Severe craniocerebral injuries and hypertensive intracerebral hemorrhages are associated with higher mortality and disability rates (1). Traumatic brain injuries lead to 1.5 million hospitalizations and 57,000 deaths in Europe annually. Hypertensive intracerebral hemorrhages exhibit a sudden onset, rapid progression, and a high fatality rate of 35%–52% within 30 days of onset, imposing a substantial burden on patients, their families, and society (2). Decompressive craniotomy, commonly performed on the frontotemporal roof, represents a primary method to alleviate refractory malignant cerebral edema and intracranial hypertension resulting from severe craniocerebral trauma and hypertensive intracerebral hemorrhage (3–6). When the patients attain a generally stable condition, and intracranial edema has largely subsided, the imperative for cranioplasty (CP) arises to reconstruct skull defects and enhance neurological function (7, 8).
CP employs various materials, including titanium skull repair materials, autologous bone, polymethyl methacrylate synthetic materials, and bioactive materials such as hydroxyapatite. Given its high strength, lightweight nature, biocompatibility, corrosion resistance, and malleability, titanium alloy for skull repair enjoys widespread use in clinical practice (9). Recent studies indicate that CP restores the original appearance of patients and alleviates the psychological burden associated with skull defects. Moreover, it enhances the cerebral blood flow and promotes nerve function recovery. Early CP may yield more favorable outcomes (10–12). Previous investigations have highlighted that decompressive craniotomy frequently correlates with abnormal glucose metabolism, compromised cerebral blood flow, and alterations in cerebrospinal fluid circulation (13, 14). Recent reports underscore the occurrence of severe complications, such as challenging incision healing, incision infection, epilepsy, facial nerve injury, exposure to skull repair materials, temporal muscle atrophy, and the need for multiple remedial operations; thereby, some patients have to remove titanium alloy material.
CP-related complications are frequently attributed to blood supply, tension, and dead space beneath the patch material of the scalp. A low-central-point titanium alloy plate and mesh were utilized to mitigate scalp tension, improve the blood supply of the skin at the defect site, and minimize dead space under the scalp. This design aims to bring the titanium alloy plate close to the dural membrane and temporal muscle below. Subsequently, we conducted a retrospective analysis of clinical data from patients with skull defects treated using low-curvature and normal-curvature titanium mesh, comparing the clinical benefits and prognoses of the two surgical modalities.
Materials and methods PatientsA retrospective analysis was conducted on 86 adult patients with skull defects who underwent skull repair surgery due to cerebral hemorrhage and craniocerebral trauma in the Department of Neurosurgery from Binhai County People's Hospital between January 2021 and December 2022. The inclusion criteria comprised patients identified with cerebral hemorrhage or craniocerebral trauma through imaging examination, aged 20 years or older, and willing to undergo surgery. Exclusion criteria included incomplete clinical data, missing information in patient follow-ups, and severe medical conditions potentially affecting the length of hospital stay.
Surgical methods of cranioplastyThe control group received normal-curvature titanium mesh for skull repair. In contrast, the observation group underwent skull repair with titanium mesh and a titanium alloy plate featuring a 5 mm central point compression of normal curvature (Figure 1). All patients underwent preoperative double-source thin-slice head computerized tomographic (CT) scans and three-dimensional reconstructions. Skull defect data were obtained and sent to the manufacturing company [Kontour (Xi'an) Medical Technology Co., Ltd], which customized titanium mesh of varying curvatures by comparing images of the healthy and defective sides. The titanium mesh was composed of a bone plate and a bone screw. The bone plates were made of TA2 pure titanium material in accordance with Chinese GB/T 13810 standard, and the bone screws were made of TC4 titanium alloy material in accordance with Chinese GB/T 13810 standard. Pure titanium and titanium alloy products have no color on the surface and are packaged with sterilization. As a preventive measure against infection, antibiotics were routinely administered 30 min before the surgical procedure. Under successful general anesthesia, the patient's head was positioned on the healthy side in the supine position. The skull repair material was initially immersed in vancomycin water, and the dura was carefully preserved intraoperatively. Any damage was closely sutured to prevent postoperative cerebrospinal fluid leakage. The titanium mesh was then covered and secured with titanium nails. Subcutaneous drainage was maintained for 3 days postoperatively, with removal based on drainage flow criteria (daily drainage volume <50 ml). Stitches were removed 7–10 days after surgery.
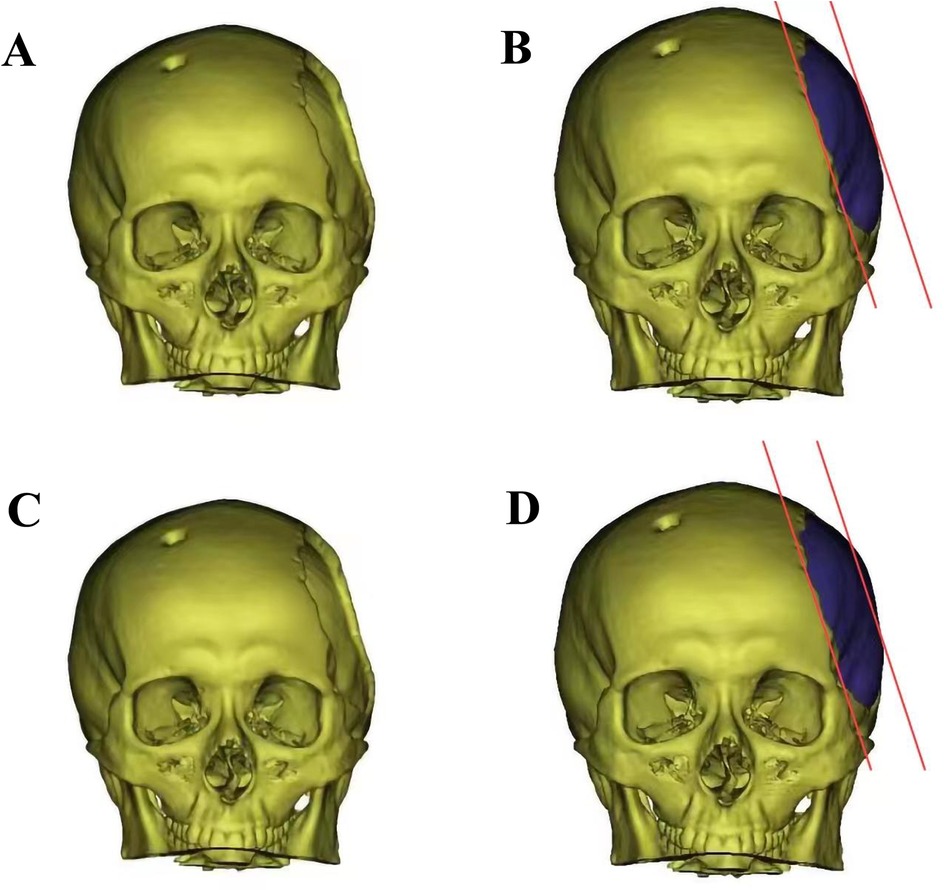
Figure 1. The diagram of skull before and after cranioplasty. (A) Left temporal skull defect before titanium mesh cranioplasty; (B) Three-dimensional model of low-curvature titanium mesh designed by factory; (C) Left temporal skull defect before titanium mesh cranioplasty; (D) Three-dimensional model of normal-curvature titanium mesh.
Study variablesThe medical records of the enrolled patients were retrospectively analyzed. The demographic information (sex and age), preoperative clinical data (etiology of bone flap removal, defect area, skull defect site, skull defect time), operative data (operation time, intraoperative blood loss, difficulty evaluation), postoperative nursing conditions and aesthetics, and postoperative complications were collected. Hospital stay duration for both groups was recorded, and patient satisfaction after CP was assessed through telephone follow-up, categorized as satisfaction, relative satisfaction, general satisfaction, and dissatisfaction. Based on patient journals, demographic characteristics, including age (<50 years old, ≥50 years old) and body mass index (BMI) (<24 kg/m2, ≥24 kg/m2), were considered.
Karnofsky's Performance Status (KPS) and quality-of-life (QOL) scores were evaluated. KPS score is widely utilized in patients with cancer, determines their functional status and predicts the likelihood of adverse postoperative outcomes or risks. Ranging from 0 to 100, a score of 0 signifies the patient's demise, 80 to 89 indicates the presence of disease symptoms with the ability to perform everyday activities independently, 90 to 99 suggests mild signs or symptoms of the disease with the capability to perform regular activities, and 100 signifies normal health. A higher score correlates with better physical condition and increased tolerance to adverse reactions post-treatment (15). QOL score is commonly employed in patients with cancer, and it is a crucial tool for evaluating the quality of life and overall health status. The QOL evaluation index encompasses 12 aspects, each categorized into five levels based on the quality of life. The total QOL score is 60 points, with less than 20 indicating poor quality of life, 21–30 as poor quality, 31–40 as average quality, 41–50 as better quality, and 51–60 as good quality of life (16).
Statistical analysisSPSS version 27.0 and GraphPad Prism 8.3.0 statistical software were employed for analysis. Measurement data were compared using a t-test, and counting data were compared using Pearson χ2 test, continuity correction χ2 test, or Fisher's Exact test based on data characteristics. Statistical significance was set at P < 0.05. The 1:1 propensity score matching (PSM) was implemented to minimize inter-group variable selection bias. PSM analysis was used to adjust for differences between patients with low-curvature and normal-curvature titanium mesh, adjusting for demographic information (sex and age), preoperative clinical data (etiology of bone flap removal, defect area, skull defect site, skull defect time), operative data (operation time, intraoperative blood loss, difficulty evaluation), postoperative nursing conditions and aesthetics, and postoperative complications.
Results Demographic and clinical characteristics of patientsThis study identified 86 eligible patients with skull defects between January 2021 and December 2022, with 22 in the low-curvature titanium mesh group and 45 in the normal-curvature group. Table 1 displayed the distribution, showing 16 men and six women in the low-curvature group, and 28 men and 17 women in the normal-curvature group. Fifteen patients in the low-curvature group were significantly older than 50 years, and seven were <50 years; 19 in the normal-curvature group were considerably older than 50 years, and 26 were <50 years (P = 0.046). Sixteen patients in the low-curvature group had a BMI >24 and six had ≤24; 20 in the normal-curvature group had a BMI between 24 and 25, with significantly different results (P = 0.030). In the low-curvature group, two complications occurred, compared to seven in the normal-curvature group, demonstrating no significant difference (P = 0.728). Furthermore, there were no significant differences between the two groups regarding the cause, site, and area of skull defects, repair operation time, intraoperative blood loss, surgical and care difficulty, and aesthetics. The 1:1 PSM method (Figure 2, Table 2) was employed to ensure baseline variable balance, enrolling 32 patients in the final study, with 16 cases each in the low-curvature and normal-curvature titanium mesh group. No significant differences were observed between the two groups in age, sex, BMI, cause of skull defect, skull defect site, skull defect area, skull repair operation time, intraoperative blood loss, surgical and care difficulty, aesthetics, and occurrence of complications.
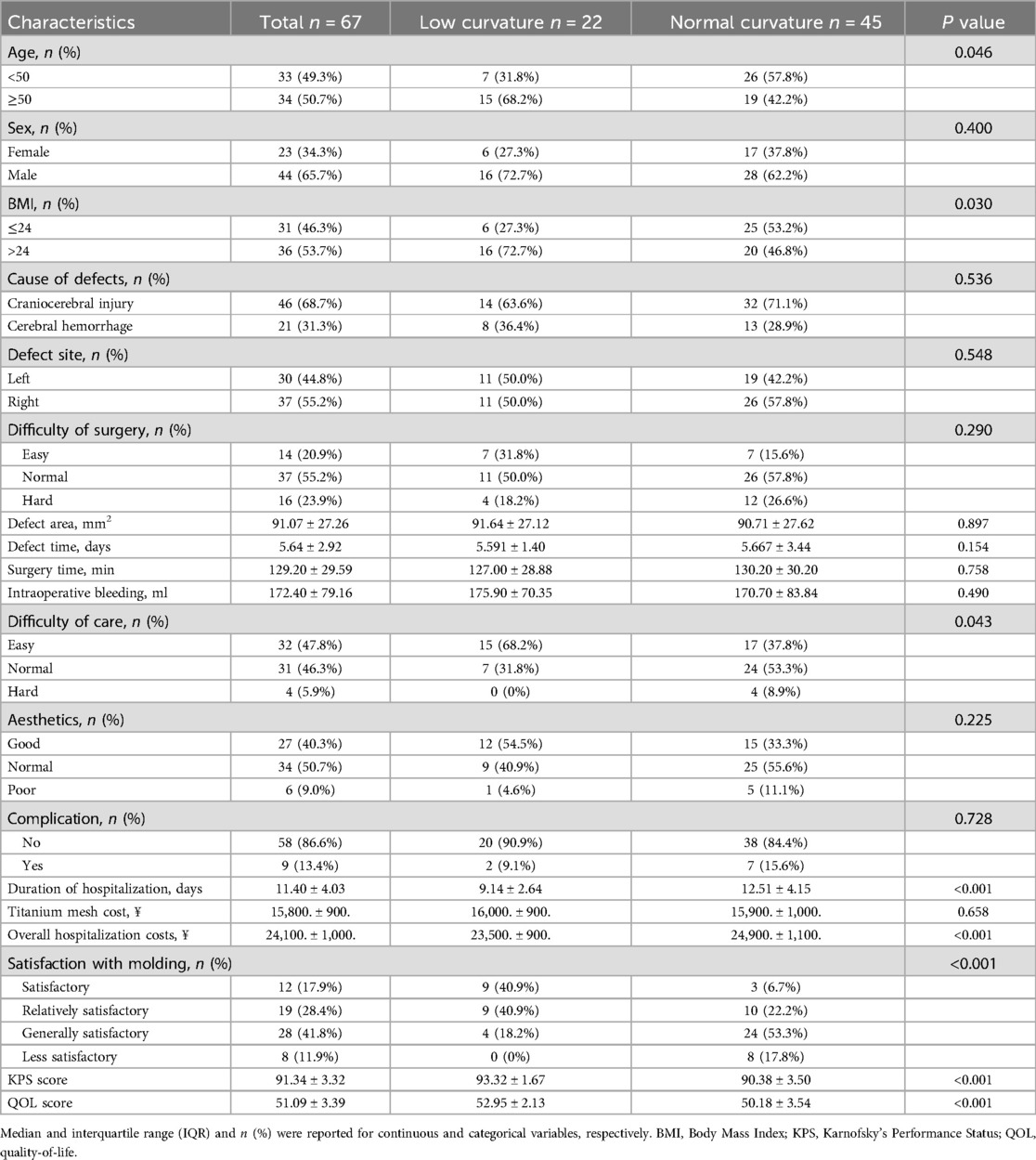
Table 1. Baseline demographic and clinical characteristics of cranioplasty in the low-curvature and normal-curvature groups before PSM.

Figure 2. PSM analysis depicting the standardized mean difference results for various variables. PSM, propensity score matching.
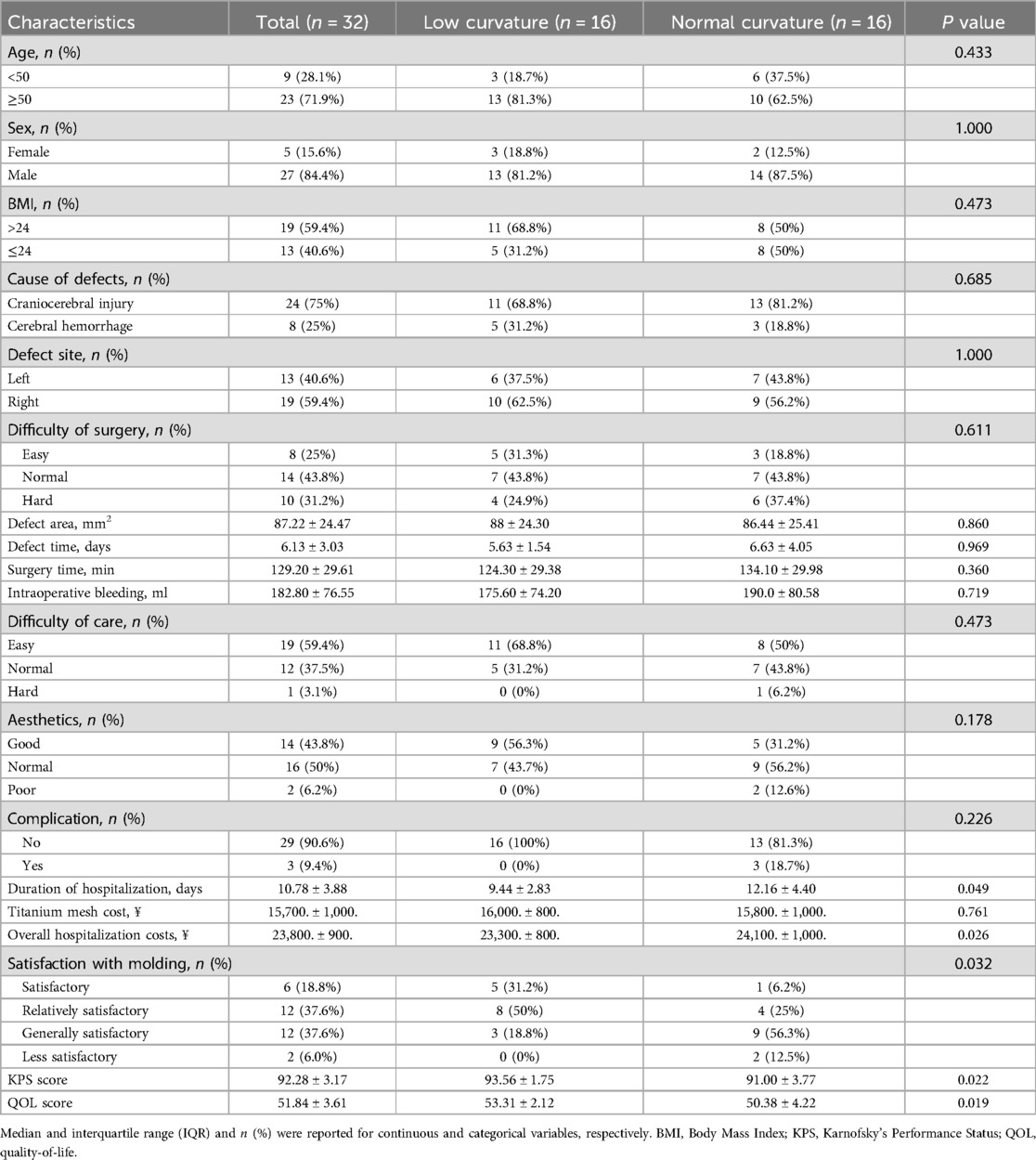
Table 2. Baseline demographic and clinical characteristics of cranioplasty in the low-curvature and normal-curvature groups after PSM.
Comparison of hospitalization duration, cost,and molding satisfactionWe investigated plastic satisfaction and hospitalization duration for all patients with skull defects. As depicted in Figure 3A, a significant difference was observed between the two groups (low-curvature group: 9.136 ± 2.642 days, normal-curvature group: 12.51 ± 4.149 days, P < 0.001), with the low-curvature group exhibiting a shorter duration than the normal-curvature group. After PSM, differences persisted between the two groups (Figure 4A) (low-curvature group: 9.438 ± 2.830 days, normal-curvature group: 12.125 ± 4.400 days, P = 0.049), with the hospitalization time for the low-curvature group remaining lower than the normal-curvature group. Pre-PSM and after PSM, the low-curvature group exhibited lower overall hospitalization costs than the normal-curvature group (Pre-PSM: 23,500. ± 900. vs. 24,900. ± 1,100., P < 0.001; after PSM: 23,300. ± 800. vs. 24,100. ± 1,000., P = 0.026). Patient-satisfaction analysis (Table 1) revealed a significant difference between the two groups (low curvature group: nine cases reported being satisfied, nine were relatively satisfied, four were generally satisfied, and none were dissatisfied; normal-curvature group: three cases reported being satisfied, ten were relatively satisfied, 24 were generally satisfied, and eight were dissatisfied, P < 0.001), with the low-curvature group expressing greater patient satisfaction than the normal-curvature group. Differences persisted between the two groups after PSM (low-curvature group: five cases reported being satisfied, eight were relatively satisfied, three were generally satisfied, and none were dissatisfied; normal-curvature group: one case reported being satisfied, four were relatively satisfied, two were generally satisfied, and two were dissatisfied, P = 0.032), with the low-curvature group exhibiting higher patient satisfaction than the normal-curvature group.

Figure 3. Comparison of hospitalization duration and molding satisfaction. (A) Differences in the expression of hospitalization duration; (B) and (C) KPS score and QOL score between the low-curvature and normal-curvature titanium mesh groups before PSM.
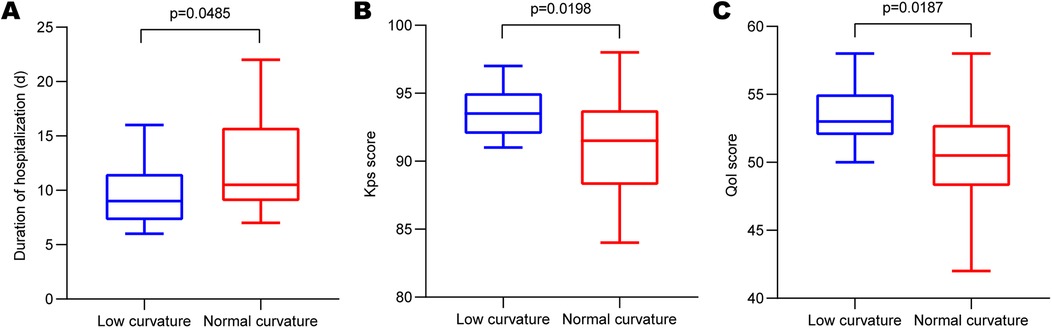
Figure 4. Comparison of hospitalization duration and molding satisfaction after PSM. (A) Differences in the expression of hospitalization duration after PSM; (B) and (C) KPS score and QOL score between the low-curvature and normal-curvature titanium mesh groups after PSM. PSM, propensity score matching; KPS, Karnofsky's Performance Status; QOL, quality-of-life.
Comparison of KPS and QOL scoresAdditionally, we performed KPS and QOL score assessments for all enrolled patients. Results revealed a significant difference in KPS scores between the two groups (Figure 3B) (low-curvature group: 93.32 ± 1.67, normal-curvature group: 90.38 ± 3.50, P < 0.001), with the KPS score higher in the low-curvature group than that of the normal-curvature group. Post-PSM, differences persisted between the two groups (Figure 4B) (low-curvature group: 93.56 ± 1.750, normal-curvature group: 91.00 ± 3.777, P = 0.022), and the KPS score of the low-curvature group remained superior to that of the normal-curvature group. There was a significant difference in QOL scores between the two groups (Figure 3C) (low-curvature group: 52.95 ± 1.67, normal-curvature group: 50.18 ± 3.537, P < 0.001), with the QOL score higher in the low-curvature group than that of the normal-curvature group. After PSM, differences persisted between the two groups (Figure 4C) (low-curvature group: 53.31 ± 2.12, normal-curvature group: 50.38 ± 4.225, P = 0.022), and the QOL score of the low-curvature group remained superior to that of the normal-curvature group.
DiscussionIn this retrospective analysis, the clinical benefits of the two surgical modalities were explored to determine the superiority of low-curvature titanium mesh CP. Pre-PSM, the low-curvature group exhibited significantly reduced hospital stays, overall hospitalization costs, superior satisfaction with plastic form, and higher KPS and QOL scores than the normal-curvature group. Similar outcomes persisted after 1:1 PSM for sex, age, BMI, defect cause, and defect site. These results showed that the significantly decreased hospital stay in the low-curvature group reduced hospital costs, alleviating the economic pressure and family burdens, and ultimately improved quality of life.
A number of synthetic materials have been used as alternatives to autologous bone flap, including metal (titanium), acrylic acid (polymethyl methacrylate), ceramic (calcium phosphate-based cement such as hydroxyapatite), and plastic (such as PEEK). The ideal cranioplasty material should promote osseointegration and be lightweight, aesthetically pleasing, durable, physiologically compatible, and cost effective (17). It has been suggested that autologous bone flap has irreplaceable advantages because the replacement of the original bone flap takes advantage of its natural biocompatibility and associated low risk of rejection, as well as the potential for reintegration with adjacent bone and subsequent growth with the patient. However, its translational application in bioengineering is still limited and aseptic bone flap resorption (BFR) is one of the most common long-term complications (18, 19). A meta-analysis investigated risk factors for resorption of aseptic bone flaps after cranioplasty with autologous bone flaps and showed that more bone flap fragments, traumatic brain injury, and younger age significantly increased the risk of resorption of aseptic bone flaps (20). In these patients, choosing a synthetic implant may be a reasonable option (19).
The widespread use of titanium alloy in skull repair surgery has been reported, and recent studies highlight the advantages of PEEK materials, such as high translucency, mechanical strength, good histocompatibility, and resistance to scalp heating in sunlight (21). PEEK materials have shown lower complications and implant failure rates in skull repair compared to titanium and autogenous bone. However, no significant difference was found between PEEK and titanium alloy in the incidence of complications post-CP and post-discharge (22), emphasizing the lack of evidence supporting the superiority of PEEK over titanium mesh repair, especially considering the substantially higher cost of PEEK materials. Overall, titanium alloy remains the preferred surgical material for skull repair procedures in patients.
While titanium alloy materials are widely utilized in neurosurgery, they present challenges, such as limited light transmission, high thermal conductivity, increased wear on covering tissues, and difficulties in intraoperative secondary molding (23). Postoperative complications, such as challenging incision healing, incision infections, epilepsy, facial nerve injuries, exposure of skull repair materials, and temporal muscle atrophy, manifest in some patients undergoing skull repair with titanium alloy materials, necessitating multiple remedial operations (24). In this study, there were 2 cases of complications in the low-curvature group, accounting for 9.1%. Both 2 patients had large skull defects and difficult healing issues. There were 7 cases of complications in the normal-curvature group, accounting for 15.6%, including 4 cases of postoperative infection and 3 cases of difficult healing issues. This study advocates using low-curvature titanium mesh to mitigate complications following skull repair. The reasons for this rationale lie in the low-curvature treatment of the titanium mesh used in skull repair, alleviating tension after scalp suturing to prevent issues like poor blood supply due to excessive tension and pulled scalp vessels, leading to difficult incision healing, scalp necrosis, and exposed titanium mesh. Moreover, the low curvature of the mesh minimizes the postoperative dead space under the scalp, lowering the incidence of postoperative epidural hematoma and intracranial infection, ultimately contributing to shortened postoperative recovery times, abridged hospital stays, and decreased overall hospitalization costs for patients.
This study reveals a shorter hospital stay for the low-curvature group than the normal-curvature group. Prior research has established a direct correlation between the length of hospital stay, postoperative complications, and the patients' baseline conditions (25). In the low-curvature group, improved surgical techniques reduce tension after scalp suturing, preventing serious complications such as poor scalp blood supply caused by traction, complicated incision healing, scalp necrosis, and titanium mesh exposure. Furthermore, the low curvature treatment decreases the space between the scalp and the dural membrane, minimizing postoperative dead space and reducing the likelihood of postoperative epidural hematoma and intracranial infection (26, 27). Consequently, this study affirms that the low-curvature titanium mesh repair group experiences a lower complication rate, leading to significantly shorter hospitalization, reduced hospitalization expenses, and improved neurological prognosis.
The KPS score is a predictive tool for adverse postoperative outcomes or risks, aiding medical personnel in evaluating treatment effectiveness and predicting patient survival. In this study, the KPS score of the low-curvature group surpasses that of the normal-curvature group. The reason behind the higher scores in the low-curvature group remains challenging to elucidate, particularly considering that both groups' scores post-skull repair exceed 80, indicating complete self-care for patients (28). In contrast, this study revealed that patients undergoing low-curvature titanium mesh repair experienced shorter hospital stays and fewer surgical complications than those with normal-curvature repair. This outcome is attributed to the low-curvature treatment of titanium mesh, which decreases scalp tension, improves scalp blood supply, and diminishes the space between the scalp and meninges. Low curvature titanium mesh was able to significantly improve KPS and whether it was associated with factors such as reduced postoperative pain and fewer complications.No other evidence supporting KPS as an influencing factor is found in existing literature, suggesting a potential avenue for exploration in subsequent studies.
The QOL score, commonly used in patients with tumor and post-neurosurgery evaluations (29), was higher in the low-curvature group than in the normal-curvature group. The results of this metric exhibited similarity between the two groups of patients undergoing surgery. However, the comprehensive nature of its scoring, encompassing 12 dimensions, including appetite, spirit, sleep, fatigue, pain, family and colleagues' understanding and cooperation, patients' perception of the disease, attitude toward treatment, daily life, treatment side effects, and facial expressions, rendered the results more robust and convincing than the classification of KPS scores. This study suggests that the primary reason for the difference between the two groups may be that postoperative pain in patients undergoing low-curvature titanium mesh skull repair is less than those with normal-curvature titanium mesh. The snugger fit resulting from the lower curvature of the skull repair reduces the tension of the incision skin, consequently alleviating patient pain. This pain reduction is accompanied by increased appetite, improved sleep quality, and a more positive attitude towards subsequent treatment. Also, patient's psychological state and postoperative rehabilitation environment may influence the differences in scores. The surgical procedures explored in this study are poised to receive further validation through expanded case studies.
To our knowledge, this is the first study to compare the clinical benefits and prognosis of low-curvature titanium mesh and normal-curvature titanium mesh in the treatment of cranioplasty. However, this study has several limitations inherent to its retrospective design. Primarily, it is a single-center, small-sample investigation, which limits the generalizability of the findings and the applications in other settings. We will needa multi-center, prospective, large-sample study for comprehensive validation in the future. The retrospective analysis employed may introduce selection bias. Additionally, the limited sample size may limit the statistical power to detect differences, particularly in less common complications or rare events. Furthermore,the study does not provide the dynamic post-discharge follow-up, a detailed examination of postoperative complications, such as infection, seizures, epidural hematoma, hardware failure, and subdural effusion, was not conducted.
ConclusionThis retrospective cohort study highlights that skull repair utilizing low-curvature titanium mesh shortens hospital stay effectively, improves patient satisfaction with surgical outcomes, and improves the postoperative functional status and quality of life for neurosurgically treated patients. These promising findings warrant further clinical exploration and promotion.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statementThe studies involving humans were approved by Institutional Review Board of the Binhai County People's Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributionsSY: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. WC: Visualization, Writing – review & editing. HT: Formal Analysis, Writing – original draft. LZ: Writing – review & editing. KJ: Writing – review & editing. HZ: Conceptualization, Funding acquisition, Supervision, Writing – original draft.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Research and Development Fund Project of Binhai People's Hospital affiliated to Kangda College, Nanjing Medical University (KD2023KYJJ157).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Oddo M, Taccone FS, Petrosino M, Badenes R, Blandino-Ortiz A, Bouzat P, et al. The neurological pupil index for outcome prognostication in people with acute brain injury (ORANGE): a prospective, observational, multicentre cohort study. Lancet Neurol. (2023) 22(10):925–33. doi: 10.1016/S1474-4422(23)00271-5
PubMed Abstract | Crossref Full Text | Google Scholar
2. Steyerberg EW, Wiegers E, Sewalt C, Buki A, Citerio G, De Keyser V, et al. Case-mix, care pathways, and outcomes in patients with traumatic brain injury in CENTER-TBI: a European prospective, multicentre, longitudinal, cohort study. Lancet Neurol. (2019) 18(10):923–34. doi: 10.1016/S1474-4422(19)30232-7
PubMed Abstract | Crossref Full Text | Google Scholar
3. Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, D’Urso P, et al. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. (2011) 364(16):1493–502. doi: 10.1056/NEJMoa1102077
PubMed Abstract | Crossref Full Text | Google Scholar
4. Chen R, Ye G, Zheng Y, Zhang Y, Zheng S, Fang W, et al. Optimal timing of cranioplasty and predictors of overall complications after cranioplasty: the impact of brain collapse. Neurosurgery. (2023) 93(1):84–94. doi: 10.1227/neu.0000000000002376
PubMed Abstract | Crossref Full Text | Google Scholar
5. Vitali M, Marasco S, Romenskaya T, Elia A, Longhitano Y, Zanza C, et al. Decompressive craniectomy in severe traumatic brain injury: the intensivist’s point of view. Diseases. (2023) 11(1):22. doi: 10.3390/diseases11010022
PubMed Abstract | Crossref Full Text | Google Scholar
6. Sahuquillo J, Dennis JA. Decompressive craniectomy for the treatment of high intracranial pressure in closed traumatic brain injury. Cochrane Database Syst Rev. (2019) 12(12):Cd003983. doi: 10.1002/14651858.CD003983.pub3
PubMed Abstract | Crossref Full Text | Google Scholar
7. Li J, Ellis DG, Kodym O, Rauschenbach L, Rieß C, Sure U, et al. Towards clinical applicability and computational efficiency in automatic cranial implant design: an overview of the AutoImplant 2021 cranial implant design challenge. Med Image Anal. (2023) 88:102865. doi: 10.1016/j.media.2023.102865
PubMed Abstract | Crossref Full Text | Google Scholar
9. Henry J, Amoo M, Taylor J, O'Brien DP. Complications of cranioplasty in relation to material: systematic review, network meta-analysis and meta-regression. Neurosurgery. (2021) 89(3):383–94. doi: 10.1093/neuros/nyab180
PubMed Abstract | Crossref Full Text | Google Scholar
11. Dang Y, Ping J, Guo Y, Yang Y, Xia X, Huang R, et al. Cranioplasty for patients with disorders of consciousness. Ann Palliat Med. (2021) 10(8):8889–99. doi: 10.21037/apm-21-1822
PubMed Abstract | Crossref Full Text | Google Scholar
12. Veldeman M, Daleiden L, Hamou H, Höllig A, Clusmann H. An altered posterior question-mark incision is associated with a reduced infection rate of cranioplasty after decompressive hemicraniectomy. J Neurosurg. (2020) 134(3):1262–70. doi: 10.3171/2020.2.JNS193335
PubMed Abstract | Crossref Full Text | Google Scholar
13. Halani SH, Chu JK, Malcolm JG, Rindler RS, Allen JW, Grossberg JA, et al. Effects of cranioplasty on cerebral blood flow following decompressive craniectomy: a systematic review of the literature. Neurosurgery. (2017) 81(2):204–16. doi: 10.1093/neuros/nyx054
PubMed Abstract | Crossref Full Text | Google Scholar
14. Jasey N, Ward I, Lequerica A, Chiaravalloti ND. The therapeutic value of cranioplasty in individuals with brain injury. Brain Inj. (2018) 32(3):318–24. doi: 10.1080/02699052.2017.1419283
PubMed Abstract | Crossref Full Text | Google Scholar
15. Reponen E, Tuominen H, Korja M. Evidence for the use of preoperative risk assessment scores in elective cranial neurosurgery: a systematic review of the literature. Anesth Analg. (2014) 119(2):420–32. doi: 10.1213/ANE.0000000000000234
PubMed Abstract | Crossref Full Text | Google Scholar
16. Temel JS, Greer JA, El-Jawahri A, Pirl WF, Park ER, Jackson VA, et al. Effects of early integrated palliative care in patients with lung and GI cancer: a randomized clinical trial. J Clin Oncol. (2017) 35(8):834–41. doi: 10.1200/JCO.2016.70.5046
PubMed Abstract | Crossref Full Text | Google Scholar
17. Feroze AH, Walmsley GG, Choudhri O, Lorenz HP, Grant GA, Edwards MS. Evolution of cranioplasty techniques in neurosurgery: historical review, pediatric considerations, and current trends. J Neurosurg. (2015) 123(4):1098–107. doi: 10.3171/2014.11.JNS14622
PubMed Abstract | Crossref Full Text | Google Scholar
18. Hersh DS, Anderson HJ, Woodworth GF, Martin JE, Khan YM. Bone flap resorption in pediatric patients following autologous cranioplasty. Oper Neurosurg (Hagerstown). (2021) 20(5):436–43. doi: 10.1093/ons/opaa452
PubMed Abstract | Crossref Full Text | Google Scholar
19. Signorelli F, Giordano M, Caccavella VM, Ioannoni E, Gelormini C, Caricato A, et al. A systematic review and meta-analysis of factors involved in bone flap resorption after decompressive craniectomy. Neurosurg Rev. (2022) 45(3):1915–22. doi: 10.1007/s10143-022-01737-z
PubMed Abstract | Crossref Full Text | Google Scholar
20. Di Rienzo A, Colasanti R, Dobran M, Carrassi E, Herber N, Paracino R, et al. Bone flap resorption after cranioplasty: risk factors and proposal of the flap integrity score. World Neurosurg. (2024) 181:e758–75. doi: 10.1016/j.wneu.2023.10.124
PubMed Abstract | Crossref Full Text | Google Scholar
21. Zhang J, Su Y, Rao X, Pang H, Zhu H, Liu L, et al. Additively manufactured polyether ether ketone (PEEK) skull implant as an alternative to titanium mesh in cranioplasty. Int J Bioprint. (2023) 9(1):634. doi: 10.18063/ijb.v9i1.634
PubMed Abstract | Crossref Full Text | Google Scholar
22. Yao S, Zhang Q, Mai Y, Yang H, Li Y, Zhang M, et al. Outcome and risk factors of complications after cranioplasty with polyetheretherketone and titanium mesh: a single-center retrospective study. Front Neurol. (2022) 13:926436. doi: 10.3389/fneur.2022.926436
PubMed Abstract | Crossref Full Text | Google Scholar
23. Rosinski CL, Patel S, Geever B, Chiu RG, Chaker AN, Zakrzewski J, et al. A retrospective comparative analysis of titanium mesh and custom implants for cranioplasty. Neurosurgery. (2020) 86(1):E15–e22. doi: 10.1093/neuros/nyz358
PubMed Abstract | Crossref Full Text | Google Scholar
24. Liu W, Zhao H, Zhang C, Xu S, Zhang F, Wei L, et al. In situ activation of flexible magnetoelectric membrane enhances bone defect repair. Nat Commun. (2023) 14(1):4091. doi: 10.1038/s41467-023-39744-3
PubMed Abstract | Crossref Full Text | Google Scholar
25. Porta FLA, Formisano R, Iaccarino C, Lavezzi S, Pompucci A, Estraneo A, et al. When the practice does not meet the theory: results from an Italian survey on the clinical and pathway management of inpatients with decompressive craniectomy or cranioplasty admitted to rehabilitation. Eur J Phys Rehabil Med. (2023) 59(3):303–16. doi: 10.23736/S1973-9087.23.07754-7
PubMed Abstract | Crossref Full Text | Google Scholar
26. Huang YH, Lee TC, Yang KY, Liao CC. Is timing of cranioplasty following posttraumatic craniectomy related to neurological outcome? Int J Surg. (2013) 11(9):886–90. doi: 10.1016/j.ijsu.2013.07.013
PubMed Abstract | Crossref Full Text | Google Scholar
27. Bader ER, Kobets AJ, Ammar A, Goodrich JT. Factors predicting complications following cranioplasty. J Craniomaxillofac Surg. (2022) 50(2):134–9. doi: 10.1016/j.jcms.2021.08.001
PubMed Abstract | Crossref Full Text | Google Scholar
28. Rashidi A, Sandalcioglu IE, Luchtmann M. Aseptic bone-flap resorption after cranioplasty - incidence and risk factors. PLoS One. (2020) 15(1):e0228009. doi: 10.1371/journal.pone.0228009
PubMed Abstract | Crossref Full Text | Google Scholar
29. Schäfer N, Proescholdt M, Steinbach JP, Weyerbrock A, Hau P, Grauer O, et al. Quality of life in the GLARIUS trial randomizing bevacizumab/irinotecan versus temozolomide in newly diagnosed, MGMT-nonmethylated glioblastoma. Neuro Oncol. (2018) 20(7):975–85. doi: 10.1093/neuonc/nox204
留言 (0)