The incidence and mortality of lung cancer are among the highest in China and the world (1). Non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) are two common histopathologic types (2). Advanced NSCLC patients with epidermal growth factor receptor (EGFR)-sensitive mutations can be treated with small-molecule tyrosine kinase inhibitors (TKIs) as first-line therapy. However, drug resistance inevitably develops within 10-13 months (3, 4). The resistance mechanisms of EGFR TKIs mainly include T790M mutation, cell-mesenchymal epithelial transition factor (C-MET) and human epidermal growth factor receptor 2 (HER2) gene amplification, kirsten rat sarcoma viral oncogene (KRAS) gene mutation, vrafmurine sarcoma viral oncegene homolog B (BRAF) gene mutation, and SCLC transformation (5–9). Some drug-resistant cases can be overcome by replacing the corresponding targeted drugs with better results, but the transformation of the pathological type brings great confusion to the clinical diagnosis and treatment process. In this paper, we report a case of advanced lung adenocarcinoma with EGFR-sensitive mutation that was transformed into small-cell lung cancer after EGFR TKIs treatment. The histological transformation from adenocarcinoma to small cell lung cancer may involve phenotypic changes in tumor cells, which could be related to genetic variations and clonal selection within the tumor. Given the heterogeneity and dynamic nature of the tumor, continuous monitoring and personalized treatment are crucial for these patients. Therefore, in the subsequent treatment process, we repeatedly performed needle biopsies and guided treatment decisions based on the pathological results. The patient received anlotinib-containing therapy together with chemotherapy and/or immunotherapy after transformation to achieve survival benefit. The case is reported as follows, along with a literature review for clinical reference.
Case presentationA 48-year-old male patient with no history of smoking or alcohol consumption. He was diagnosed with right lung adenocarcinoma cT2N0M1a (pleural metastasis) stage IV A in June 2020, with EGFR 19 exon deletion mutation, negative for anaplastic lymphoma kinase (ALK), KRAS, proto-oncogene 1, receptor tyrosine kinase (ROS1), MET, BRAF. Gefitinib was started on June 3, 2020 and continued with good response.
Disease progression was confirmed in September 2021. Subsequently, right lung biopsy was performed and pathological diagnosis was small cell carcinoma with immunohistochemistry: Pan-cytokeratin (PCK) (partial +), chromogranin A (CgA) (+), synaptophysin (Syn) (+), neural cell adhesion molecule-1 (NCAM-1/CD56) (+), thyriod transcription factor-1 (TTF-1) (+), cytokeratin-7 (CK7) (-), napsinA (-), Ki-67 (Li:70%). Etoposide and platinum (EP) regimen is commonly used in the treatment of classical SCLC. Meanwhile, considering the poor treatment efficacy and rapid disease progression often observed after SCLC transformation, we choose the treatment regimen of EP combined with anlotinib, an oral tyrosine kinase inhibitor. The patient started treatment with anlotinib together with EP regimen on October 2, 2021. After six cycles of anlotinib in combination with EP, patients with a partial response received oral anlotinib as maintenance therapy (Figures 1–3).
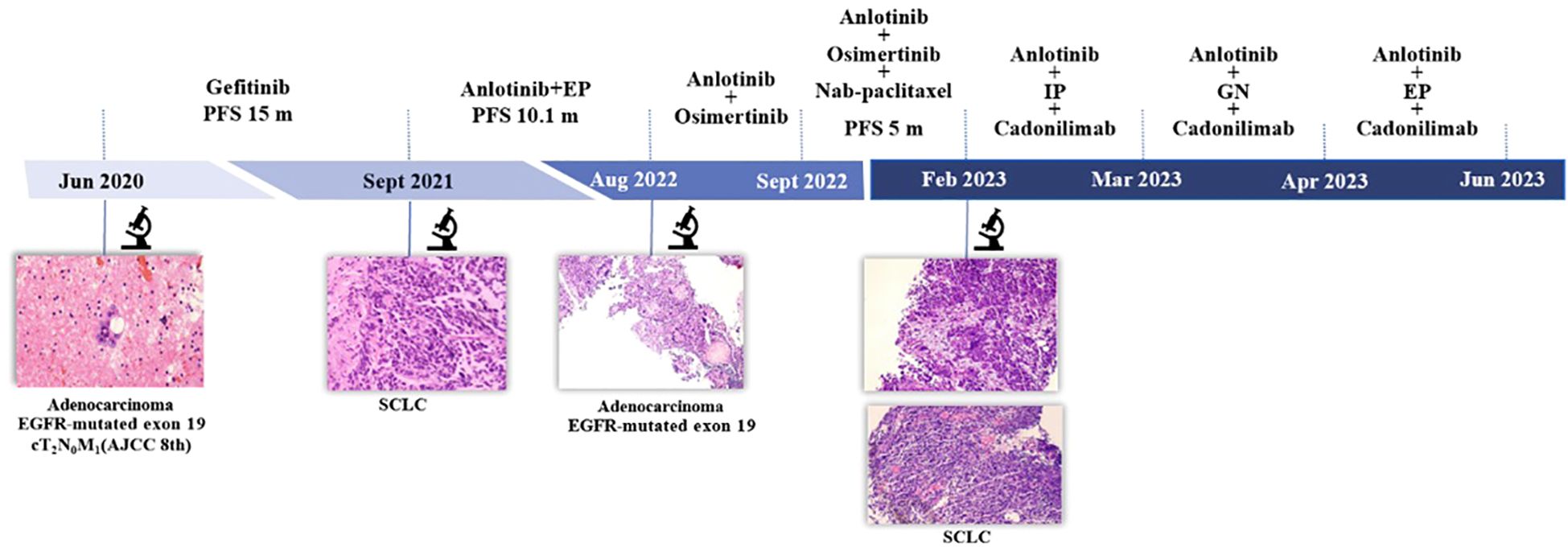
Figure 1. Timeline. AJCC 8th, American Joint Committee on Cancer; EP, etoposide and cisplatin; IP, irinotecan and cisplatin; GN, gemcitabine and vinorelbine; PFS, progression-free survival; SCLC, small cell lung cancer.
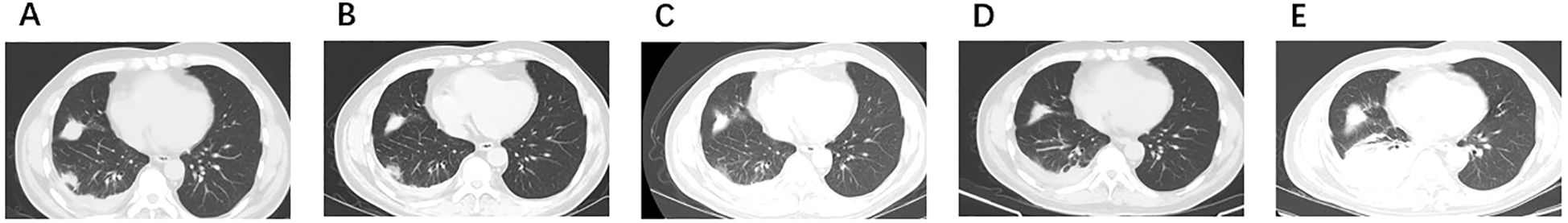
Figure 2. Radiologic images. (A) September 2021: SCLC transformation. (B) November 2021: PR to anlotinib combined with EP. (C) February 2022: PR to anlotinib combined with EP. (D) June 2022: SD to anlotinib. (E) August 2022: PD to anlotinib. PR, partial response; SD, stable disease; PD, progressive disease.
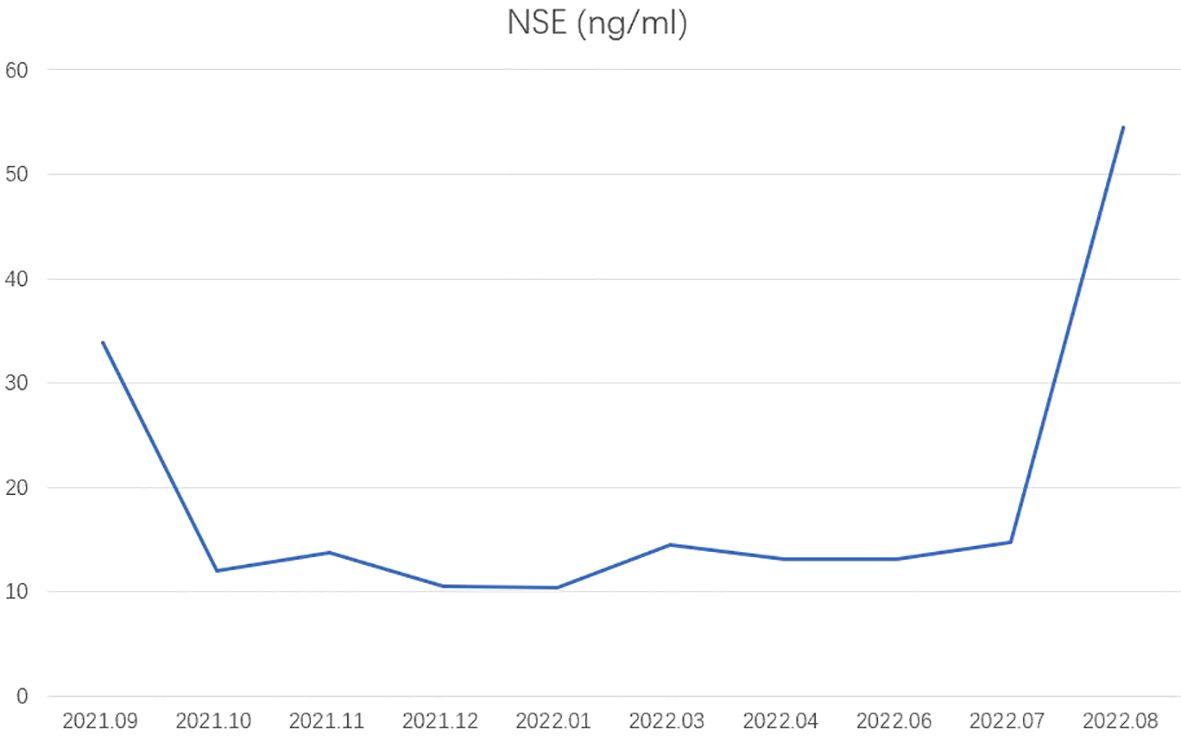
Figure 3. Tumor markers. NSE, neuron-specific enolase.
In August 2022, due to disease progression (axillary lymph node metastasis), a right axillary lymph node biopsy was performed, which revealed metastatic adenocarcinoma with EGFR19 deletion mutation along with phosphatidylinositol-4, 5-bisphosphate 3-kinase, catalytic subunit alpha (PIK3CA) mutation, tumor protein p53 (TP53) mutation, phosphatase and tensin homologue deleted on chromosome 10 (PTEN) deletion, retinoblastoma1 (RB1) deletion. According to the mutation of EGFR 19 deletion, anlotinib together with osimertinib were given on August 11, 2022. After a short period of 21 days, the significant reduction in axillary lymph nodes, but a new metastasis was found. However, the new lesion was too small to be biopsied. Chemotherapy, nab-paclitaxel, was added to the previous regimen of anlotinib and osimertinib for this patient who had a good performance status. This combination was proven to have a good response and had been given for 6 cycles.
In February 2023, two new lesions were confirmed as new metastases with small cell carcinoma both in the right lower lung and left supraclavicular lymph node. We tried several chemotherapy regimens along with anlotinib and cadonilimab, but all of these regimens failed to achieve a response in this patient, and he died on June 9, 2023 (Figure 1).
DiscussionSCLC transformation is one of the mechanisms of drug resistance in TKIs-treated NSCLC patients with EGFR-sensitive mutations, and relevant studies have shown that the incidence of SCLC transformation is 5% ~ 14%, which generally occurs 14-26 months after TKIs treatment, and the median time of transformation is 18 months (4, 10–12). Transformed SCLC shares similarities with classical SCLC in terms of pathology, clinical manifestations, and drug sensitivity, but cannot be fully classified as classical SCLC (13). Transformed SCLC is often poorly treated with rapid disease progression, and the standard chemotherapy regimen for SCLC, EP, is the mainstay of treatment. Marcoux et al. retrospectively found (14) that treatment with etoposide in combination with a platinum regimen after SCLC transformation resulted in a clinical response rate of 54%, with a median progression-free survival (mPFS) of 3.4 months. Median overall survival (mOS) after SCLC transformation was only 10.9 months as reported. Transformed SCLC after resistance to third-generation EGFR TKIs had a better prognosis than resistance to first- and second-generation EGFR TKIs (12). In this case, the treatment regimen of anlotinib combined with EP in the transformation of SCLC after resistance to first-generation EGFR TKIs therapy made the patient have a good clinical outcome with PFS of 10.1 months, and the OS more than 36 months.
The mechanism of SCLC transformation is uncertain. Current mechanisms involved in SCLC transformation include lineage plasticity and tumor heterogeneity. (I) Lineage plasticity is the ability of cells to transdifferentiate from one fixed lineage to another. Inhibition of EGFR signaling by TKIs can selectively promote histologic transformation in tumor cells with specific genomic backgrounds (e.g., TP53 mutations and RB1 deletions), i.e., from epithelial lineage adenocarcinoma to neuroendocrine lineage SCLC. (II) Pre-treatment tumor heterogeneity. Nicholson et al. (15) analyzed a cohort of 100 SCLC patients and found that 28% of SCLC cases were of mixed histology. Zhao et al. (16) also found 10 cases of SCLC combined with mixed histology in a cohort of 170 SCLC patients. These results support the theory that pre-treatment tumor heterogeneity leads to transformation to SCLC. In our case, the patient’s initial diagnosis was established through pleural fluid cytology without needle biopsy of the primary site. Upon demonstrating resistance to EGFR-TKI therapy, a needle biopsy of the primary site subsequently identified the presence of small cell lung cancer. As treatment progressed, puncture biopsies conducted at various metastatic sites revealed the coexistence of both adenocarcinoma and small cell carcinoma. The possible factors leading to this phenomenon include: (I) Tumor Heterogeneity: Within the tumor, there may exist different subpopulations of cells that vary in genetics, epigenetics, or phenotype. This heterogeneity can lead to different metastatic sites exhibiting distinct pathological features. (II) Lineage Plasticity: Cancer cells may transition from adenocarcinoma to small cell carcinoma, possibly to adapt to a new microenvironment or evade treatment. (III) Genetic and Epigenetic Variations: Tumor cells may accumulate genetic and epigenetic variations during growth and metastasis, which can lead to changes in cell phenotype. For example, mutations in certain genes or alterations in epigenetic modifications can cause adenocarcinoma cells to transform into small cell carcinoma cells. (IV) Influence of the Microenvironment: The microenvironment in which tumor cells reside can significantly impact their phenotype. Different microenvironments may promote or inhibit the growth and differentiation of specific types of cancer cells, resulting in metastatic sites exhibiting different pathological types. (V) Treatment-induced Adaptive Changes: Treatment can exert selective pressure on tumor cells, leading to the emergence of drug-resistant subpopulations. These resistant cells may have different phenotypes, including a transition from adenocarcinoma to small cell carcinoma. It was found that RB1 deletion and TP53 mutation were prevalent in patients who developed SCLC transformation, and the risk of SCLC transformation in patients with EGFR-mutated lung adenocarcinoma combined with RB1 deletion and TP53 mutation was 42.8 times higher than that in patients without RB1 deletion and TP53 mutation (13). In clinical practice, NSCLC patients with RB1 and TP53 inactivation need to be aware of the possibility of SCLC transformation during treatment. In addition, myelocytomatosis viral oncogene homolog (MYC) amplification, Notch pathway activation, Achaete-scute homolog-1 (AscL1) gene expression, PIK3CA mutations, and catalytic polypeptide hypermutation by apolipoprotein B (ApoB) mRNA editing enzyme have been implicated in SCLC transformation (11, 17–20).
Anlotinib is an oral tyrosine kinase inhibitor that can effectively inhibit the kinases of vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor (PDGFR), fibroblast growth factor receptor (FGFR), stem cell factor receptor (c-Kit), etc. Anlotinib has the potential to be an anti-kinase and has the ability to inhibit the kinases of FGFR and c-Kit, which have dual roles of anti-tumor angiogenesis and tumor growth inhibition (21–23). Anlotinib has a potent inhibitory effect on VEGFR, PDGFR and FGFR signaling pathways. Therefore, compared with other anti-angiogenic drugs, anlotinib can simultaneously inhibit the three signaling pathways related to angiogenesis and comprehensively block tumor neovascularization. c-Kit-mediated signaling pathway plays an important role in the initiation, development and recurrence of many malignant tumors, and anlotinib has also shown strong inhibitory activity on c-Kit. Currently, some studies indicated that anlotinib combined with etoposide and platinum as first-line treatment for extensive-stage SCLC also showed good efficacy and tolerability (24, 25). Hu et al. (24) showed that the mPFS of anlotinib combined with platinum-etoposide chemotherapy in the first-line treatment of extensive-stage SCLC was up to 8.02 months, and the mOS was up to 15.87 months. A Chinese multicenter real-world study (25) showed that the mPFS was 6.0 months and the mOS was 10.5 months. In this case, we innovatively adopted the treatment regimen of anlotinib combined with EP in the transformation of SCLC after resistance to EGFR TKIs therapy, and the PFS reached 10.1 months, which was a longer PFS compared with the data from previous case reports and retrospective analyses, suggesting that the anlotinib combined with EP regimen could be the first choice of treatment after transformation to SCLC. This case was transformed to SCLC after treatment of a first-generation EGFR TKIs, and the combination treatment modality (chemotherapy, target therapy and immunotherapy) with anlotinib was continued in the subsequent treatment, which enabled this patient to have an OS of more than 20 months after transformation to SCLC.
A study by Wang et al. (26) retrospectively analyzed 29 patients with transformed SCLC. Except for one patient who died shortly after transformation, the other 28 patients received at least one treatment after transformation. Sixteen patients received chemotherapy in combination with EGFR TKIs and 8 patients received chemotherapy without EGFR TKIs. Compared to chemotherapy without EGFR TKIs, chemotherapy with EGFR TKIs improved mPFS (5.2 months vs. 3.0 months, p=0.014). However, there was no significant effect on mOS (14.8 vs. 13.0 months, p=0.474). Thirteen patients received local radiotherapy and the results showed that radiotherapy improved OS in patients who transformed to SCLC compared to no radiotherapy (15.5 months vs. 13.9 months, p=0.045). In addition, 18 patients received anti-angiogenesis inhibitor monotherapy or combination therapy, of which 83.3% were treated with anlotinib, 6 patients were treated with anlotinib monotherapy, and the rest were treated with chemotherapy and/or EGFR TKIs. The results showed that anti-angiogenesis inhibitors significantly improved OS in patients who converted to SCLC. In another retrospective study by Wang et al. (12), 5 patients with transformed SCLC were treated with anlotinib, with an objective response rate (ORR) of 66.7% and a mPFS of 6.2 months, which also showed the efficacy of anlotinib in the treatment of transformed SCLC (Table 1).
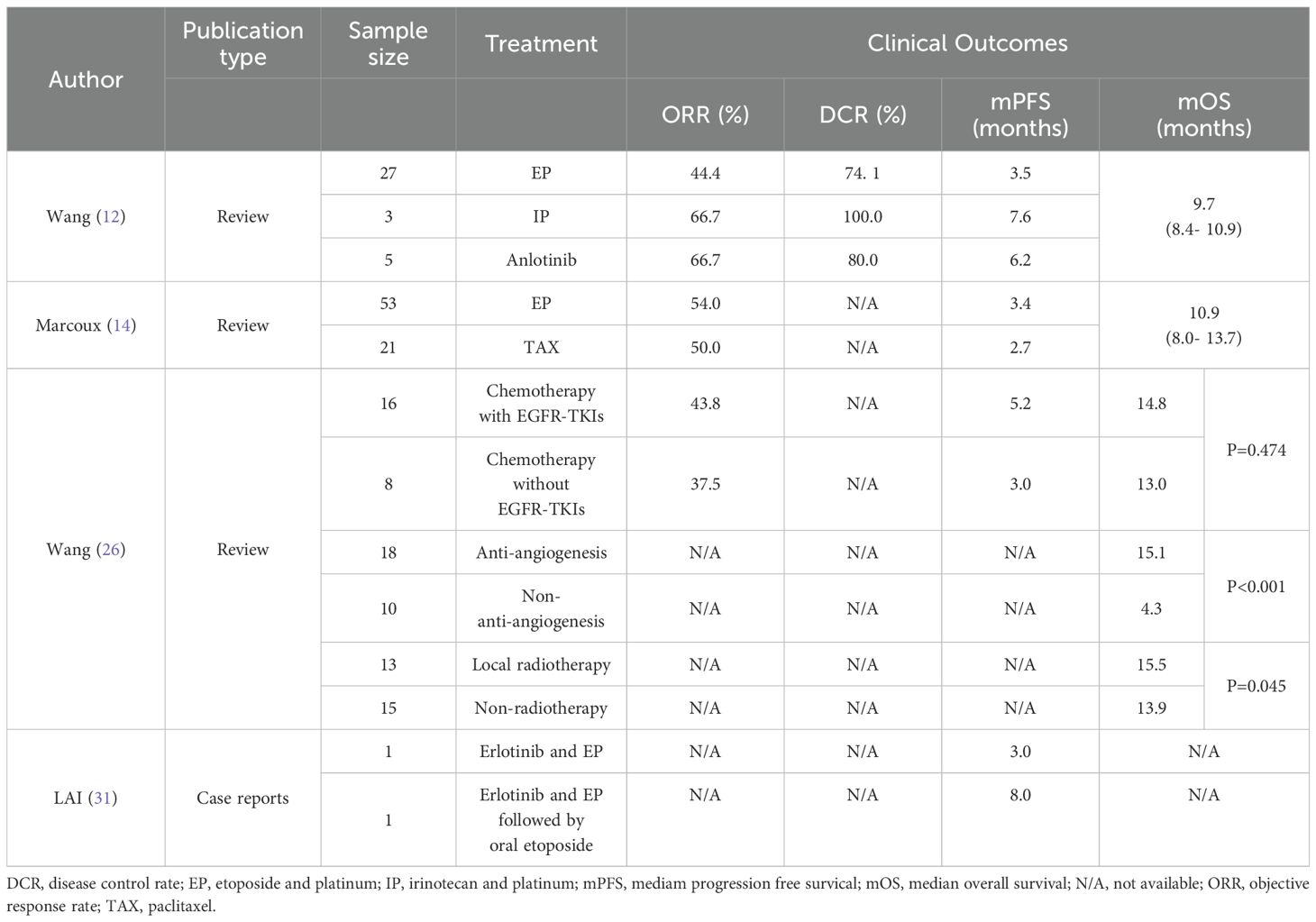
Table 1. Literature review of small cell lung cancer transformation.
The efficacy of immunotherapy in patients with SCLC transformation has been unclear. Sehgal et al. (27) showed that transformed SCLC patients treated with nivolumab or pembrolizumab monotherapy could achieve a mOS of 13.0 months after transformation to SCLC, suggesting that immunotherapy may provide a survival benefit to transformed SCLC patients. However, a retrospective study showed that immunotherapy were ineffective in 17 patients with SCLC transformation (14). Related studies have found that EGFR-mutant NSCLC often presents an immunosuppressive phenotype, while most SCLC are also immunosuppressive with a lack of major histocompatibility complex-I (MHC-I) expression, and transformed SCLC also exhibit reduced MHC-I expression (28–30). The present patient also received immunotherapy, but it did not appear to be beneficial. Therefore, immunotherapy for transformed SCLC needs to be further investigated at this time.
ConclusionBased on the pathological findings, the case presents a complex scenario of tumor heterogeneity and transformation. This case highlights the importance of continuous monitoring and re-evaluation of tumor characteristics, as well as the challenges in managing patients with evolving tumor biology. The differential diagnosis and tailored treatment strategies are crucial in such cases to address the diverse therapeutic needs arising from the presence of different histological subtypes within the same patient.
This case of advanced lung adenocarcinoma patient was transformed to SCLC resistant to one generation EGFR TKIs treatment, and the front-line treatment after transformation was a regimen of anlotinib in combination with EP, which resulted in the patient achieving a long PFS. After the first progression with anlotinib in combination with EP, anlotinib was shown to be effective with nab-paclitaxel. Subsequent treatment with an anlotinib-containing regimen resulted in an OS of more than 20 months for the patient. It is suggested that for transformed SCLC patients, the treatment containing anlotinib may provide survival benefit and is a feasible option.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statementWritten informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributionsJW: Writing – original draft, Visualization. XL: Data curation, Writing – review & editing. SD: Software, Validation, Writing – review & editing. SH: Supervision, Writing – review & editing. FR: Conceptualization, Formal analysis, Writing – review & editing. YQ: Investigation, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
AcknowledgmentsThe patient involved in this case report gave his informed consent authorizing use and disclosure of his health information.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Sung H, Ferlay J, Siegel RL, Laversanne M, Jemal A, Bray F, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
PubMed Abstract | Crossref Full Text | Google Scholar
2. Zeng Q, Vogtmann E, Jia M-M, Parascandola M, Zou X-N. Tobacco smoking and trends in histological subtypes of female lung cancer at the Cancer Hospital of the Chinese Academy of Medical Sciences over 13 years. Thorac Cancer. (2019) 10:1717–24. doi: 10.1111/1759-7714.13141
PubMed Abstract | Crossref Full Text | Google Scholar
3. Fukuoka M, Wu Y-L, Thongprasert S, Sunpaweravong P, Leong SS, Sriuranpong V, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol. (2011) 29:2866–74. doi: 10.1200/JCO.2010.33.4235
PubMed Abstract | Crossref Full Text | Google Scholar
4. Oser MG, Niederst MJ, Sequist LV, Engelman DJA. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol. (2015) 16:e165–72. doi: 10.1016/S1470-2045(14)71180-5
PubMed Abstract | Crossref Full Text | Google Scholar
5. Lim SM, Syn NL, Cho BC, Soo RA. Acquired resistance to EGFR targeted therapy in non-small cell lung cancer: Mechanisms and therapeutic strategies. Cancer Treat Rev. (2018) 65:1–10. doi: 10.1016/j.ctrv.2018.02.006
PubMed Abstract | Crossref Full Text | Google Scholar
7. Mainardi S, Mulero-Sánchez A, Prahallad A, Astrid B, Paul K, et al. SHP2 is required for growth of KRAS-mutant non-small-cell lung cancer in vivo. Nat Med. (2018) 24:961–7. doi: 10.1038/s41591-018-0023-9
PubMed Abstract | Crossref Full Text | Google Scholar
8. Minari R, Bordi P, La Monica S, Squadrilli A, Leonetti A, Bottarelli L, et al. Concurrent acquired BRAF V600E mutation and MET amplification as resistance mechanism of first-line osimertinib treatment in a patient with EGFR-mutated NSCLC. J Thorac Oncol. (2018) 13:e89–91. doi: 10.1016/j.jtho.2018.03.013
PubMed Abstract | Crossref Full Text | Google Scholar
9. Pillai RN, Behera M, Berry LD, Rathi N, Pillai M, Behera L, et al. HER2 mutations in lung adenocarcinomas: A report from the Lung Cancer Mutation Consortium. Cancer. (2017) 123:4099–105. doi: 10.1002/cncr.v123.21
PubMed Abstract | Crossref Full Text | Google Scholar
10. Piotrowska Z, Niederst MJ, Karlovich CA, Wakelee HA, Sequist LV. Heterogeneity underlies the emergence of EGFRT790 wild-type clones following treatment of T790M-positive cancers with a third-generation EGFR inhibitor. Cancer Discovery. (2015) 5:713–22. doi: 10.1158/2159-8290.CD-15-0399
PubMed Abstract | Crossref Full Text | Google Scholar
11. Lee J-K, Lee J, Kim S, Kim S, Youk J, Park S, et al. Clonal history and genetic predictors of transformation into small-cell carcinomas from lung adenocarcinomas. J Clin Oncol. (2017) 35:3065–74. doi: 10.1200/JCO.2016.71.9096
PubMed Abstract | Crossref Full Text | Google Scholar
12. Wang W, Xu C, Chen H, Jia J, Wang L, Feng H, et al. Genomic alterations and clinical outcomes in patients with lung adenocarcinoma with transformation to small cell lung cancer after treatment with EGFR tyrosine kinase inhibitors: A multicenter retrospective study. Lung Cancer. (2021) 155:20–7. doi: 10.1016/j.lungcan.2021.03.006
PubMed Abstract | Crossref Full Text | Google Scholar
13. Niederst MJ, Sequist LV, Poirier JT, Mermel CH, Lockerman EL, Garcia AR, et al. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat Commun. (2015) 6:6377. doi: 10.1038/ncomms7377
PubMed Abstract | Crossref Full Text | Google Scholar
14. Marcoux N, Gettinger SN, O’Kane G, Arbour KC, Neal JW, Husain H, et al. EGFR-mutant adenocarcinomas that transform to small-cell lung cancer and other neuroendocrine carcinomas: clinical outcomes. J Clin Oncol. (2019) 37:278–85. doi: 10.1200/JCO.18.01585
PubMed Abstract | Crossref Full Text | Google Scholar
15. Nicholson SA, Beasley MB, Brambilla E, Hasleton PS, Colby TV, Sheppard MN, et al. Small cell lung carcinoma (SCLC): a clinicopathologic study of 100 cases with surgical specimens. Am J Surg Pathol. (2002) 26:1184–97. doi: 10.1097/00000478-200209000-00009
PubMed Abstract | Crossref Full Text | Google Scholar
16. Zhao X, McCutcheon JN, Kallakury B, Chahine JJ, Pratt D, Raffeld M, et al. Combined small cell carcinoma of the lung: is it a single entity? J Thorac Oncol. (2018) 13:237–45. doi: 10.1016/j.jtho.2017.10.010
PubMed Abstract | Crossref Full Text | Google Scholar
17. Tenjin Y, Nakamura K, Ishizuka S, Saruwatari K, Sato R, Tomita Y, et al. Small cell lung cancer derived from adenocarcinoma with mutant epidermal growth factor receptor provides a signature of transcriptional alteration in tumor cells. Intern Med. (2019) 58:3261–5. doi: 10.2169/internalmedicine.2988-19
PubMed Abstract | Crossref Full Text | Google Scholar
18. Xie T, Li Y, Ying J, Cai W, Li J, Lee KY, et al. Whole exome sequencing (WES) analysis of transformed small cell lung cancer (SCLC) from lung adenocarcinoma (LUAD). Transl Lung Cancer Res. (2020) 9:2428–39. doi: 10.21037/tlcr-20-1278
PubMed Abstract | Crossref Full Text | Google Scholar
19. Popat S. Histologically transformed SCLC from EGFR-mutant NSCLC: understanding the wolf in sheep’s clothing. J Thorac Oncol. (2019) 14:1689–91. doi: 10.1016/j.jtho.2019.07.010
PubMed Abstract | Crossref Full Text | Google Scholar
20. Dorantes-Heredia R, Ruiz-Morales JM, Cano-García F. Histopathological transformation to small-cell lung carcinoma in non-small cell lung carcinoma tumors. Transl Lung Cancer Res. (2016) 5:401–12. doi: 10.21037/tlcr.2016.07.10
PubMed Abstract | Crossref Full Text | Google Scholar
21. Shen G, Zheng F, Ren D, Du F, Dong Q, Wang Z, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol. (2018) 11:120. doi: 10.1186/s13045-018-0664-7
PubMed Abstract | Crossref Full Text | Google Scholar
22. Lin B, Song X, Yang D, Bai D, Yao Y, Lu N. Anlotinib inhibits angiogenesis via suppressing the activation of VEGFR2, PDGFRβ and FGFR1. Gene. (2018) 654:77–86. doi: 10.1016/j.gene.2018.02.026
PubMed Abstract | Crossref Full Text | Google Scholar
23. Wang G, Sun M, Jiang Y, Zhang T, Sun W, Wang H, et al. Anlotinib, a novel small molecular tyrosine kinase inhibitor, suppresses growth and metastasis via dual blockade of VEGFR2 and MET in osteosarcoma. Int J Cancer. (2019) 145:979–93. doi: 10.1002/ijc.v145.4
PubMed Abstract | Crossref Full Text | Google Scholar
24. Deng P, Hu C, Chen C, Cao L, Gu Q, An J, et al. Anlotinib plus platinum-etoposide as a first-line treatment for extensive-stage small cell lung cancer: A single-arm trial. Cancer Med. (2022) 11:3563–71. doi: 10.1002/cam4.v11.19
PubMed Abstract | Crossref Full Text | Google Scholar
25. Zheng H, Jiang A, Gao H, Liu N, Zheng XQ, Fu X, et al. The efficacy and safety of anlotinib combined with platinum-etoposide chemotherapy as first-line treatment for extensive-stage small cell lung cancer: A Chinese multicenter real-world study. Front Oncol. (2022) 12:894835. doi: 10.3389/fonc.2022.894835
PubMed Abstract | Crossref Full Text | Google Scholar
26. Wang S, Xie T, Hao X, Wang Y, Hu X, Wang L, et al. Comprehensive analysis of treatment modes and clinical outcomes of small cell lung cancer transformed from epidermal growth factor receptor mutant lung adenocarcinoma. Thorac Cancer. (2021) 12:2585–93. doi: 10.1111/1759-7714.14144
PubMed Abstract | Crossref Full Text | Google Scholar
27. Sehgal K, Varkaris A, Viray H, VanderLaan PA, Rangachari D, Costa DB. Small cell transformation of non-small cell lung cancer on immune checkpoint inhibitors: uncommon or under-recognized? J Immunother Cancer. (2020) 8(1):e000697. doi: 10.1136/jitc-2020-000697
PubMed Abstract | Crossref Full Text | Google Scholar
28. Le X, Negrao MV, Reuben A, Federico L, Diao L, McGrail D, et al. Characterization of the immune landscape of EGFR-mutant NSCLC identifies CD73/adenosine pathway as a potential therapeutic target. J Thorac Oncol. (2021) 16:583–600. doi: 10.1016/j.jtho.2020.12.010
PubMed Abstract | Crossref Full Text | Google Scholar
29. Mahadevan NR, Knelson EH, Wolff JO, Vajdi A, Saigí M, Campisi M, et al. Intrinsic immunogenicity of small cell lung carcinoma revealed by its cellular plasticity. Cancer Discovery. (2021) 11:1952–69. doi: 10.1158/2159-8290.CD-20-0913
PubMed Abstract | Crossref Full Text | Google Scholar
30. Burr ML, Sparbier CE, Chan KL, Chan YC, Kersbergen A, Lam EYN, et al. An evolutionarily conserved function of polycomb silences the MHC class I antigen presentation pathway and enables immune evasion in cancer. Cancer Cell. (2019) 36(4):385–401.e8. doi: 10.1016/j.ccell.2019.08.008
PubMed Abstract | Crossref Full Text | Google Scholar
31. Lai L, Meng WT, Wei JL, Zhang X, Tan Z, Lu Y, et al. Transformation of NSCLC to SCLC after 1st-and 3rd-generation EGFR-TKI resistance and response to EP regimen and erlotinib:2CARE-compliant case reports[J. Med (Baltimore). (2021) 100:e25046. doi: 10.1097/MD.0000000000025046
留言 (0)