). Moreover, PWS is often associated with type 2 diabetes (T2D), that occurs in 10-25% of PWS subjects, usually in adulthood (6). The management of obesity and T2D in these subjects is still a matter of debate and no specific treatment has been established yet.
Due to their concomitant effect on glycemic control and body weight (BW), Glucagon-like Peptide 1 receptor agonists (GLP-1 RA) may represent an appealing option. Yet, literature is still limited to few reports of PWS subjects treated with exenatide or liraglutide (7–18), and with no more that 5 cases (19, 20) using with once weekly semaglutide as an add-on therapy. To the best of our knowledge, no data are currently available on semaglutide monotherapy in PWS. We herein describe the efficacy and safety of 24 months of once-weekly semaglutide monotherapy in a young Caucasian male with PWS, obesity and T2D.
Case descriptionIn December 2019, a 27-years-old Caucasian male was admitted to the Emergency Department of the University Hospital of Pisa for polyuria and polydipsia in the past 4-6 weeks, associated with fever and vomiting in the last 5 days. Upon admission, lab tests documented severe hyperglycemia (fasting plasma glucose 22.5 mmol/L) with no evidence of acidosis (blood gas analysis: pH of 7.42, pO2 59 mmHg, pCO2 of 37 mmHg, HCO3- of 24 mmol/L). Serum C-reactive protein level was 9.91 mg/dl (normal value <0.5 mg/dl) and procalcitonin level 11.14 ng/mL (normal value <0.05 ng/mL). Chest X-rays revealed reticular and micronodular interstitial involvement with pleural effusion at the bases, suggestive of pneumonia. The patient was transferred to our Section of Diabetes and Metabolic Diseases with a diagnosis of severe hyperglycemia in new-onset diabetes mellitus and sepsis. On admission to the ward, the patient was conscious. The clinical picture was characterized by dysmorphic facies with narrow minimal frontal diameter, almond-shaped eyes and thin upper lip, small hands and feet, scoliosis, gynecomastia, testicular hypoplasia, and bilateral lower limb swelling. His BW was 66 kg, and height was 148 cm, with a body mass index (BMI) of 30.1 kg/m2. Systolic blood pressure was 125 mmHg, diastolic blood pressure 80 mmHg, pulse rate 90 beats per minute, body temperature 38,5°C. The rest of the physical examination was unremarkable. Fasting plasma glucose (FPG) was 23.3 mmol/L and HbA1c 116 mmol/mol. The search of islet autoantibodies (i.e., glutamic acid decarboxylase autoantibodies, anti-GAD and islet tyrosine phosphatase autoantibodies, anti-IA2) was negative. Urine albumin to creatinine ratio (ACR) in a spot urine sample was 104.5 mg/g (normal value < 30 mg/g). Liver and renal function tests were within the normal range. Insulin-like Growth Factor 1 (IGF-1) values were within the lower limits of the reference range for age (96.6 µg/L). Gonadotropin and testosterone levels were consistent with mixed hypogonadism (FSH 22.6 UI/L; LH 7.1 UI/L; testosterone 1.69 μg/L; sex hormone binding globulin, SHBG, 42 nmoL/L), while thyroid and adrenal function was normal (data not shown). Blood cultures were positive for Candida glabrata. Microscopic urinalysis excluded urinary tract infections. Screening for retinopathy, hypertension and cardiovascular disease was uneventful. No foot problems were observed. Abdominal ultrasound was normal, while testicular ultrasound described small testes with hypoechoic echotexture and breast ultrasound confirmed the presence of true gynecomastia. Bone mineral density (BMD), measured by dual-energy X-ray absorptiometry (DXA) at the lumbar spine, femoral neck and total hip, was consistent with osteopenia (lumbar Z-score -2.3, femoral neck Z-score -2.3, total hip Z-score -2.2). Familial history was negative for neurodevelopmental delay, genetic syndromes, and diabetes mellitus. Consanguinity was not present in the family. The patient was treated with i.v. fluid and insulin infusion, and caspofungin was started to target C. glabrata. Upon hyperglycemia and sepsis resolution, a basal-bolus insulin therapy was initiated (total daily insulin requirement 0.4 unit per kg of BW, approximately 26 U/day) yielding a progressive improvement of daily glucose profiles. Insulin was subsequently managed by the parents because the patient was not self-sufficient. At discharge, the patient was equipped with the FreeStyle Libre Flash Glucose Monitoring System (Abbot Diabetes Care, Alameda, California, USA). In the meantime, a PWS was suspected and, after obtaining written informed consent from both parents, the genetic test was performed. The methylation-specific multiplex ligation-dependent probe amplification (MS-MLPA) identified 2 copies of chromosome 15q11, and an abnormal DNA methylation with 2 methylated copies. Short tandem repeat (STR) linkage analysis excluded uniparental disomy and suggested the imprinting defect by epimutation. At discharge the patient was prescribed testosterone but no GH replacement therapy, in accordance with the Endocrinology consultant. Because of the COVID-19 pandemic, the patient was only seen in the outpatient clinic in March 2022 when glycemic control was at target (FPG 4.89 mmol/L; HbA1c 41 mmol/mol) despite discontinuation of the Flash Glucose Monitoring. Several non-severe hypoglycemic events were reported. A 13 kg body weight gain (from 66 to 79 kg with a BMI of 36 kg/m2) was observed. Given the poor adherence to diet and family burden in the management of insulin treatment and in the light of negative antibodies, insulin therapy was discontinued, and once-weekly semaglutide started at the initial dose of 0.25 mg per week, gradually increased to 0.5 mg per week. No metformin was given due to the swallowing difficulties of the patient. Twelve months after starting semaglutide, BW dropped from 79 to 73 kg (absolute change -6 kg; -7.6%; Figure 1), with a final BMI of 33.3 kg/m2, and glycemic control remained at target (FPG 5.9 mmol/L; HbA1c 40 mmol/mol; Figure 2). Neither hypoglycemia nor side effects were reported. At 18-month follow-up, a slight weight gain was observed (+ 2 kg; 75 kg; Figure 1) without deterioration in glycemic control (FPG 5.9 mmol/L; HbA1c 42 mmol/mol; Figure 2). Semaglutide was continued at 0.5 mg once weekly, with a personalized physical activity program and nutritional intervention. To support family members in diabetes management, FreeStyle Libre 2 sensor was also prescribed. The 14 days ambulatory glucose profile (AGP) before the 18-month visit showed a time in range (TIR) of 94%, a time below range (TBR) of 3%, and a time above range (TAR) of 3%, time with active data 71%, glucose management indicator (GMI) 41 mmol/mol and coefficient of variation (CV) 23.5%. After 24 months semaglutide treatment, BW was 71 kg (absolute change -4 kg vs. 18-month follow-up and -8 kg vs. baseline; Figure 1) with persistent optimal glycemic control (FPG 5.4 mmol/L; HbA1c 38 mmol/mol) (Figure 2). No foot, micro- and macrovascular complications were observed. Urine ACR was within the normal range (11.4 mg/g). No hypoglycemia nor gastro-intestinal or psychiatric adverse events were reported; adherence to semaglutide was optimal and caregivers’ satisfaction high.
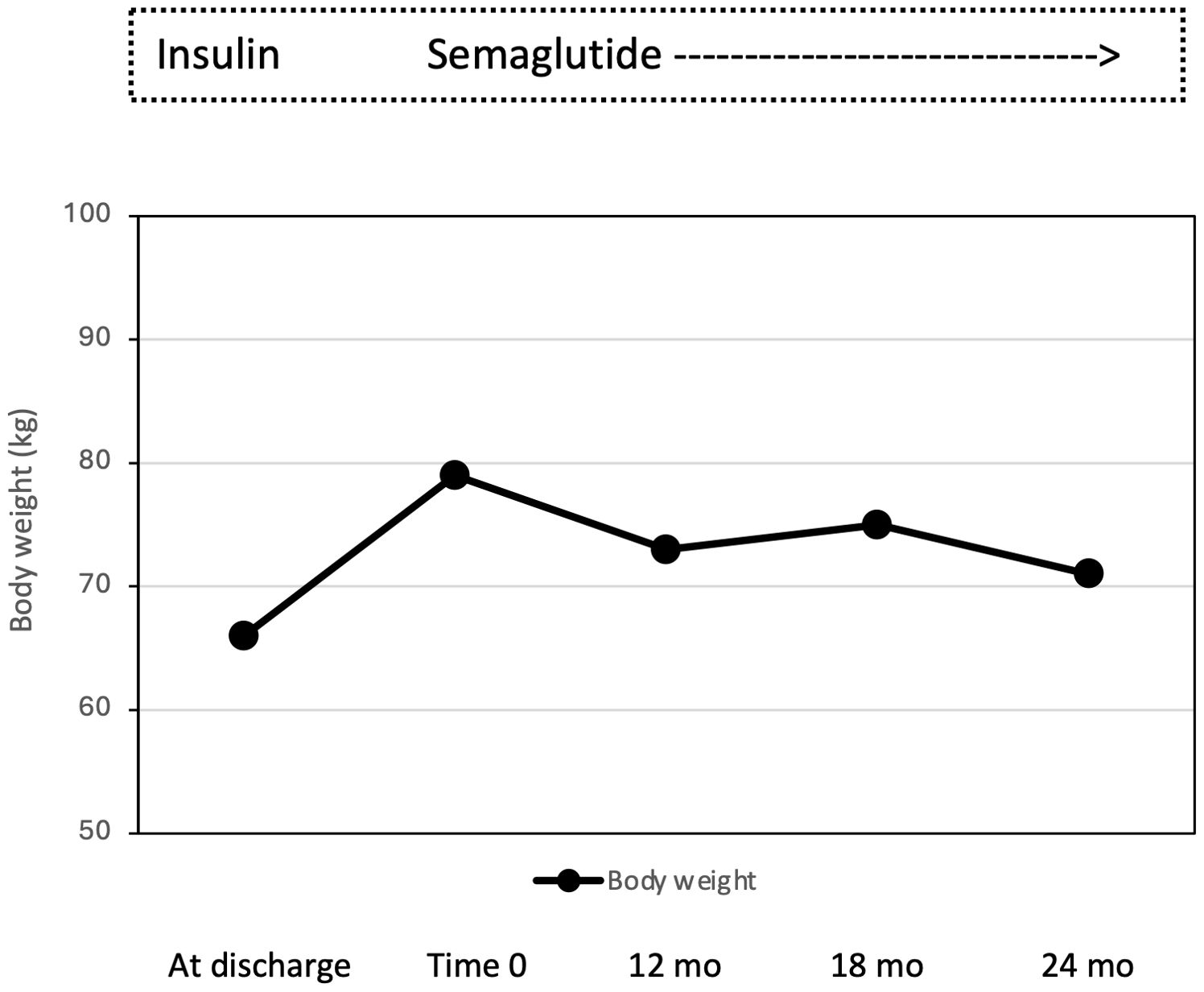
Figure 1. Effect of semaglutide monotherapy on body weight from time 0 to 12, 18 and 24 months (mo).
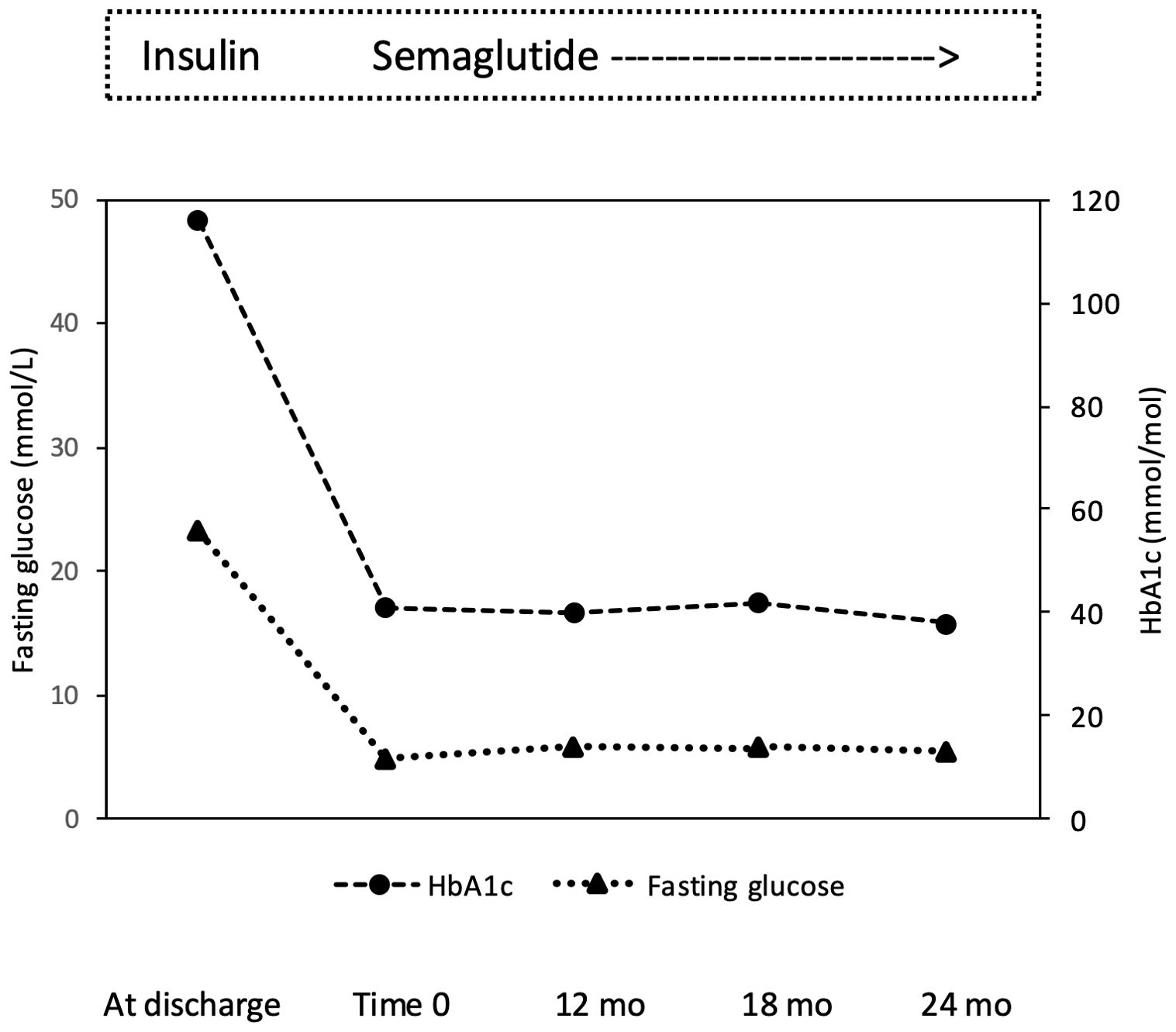
Figure 2. Effect of semaglutide monotherapy on fasting plasma glucose and HbA1c from time 0 to 12, 18 and 24 months (mo).
Materials and methodsBlood samples were obtained between 8 and 9 a.m. after an overnight fast. Urine samples were collected as first morning spot urine sample. All laboratory and hormonal parameters were determined according to standard methods. Anti-GAD and anti-IA2 autoantibodies were analyzed by a radioimmunoassay using a commercial kit (Medipan, Berlin, Germany). GFR was estimated from creatinine by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (21). Albuminuria was evaluated by measuring the ration between albumin concentration in milligrams by creatinine concentration in grams in a spot urine sample. Multiplex ligation probe amplification was applied to identify abnormal methylation of the PWS region of chromosome 15. MLPA reagents were obtained from MRC-Holland (Amsterdam, The Netherlands; SALSA MLPA kit ME028). Data analysis was performed with Coffalyser.Net software obtained from MRC-Holland (Amsterdam, The Netherlands). The proband and his parents were genotyped for 9 short tandem repeats (STR) located in the typical PWS and Angelman syndrome (AS) deletion region and for 3 STRs located in other chromosomes by polymerase chain reaction amplification and separation on an automated ABI-3500 DNA sequencer. The polymorphic markers were analyzed by GeneScan3.1 software (Applied Biosystems, Foster City, CA, USA). The location of the STRs was obtained from UCSC Genome Bioinformatics (https://genome-euro.ucsc.edu; build 37/hg19).
DiscussionTo the best of our knowledge, this is the first case to evaluate the efficacy and safety of once-weekly semaglutide monotherapy in a young subject with PWS, obesity, and T2D.
In PWS, controlling weight and T2D remains critical to mitigate associated morbidity and mortality. However, no definitive treatment strategy has been established (22). GLP-1 RA have been shown to be quite effective in lowering plasma glucose levels with no risk of hypoglycemia and to favor body weight reduction (23). Because of the latter, GLP-1 RA, in particular liraglutide and semaglutide, are currently indicated for the pharmacologic treatment of obesity in individuals with and without diabetes (24). A such GLP-1 RA may be a therapeutic option in PWS subjects, yet the literature on this use is still scanty. The efficacy and safety profile of exenatide and liraglutide in PWS have been explored in few studies (25) and, to the best of our knowledge, the use of semaglutide as an add-on to other antidiabetic drugs has been reported only in 5 cases of PWS (19, 20). We now report data on the efficacy and safety of once-weekly semaglutide monotherapy in a young adult with PWS, obesity and T2D showing a meaningful body weight reduction, attainment of optimal glycemic control, good tolerability and no hypoglycemic events.
The use of exenatide and liraglutide in PWS has resulted in high variability of body weight changes, with BMI reduction ranging from 1.5 to 16.0 kg/m2 (25) or without significant effects, as showed in a recent trial that investigated the effect of liraglutide on weight management in children and adolescents with PWS and obesity (26). On the other hand, obesity in PWS subjects shows distinct phenotypic and metabolic characteristics that are not common to simple obesity (1). Moreover, several underlying mechanisms have been hypothesized (4), probably accounting for the variability in response to therapies.
In the case reported by Sani et al. (19), where semaglutide was added to insulin therapy, after 12 months, body weight went from 99.5 to 94.3 kg, along with a reduction in fat mass and insulin requirements. More heterogeneous are the weight loss reported by Giménez-Palop et al. (20). In our case, semaglutide (0.5 mg per week) allow body weight to go from 79 to 71 kg with an absolute change -8 kg, i.e., -10.1%. Given that a ≥5% weight loss is currently considered as clinically meaningful, our results are then of solid clinical relevance. The variability between our study as well as among the few reported observation should not surprise given the interindividual variability among different GLP-1 RA (27); rather all the so far available evidence support the potential beneficial effect of this class of agents in PWS. In our patient, along with marked body weight reduction, semaglutide monotherapy was highly effective in controlling plasma glucose levels over 2-year treatment. Moreover, we replaced insulin by once weekly semaglutide yet ensuring excellent glucose metabolic profile in a person fully dependent and with intellectual disability, without increasing the risk of hypoglycemic events and the burden to treatment management for the family members. Our subject had no sign of diabetes complication at the time of diagnosis apart from an increased ACR. Interestingly enough, ACR was normalized at the end of the observation period which may reflect the reduction in body weight and the persistent glycemic control as obtained with semaglutide, although a renal protective effect of semaglutide has been demonstrated in randomized clinical trials (28).
There are some limitations to this study. First, this is a single case report, thus, the results may be partially different from other studies and should be interpreted with caution. Second, changes in body composition were not assessed during the study. As a final limitation of the study, circulating levels of orexigenic and anorexigenic hormones were also not evaluated. However, unless of important clinical reasons, the assessment of body composition and the evaluation of hormone milieu in a single case may be limited by a variety of reasons (i.e., radioprotection, economic reasons, lack of facilities).
ConclusionIn conclusion, this PWS case report shows a persistent effect of semaglutide 0,5 mg as monotherapy on body weight reduction and glycemic control. Our case differs to some extent with what obtained in other PWS cases highlighting the need of randomized controlled trials exploring long-term efficacy and safety of semaglutide, even at higher doses, in these patients. Understanding the role of GLP-1 RA in PWS may offer a new and effective therapeutic opportunity for a condition still orphan of an evidence-based recommendation for the treatment of PWS with obesity and type 2 diabetes.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementEthical approval was not required for the studies involving humans for a case report study in accordance with the local legislation and institutional requirements. Informed written consent was expressed by the parents, with the support of a linguistic mediator. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributionsED: Data curation, Investigation, Validation, Writing – original draft, Writing – review & editing, Visualization. GD: Data curation, Visualization, Writing – review & editing, Validation. AM: Writing – review & editing, Data curation, Validation, Visualization. FB: Data curation, Validation, Visualization, Writing – review & editing. FC: Data curation, Validation, Visualization, Writing – review & editing. PM: Validation, Supervision, Visualization, Writing – review & editing. SDP: Supervision, Validation, Visualization, Writing – review & editing. AD: Conceptualization, Supervision, Validation, Visualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
AcknowledgmentsWe appreciate our patient and his family for the participation in this study. We also thank all nurses of the Section of Diabetes and Metabolic Diseases for the clinical support.
Conflict of interestED, AD, AM, FB, FC: no duality of interest. GD: Eli lilly Italia. PM: Eli lilly Italia, Novo Nordisk, Gruppo Menarini, Laboratori Guidotti. SDP: Abbott, Amarin Corporation, AstraZeneca, Berlin Chemie AG, Biomea Fusion, Boehringer Ingelheim, Laboratori Guidotti, Menarini International, Novartis Pharmaceuticals Co., Merck Sharpe & Dohme, Eli Lilly and Company, Novo Nordisk, Sanofi, Sun Pharmaceuticals.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References2. Butler MG, Hartin SN, Hossain WA, Manzardo AM, Kimonis V, Dykens E, et al. Molecular genetic classification in Prader–Willi syndrome: a multisite cohort study. J Med Genet. (2019) 56:149–53. doi: 10.1136/jmedgenet-2018-105301
PubMed Abstract | Crossref Full Text | Google Scholar
3. Tauber M, Hoybye C. Endocrine disorders in Prader-Willi syndrome: a model to understand and treat hypothalamic dysfunction. Lancet Diabetes Endocrinol. (2021) 9:235–46. doi: 10.1016/S2213-8587(21)00002-4
PubMed Abstract | Crossref Full Text | Google Scholar
4. Muscogiuri G, Barrea L, Faggiano F, Maiorino MI, Parrillo M, Pugliese G, et al. Obesity in Prader-Willi syndrome: physiopathological mechanisms, nutritional and pharmacological approaches. J Endocrinol Invest. (2021) 44:2057–70. doi: 10.1007/s40618-021-01574-9
PubMed Abstract | Crossref Full Text | Google Scholar
5. Salvatore M, Torreri P, Grugni G, Rocchetti A, Maghnie M, Patti G, et al. The Italian registry for patients with Prader-Willi syndrome. Orphanet J Rare Dis. (2023) 18:28. doi: 10.1186/s13023-023-02633-5
PubMed Abstract | Crossref Full Text | Google Scholar
7. Sze L, Purtell L, Jenkins A, Loughnan G, Smith E, Herzog H, et al. Effects of a single dose of exenatide on appetite, gut hormones, and glucose homeostasis in adults with Prader-Willi syndrome. J Clin Endocrinol Metab. (2011) 96:E1314–9. doi: 10.1210/jc.2011-0038
PubMed Abstract | Crossref Full Text | Google Scholar
8. Cyganek K, Koblik T, Kozek E, Wojcik M, Starzyk J, Malecki MT. Liraglutide therapy in Prader-Willi syndrome. Diabetes Med. (2011) 28:755–6. doi: 10.1111/j.1464-5491.2011.03280.x
PubMed Abstract | Crossref Full Text | Google Scholar
9. Paisey R, Bower L, Rosindale S, Lawrence C. Successful treatment of obesity and diabetes with incretin analogue over four years in an adult with Prader–Willi syndrome. Pract Diabetes. (2011) 28:306–7. doi: 10.1002/pdi.1621
Crossref Full Text | Google Scholar
10. Seetho IW, Jones G, Thomson GA, Fernando DJ. Treating diabetes mellitus in Prader-Willi syndrome with Exenatide. Diabetes Res Clin Pract. (2011) 92:e1–2. doi: 10.1016/j.diabres.2010.12.009
PubMed Abstract | Crossref Full Text | Google Scholar
11. Senda M, Ogawa S, Nako K, Okamura M, Sakamoto T, Ito S. The glucagon-like peptide-1 analog liraglutide suppresses ghrelin and controls diabetes in a patient with Prader-Willi syndrome. Endocr J. (2012) 59:889–94. doi: 10.1507/endocrj.ej12-0074
PubMed Abstract | Crossref Full Text | Google Scholar
12. Fintini D, Grugni G, Brufani C, Bocchini S, Cappa M, Crinò A. Use of GLP-1 receptor agonists in Prader-Willi Syndrome: report of six cases. Diabetes Care. (2014) 37:e76–7. doi: 10.2337/dc13-2575
PubMed Abstract | Crossref Full Text | Google Scholar
13. Salehi P, Hsu I, Azen CG, Mittelman SD, Geffner ME, Jeandron D. Effects of exenatide on weight and appetite in overweight adolescents and young adults with Prader-Willi syndrome. Pediatr Obes. (2017) 12:221–8. doi: 10.1111/ijpo.12131
PubMed Abstract | Crossref Full Text | Google Scholar
14. Horikawa Y, Enya M, Komagata M, Hashimoto KI, Kagami M, Fukami M, et al. Effectiveness of sodium-glucose cotransporter-2 inhibitor as an add-on drug to GLP-1 receptor agonists for glycemic control of a patient with prader-willi syndrome: A case report. Diabetes Ther. (2018) 9:421–6. doi: 10.1007/s13300-018-0369-5
PubMed Abstract | Crossref Full Text | Google Scholar
15. Kim YM, Lee YJ, Kim SY, Cheon CK, Lim HH. Successful rapid weight reduction and the use of liraglutide for morbid obesity in adolescent Prader-Willi syndrome. Ann Pediatr Endocrinol Metab. (2020) 25:52–6. doi: 10.6065/apem.2020.25.1.52
PubMed Abstract | Crossref Full Text | Google Scholar
16. Sano H, Kudo E, Yamazaki T, Ito T, Hatakeyama K, Kawamura N. Efficacy of sodium-glucose cotransporter 2 inhibitor with glucagon-like peptide-1 receptor agonist for the glycemic control of a patient with Prader-Willi syndrome: a case report. Clin Pediatr Endocrinol. (2020) 29:81–4. doi: 10.1297/cpe.29.81
PubMed Abstract | Crossref Full Text | Google Scholar
17. Candler T, McGregor D, Narayan K, Moudiotis C, Burren CP. Improvement in glycaemic parameters using SGLT-2 inhibitor and GLP-1 agonist in combination in an adolescent with diabetes mellitus and Prader-Willi syndrome: a case report. J Pediatr Endocrinol Metab. (2020) 33:951–5. doi: 10.1515/jpem-2019-0389
PubMed Abstract | Crossref Full Text | Google Scholar
18. Ahmed S, Naz A KM. Weight loss of over 100 lbs in a patient of prader-willi syndrome treated with glucagon-like peptide-1 (GLP-1) agonists. Cureus. (2023) 15:e35102. doi: 10.7759/cureus.35102
PubMed Abstract | Crossref Full Text | Google Scholar
19. Sani E, Prato GD, Zenti MG, Bordugo A, Trombetta M, Bonora E. Effects of semaglutide on glycemic control and weight loss in a patient with prader-willi syndrome: A case report. Endocr Metab Immune Disord Drug Targets. (2022) 22:1053–7. doi: 10.2174/1871530322666220509225637
PubMed Abstract | Crossref Full Text | Google Scholar
20. Giménez-Palop O, Romero A, Casamitjana L, Pareja R, Rigla M, Caixàs A. Effect of semaglutide on weight loss and glycaemic control in patients with Prader-Willi Syndrome and type 2 diabetes. Endocrinol Diabetes Nutr (Engl Ed). (2024) 71:83–7. doi: 10.1016/j.endien.2023.12.001
PubMed Abstract | Crossref Full Text | Google Scholar
21. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
PubMed Abstract | Crossref Full Text | Google Scholar
22. Crinò A, Fintini D, Bocchini S, Grugni G. Obesity management in Prader-Willi syndrome: current perspectives. Diabetes Metab Syndr Obes. (2018) 11:579–93. doi: 10.2147/DMSO.S141352
PubMed Abstract | Crossref Full Text | Google Scholar
24. Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art. Mol Metab. (2021) 46:101102. doi: 10.1016/j.molmet.2020.101102
PubMed Abstract | Crossref Full Text | Google Scholar
25. Ng NBH, Low YW, Rajgor DD, Low JM, Lim YY, Loke KY, et al. The effects of glucagon-like peptide (GLP)-1 receptor agonists on weight and glycaemic control in Prader-Willi syndrome: A systematic review. Clin Endocrinol (Oxf). (2022) 96:144–54. doi: 10.1111/cen.14583
PubMed Abstract | Crossref Full Text | Google Scholar
26. Diene G, Angulo M, Hale PM, Jepsen CH, Hofman PL, Hokken-Koelega A, et al. Liraglutide for weight management in children and adolescents with prader-willi syndrome and obesity. J Clin Endocrinol Metab. (2022) 108:4–12. doi: 10.1210/clinem/dgac549
PubMed Abstract | Crossref Full Text | Google Scholar
27. Nauck MA, Meier JJ. Management Of Endocrine Disease: Are all GLP-1 agonists equal in the treatment of type 2 diabetes? Eur J Endocrinol. (2019) 181:R211–34. doi: 10.1530/EJE-19-0566
PubMed Abstract | Crossref Full Text | Google Scholar
28. Perkovic V, Tuttle KR, Rossing P, Mahaffey KW, Mann JFE, Bakris G, et al. Effects of semaglutide on chronic kidney disease in patients with type 2 diabetes. N Engl J Med. (2024) 391:109–21. doi: 10.1056/NEJMoa240334
留言 (0)