Oligodendroglioma is a rare primary brain tumor that is challenging to cure, and it originates from oligodendrocytes or glial precursor cells (1), constituting 2%–5% of all central nervous system (CNS) tumors (2). According to the World Health Organization (WHO) classification guidelines, oligodendrogliomas are characterized by the presence of an IDH mutation and 1p/19q codeletion (3). Based on tumor cells' integrated histological and molecular features, oligodendrogliomas can be divided into well-differentiated WHO grade II and anaplastic WHO grade III categories (4).
Given their infrequent occurrence, grade II and III oligodendrogliomas are often combined into an entity or grouped with astrocytic tumors during clinical investigations (5). Limited studies have identified the clinical and biological prognostic factors to predict the outcome of WHO II oligodendrogliomas (OG/II) patients (6), which exhibit lower malignancy compared to anaplastic oligodendrogliomas (AOG). Meanwhile, the therapeutic approaches for OG/II and AOG are usually different. For AOG, the recommended treatment protocol includes maximal safe surgical resection followed by radiation and chemotherapy (3, 7). However, the therapy for OG/II remains controversial. According to the 2022 National Comprehensive Cancer Network (NCCN) guidelines, OG/II is divided into low- and high-risk groups depending on the age and extent of resection (8). Nonetheless, as one of the important clinical features, tumor size was not considered a risk factor above.
In this study, a retrospective analysis was conducted utilizing data from the Surveillance, Epidemiology, and End Results (SEER) database. Clinical characteristics and independent prognostic factors were analyzed in OG/II patients. Furthermore, an optimal cut-off value for tumor size was established to identify patients with a poor prognosis, leading to the stratification of patients into two subgroups based on tumor size. Subsequent analyses were performed to elucidate the impacts of tumor size on the prognosis of OG/II patients, which is instructive for therapeutic strategies, such as the extent of resection, radiation, and chemotherapy.
Material and methods Data collectionData were collected from the SEER database (version 8.4.1), and patients diagnosed with WHO-II grade oligodendroglioma (ICD-O-3 histologic code 9450) from 1975 to 2020 were chosen in this study. Our data included age, sex, race, primary site, tumor size, surgery, radiation, survival time, and vital status.
An extraction workflow of cases is presented in Figure 1. A total of 2,952 cases were found in the SEER database, and we cleaned data as follows (1): cases that lacked surgical information or were not surgical were excluded. The surgical information was classified into biopsy, subtotal resection, total resection, and extended resection. (2) Tumor size was collected according to the terms “CS Tumor Size”, and cases with unknown tumor sizes were cleaned, including the CS tumor size codes 999, 990, and 000. (2) cases that died due to other diseases were omitted. The endpoint of this study was cancer-specific survival. Finally, we confirmed the grades of selected cases, and all cases were moderately differentiated, meaning grade II. All data were collected and analyzed by two independent researchers and verified by the third one.
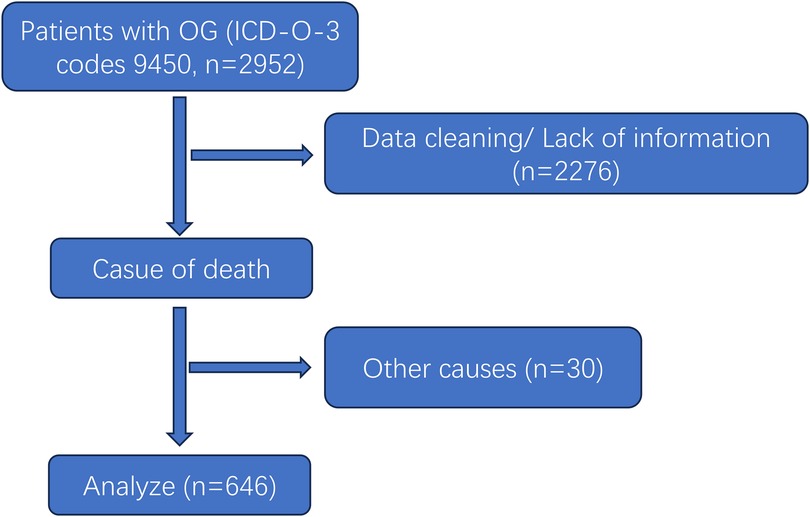
Figure 1. Flowchart of OG/II patient selection.
Statistical analysisAll statistical analyses were performed with R (version 4.1.3), and the following R packages were used: “rms,” “foreign,” “survival,” “ggplot2,” “survminer,” and “forestplot.” Statistical significance was set at P < 0.05. Univariable and multivariable regression analyses were performed in all patients using a Cox proportional hazard model, and the results were presented as hazard ratios (HR) with corresponding 95% confidence intervals (CIs). Variables considered clinically relevant or showed a univariate relationship with outcome were entered into a multivariate Cox regression model. Survival was assessed using Kaplan-Meier models, and statistical significance was determined using the log-rank test. A prognostic nomogram was constructed by R to predict the survival of patients, and calibration curves were formulated to evaluate the judgment ability of the nomogram.
Results Patients characteristicsA total of 676 postoperative WHO-II grade oligodendroglioma (OG/II) patients were collected in this study, with the diagnostic years ranging from 2004 to 2020 (Figure 1). The clinical characteristics of selected patients are listed in Table 1. Among them, 47.52% of patients were <40 years old, 87.15% were white, and 56.97% were male. Concerning tumor location, the majority of OG/II cases (62.23%) were situated in the frontal lobe.
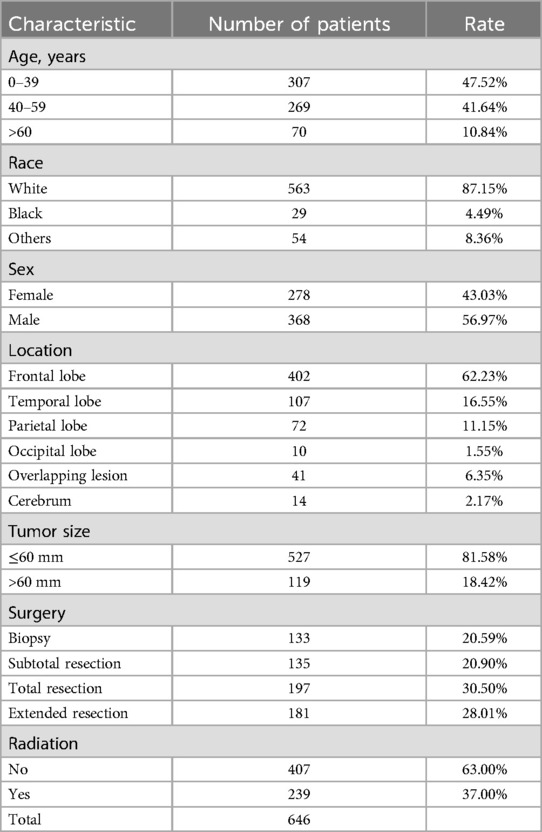
Table 1. Characteristics of included patients.
Identification and validation of cut-off value for tumor sizeThe X-tile software was applied to analyze the optimal tumor size cut-off based on survival information. The cut-off value was identified by maximizing the chi-square score and minimizing the P value. As shown in Figure 2, 60 mm was identified as a suitable cut-off value. Furthermore, we also validated the cut-off value in increments of 5 mm using both univariate and multivariate Cox regression analyses (Table 2). Multivariate analysis showed that the P values of cut-off values from 30 to 75 mm were significant, and the tumor size cut-off at 60 mm had a high HR (2.035, 95% CI 1.390–2.981) for CSS. Although the largest HR was a cut-off value of 20 mm, univariate analysis has no significance. Thus, 60 mm was confirmed to be the optimal cut-off value for tumor size.
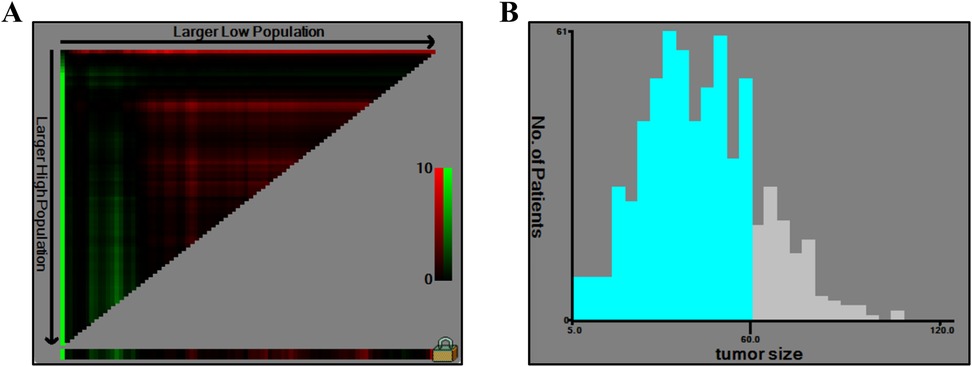
Figure 2. X-tile analysis of tumor size. (A) The graph shows that the optimal cutoff value was determined by X-tile software. (B) A histogram shows the distribution of tumor size values among patients.
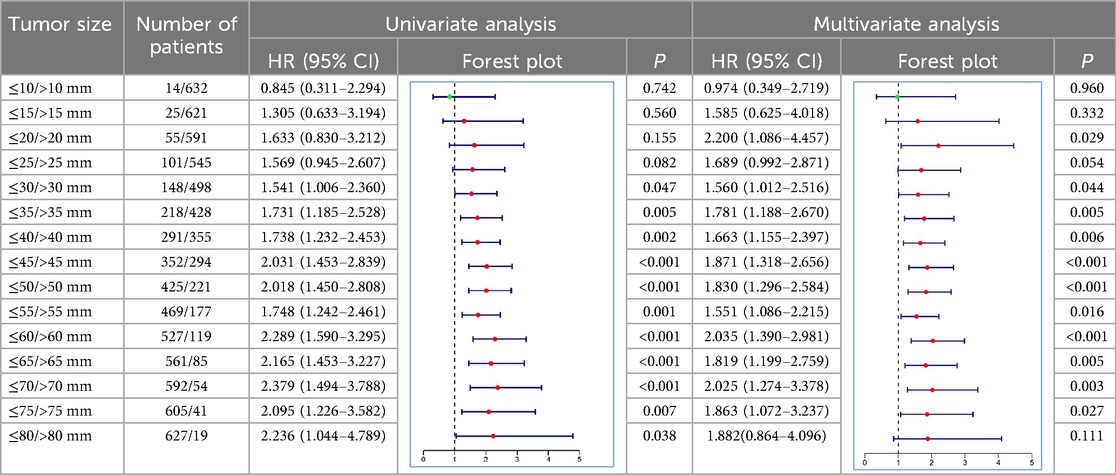
Table 2. Univariate and multivariate Cox analysis of different tumor size cutoffs in OG/II patients.
Factors associated with cause-specific survivalUnivariate and multivariate COX analyses evaluated the associations of characteristics in Table 1 with cause-specific survival (CSS). Univariate analysis indicated that age (>60 years vs. <40 years, HR 3.82, 95% CI 2.45–5.97, P < 0.01), sex (male vs. female, HR 1.45, 95% CI 1.03–2.04, P = 0.031), tumor size (>60 mm vs. ≤60 mm, HR 2.29, 95% CI 1.59–3.30, P < 0.01), surgery (total resection vs. biopsy, HR 0.36, 95% CI 0.21–0.64, and extended resection vs. biopsy, HR 0.62, 95% CI 0.42–0.82, P = 0.019), and radiation (no vs. yes, HR 1.70, 95% CI 1.22–2.38, P = 0.002) were significantly related to CSS of OG/II patients. Meanwhile, a similar result was found in multivariate COX analyses, except for radiation (Table 3). The result suggested that age, sex, tumor size, and surgery were the key prognostic factors for OG/II patients.
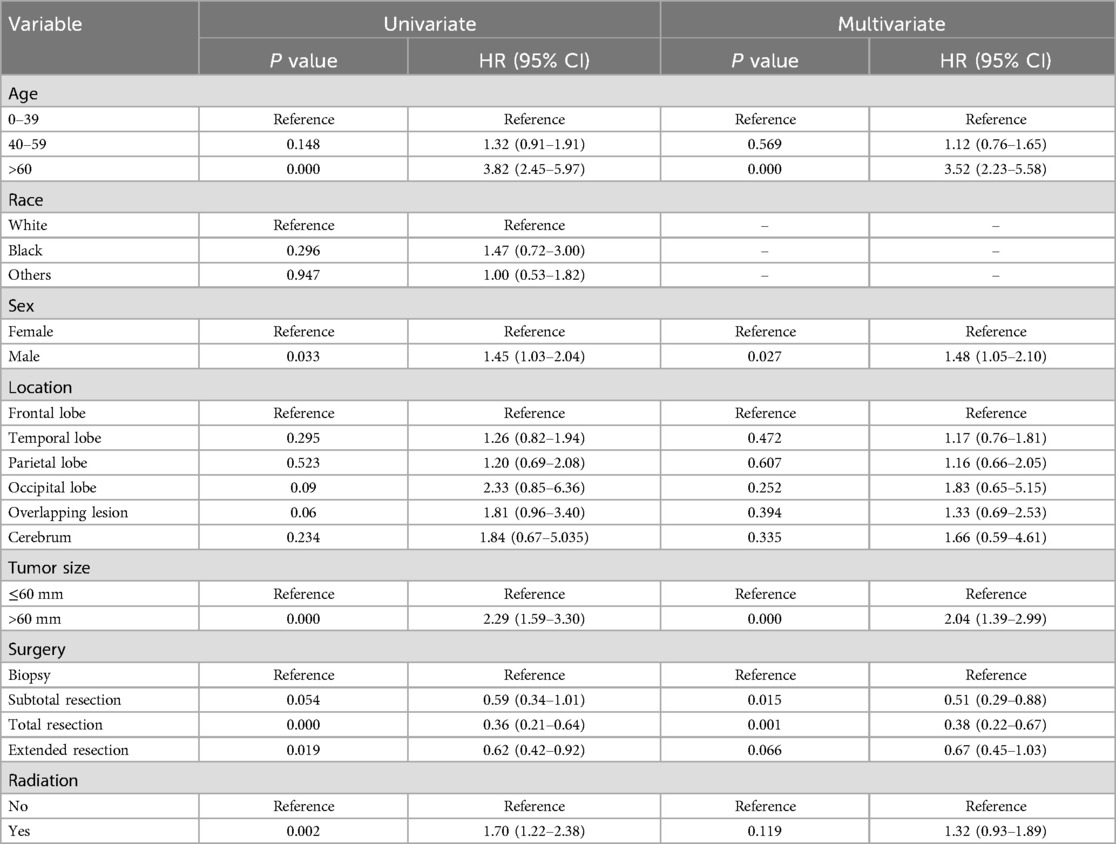
Table 3. Univariate and multivariate analyses of cause-specific survival.
Kaplan-Meier curves were subsequently conducted on these four factors, and the result indicated that patients older than 60 years (Figure 3A) or males (Figure 3B) lived shorter. In contrast, those with tumor sizes smaller than 60 mm (Figure 3C) or operated with total resection (Figure 3D) had a longer survival time.
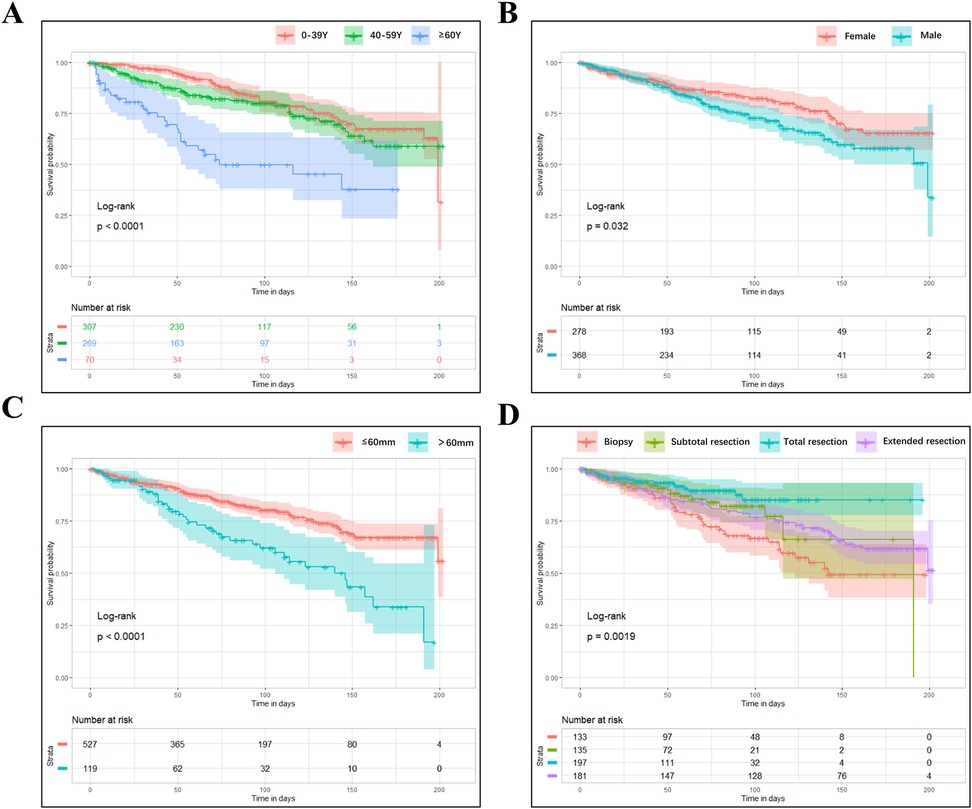
Figure 3. The kaplan-meier curves of CSS for OG/II patients. (A) age; (B) sex; (C) tumor size; (D) the extent of recession.
Impacts of tumor size on treatment outcomesBased on the tumor size, the OG/II patients were categorized into a low-risk group (n = 527) and a high-risk group (n = 119). The characteristics of patients in these groups are detailed in Table 4. Univariate and multivariate COX analyses were conducted (Table 5). In the low-risk group, multivariate analyses indicated that an age greater than 60 years (HR 4.05) was associated with poor CSS. In contrast, surgery, especially for total resection (HR 0.35), was related to improved CSS. In the high-risk group, only age and sex were considered independent predictors for CSS. Furthermore, an unfavorable role of radiation (HR 1.58, P = 0.033) was found in the low-risk group, and a favorable tendency of radiation (HR 0.98) was observed in the high-risk group. However, the tendency lacked statistical significance.
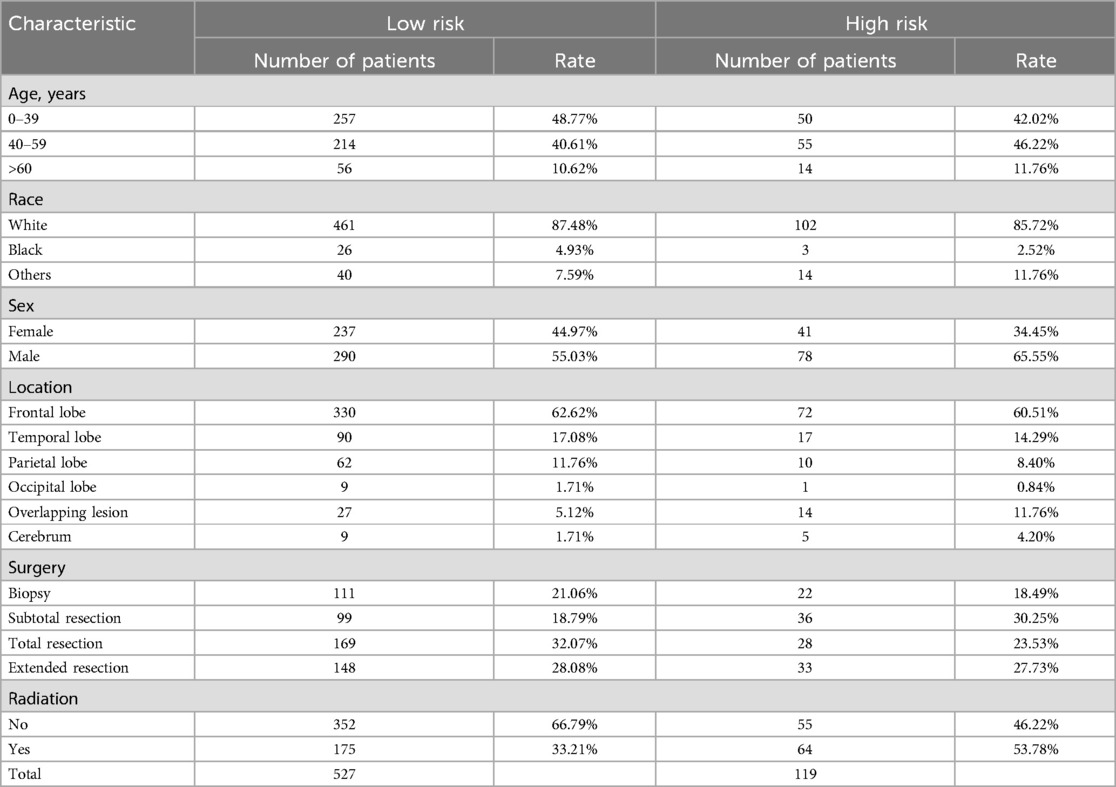
Table 4. Characteristics of patients in two groups.
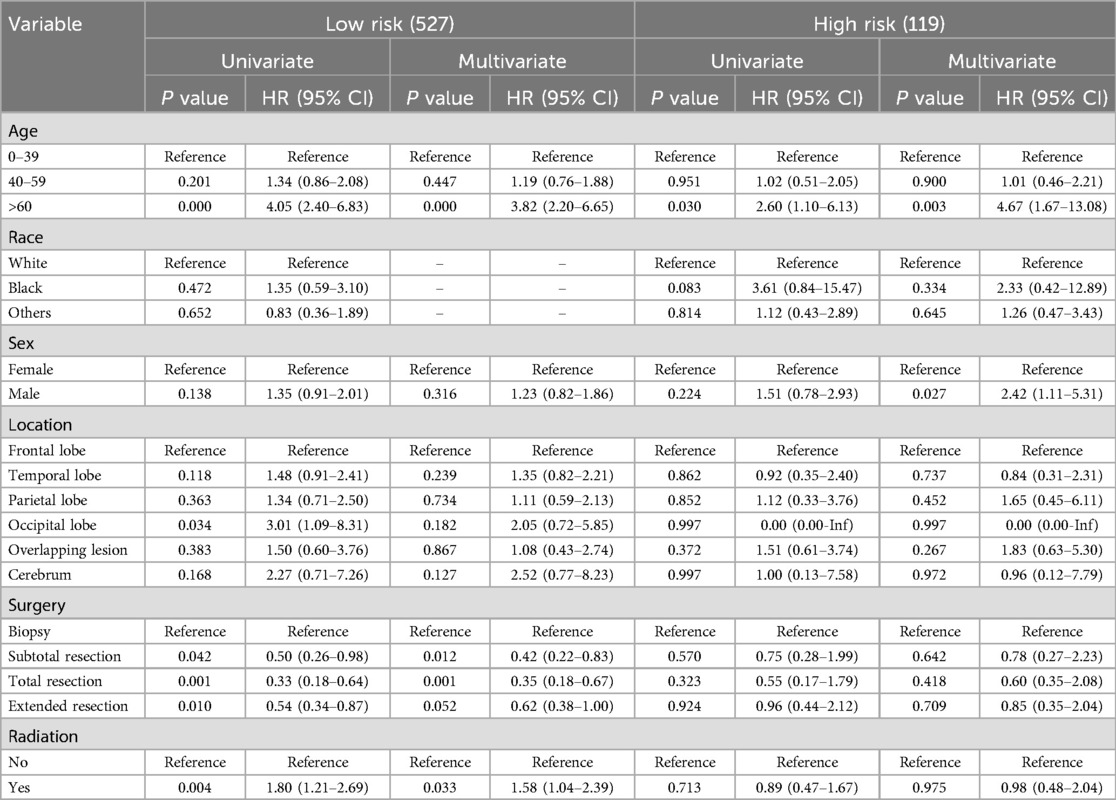
Table 5. Univariate and multivariate analyses in two groups.
Construction of nomogram for the low-risk groupA nomogram was constructed to predict the survival of postoperative OG/II patients with a tumor size less than 60 mm. As shown in Figure 4A, each patient's corresponding survival probability could be obtained by summing each predictor's total scores. For example, a 60-year-old male white patient was diagnosed with an oligodendroglioma in the occipital lobe, and he underwent a subtotal resection of the tumor without additional radiation. According to the nomogram, the predicted 3-,5-, and 10-year survival rates are about 81%, 70%, and 50% respectively. Furthermore, The calibration curves of the 3-, 5-, and 10-year survival rates showed good agreement between the nomogram predictions and actual observations (Figures 4B–D).
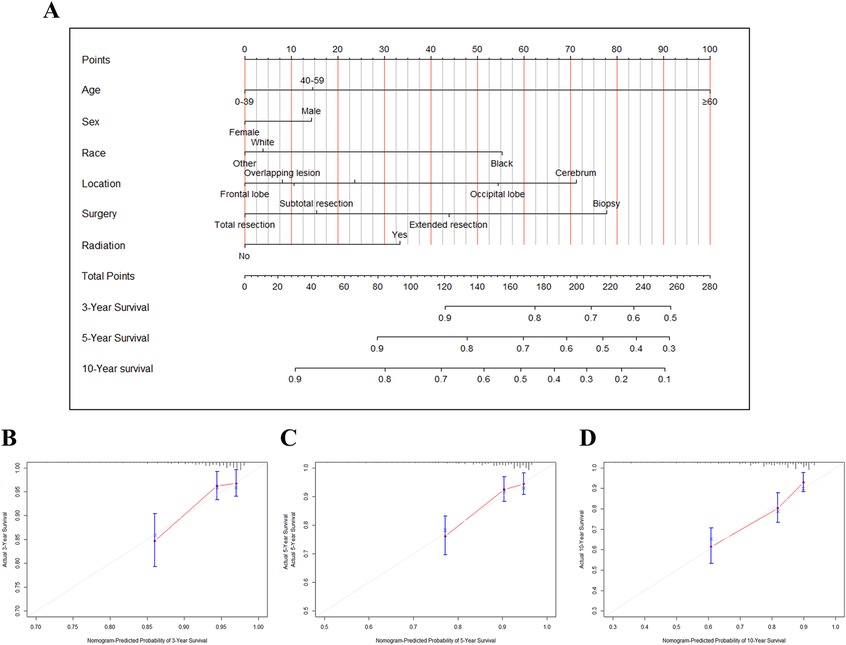
Figure 4. The nomogram and calibration plots for predicting survival of OG/II patients with tumor size ≤ 60 mm. (A) The nomogram for predicting 3-,5-, and 10-year survival. Calibration plots for 3-year (B), 5-year (C), and 10-year (D) survival prediction.
DiscussionThe clinic's prediction of oligodendroglioma outcomes remains challenging due to its rare incidence. Specifically, accurate and effective prognostication of OG/II, a subtype of oligodendroglioma, is critical for personalized therapeutic approaches and may present additional difficulties. Thus, we conducted a retrospective analysis on SEER, a database that offers an opportunity to investigate rare diseases.
Our study collected and analyzed 646 postoperative OG/II cases from SEER. Four key prognostic factors related to CCS in postoperative OG/II patients were identified, including age, sex, excision extension, and tumor size. OG/II can be classified into low- and high-risk groups based on tumor size. Total recession is recommended in the low-risk group, while extended resection and radiation may not be beneficial. Tumor size can be a valuable factor for predicting prognosis and making therapeutic schedules, and a nomogram was established (Figure 5).
Figure 5. Schematic of study.
Nearly half of our cases were younger than 40 years, with the incidence peak at 30–39 years. A similar result, 36–40 years, was reported in a study based on the Central Brain Tumor Registry of the United States (CBTRUS) from 2000 to 2013 (9). Our analysis indicated that age is an important factor affecting the prognosis of OG/II patients, and patients over 60 years had worse survival when compared to those younger than 40 years. Age is also considered an essential prognostic factor in other types of glioma (10). Research has demonstrated that glioma is more aggressive in elderly patients (11). Older men usually do not recommend surgery and adjuvant treatment (12). Thus, younger patients are more likely to have a better prognosis. Elderly patients are more prone to have comorbidities, which makes them more susceptible to death from factors other than tumors. During the process of organizing our data, we found that patients over the age of 60 have a 23.3% chance of dying from non-tumor-related causes. In contrast, in patients under the age of 60, the rate of non-tumor-related mortality is only 7.5%. Thus, we chose to focus on cancer-specific survival (CCS) to mitigate the potential impact of comorbidities.
Sex is another independent prognostic factor in our study, as shown by univariate and multivariate analysis. In the present study, the male gender was related to high tumor-specific mortality compared to females. As reported, sex differences have been well-identified in many brain tumors, such as glioblastoma. Glioma patients usually present a greater tumor incidence and worse outcomes in males, which may be caused by differences in pathophysiological mechanisms such as hormonal influences, metabolic pathways, immune responses, and molecular changes (13). Understanding the role of gender in OG/II may help to create a sex-specific therapy to improve the survival of patients.
Maximal safe resection of the tumor is the first and most recommended therapy for glioma. However, for OG/II, the influence of tumor resection on the prognosis seems very mild (14). The extent of surgical tumor resection remains controversial. Shawn L. et al. conducted a retrospective study on a multicenter and multinational cohort of 757 diffuse low-grade glioma (LGG) patients. Their result indicated that the extent of surgical tumor resection beginning at 75% improves over survival while beginning at 80% improves progression-free survival of LLG patients (15). However, this result relied on the combined analysis of oligodendrogliomas and astrocytomas. Connor J. et al. reported that a greater extent of surgical tumor resection is associated with improved survival in oligodendrogliomas (16), which include OG/II and AOG. Our study focused on OG/II and found that extended resection of tumors can not benefit more than subtotal resection. And patients with total resection had better cause-specific survival. Thus, we recommended a total tumor resection for OG/II.
Tumor size is the maximum diameter of the tumor and has been proven to be a critical prognostic factor for many tumors, such as lung cancer (17), uterine sarcoma (18), and hepatoma (19). In neuroblastoma, Wang et al. identified a cut-off value of 4 cm for tumor size and suggested that tumor size >4 cm might predict poor prognosis (20). Lin et al. reported a tumor size of 59 mm as a critical cut-off value for low-grade supratentorial glioma, and they suggest that a tumor size >59 mm represents a high risk and indicates a worsened outcome (21). However, few studies investigate the value of tumor size for OG/II patients. We identified and verified a cut-off value of 60 mm for OG/II, which indicated that tumor size >60 mm was a high risk for postoperative OG/II patient survival.
Therapeutic schedules for OG/II patients remain controversial and should mainly focus on prognostic factors. ASCO-SNO Guidelines suggest surgical resection accompanied by radiation and chemotherapy for oligodendroglioma, including OG/II and AOG, but the strength of the recommendation is weak (3). A risk classification system was introduced in 2022 NCCN guidelines, and OG/II is divided into low- and high-risk groups relying on the age and extent of resection (8). However, this classification did not include the tumor size, an essential prognostic factor in our analysis. Thus, we further divided the patients into a low-risk group (tumor size ≤60 mm) and a high-risk group (tumor size >60 mm). The result of univariate and multivariate analyses suggested subtotal or total resection in low-risk patients. However, extended resection and radiation were not recommended in patients with tumor sizes smaller than 60 mm. The result agrees with the biological behavior of OG/II, which is a benign tendency with relatively slow tumor growth (15, 22). When making a therapeutic schedule, it is important to consider the benefits and potential damage of treatment such as surgery, radiation, and chemotherapy (23). In patients with tumor sizes larger than 60 mm, we found beneficial roles in CSS of radiation with HR < 1, which suggested an adjuvant treatment. However, the results were not statistically significant with P values > 0.05, possibly due to the small sample size. More studies are needed to clarify the results.
There are several limitations to our study. Firstly, this is a retrospective study performed on SEER data, and some potential biases can not be avoided, such as incomplete data and misclassification of variables. We are eager for more studies to confirm our idea, especially for randomized controlled trials. Secondly, many variables do not exist or are incomplete in the SEER database. Still, they are closely related to survival, such as the details of chemotherapy, duration of symptoms, etc. Thirdly, the cases in the high-risk group are not big enough, and we can not get a useful and significant result in this group. Lastly, we established a nomogram to predict the survival of OG/II patients with tumor sizes less than 60 mm. An external validation cohort may be needed to assess the applicability in the patients. Other variables, like background disease, need to be considered in the nomogram in future studies.
ConclusionIn summary, our study identified four critical prognostic factors in postoperative WHO-II grade oligodendroglioma: age, sex, the extent of recession, and tumor size. We established a cut-off value of 60 mm for tumor size, which allowed us to classify OG/II into low- and high-risk groups. Further analysis indicates that total resection is advantageous for patients with tumor sizes less than 60 mm, and subtotal recession also appears to be favorable. However, extended resection and radiation therapy may not confer additional benefits on these patients. Furthermore, A nomogram established in the present study could predict the prognosis for OG/II patients with tumor size less than 60 mm objectively and accurately. However, more studies are required to confirm our conclusion.
Data availability statementPublicly available datasets were analyzed in this study. This data can be found here: Our original data are obtained from a public database(SEER), and the analyzed data are available from the corresponding author on reasonable request.
Author contributionsQL: Data curation, Funding acquisition, Writing – original draft. YW: Methodology, Software, Writing – original draft. YX: Data curation, Formal Analysis, Methodology, Writing – original draft. SY: Supervision, Writing – review & editing. HJ: Conceptualization, Supervision, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Medical Science and Health Technology of Zhejiang Province, China (2023KY786).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Rincon-Torroella J, Rakovec M, Materi J, Raj D, Vivas-Buitrago T, Ferres A, et al. Current and future frontiers of molecularly defined oligodendrogliomas. Front Oncol. (2022) 12:934426. doi: 10.3389/fonc.2022.934426
PubMed Abstract | Crossref Full Text | Google Scholar
3. Mohile NA, Messersmith H, Gatson NT, Hottinger AF, Lassman A, Morton J, et al. Therapy for diffuse astrocytic and oligodendroglial tumors in adults: ASCO-SNO guideline. J Clin Oncol. (2022) 40(4):403–26. doi: 10.1200/JCO.21.02036
PubMed Abstract | Crossref Full Text | Google Scholar
4. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro-oncology. (2021) 23(8):1231–51. doi: 10.1093/neuonc/noab106
PubMed Abstract | Crossref Full Text | Google Scholar
5. Chaichana KL, McGirt MJ, Laterra J, Olivi A, Quinones-Hinojosa A. Recurrence and malignant degeneration after resection of adult hemispheric low-grade gliomas. J Neurosurg. (2010) 112(1):10–7. doi: 10.3171/2008.10.JNS08608
PubMed Abstract | Crossref Full Text | Google Scholar
6. Harary M, Kavouridis VK, Torre M, Zaidi HA, Chukwueke UN, Reardon DA, et al. Predictors and early survival outcomes of maximal resection in WHO grade II 1p/19q-codeleted oligodendrogliomas. Neurooncology. (2020) 22(3):369–80. doi: 10.1093/neuonc/noz168
PubMed Abstract | Crossref Full Text | Google Scholar
7. Berger TR, Wen PY, Lang-Orsini M, Chukwueke UN. World health organization 2021 classification of central nervous system tumors and implications for therapy for adult-type gliomas: a review. JAMA Oncol. (2022) 8(10):1493–501. doi: 10.1001/jamaoncol.2022.2844
PubMed Abstract | Crossref Full Text | Google Scholar
8. Horbinski C, Nabors LB, Portnow J, Baehring J, Bhatia A, Bloch O, et al. NCCN Guidelines(R) insights: central nervous system cancers, version 2.2022. J Natl Compr Canc Netw. (2023) 21(1):12–20. doi: 10.6004/jnccn.2023.0002
PubMed Abstract | Crossref Full Text | Google Scholar
9. Achey RL, Khanna V, Ostrom QT, Kruchko C, Barnholtz-Sloan JS. Incidence and survival trends in oligodendrogliomas and anaplastic oligodendrogliomas in the United States from 2000 to 2013: a CBTRUS report. J Neuro-Oncol. (2017) 133(1):17–25. doi: 10.1007/s11060-017-2414-z
PubMed Abstract | Crossref Full Text | Google Scholar
10. Wang T, Niu X, Gao T, Zhao L, Li J, Gan Y, et al. Prognostic factors for survival outcome of high-grade multicentric glioma. World Neurosurg. (2018) 112:e269–e77. doi: 10.1016/j.wneu.2018.01.035
PubMed Abstract | Crossref Full Text | Google Scholar
11. Krishnatry R, Zhukova N, Guerreiro Stucklin AS, Pole JD, Mistry M, Fried I, et al. Clinical and treatment factors determining long-term outcomes for adult survivors of childhood low-grade glioma: a population-based study. Cancer. (2016) 122(8):1261–9. doi: 10.1002/cncr.29907
PubMed Abstract | Crossref Full Text | Google Scholar
15. Hervey-Jumper SL, Zhang Y, Phillips JJ, Morshed RA, Young JS, McCoy L, et al. Interactive effects of molecular, therapeutic, and patient factors on outcome of diffuse low-grade glioma. J Clin Oncol. (2023) 41(11):2029–42. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I=Immediate Family Member, Inst=My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwcorascopubs.org/jco/authors/author-center Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments). Michael McDermott Honoraria: Insightec Consulting or Advisory Role: Stryker Patents, Royalties, Other Intellectual Property: Royalties from Limitorr CSF reservoir sales excluding my institution; manufacturer Integra Lifesciences; patent rights signed over to University of California many years ago No other potential conflicts of interest were reported. doi: 10.1200/JCO.21.02929
PubMed Abstract | Crossref Full Text | Google Scholar
16. Kinslow CJ, Garton ALA, Rae AI, Marcus LP, Adams CM, McKhann GM, et al. Extent of resection and survival for oligodendroglioma: a U.S. population-based study. J Neuro-Oncol. (2019) 144(3):591–601. doi: 10.1007/s11060-019-03261-5
PubMed Abstract | Crossref Full Text | Google Scholar
17. Gulack BC, Yang CF, Speicher PJ, Meza JM, Gu L, Wang X, et al. The impact of tumor size on the association of the extent of lymph node resection and survival in clinical stage I non-small cell lung cancer. Lung Cancer. (2015) 90(3):554–60. doi: 10.1016/j.lungcan.2015.10.011
PubMed Abstract | Crossref Full Text | Google Scholar
18. Cao S, Liao X, Xu K, Xiao H, Shi Z, Zou Y, et al. Development and validation of tumor-size-stratified prognostic nomograms for patients with uterine sarcoma: a SEER database analysis. Cancer Med. (2023) 12(2):1339–49. doi: 10.1002/cam4.5014
PubMed Abstract | Crossref Full Text | Google Scholar
19. Yang A, Xiao W, Chen D, Wei X, Huang S, Lin Y, et al. The power of tumor sizes in predicting the survival of solitary hepatocellular carcinoma patients. Cancer Med. (2018) 7(12):6040–50. doi: 10.1002/cam4.1873
PubMed Abstract | Crossref Full Text | Google Scholar
20. Wang JX, Cao ZY, Wang CX, Zhang HY, Fan FL, Zhang J, et al. Prognostic impact of tumor size on patients with neuroblastoma in a SEER-based study. Cancer Med. (2022) 11(14):2779–89. doi: 10.1002/cam4.4653
PubMed Abstract | Crossref Full Text | Google Scholar
21. Lin DD, Deng XY, Zheng DD, Gu CH, Yu LS, Xu SY, et al. The effects of tumor size and postoperative radiotherapy for patients with adult low-grade (WHO grade II) infiltrative supratentorial astrocytoma/oligodendroglioma: a population-based and propensity score matched study. Cancer Med. (2018) 7(12):5973–87. doi: 10.1002/cam4.1853
PubMed Abstract | Crossref Full Text | Google Scholar
22. Gorovets D, Kannan K, Shen R, Kastenhuber ER, Islamdoust N, Campos C, et al. IDH Mutation and neuroglial developmental features define clinically distinct subclasses of lower grade diffuse astrocytic glioma. Clin Cancer Res. (2012) 18(9):2490–501. doi: 10.1158/1078-0432.CCR-11-2977
PubMed Abstract | Crossref Full Text | Google Scholar
23. Delev D, Heiland DH, Franco P, Reinacher P, Mader I, Staszewski O, et al. Surgical management of lower-grade glioma in the spotlight of the 2016 WHO classification system. J Neuro-Oncol. (2019) 141(1):223–33. doi: 10.1007/s11060-018-03030-w
留言 (0)