Prostate cancer (PrCa) is a prevalent malignancy among men worldwide, and understanding its statistics is crucial for assessing its impact on public health (1). In 2020, there were an estimated 1.4 million new cases of PrCa diagnosed globally (2). It primarily affects older men, with the majority of cases diagnosed in individuals over the age of 65 (2). However, it is relatively rare in men under the age of 40. PrCa is a significant cause of cancer-related deaths in men, accounting for nearly 4% of all cancer-related deaths in 2020 (2). Several risk factors influence the development of PrCa, including age, family history, genetic mutations, and ethnicity, with African American men having a higher incidence and mortality rate (2). Screening for PrCa often involves prostate-specific antigen (PSA) testing, although its effectiveness and potential harms are still a subject of debate (3). Treatment options for prostate cancer depend on various factors, including disease stage, patient age, overall health, and personal preferences (4).
MicroRNAs (MiRNAs) are small non-coding RNA molecules that functionally regulate gene expression through degradation or translational repression of mRNA targets (5). These molecules play important regulatory functions during cellular processes, including cell cycle entry and progression, differentiation, and apoptosis (6). The dysregulation of miRNAs has been causatively involved with tumor initiation, aggressiveness, and therapeutic evasion observed in cancer, particularly with prostate cancer (PrCa). They control proliferation and regression of a tumor with metastasis and drug sensitivity mainly through modulating complex signaling pathways and networks controlling gene expression (7, 8).
MiRNAs in PrCa exert oncogenic and tumor-suppressive functions (9). For instance, some of them are overexpressed, thereby enhancing tumor proliferation and survival, while others are downregulated with lost tumor-suppressive functions (10). Further evidence for the critical roles they play in prostate tissue comes from tissue-specific expression patterns, since aberrant miRNA profiles have been associated with disease progression, aggressiveness, and prognosis in prostate cancer (7, 9). Modified MiRNA expression patterns shed light on their potential use as a diagnostic biomarker, therapeutic target, and prognostic indicator in PrCa (10).
Despite all such developments, translation of MiRNA studies to the clinic remains a challenge. Many studies have small sample sizes, non-standardized methodologies, and heterogeneity in MiRNA selection and analysis, which makes it difficult to compare studies and have robust meta-analyses (14, 15). Most of the studies conducted focus on single MiRNAs without taking into account their interactions in complex regulatory networks, thereby limiting understanding of their roles in broader PrCa (3, 9). Sample collection, RNA extraction, and detection platforms also vary across studies (10).
Variations in study designs, including sample collection methods, RNA extraction techniques, and MiRNA detection platforms, contribute to inconsistencies and make comparisons challenging. Addressing these literature gaps through well-designed studies with larger sample sizes, standardized methodologies, and prospective designs would enhance the reliability and generalizability of findings. Therefore, the objectives of this investigation were to comprehensively evaluate the existing literature and summarize the evidence regarding the role of specific MiRNAs in PrCa progression. These studies aimed to identify and synthesize relevant scientific research to investigate the association between specific MiRNAs and PrCa progression, providing an overview of the current knowledge in the field. Additionally, the investigation secondarily aimed to explore potential sources of heterogeneity across studies, such as study design, sample size, methodology, and patient characteristics, and assess the quality and reliability of the included studies.
Materials and methodsReview protocolIn this investigation, the study design adhered to the rigorous PRISMA guidelines (11, 12). By adhering to these guidelines, this systematic review ensured transparency, minimized bias, and provided a comprehensive scientific overview of the existing evidence pertaining to the objectives of this study as represented in Figure 1. The protocol was registered in the PROSPERO database with the registration number CRD42023428460 being assigned to it.
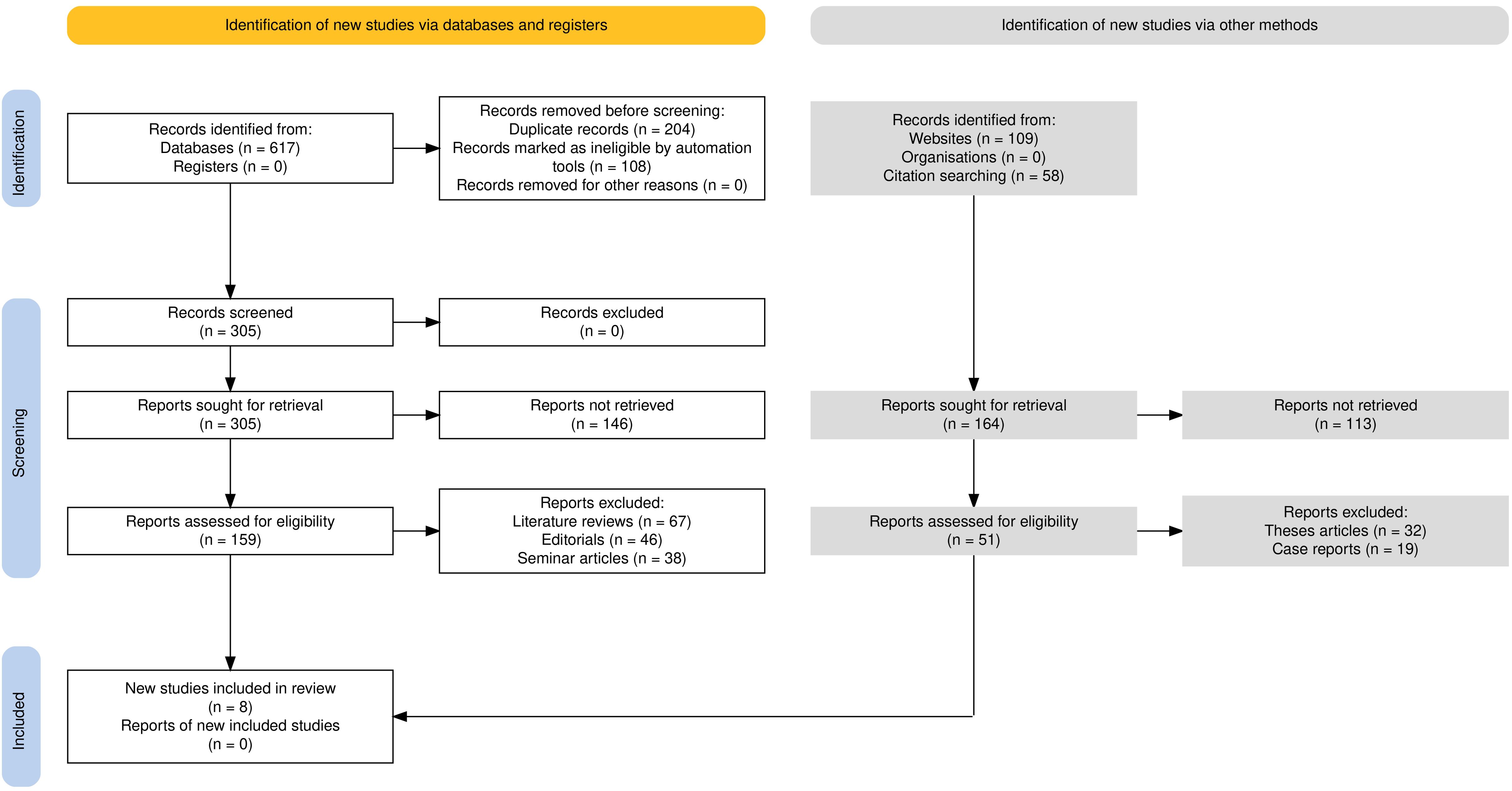
Figure 1. Flowchart representing the PRISMA guideline application in this review.
PICOS strategyFor the purpose of carrying out this review, a clearly outlined PECOS (Population, Exposure, Comparison, Outcome, Study design) strategy was applied for setting up the research criteria, as mentioned below-
● Population: Cases with prostate cancer (PrCa), diagnosed by tissue analysis or cell cultures.
● Exposure: Expression levels of specific microRNAs (MiRNAs) associated with PrCa progression.
● Comparator: The differences in MiRNA expressions between normal and cancerous tissues or between stages of PrCa.
● Outcomes: Prostate cancer progression markers such as tumor proliferation, metastasis, lymph node dissemination, gene regulation and potential as biomarkers of MiRNA.
● Study design: Experimental studies using quantitative polymerase chain reaction (qPCR) to assess the expression of MiRNA and its impact on PrCa progression.
Database search strategyAn extensive database search strategy was implemented across seven major online databases. The databases utilized included PubMed/MEDLINE, Embase, Web of Science, Scopus, Cochrane Library, CINAHL, and PsycINFO. A combination of MeSH (Medical Subject Headings) keywords and Boolean operators were employed to ensure a comprehensive search. The MeSH keywords included terms related to PrCa, microRNAs, and cancer progression, such as “prostatic neoplasms,” “microRNAs,” and “disease progression.” Boolean operators (AND, OR) were used to combine these keywords and refine the search results. Additionally, truncation and wildcard symbols were utilized to account for variations in spelling and word endings. Table 1 displays the search strategy across these databases.
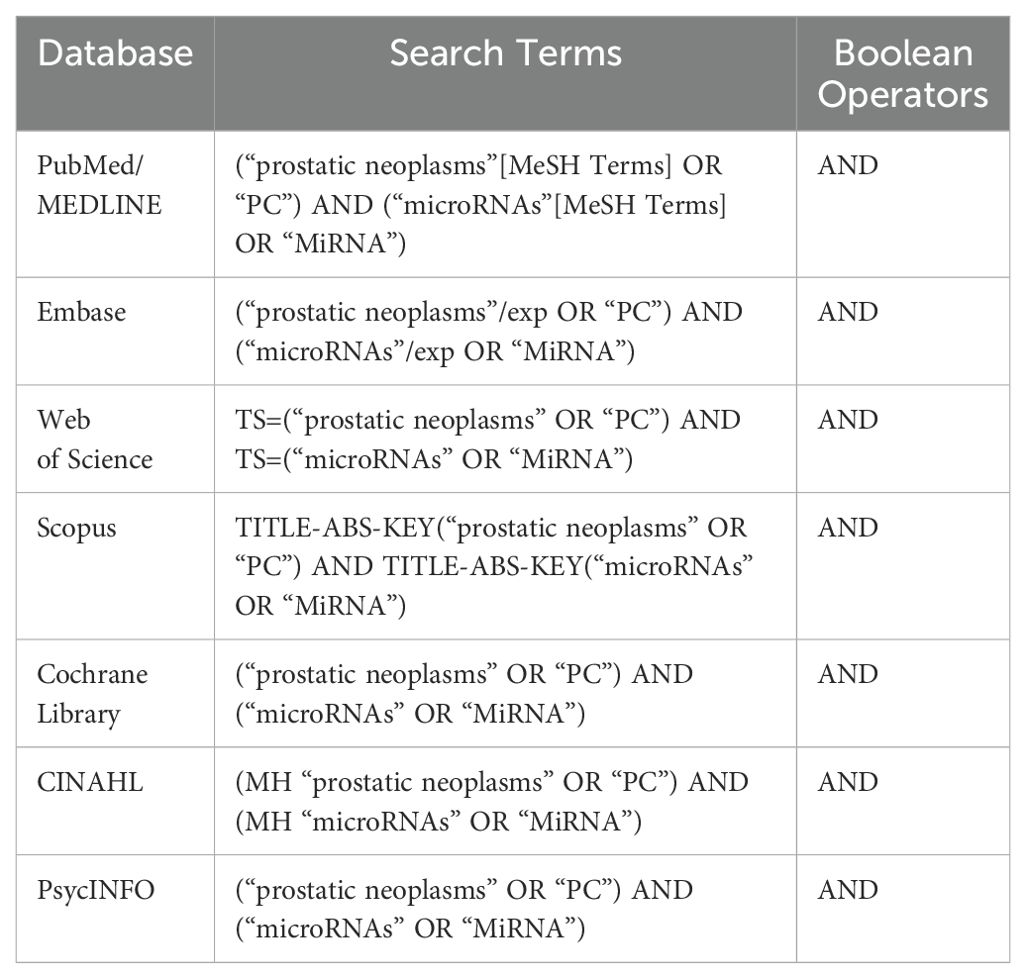
Table 1. Online database search using Boolean operators and MeSH keywords.
Selection criterion for the reviewThe inclusion and exclusion criteria for this review were defined to ensure the selection of relevant studies while maintaining a focus on the research question. The inclusion criteria encompassed studies that met the following criteria: (1) focused on human subjects diagnosed with PrCa, (2) examined the expression levels of specific MiRNAs in PrCa tissue/tissue samples, (3) investigated the association between MiRNA expression and PrCa progression or clinical outcomes, (4) included a control or comparison group for comparison purposes, and (5) reported sufficient data or statistical measures to assess the association. Studies that were not published in English, involved animal models, lacked relevant outcomes or data, or were conference abstracts, case reports, reviews, or editorial articles were excluded from the review. The criteria were designed to ensure the inclusion of high-quality studies that provided relevant insights into the association between specific MiRNA expression and PrCa progression while excluding studies that may introduce bias or lack appropriate data for analysis.
Data extraction and reviewer protocolThe data extraction protocol for this investigation employed multiple reviewers to ensure accuracy and minimize bias. The reviewers were trained in the data extraction process and were provided with a standardized data extraction form. Initially, a pilot test was conducted to ensure consistency and clarity in the extraction process. The reviewers independently extracted relevant data from the selected studies, including study characteristics (e.g., author, year, country), participant characteristics (e.g., sample size, age), methodology (e.g., study design, MiRNA assessment methods), and outcomes (e.g., MiRNA expression levels, PrCa progression measures). Any discrepancies or uncertainties were resolved through discussion and consensus among the reviewers. Additionally, a quality assessment of the included studies was conducted to evaluate the risk of bias and methodological quality. The data extraction process was carefully conducted to ensure the systematic collection of relevant information from each study, and the involvement of multiple reviewers helped to enhance the reliability and validity of the extracted data for further analysis and synthesis in the systematic review.
Bias assessment of included studiesThe bias assessment for this study utilized the Newcastle-Ottawa Scale (NOS). Two independent reviewers assessed the included studies using the NOS criteria (13, 14), which evaluate three main domains: selection of study groups, comparability of groups, and ascertainment of exposure or outcome. Each criterion was scored, and the total scores were used to determine the overall risk of bias for each study. Any disagreements or discrepancies in the assessment were resolved through discussion and consensus between the reviewers. The NOS allowed for a comprehensive evaluation of the included studies’ quality and risk of bias, providing valuable insights into their internal validity and potential sources of bias. This rigorous bias assessment using the NOS ensured that only studies with a high methodological quality and low risk of bias were included in the systematic review, enhancing the reliability and validity of the findings.
Statistical protocolThe meta-analysis of the studies included in this review was conducted using RevMan 5, a specialized software for meta-analyses in systematic reviews. Data from eligible studies were extracted and synthesized using robust statistical methods. Effect sizes, such as odds ratios (ORs) and risk ratios (RRs), along with their corresponding 95% confidence intervals (CIs), were calculated for the outcomes of interest. The influence of MiRNAs on PrCa progression was assessed based on the total number of tissue samples analyzed for changes associated with MiRNA expression. Statistical heterogeneity among the included studies was evaluated using the I² statistic. In cases of substantial heterogeneity, a random-effects model was employed to pool the data. Sensitivity analyses were performed to assess the impact of individual studies on the overall effect estimates, while subgroup analyses were conducted to explore sources of heterogeneity based on patient characteristics, study design, or other relevant factors. Forest plots were generated to visually represent the meta-analysis results. Overall effect estimates were calculated using the inverse variance method, and statistical significance was evaluated using p-values. The study was registered in PROSPERO with acknowledgement number 428460.
ResultsInitially, a total of 617 records were identified from database searches. No study was retrieved from registers. Before screening, 204 duplicate records were removed and 108 records were excluded as ineligible by automation tools. This left 305 records for screening. All of them were excluded since they did not meet the inclusion criteria based on title and abstract screening. At the screening stage, an attempt was made to retrieve all the 305 records. However, owing to access issues or incomplete publication, retrieval of 146 reports became impossible. The remaining 159 reports were screened for inclusion. At this stage, 67 literature reviews were excluded due to lack of original data; 46 editorials were removed because they did not contain experimental results; and 38 seminar articles were removed from the list as they did not attain methodological standards. In addition, 109 records were found through website searches and 58 through citation searching, for a total of 164 reports retrieved. Of these, 113 could not be obtained because they were unavailable or restricted. The remaining 51 reports were reviewed for inclusion; however, 32 thesis articles were excluded because they were not peer-reviewed articles, and 19 case reports were excluded because they did not present experimental outcomes related to the review. Ultimately, 8 studies (15–22) met the predefined inclusion criteria and were included in the review.
Figure 2 presents the assessment of bias of the 8 selected studies (15–22) for the review using the NOS across different domains. The statistical analysis was often rated as low risk of bias, indicating an appropriate and adequate analysis of the data. The assessment of whether the study controls for any additional factors that could influence the association between exposure and outcome was generally rated as unclear, indicating insufficient information on potential confounders. Overall, the studies exhibited some variations in the risk of bias across the different domains as assessed by the NOS. The representativeness of the exposed cohort, assessment of outcome, and control for additional factors were areas where many studies lacked clarity and provided an uncertain risk of bias. However, there were domains, such as selection of the non-exposed cohort, comparability of cohorts, and statistical analysis, where the risk of bias was generally low.
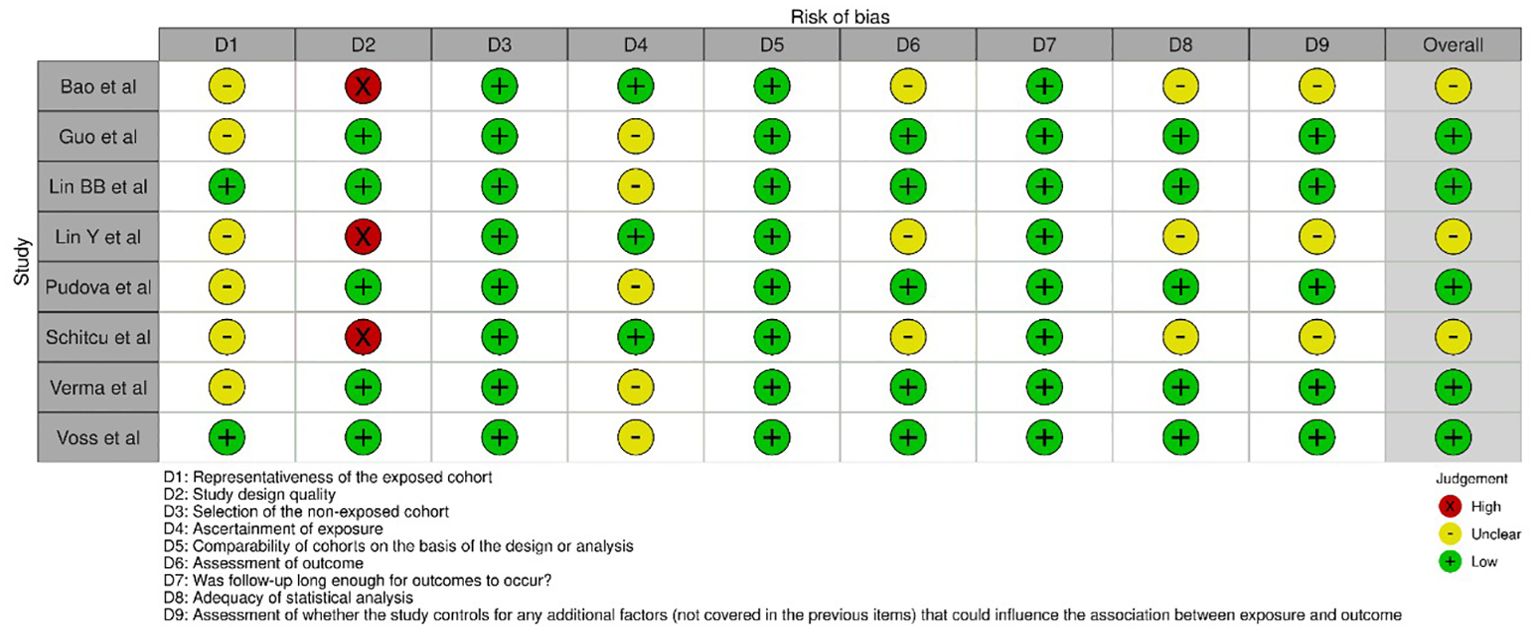
Figure 2. Evaluation of intra-review bias in the included studies.
Table 2 provides a comparison of major assessments conducted across the selected studies. The studies were conducted in different regions and varied in sample size and mean age. The studies conducted in China (15–18) had sample sizes ranging from 60 to 478, and the mean age was unspecified for all of them. Pudova et al’s study (19) in Russia had a smaller sample size of 44 tissue samples, with a mean age of 64 years. Schitcu et al’s study (20) in Romania also had a sample size of 44 participants, with a mean age of 66 ± 4.9 years. Verma et al’s study (21) in the USA utilized 292 MiRNA counts, but the sample mean age was unspecified. Voss et al’s study (22) in Sweden had a sample size of 136 participants, and the mean age was unspecified. These differences in sample size and mean age across the studies reflect the variability in the populations and settings in which the research was conducted.

Table 2. Included papers and their characteristics.
Table 3 presents a comparison of findings and other variables assessed across the selected studies. Regarding the protocol, all studies utilized an experimental approach, indicating a consistent methodology in examining the impact of MiRNAs on PrCa. Multiple MiRNAs were identified across the studies. This indicates a wide exploration of MiRNA involvement in PrCa. The target genes assessed varied across the studies and included FOXO1, FOXO3a, PTEN, TMPRSS2, ERG, TGFβ, PP2A, EGR1, PDCD4, TP53, AGO2, BIRC5, EGFR, DICER1, SSB, NF2, PPARA, and several others. This suggests a comprehensive evaluation of the genes influenced by MiRNAs in PrCa. The gene sequencing method predominantly employed was quantitative polymerase chain reaction (qPCR). This widely used method provided consistent and reliable results across the studies for analyzing MiRNA expression and its impact on target genes. In terms of inference, the studies examined various aspects related to MiRNA involvement in PrCa. These included the effect of MiRNAs on gene expression to prevent or promote PrCa growth, upregulation of MiRNAs in PrCa tissues, potential MiRNAs as biomarkers for early detection of PrCa metastases, correlation between specific MiRNAs and PrCa proliferation and lymph node dissemination, and the influence of MiRNAs on gene regulation in different stages of PrCa. By comparing these variables across the studies, it becomes evident that there is a comprehensive exploration of MiRNA’s role in PrCa. The studies collectively provide insights into the impact of MiRNAs on gene expression, their potential as biomarkers, and their association with PrCa progression. Additionally, some studies specifically highlight the influence of MiRNAs on osteoblast binding and cell adhesion between PrCa cells, indicating their potential impact on PrCa metastasis.
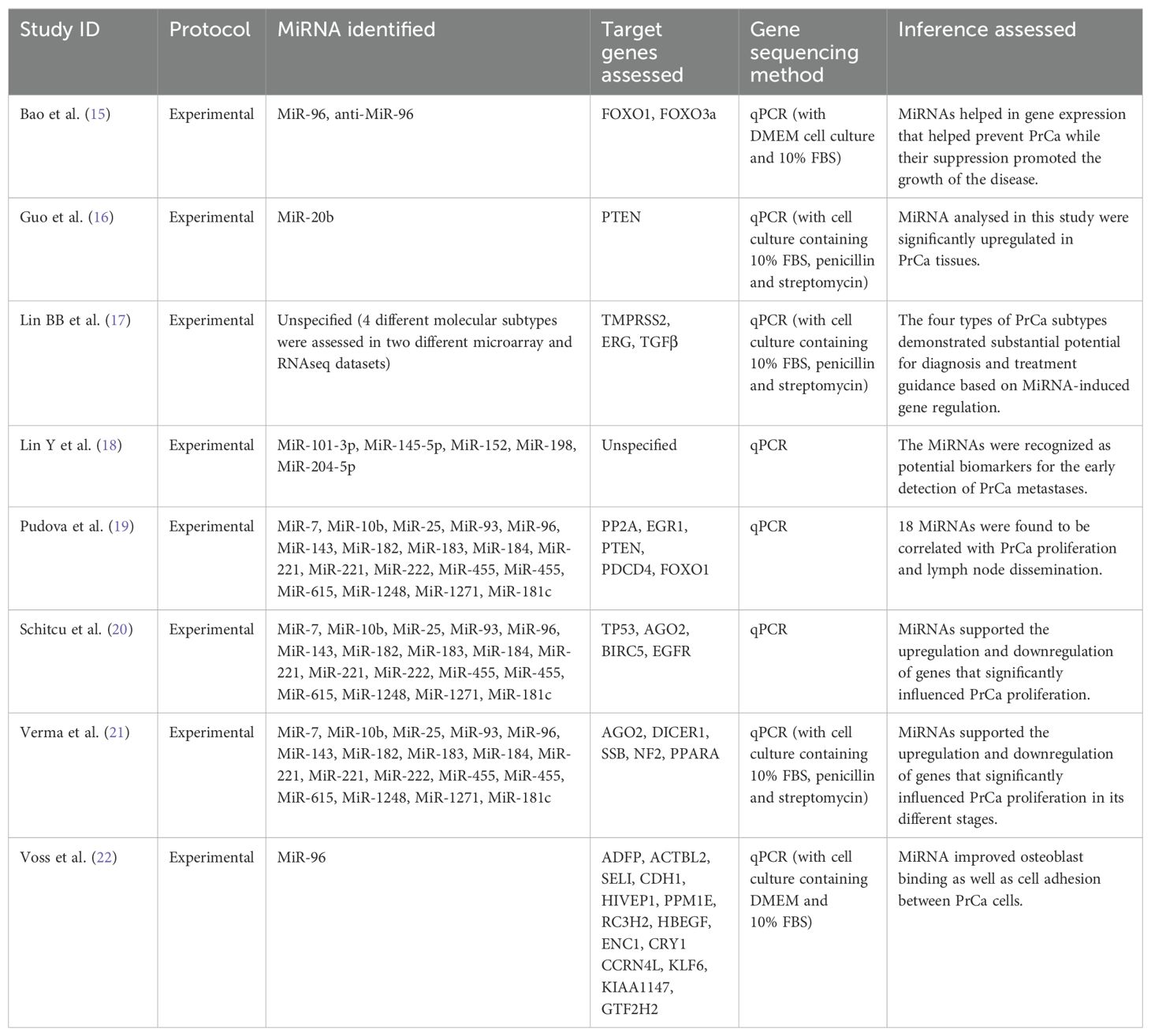
Table 3. Results and other different variables assessed pertaining to MiRNA in the included papers.
As depicted in Figure 3, the statistical analysis was conducted for a forest plot demonstrating an OR of 0.28, with a 95% CI ranging from 0.21 to 0.39. This analysis incorporated data from five studies (15, 16, 19, 20, 22), which evaluated the impact of microRNAs (MiRNAs) on prostate cancer (PrCa) proliferation. Proliferation was defined as the measurable increase in tumor growth, assessed using standardized methods such as cell proliferation assays (e.g., MTT assay, BrdU incorporation) or qPCR-based analyses. The results indicated a statistically significant effect of MiRNAs on reducing PrCa proliferation, as evidenced by the OR estimate. Heterogeneity analysis revealed a Chi-square value of 9.36 with 4 degrees of freedom (df) at a significance level of P = 0.05, and an I-squared (I²) statistic of 57%, indicating moderate heterogeneity. This heterogeneity suggested that variations in study design, participant characteristics, or other methodological factors contributed to differences in effect sizes across studies. Furthermore, the overall effect test produced a Z-value of 8.01 with a highly significant P-value of less than 0.00001, confirming a statistically significant overall effect of MiRNAs on PrCa proliferation.
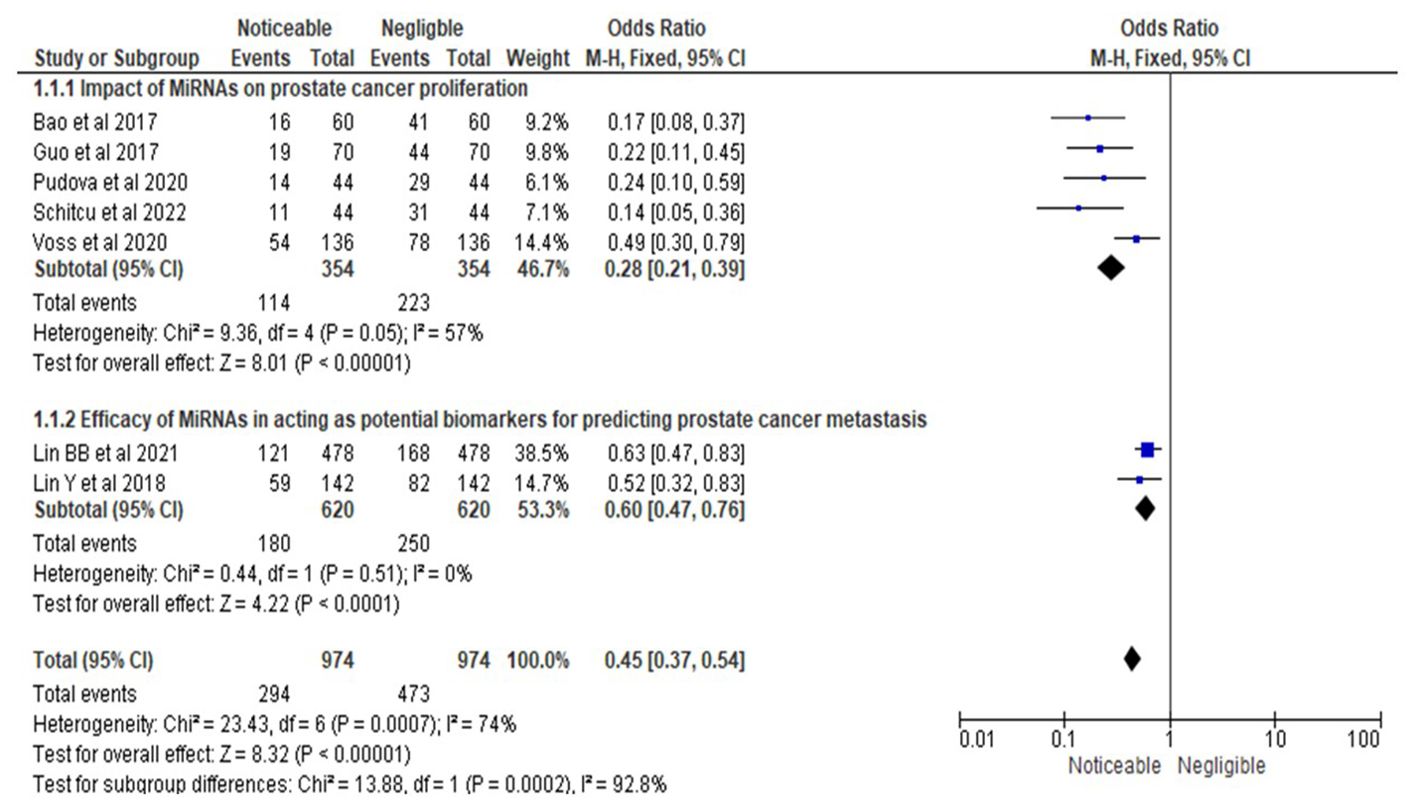
Figure 3. Impact of MiRNAs on PrCa progression in terms of ORs; Efficacy of MiRNAs in acting as potential biomarkers for predicting PrCa metastasis.
In the second subsection of Figure 3, the forest plot revealed an OR of 0.60 with a 95% CI ranging from 0.47 to 0.76, based on two studies (17, 18) investigating the efficacy of MiRNAs as potential biomarkers for predicting PrCa metastasis. Metastasis prediction was defined as the ability of MiRNAs to accurately forecast the spread of cancer cells to secondary locations such as lymph nodes or distant organs, validated through clinical outcomes (e.g., lymph node involvement, progression-free survival) or diagnostic tools such as qPCR or imaging studies. The results demonstrated a statistically significant effect of MiRNAs in predicting metastasis, as indicated by the OR estimate. The heterogeneity analysis reported a Chi-square value of 0.44 with 1 degree of freedom (P = 0.51) and an I² statistic of 0%, indicating no observed heterogeneity. This finding suggested a high degree of consistency in effect sizes across the two studies, likely due to similarities in study design or participant characteristics. The overall effect test produced a Z-value of 4.22 with an extremely significant P-value of less than 0.0001, providing robust evidence for the potential utility of MiRNAs as biomarkers for predicting PrCa metastasis.
Similarly, as shown in Figure 4, the statistical analysis of the RR for MiRNAs’ impact on PrCa proliferation yielded an RR of 0.51, with a 95% CI ranging from 0.43 to 0.61. This analysis included data from five studies (15, 16, 19, 20, 22) and confirmed a statistically significant effect of MiRNAs in reducing PrCa proliferation. Proliferation was assessed using standardized definitions and methods, focusing on measurable changes in cell number or tumor growth, as validated through assays or qPCR techniques. Moderate heterogeneity was observed (I² = 57%, Chi-square = 9.27, df = 4, P = 0.05), suggesting some variability in effect sizes attributable to methodological or population-related differences. The overall effect test produced a Z-value of 7.65 with a highly significant P-value of less than 0.00001, supporting the conclusion that MiRNAs significantly influence PrCa proliferation.
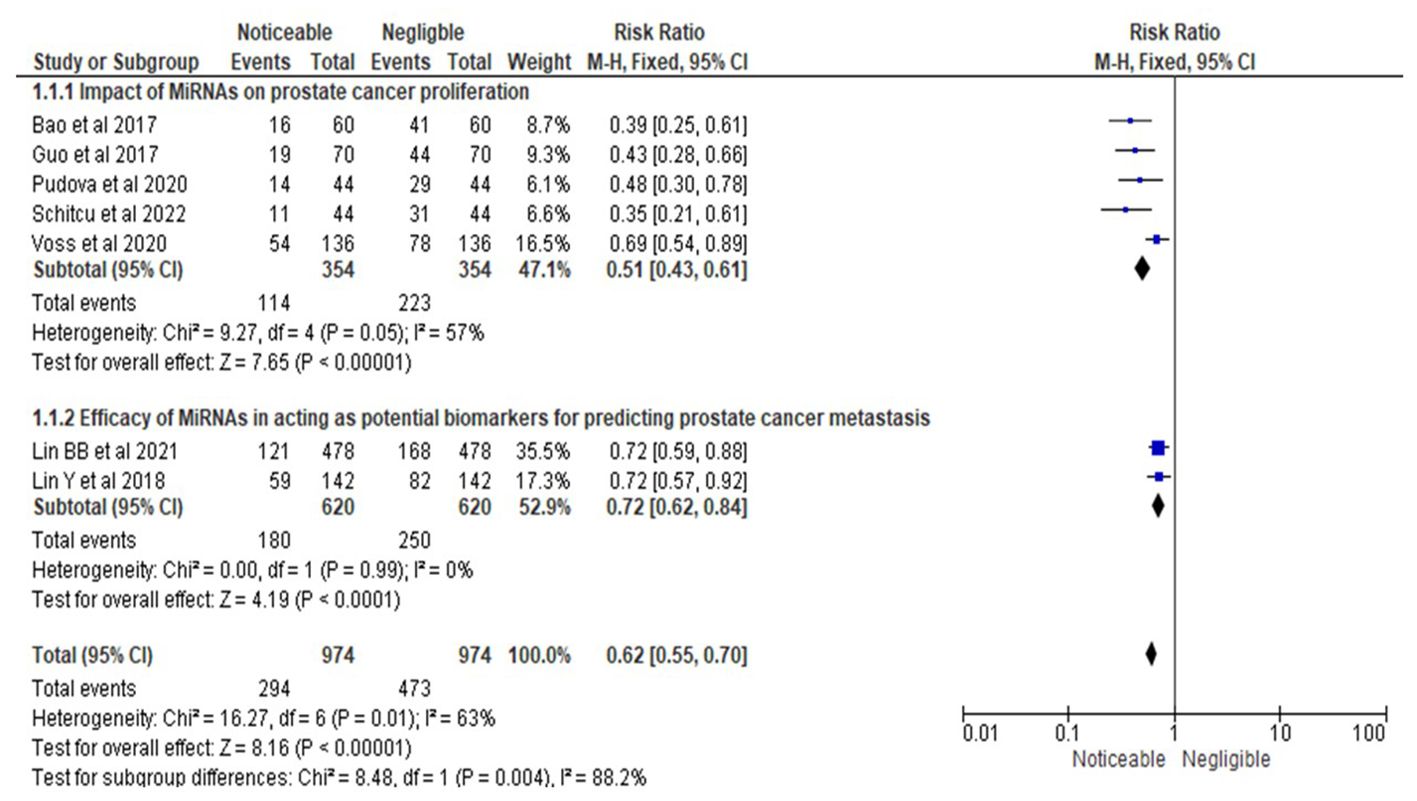
Figure 4. Impact of MiRNAs on PrCa progression in terms of RRs; Efficacy of MiRNAs in acting as potential biomarkers for predicting PrCa metastasis.
In the second subsection of Figure 4, the RR for the efficacy of MiRNAs as biomarkers for predicting PrCa metastasis was 0.72, with a 95% CI ranging from 0.62 to 0.84. This analysis incorporated two studies (17, 18), which demonstrated a statistically significant effect of MiRNAs in predicting metastasis. Metastasis prediction was defined and evaluated through standardized criteria, including the ability to predict cancer cell spread using validated diagnostic tools and clinically relevant outcomes. No heterogeneity was detected (I² = 0%, Chi-square = 0.00, df = 1, P = 0.99), indicating consistent findings across the studies. The overall effect test yielded a Z-value of 4.19 with an extremely significant P-value of less than 0.0001, providing strong evidence of MiRNAs’ efficacy as biomarkers for metastasis prediction.
Sensitivity analysis observationsDespite differences in study settings, all studies employed a qPCR-based experimental approach to evaluate the expression of specific miRNAs and their associations with prostate cancer (PrCa) progression. This methodological consistency minimized the likelihood of bias related to gene expression measurement techniques. The sensitivity analysis was performed by systematically removing each study, one at a time, to observe its impact on the pooled effect sizes for proliferation and metastasis prediction.
1. Impact on Proliferation Results (OR = 0.28, 95% CI: 0.21–0.39)
● The removal of Lin BB et al. (17), which had the largest sample size (n = 478), slightly increased the heterogeneity (I² = 62%) but did not significantly alter the overall effect size. This finding confirmed that the large sample size contributed to the precision of the pooled result but did not disproportionately influence the outcome.
● Studies with smaller sample sizes, such as Pudova et al. (19) and Schitcu et al. (20), had minimal impact on the effect estimate when excluded, reinforcing the stability of the pooled odds ratio.
● The heterogeneity remained at a moderate level (I² = 57%) throughout, indicating that the observed variability was due to intrinsic study differences such as sample size, target genes assessed, or population characteristics rather than any single study’s influence.
2. Impact on Metastasis Prediction Results (OR = 0.60, 95% CI: 0.47–0.76)
● The exclusion of each individual study in this analysis, including Lin Y et al. (18) and Lin BB et al. (17), resulted in no significant changes to the pooled odds ratio or heterogeneity.
● Heterogeneity remained at 0% across all iterations, reflecting a high degree of consistency among the included studies investigating metastasis. This uniformity was likely due to similarities in study design and participant characteristics across these studies.
3. Impact on Risk Ratios for Proliferation (RR = 0.51, 95% CI: 0.43–0.61)
● The analysis for risk ratios showed similar trends, with minimal shifts in the pooled effect size and moderate heterogeneity (I² = 57%). Exclusion of any study, including the larger studies such as Verma et al. (21) and Voss et al. (22), did not undermine the statistical significance of the results.
● The findings highlighted that the pooled risk ratios remained robust, further validating the association between miRNA expression and reduced prostate cancer proliferation.
DiscussionThe comprehensive exploration of MiRNA involvement in prostate cancer through various experimental studies and the identification of multiple MiRNAs and target genes contribute to our understanding of the molecular mechanisms underlying PrCa development and progression. The studies address various literature gaps by investigating the effects of MiRNAs on gene expression, their potential as biomarkers for early detection of PrCa metastases, their correlation with prostate cancer proliferation and lymph node dissemination, and their influence on gene regulation in different stages of PrCa. The statistical analysis revealed a noticeable effect of MiRNAs on prostate cancer proliferation, as indicated by the OR and RR estimate. Although heterogeneity exists among the studies, the overall effect test confirms a statistically significant association between MiRNAs and PrCa proliferation. Similarly, the efficacy of MiRNAs as potential biomarkers for predicting PrCa metastasis is again demonstrated by the forest plots. The low heterogeneity and highly significant overall effect test provide strong evidence of the potential of MiRNAs as biomarkers for predicting PrCa metastasis. These findings have important implications for future research and clinical practice. The identified MiRNAs and their target genes can serve as potential therapeutic targets or diagnostic biomarkers for PrCa. Further investigation into the specific mechanisms through which MiRNAs regulate gene expression in different stages of PrCa can provide insights into the underlying molecular pathways and potentially lead to the development of targeted therapies. Additionally, the differential efficacy of MiRNAs in predicting PrCa metastasis highlights the need for further research to identify specific MiRNAs with higher accuracy and reliability as biomarkers.
The interplay between miRNAs and key regulators, such as AGO2 (23), involves intricate mechanisms (24). TP53 is involved in regulating miRNA association with AGO2, highlighting a novel pathway for miRNA regulation in carcinogenesis (25, 26). Recent investigations have uncovered the cytoplasmic incorporation of AGO2 and miR-96 (27), shedding light on their potential therapeutic targeting (28). Further exploration has identified miR-542-5p as a notable regulator of AGO2 and EGFR (19, 29, 30). Within the miR-183-96-182 oncogenic cluster, miR-183-5p emerges as a promising diagnostic biomarker for prostate cancer (31–34), while miR-96-5p influences the tumor suppressor PTEN (35). Notably, miR-96 exhibits higher expression levels in prostate cancers with mutant TP53 (36), and its role extends to cell-cell adhesion and androgen signaling regulation (37). Additional investigations implicate miR-20b in prostate cancer progression, with a particular focus on cellular proliferation, epithelial-mesenchymal transition (EMT), and migration (38, 39). Conversely, downregulation of miR-542, especially in TP53 mutated samples, relates to the regulation of EMT, metastasis, and TP53 expression (40). Hypoxic conditions (41) and DNA repair/Notch pathway regulation further influence the expression level of miR-542 (42, 43).
The study has several limitations that should be considered. Firstly, although the protocol of the included studies was consistent in utilizing an experimental approach, there may be variations in the study design and participant characteristics that could contribute to heterogeneity in the findings. Secondly, the assessment of multiple MiRNAs and target genes across the studies provides a comprehensive evaluation, but it may also lead to differences in the specific MiRNAs and genes examined, making it challenging to draw definitive conclusions about the overall role of MiRNAs in PrCa. Additionally, the studies primarily focused on examining MiRNA involvement in PrCa proliferation and its potential as biomarkers, but other important aspects such as MiRNA function in metastasis could be further explored. Furthermore, using ORs and RRs introduced the potential for ORs to overestimate associations when outcomes were common, and RRs being unsuitable for case-control studies. Both measures could have affected by confounding variables if not properly adjusted and provided limited insight into causality. Additionally, heterogeneity across studies could impact their reliability and generalizability. Future research should address these limitations by considering larger sample sizes, standardized study designs, and comprehensive assessments of MiRNA functions in various aspects of PrCa.
ConclusionThe findings suggest that MiRNAs have a noticeable impact on PrCa proliferation and highlight their potential as biomarkers for predicting PrCa metastasis. However, the presence of heterogeneity among the studies indicates variations in effect sizes that may arise from differences in study design and participant characteristics. Despite this heterogeneity, the statistical analyses confirm a statistically significant overall effect of MiRNAs on PrCa proliferation and their potential as biomarkers for predicting PrCa metastasis. Further research addressing the limitations and exploring MiRNA functions in different aspects of PrCa is warranted to enhance our understanding of MiRNA’s role in this disease.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributionsJA: Conceptualization, Data curation, Methodology, Software, Validation, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (grant number IMSIU-DDRSP2501).
Conflict of interestThe author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Chys B, Devos G, Everaerts W, Albersen M, Moris L, Claessens F, et al. Preoperative risk-stratification of high-risk prostate cancer: a multicenter analysis. original research. Front Oncol. (2020) 10:246. doi: 10.3389/fonc.2020.00246
PubMed Abstract | Crossref Full Text | Google Scholar
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
PubMed Abstract | Crossref Full Text | Google Scholar
3. Salciccia S, Capriotti AL, Laganà A, Fais S, Logozzi M, De Berardinis E, et al. Biomarkers in prostate cancer diagnosis: from current knowledge to the role of metabolomics and exosomes. Int J Mol Sci. (2021) 22:4367. doi: 10.3390/ijms22094367
PubMed Abstract | Crossref Full Text | Google Scholar
4. Ditonno F, Franco A, Manfredi C, Fasanella D, Abate M, La Rocca R, et al. The role of miRNA in testicular cancer: current insights and future perspectives. Medicina (Kaunas). (2023) 59:2033. doi: 10.3390/medicina59112033
PubMed Abstract | Crossref Full Text | Google Scholar
5. Braicu C, Raduly L, Morar-Bolba G, Cojocneanu R, Jurj A, Pop LA, et al. Aberrant miRNAs expressed in HER-2 negative breast cancers patient. J Exp Clin Cancer Res. (2018) 37:257. doi: 10.1186/s13046-018-0920-2
PubMed Abstract | Crossref Full Text | Google Scholar
6. Braicu C, Gulei D, Raduly L, Harangus A, Rusu A, Berindan-Neagoe I. Altered expression of miR-181 affects cell fate and targets drug resistance-related mechanisms. Mol Aspects Med. (2019) 70:90–105. doi: 10.1016/j.mam.2019.10.007
PubMed Abstract | Crossref Full Text | Google Scholar
7. Tarantino G, Crocetto F, Vito CD, Martino R, Pandolfo SD, Creta M, et al. Clinical factors affecting prostate-specific antigen levels in prostate cancer patients undergoing radical prostatectomy: a retrospective study. Future Sci OA. (2021) 7:FSO643. doi: 10.2144/fsoa-2020-0154
PubMed Abstract | Crossref Full Text | Google Scholar
8. Tomuleasa C, Braicu C, Irimie A, Craciun L, Berindan-Neagoe I. Nanopharmacology in translational hematology and oncology. Int J Nanomed. (2014) 9:3465–79. doi: 10.2147/ijn.S60488
PubMed Abstract | Crossref Full Text | Google Scholar
9. Corsten MF, Dennert R, Jochems S, Kuznetsova T, Devaux Y, Hofstra L, et al. Circulating MicroRNA-208b and MicroRNA-499 reflect myocardial damage in cardiovascular disease. Circ Cardiovasc Genet. (2010) 3:499–506. doi: 10.1161/CIRCGENETICS.110.957415
PubMed Abstract | Crossref Full Text | Google Scholar
10. Condrat CE, Thompson DC, Barbu MG, Bugnar OL, Boboc A, Cretoiu D, et al. miRNAs as biomarkers in disease: latest findings regarding their role in diagnosis and prognosis. Cells. (2020) 9:276. doi: 10.3390/cells9020276
PubMed Abstract | Crossref Full Text | Google Scholar
11. Haddaway NR, Page MJ, Pritchard CC, McGuinness LA. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis Campbell Syst Rev. (2022) 18:e1230. doi: 10.1002/cl2.1230
PubMed Abstract | Crossref Full Text | Google Scholar
12. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PloS Med. (2021) 18:e1003583. doi: 10.1371/journal.pmed.1003583
PubMed Abstract | Crossref Full Text | Google Scholar
13. McGuinness LA, Higgins JPT. Risk-of-bias Visualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Syn Meth. (2020) 12(1):55–61. doi: 10.1002/jrsm.1411
PubMed Abstract | Crossref Full Text | Google Scholar
15. Bao YH, Wang Y, Liu Y, Wang S, Wu B. MiR-96 expression in PrCa and its effect on the target gene regulation. Eur Rev Med Pharmacol Sci. (2017) 21:4548–56.
PubMed Abstract | Google Scholar
16. Guo J, Xiao Z, Yu X, Cao R. miR-20b promotes cellular proliferation and migration by directly regulating phosphatase and tensin homolog in PrCa. Oncol Lett. (2017) 14:6895–900. doi: 10.3892/ol.2017.7041
PubMed Abstract | Crossref Full Text | Google Scholar
17. Lin BB, Lei HQ, Xiong HY, Fu X, Shi F, Yang XW, et al. MicroRNA-regulated transcriptome analysis identifies four major subtypes with prognostic and therapeutic implications in PrCa. Comput Struct Biotechnol J. (2021) 19:4941–53. doi: 10.1016/j.csbj.2021.08.046
PubMed Abstract | Crossref Full Text | Google Scholar
18. Lin Y, Chen F, Shen L, Tang X, Du C, Sun Z, et al. Biomarker microRNAs for PrCa metastasis: screened with a network vulnerability analysis model. J Transl Med. (2018) 16:134. doi: 10.1186/s12967-018-1506-7
PubMed Abstract | Crossref Full Text | Google Scholar
19. Pudova EA, Krasnov GS, Nyushko KM, Kobelyatskaya AA, Savvateeva MV, Poloznikov AA, et al. MiRNAs expression signature potentially associated with lymphatic dissemination in locally advanced PrCa. BMC Med Genomics. (2020) 13:129. doi: 10.1186/s12920-020-00788-9
PubMed Abstract | Crossref Full Text | Google Scholar
21. Verma S, Pandey M, Shukla GC, Singh V, Gupta S. Integrated analysis of MiRNA landscape and cellular networking pathways in stage-specific PrCa. PloS One. (2019) 14:e0224071. doi: 10.1371/journal.pone.0224071
PubMed Abstract | Crossref Full Text | Google Scholar
22. Voss G, Haflidadóttir BS, Järemo H, Persson M, Catela Ivkovic T, Wikström P, et al. Regulation of cell-cell adhesion in PrCa cells by microRNA-96 through upregulation of E-Cadherin and EpCAM. Carcinogenesis. (2020) 41:865–74. doi: 10.1093/carcin/bgz191
PubMed Abstract | Crossref Full Text | Google Scholar
23. Verma S, Pandey M, Shukla GC, Singh V, Gupta S. Integrated analysis of miRNA landscape and cellular networking pathways in stage-specific prostate cancer. PloS One. (2019) 14:e0224071. doi: 10.1371/journal.pone.0224071
PubMed Abstract | Crossref Full Text | Google Scholar
24. Krell J, Stebbing J, Carissimi C, Dabrowska AF, de Giorgio A, Frampton AE, et al. TP53 regulates miRNA association with AGO2 to remodel the miRNA-mRNA interaction network. Genome Res. (2016) 26:331–41. doi: 10.1101/gr.191759.115
PubMed Abstract | Crossref Full Text | Google Scholar
25. Krell J, Stebbing J, Frampton AE, Carissimi C, Harding V, De Giorgio A. The role of TP53 in miRNA loading onto AGO2 and in remodelling the miRNA-mRNA interaction network. Lancet. (2015) 385:S15. doi: 10.1016/s0140-6736(15)60330-0
留言 (0)