Oncolytic viruses offer a novel and promising approach to cancer therapy. They selectively infect and destroy tumor cells, sparing normal cells in the process (1). As the number of oncolytic viruses approved by the Food and Drug Administration (FDA) for clinical use continues to grow, interest in this therapeutic strategy has markedly increased. Oncolytic viruses can be administered as monotherapy or combined with radiotherapy, chemotherapy, immunotherapy, or cell-based therapies, presenting promising prospects for cancer treatment. Currently, Several oncolytic viruses are employed, such as adenovirus (Ad) (2), herpes simplex virus (HSV) (3), vaccinia virus (VV) (4), reovirus (5), poliovirus (6), coxsackie virus (CV) (7), Newcastle disease virus (NDV) (8), vesicular stomatitis virus (VSV) (9), myxoma virus (10) and some Senteroviruses (11).
Research on oncolytic viruses began in the early twentieth century, revealing that certain wild-type or naturally attenuated viral strains could effectively treat cancer (12). Over recent decades, significant advancements had been achieved in cancer therapy with oncolytic viruses, and the milestones are summarized in Figure 1.
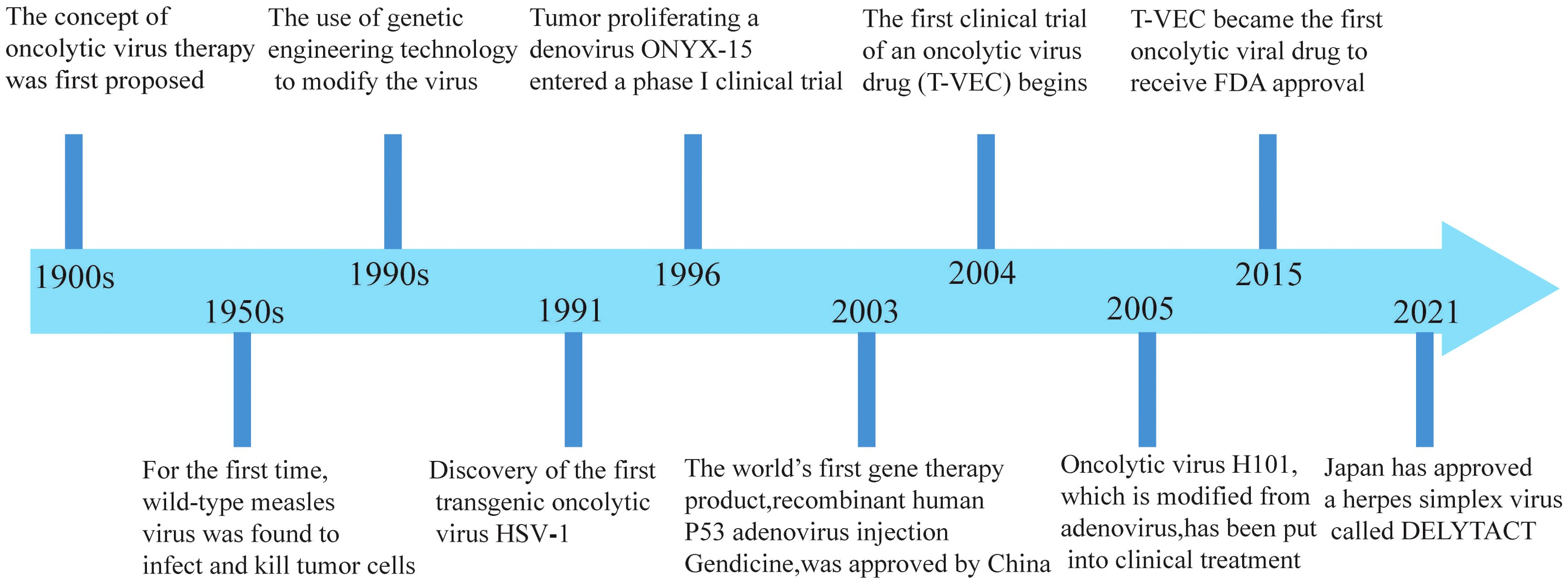
Figure 1. Significant milestones in the development of oncolytic viruses.
The antitumor effects of oncolytic viruses extend beyond receptor expression, potential mutations or transcriptional resistance. Furthermore, these viruses can stimulate non-autoantigen responses and amplify antitumor immune (13, 14).
Preclinical and clinical evidence demonstrates that oncolytic viruses can inhibit tumor growth through via multiple mechanisms. Nonetheless, several challenges limit their clinical translation. However, the clinical application of oncolytic viruses is hindered by challenges such as safety concerns, immune evasion, large-scale production, and clinical trials design (1, 15). Integrating oncolytic viruses with complementary therapies may enhance their efficacy and address existing challenges.
2 Factors influencing the anti-tumor effects of oncolytic viruses2.1 Induction of tumor cell lysisOncolytic viruses proliferate rapidly within tumor cells competing for biomolecules and energy, ultimately leading to host cell damage. Oncolytic virus releases progeny viruses after lysis of tumor cells, which infect nearby tumor cells and gradually metastasize to distal tumor cells until cleared by the host immune system (16). Viral replication of whin tumor cells alters their metabolic profile, inhibiting DNA repair, disrupts cell cycle regulation, and promotes apoptosis.
Finally, tumor cells destroyed by oncolytic viruses also release damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs), which intensify the immune response against the surrounding tumor cells (Figure 2).
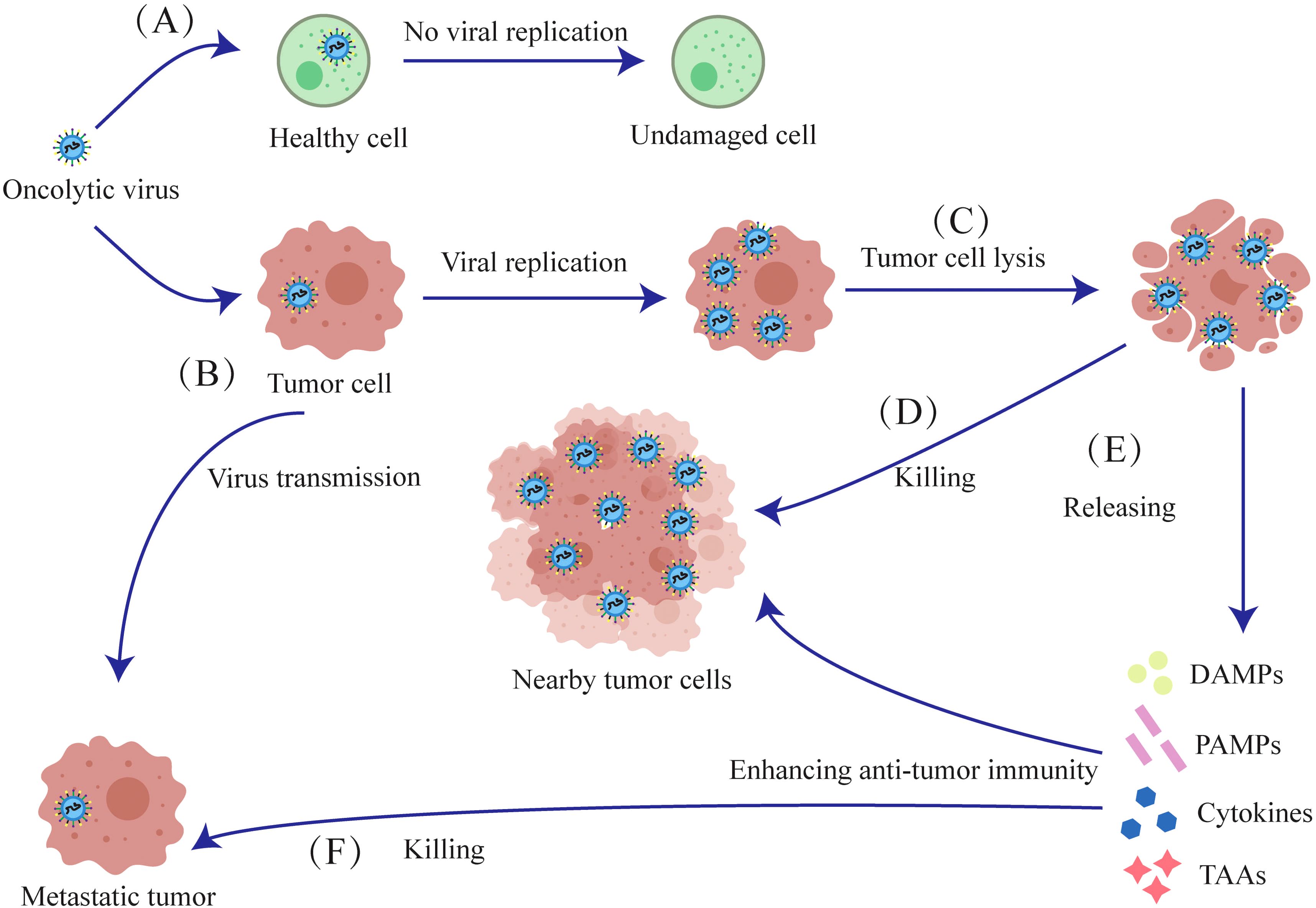
Figure 2. Mechanisms of oncolytic viruses. (A) The oncolytic virus has no destructive effect on normal cells. (B) The oncolytic virus selectively infects tumor cells, initiating viral replication within them. (C) Oncolytic viruses proliferate and lyse tumor cells. (D) The released oncolytic viruses then infect nearby tumor cells and lyse them. (E) Tumor cells lysed by oncolytic viruses release Cytokines, TAAs, DAMPs and PAMPs, which induce anti-tumor immunity against proximal and metastatic tumors. (F) After lysing tumor cells to release antigens and cytokines, oncolytic viruses further infect and kill metastatic tumors.
While oncolytic virus generally spare healthy cells, excessive oncolytic activity may result in off-target effects or heightened toxicity, potentially causing damage to normal tissues (17). The efficacy of oncolytic viruses is affected by factors such as virus type, injection dose, tumor cells sensitivity, and genetic modifications (18, 19).
2.2 Tumor immune responseOncolytic viruses can elicit specific B-cell and T-cell responses against tumor antigens, and potentially prevent long-term tumor recurrence. B lymphocytes primarily secrete antibodies and eliminate tumor cells through antibody-mediated mechanisms. Cytotoxic T lymphocytes (CTLs) directly recognize and destroy tumor cells, while helper T cells regulate and amplify the immune response. Innate immune cells, such as macrophages, natural killer (NK) cells and dendritic cells (DCs) can also directly kill tumor cells and secrete cytokines that amplify adaptive immune responses or sensitize tumor cells to viruses (20, 21).
Oncolytic viruses induce the release of toll-like receptor (TLR) ligands, PAMPs and DAMPs from the infected tumor cells, which then activate antigen-presenting cells (APCs), NK cells and T cells. TLR ligands can counteract tumor-induced immunosuppression by modulating cytokines (22). Furthermore, DCs expressing the Major Histocompatibility Complex-1(MHC-1) and MHC-2 receptors respectively activate the CD8+ and CD4+T cells by presenting antigens. NK cells and activated CD8+T cells synergistically release perforin, granzyme and cytokines that directly kill tumor cells (Figure 3).
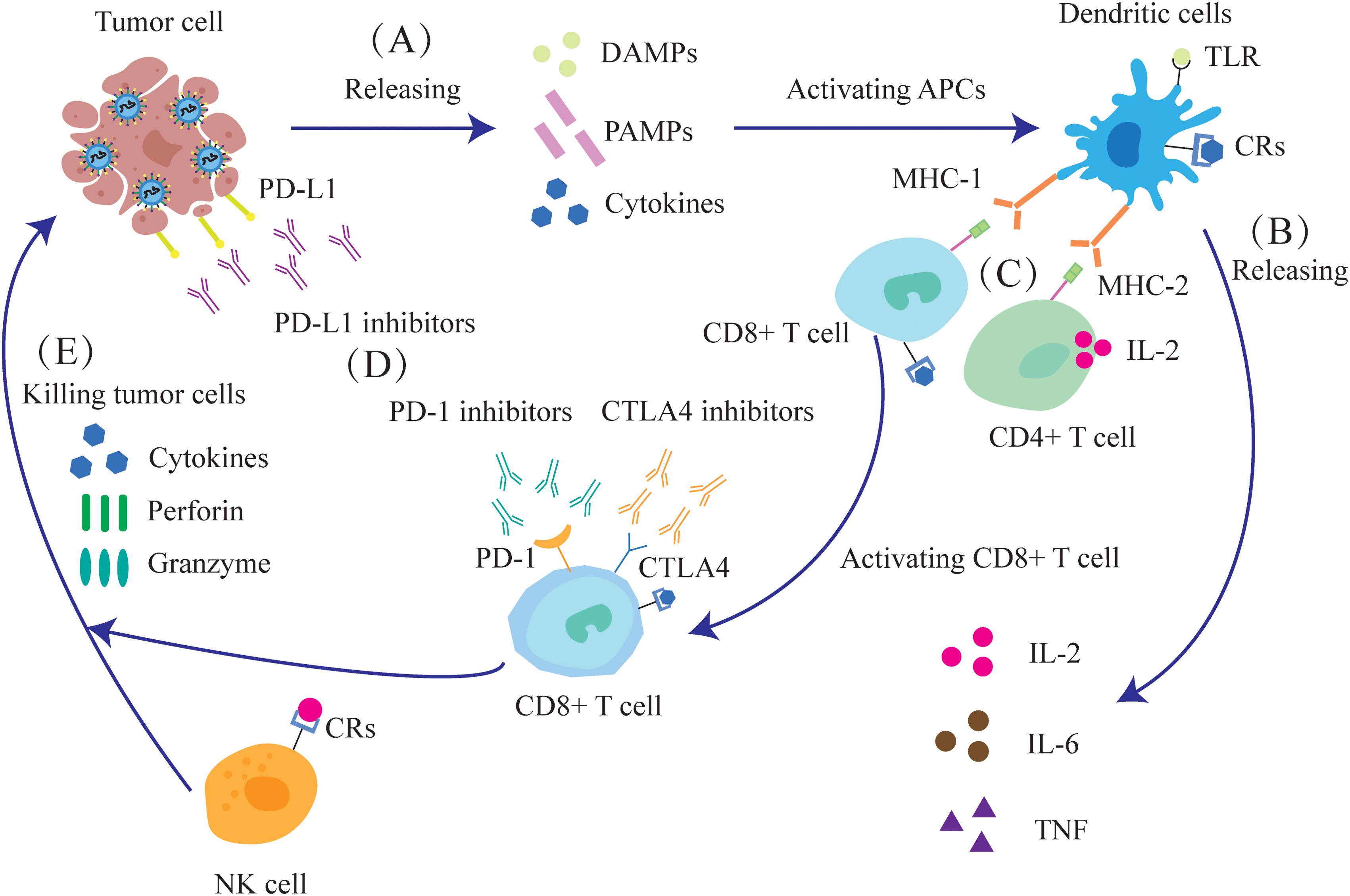
Figure 3. Oncolytic viruses can enhance the anti-tumor immune response and improve the effect of immunotherapy. (A) The destruction of tumor cells releases DAMPs, PAMPs and cytokines. (B) APCs, such as DCs, are activated and release IL-2, IL-6, and tumor necrosis factor (TNF). (C) After dendritic cells mature, they activate CD4+ and CD8+ T cells through MHC. (D) Oncolytic virus infection leads to increased expression of the immune checkpoint molecules PD-1, PD-L1 and CTLA-4, as well as enhanced sensitivity of tumor cells to immune checkpoint inhibitors (ICIs). (E) NK cells, together with activated CD8+T cells, can release cytokines, perforin and granzyme to kill tumor cells.
Oncolytic viruses elicit a host immune response that suppresses the tumor-killing effects, but this can be mitigated by combining oncolytic virus with low-dose chemotherapy or transforming growth factor beta (TGF-β) (23, 24). These agents can transiently suppress immune response, thus ensuring survival of the virus particles till they reach the tumor site. However, the precise safe dosage of these drugs still required further investigation.
While anti-viral immunity impairs efficacy, tumor-killing responses can be enhanced by immune stimulation. Tumor regression can be enhanced by increasing their therapeutic dose, immunostimulants immunostimulants as adjuvants (25, 26), or engineering recombinant viruses to express immunostimulatory genes or modified promoter elements (27). Interestingly, a virus that is oncogenic in one host may exert oncolytic properties against various tumors in another host (28).
The expression of cytotoxic proteins can also boost the efficacy of oncolytic viruses. For instance, adenovirus death protein (ADP) is a glycoprotein that effectively lyses cells and releases viral particles during advanced stages of infection. Consequently, viruses overexpressing ADP can spread more rapidly and efficiently within tumors (29).
In a Phase I clinical trial, patients with malignant pleural mesothelioma received intrathoracic oncolytic virus therapy, which reduced tumor cell density and increase the density of multiple immune cells (30).
2.3 Recombinant oncolytic virusRecombinant oncolytic virus strains are develop by incorporating target genes or expression elements in the viral genome. These engineered viruses produce supplementary proteins in the host tumor cells, which enhance the anti-tumor efficacy of oncolytic viruses or confer additional therapeutic attributes (31).
The oncolytic vaccinia virus represents a promising recombinant strain. Studies have shown that vaccinia virus, when armed with IL-2, IL-15 or HBD2 is highly effective at recognizing and killing tumor cells (32–34). Notably, recombinant vaccinia virus strains expressing bacterial flagellin have demonstrated oncolytic effects in solid tumor models (35).
Newcastle disease virus (NDV) is the longest-used oncolytic virus in clinical trials and has a well-established safety record as a monotherapy, attributed to its robust induction of antiviral responses in non-transformed mammalian cells (36). NDV can also be recombined in many ways, for instance, an NDV strain expressing matrix metalloproteinase (MMP) 8 enhances viral accumulation in tumors, thereby improving oncolytic efficacy. Additionally, NDV strains have been engineered to promote the release of IFNγ from virus-infected melanomas cells (37, 38).
In addition, many oncolytic viruses have been successfully reprogrammed, and it is believed that more successful novel oncolytic viruses will be designed for preclinical and clinical trials in the future.
2.4 The tumor microenvironmentThe tumor microenvironment (TME) is a complex network of tumor and stromal cells that fosters the growth and survival of cancer cells. Depending on cytokine profile and infiltration of immune cells, the TME can be categorized as immunologically “cold” or “hot”.
A cold TME is defined by elevated levels of inhibitory cytokines and immune checkpoint molecules, along with high infiltration of immunosuppressive cells, which hinder the immune system from accurately identifying and killing tumor cells (39). Conversely, a hot TME facilitates detection and elimination of tumor cells via immune effector cells, pro-inflammatory cytokines, and immunostimulatory molecules.
Since hot tumors respond better to immunotherapy, transforming the TME from “cold” to “hot” can increase the effectiveness of oncolytic viruses by enhancing immune recognition and destruction of tumor cells (40).
Oncolytic viruses can transform the TME from “cold” to “hot”, and evoke an adaptive antitumor immune response by releasing tumor-associated antigens (TAAs), PAMPS, and DAMPS. These immunostimulatory molecules recruit antigen-presenting cells (APCs) to the tumor site (41), resulting in antitumor and antiviral responses (Figure 4). Thus, oncolytic viruses are a promising immunotherapeutic agent for tumor control.
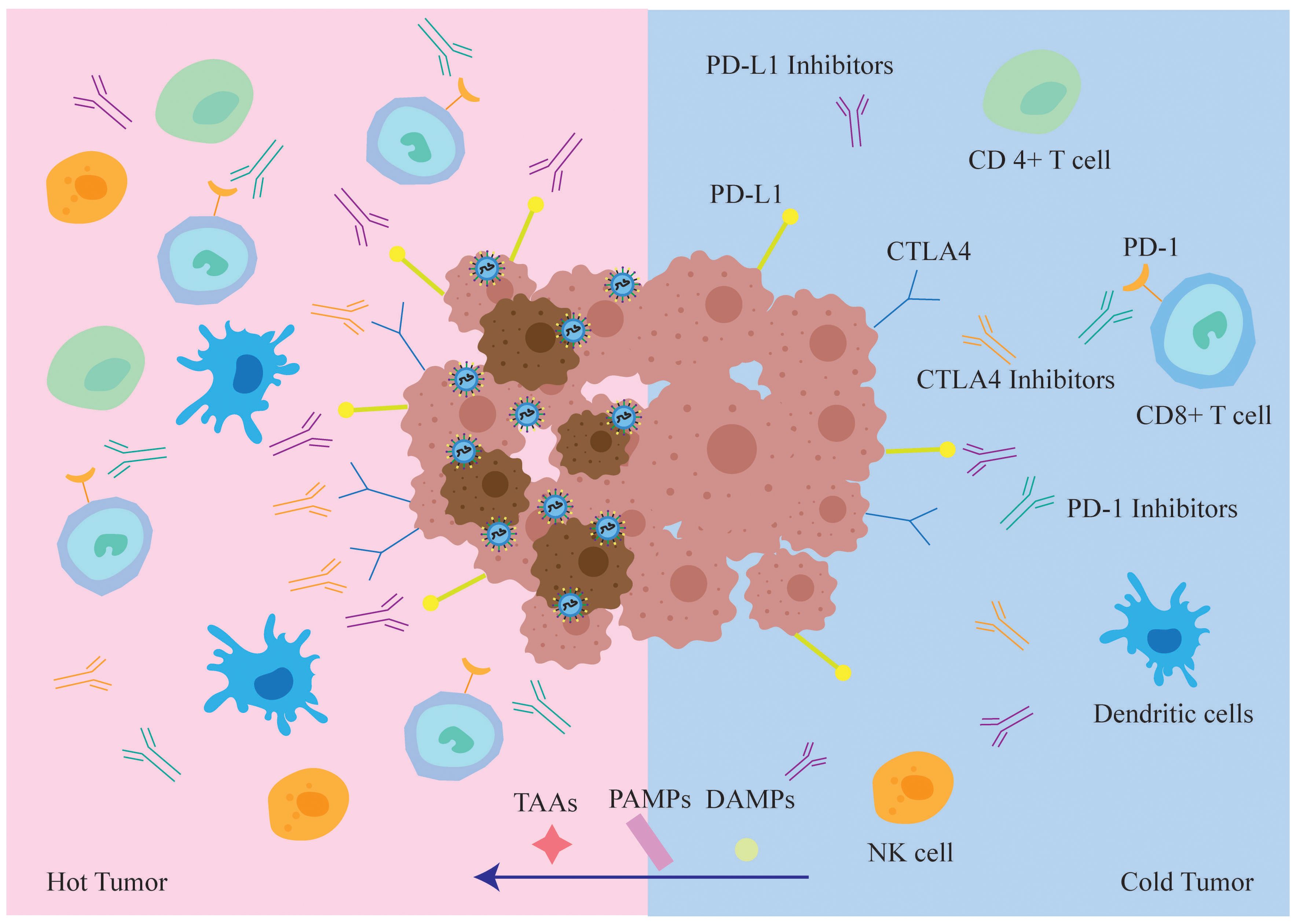
Figure 4. In immune “hot” TME, tumor cells infected with oncolytic virus are more easily recognized and cleared by the immune system. While oncolytic virus lyses tumor cells, it also draws various types of immune cells into the tumor microenvironment, transforming “cold” tumors into “hot” tumors and increasing the sensitivity of tumor cells to the immune checkpoint inhibitors ICIs. At the same time, TAA, PAMPs and DAMPs will also enter the “hot” tumor, TAA enhances the recognition and killing of tumor cells by the host immune system, whereas PAMPs and DAMPs enhance anti-tumor cellular immune responses.
Evidence indicates that viruses can transport genes encoding immunostimulatory molecules, such as cytokines, chemokines, and co-stimulatory receptors, to the TME, thereby enhancing antitumor immunity (42).
Viral particles and gene products released upon tumor cell lysis can also activate the immune system. Furthermore, oncolytic viruses can inhibit tumor angiogenesis, reduce tumor blood supply, and induce tumor hypoxia and nutrient deficiency by inactivating pro-angiogenic factors secreted by tumor cells. Virus-infected cells often trigger an inflammatory response that can lead to tissue damage. Oncolytic viruses can modulate the tumor microenvironment by regulating the production and release of inflammatory factors, reducing surrounding tissue damage, and thereby enhancing therapeutic efficacy.
Oncolytic adenoviruses engineered to locally express inflammatory cytokines IL-12 and PD-L1 blocking antibodies can repolarize TME, enhance CD8 T cell activity, and can kill tumor cells by altering the tumor microenvironment (43, 44).
3 Limitations of oncolytic virus monotherapyOncolytic viruses, as a novel anticancer approach, function by selectively infecting and lysing tumor cells. Despite their potential, the clinical efficacy of oncolytic virus monotherapy is significantly constrained by several factors:
3.1 Tumor heterogeneityOncolytic virus infection depends on specific viral receptors, the expression of which varies widely among tumor types and patients. This heterogeneity markedly impacts the efficacy of oncolytic viruses, complicating efforts to achieve consistent outcomes across tumors. Moreover, the high mutation rates of solid tumors frequently undermine the long-term effectiveness of oncolytic virus monotherapy (45).
3.2 Immune responseInfection of tumor cells by oncolytic viruses activates host immune responses, resulting in viral clearance and diminished tumor-killing efficacy. Tumors suppress immune responses via mechanisms like elevated PD-L1 expression or recruitment of regulatory T cells (Tregs), further reducing oncolytic virus efficacy (43, 46, 47).
3.3 Limitations in local and systemic efficacyWhile effective at lysing local tumors, oncolytic viruses show limited efficacy in controlling metastatic tumors. Monotherapy frequently fails to comprehensively target metastatic lesions.
3.4 Balancing viral load and safetyIncreasing the viral dose may improve therapeutic efficacy but also heightens the risk of adverse effects, including inflammatory responses, organ failure, and, in severe cases, death (17). Monotherapy struggles to balance the need for therapeutic efficacy with ensuring patient safety.
4 Combination of oncolytic virus therapy with other treatment approachesGiven the limitations of oncolytic virus monotherapy, combination therapy has become a pivotal approach in cancer treatment. Integrating multiple therapeutic approaches achieves synergistic effects, effectively killing tumor cells while addressing the limitations of monotherapy.
For example, radiotherapy and chemotherapy disrupt tumor barriers, enhancing viral penetration; immune checkpoint inhibitors modulate host immune responses, reducing the clearance of oncolytic viruses. Furthermore, the combination of oncolytic viruses with CAR-T or CAR-NK cells not only improves the tumor microenvironment but also releases viral particles and cytokines that further activate and enhance the functions of CAR-T and CAR-NK cells, ultimately improving the overall therapeutic efficacy.
4.1 Combination of oncolytic viruses with radiotherapy or chemotherapyAlthough radiotherapy is routinely used for local tumors and early-stage disease, it has several disadvantages, including off-target effects on normal cells and the development of radio-resistance in the tumor cells, which compromise therapeutic outcomes. Furthermore, radiotherapy is largely ineffective against metastatic growth. Similarly, chemotherapeutic agents are typically non-selective, affecting normal tissues and resulting in systemic toxicity and side effects that can significantly impact patients’ quality of life. In addition, tumor cells frequently mutate, generating drug-resistant clones that diminish or nullify the therapeutic effects of chemotherapeutic drugs.
On the other hand, oncolytic viruses selectively infect and lyse tumor cells. Furthermore, while radiotherapy and chemotherapy are typically localized, oncolytic viruses can target metastatic tumors due to their ability to infect tumor cells. Thus, combining oncolytic viruses with chemotherapy or radiotherapy can provide both local and systemic disease control (48, 49).
Numerous preclinical studies have investigated the combination of oncolytic virus with radiotherapy and chemotherapy. Recent studies have shown that the combination of temozolomide or vincristine with oncolytic viruses can significantly kill mouse tumors (50, 51). At the same time, oncolytic viruses have been shown to enhance the efficacy of mitomycin and hydroxycamptothecin (52).
In clinical trials, H101, an oncolytic virus approved by China’s State Drug Administration, has been widely tested in combination with chemoradiotherapy. A number of clinical studies demonstrate the significant efficacy of oncolytic viruses in treating various tumors when combined with chemoradiotherapy (53–56).
Researchers have also combined a novel telomerase oncolytic virus with radiotherapy to treat patients with esophageal cancer, integrating recombinant technology with combination therapy (57).
Additionally, a prospective randomized phase 2 trial combining oncolytic viruses with radiotherapy reported a significant reduction in positivity in local biopsies (58). Reovirus has also demonstrated efficacy in clinical patients when combined with radiotherapy and chemotherapy (59, 60).
Numerous clinical studies on the combination of oncolytic viruses with chemoradiotherapy have reported favorable therapeutic outcomes with minimal side effects. Some clinical trials of oncolytic virus combined with chemoradiotherapy have been listed in Table 1.
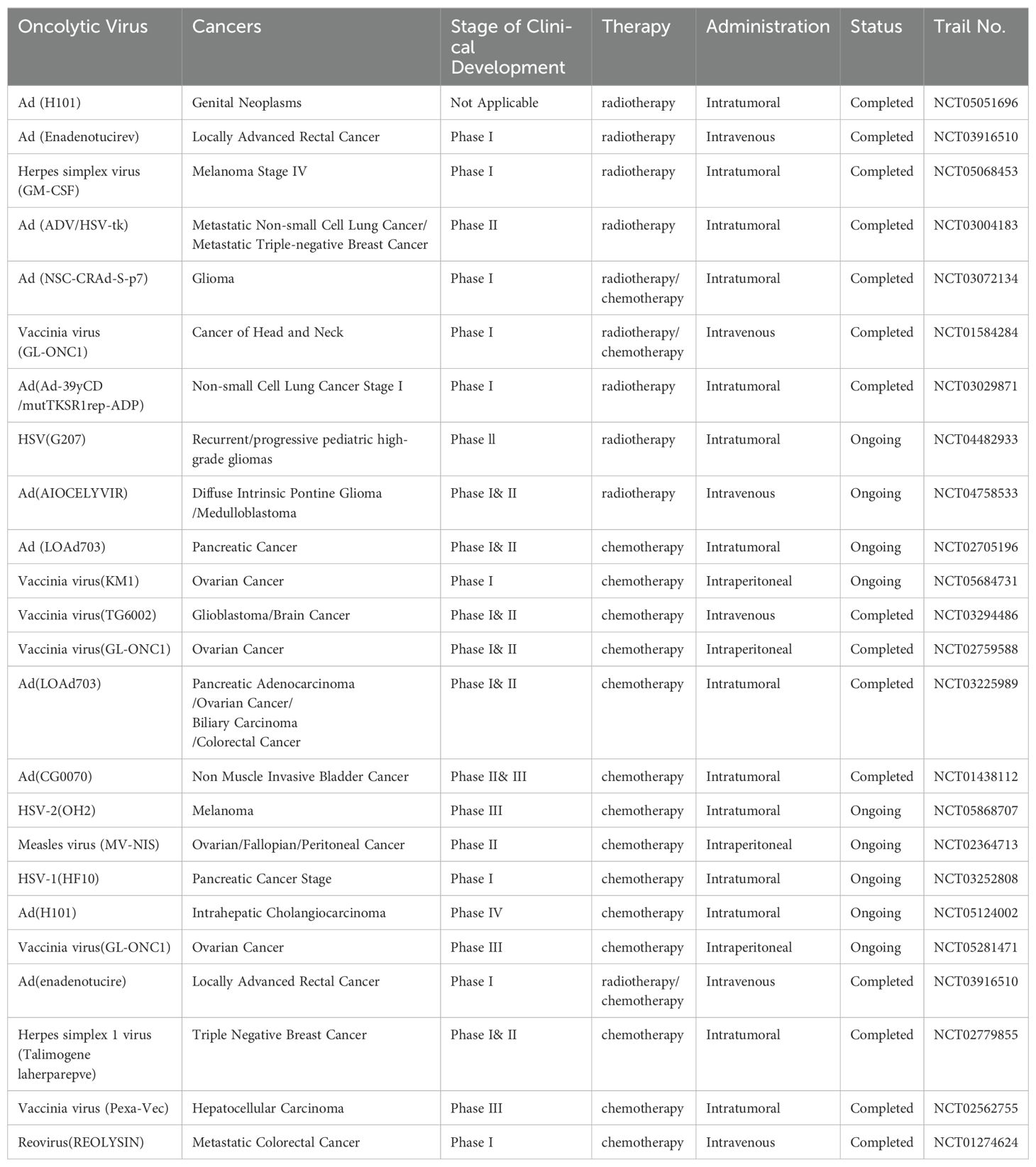
Table 1. Clinical trials of oncolytic virus combined with radiotherapy and chemotherapy.
4.2 Combination of oncolytic viruses and immune checkpoint inhibitorsThe expression of immune checkpoint molecules on immune cells will inhibit their function preventing the body from generating an effective antitumor immune response. These checkpoints can be exploited by tumors to evade immune surveillance. can be exploited by tumors to evade immune surveillance. Immune checkpoint inhibitors (ICIs), also referred to as immune system modulators, target immune checkpoints to enhance the immune response or to relieve immune suppression. Commonly used ICIs include Nivolumab, Ipilimumab, Pembrolizumab and Atezolizumab.
ICIs re-engage T cell anti-tumor activity of T cells by reversing the immunosuppressive tumor microenvironment. Oncolytic viruses can stimulate immune response and promote immune cell infiltration, while ICIs amplify this effect by reducing inhibitory signals, thereby enhancing immune response and therapeutic effect. The response to ICIs depends heavily on the TME, where “hot” tumors respond better to treatment, whereas “cold” tumors are less responsive. Therefore, improving TME is a key strategy for enhancing treatment efficacy (61).
At present, the combination of oncolytic viruses and ICIs has demonstrated significant anti-tumor effects in preclinical studies across various tumor types. Recent studies have further validated this approach (62–64).
Numerous clinical trials have been conducted on combination therapies. At present, a variety of ICIs combined with oncolytic viruses are currently being evaluated in clinical trials.
Nivolumab, a PD-1 inhibitor, was the first approved immunotherapy drug in China. Nivolumab has been combined with various recombinant oncolytic viruses in multiple tumor types, demonstrating sufficient safety and significant tumor regression (65–67).
Ipilimumab, a monoclonal antibody targeting CTLA-4, enhances the immune system’s ability to kill cancer cells by inhibiting an immunosuppressive checkpoint. HF10 is a biologically selected replicating oncolytic virus derived from herpes simplex virus type 1 (HSV-1). So far, numerous clinical trials have evaluated the combination of HF10 and Ipilimumab demonstrating remarkable efficacy (68–70).
Pembrolizumab is the only PD-1 inhibitor globally and in China that has received first-line three indications and single-agent indications for advanced non-small cell lung cancer (NSCLC). Numerous clinical trials combining Pembrolizumab with oncolytic viruses have shown sustained responses, with clinical benefits observed even in refractory patients (71–73).
Atezolizumab, a PD-L1 inhibitor, was approved by the US FDA in 2016. Atezolizumab binds to PD-L1 on tumor cells and block its interaction with PD-1 on T cells and antigen presenting cells, thereby relieving immunosuppression and enhancing T cell-mediated tumor cell destruction. At present, clinical studies have proved that Atezolizumab combined with oncolytic virus is very effective and safe (74, 75).
Of course, in addition to the above major classes of immune checkpoint inhibitors, other ICIs such as the PD-1 antibody Camrelizumab and the PD-L1 antibodies Durvalumab and Avelumab also show promising research prospects, with several ongoing preclinical and clinical trials. The results of the experiment are also being expected.
Therefore, combining oncolytic virus with ICIs may enhance anti-tumor immune responses. Some clinical trials of oncolytic viruses combined with immune checkpoint inhibitors are listed in Table 2.
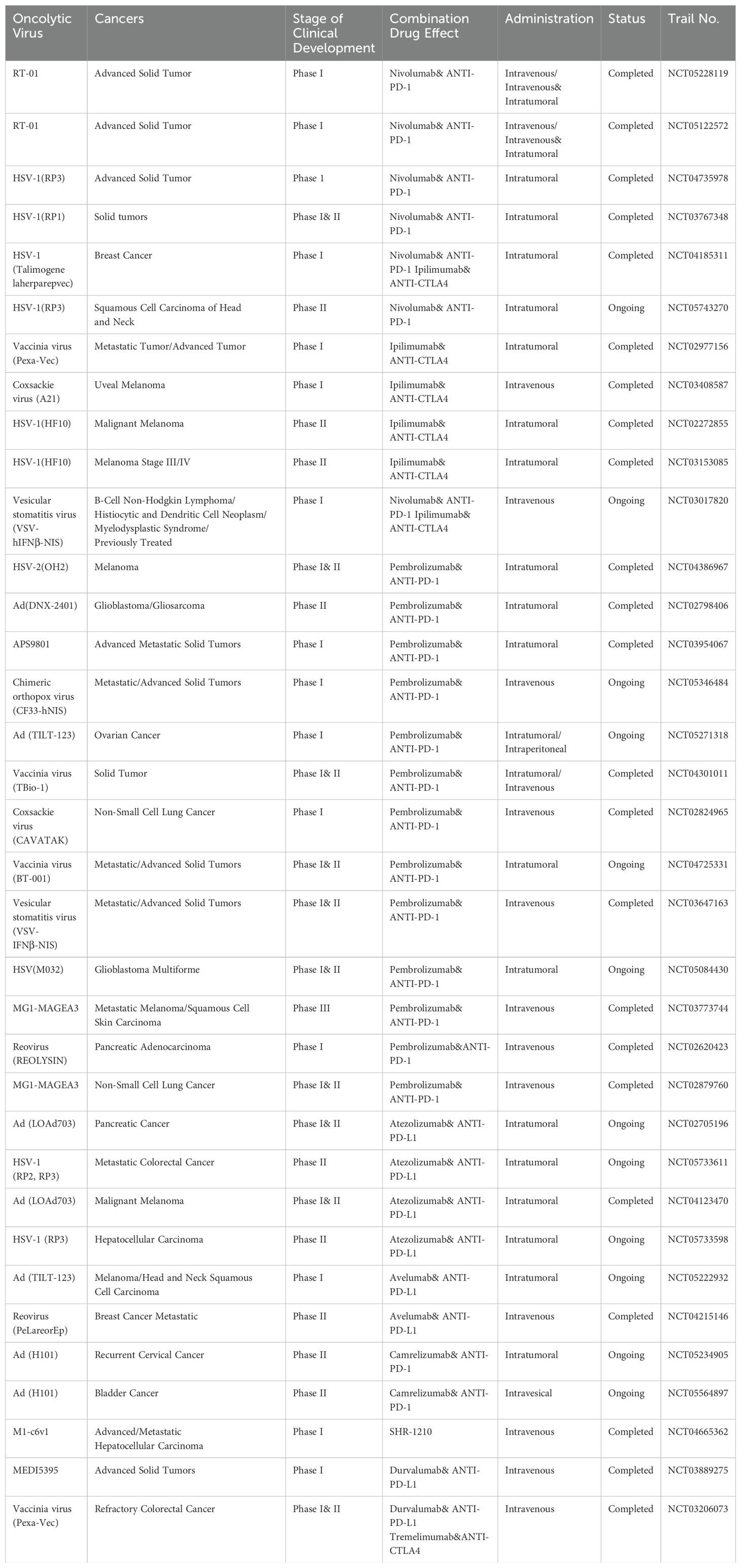
Table 2. Clinical trials of oncolytic viruses combined with immune checkpoint inhibitors.
4.3 Combination of oncolytic viruses with CAR-T cellsT cells are genetically engineered to express a chimeric antigen receptor (CAR) transgene and become chimeric antigen receptor T cells (CAR-T cells). CAR proteins are comprised of three components: the extracellular antigen-recognition domain of the single-chain fragment variable region (scFv), a transmembrane domain, and the intracellular CD3ζ domain (76). The design of CAR-T cells is complex and has evolved through five generations.
Oncolytic viruses activate CAR-T cells and also guide them to the infected tumor cells. In turn, the activated CAR-T cells release cytokines, such as IL-2, IFN-γ and TNF-α, which enhance replication and infectivity of oncolytic viruses, thereby amplifying the overall therapeutic effect (Figure 5).

Figure 5. The anti-tumor mechanism of oncolytic viruses and CAR-T cells. (A) T cells are genetically modified to express CAR transgenes. (B) CAR-T cells can release IL-2, IFN-γ, and TNF-α after activation. (C) These molecules can intensify the inflammatory response in tumor cells, thereby increasing their susceptibility to viral infection. (D) CAR-T cells can release perforin to kill tumor cells. (E) Oncolytic viruses can release viral particles after they replicate and lyse tumor cells. (F) These viral particles can stimulate and activate CAR-T cells to produce effects.
CAR-T cell therapy is a new cellular immunotherapy technique that integrates synthetic receptors into T cells, enabling them to recognize and kill tumor cells using homologous targeting ligands (77, 78). However, it was soon discovered that CAR-T therapy has limitations and side effects such as systemic toxicity and neurotoxicity, and the therapeutic effect may not be durable. At the same time, the complex construction process of CAR-T cells presents significant challenges for their application (79, 80).
Experimental data demonstrate the substantial clinical efficacy of CAR-T cells in treating hematologic malignancies (81). However, significant challenges remain in using CAR-T cells to treat advanced solid tumors. These challenges are primarily due to the low transport efficiency of CAR-T cells to TME, issues with antigen recognition, and structural differences between the structure of other solid tumors and hematologic tumors. Single therapy with CAR-T cells may not overcome these problems smoothly (82). However, with the FDA approval of the first oncolytic virus, T-VEC,the combination of oncolytic virus and CAR-T cells has officially opened the prelude. Recombinant oncolytic viruses may suppress local immunity, resulting in better therapeutic efficacy and persistence of CAR-T cells (83).
Oncolytic virus serve as reliable adjuvant for CAR-T cell therapy in solid tumors (84). Preclinical experiments have shown that oncolytic viruses loaded with IL-7 and CXCL11, in combination with CAR-T cells, significantly enhance tumor cell killing (85, 86).
At the same time, an interesting study in which oncolytic viruses expressing PD-L1 blocking micro-antibodies with CAR-T cells successfully controlled solid tumor growth (87). Another study combined CAR-T cells with oncolytic viruses equipped with the chemokine RANTES and the cytokine IL15, which improved the survival rate of tumor-bearing mice (88). Additionally, innovative strategies have been developed to encapsulate oncolytic viruses within CAR-T cells for treating solid tumors, yielding promising results (89).
Current studies suggest that the combination of oncolytic virus and CAR-T cells enhances therapeutic efficacy by enabling the virus to replicate and destroy tumor cells, stimulate immunity, and modulate the tumor’s immunosuppressive microenvironment, thus promoting CAR-T cell survival and activity.
Despite encouraging preclinical results, only one clinical trial investigating the combination of CAR-T cells and oncolytic viruses is ongoing, and further clinical trials are expected to follow. This may be due to the immunosuppressive tumor microenvironment, T cell depletion, or the absence of suitable antigen targets.
The summary of clinical trials of oncolytic virus combined with CAR-T cells for cancer treatment is shown in Table 3.

Table 3. Clinical trials of oncolytic viruses combined with CAR-T cells.
The slow progress of clinical trials combining CAR-T cells and oncolytic virus may be attributed to the systemic toxicity and neurotoxicity caused by CAR-T cells, which compromise their safety. In recent years, numerous patient deaths in CAR-T cells clinical trials have led to the emergency suspension of the experiment by FDA. In this way, attention has shifted to the carrier function of CAR T cells, using them to deliver oncolytic viruses to tumors to produce the effect of killing tumor cells. Studies have shown that CAR-T cells can deliver low doses of oncolytic viruses without affecting T cells quantity or function. CAR-T cells can be used as a carrier to release the virus to multiple tumor targets, thus further enhancing the role of killing tumor cells (90).
4.4 Combination of oncolytic viruses with CAR-NK cellsDue to concerns about the safety of CAR-T cells, attention has shifted to NK cells that express a low level of PD-1, which promote the migration of dendritic cells and cause less immunosuppression. Genetically modified NK cells express CAR transgenes are termed Chimeric Antigen Receptor NK cells (CAR-NK cells).CAR-NK cells are engineered to recognize and annihilate cancer cells (Figure 6). The viral particles released from the lysed tumor cells can stimulate CAR-NK cells in a manner similar to the CAR-T cells.
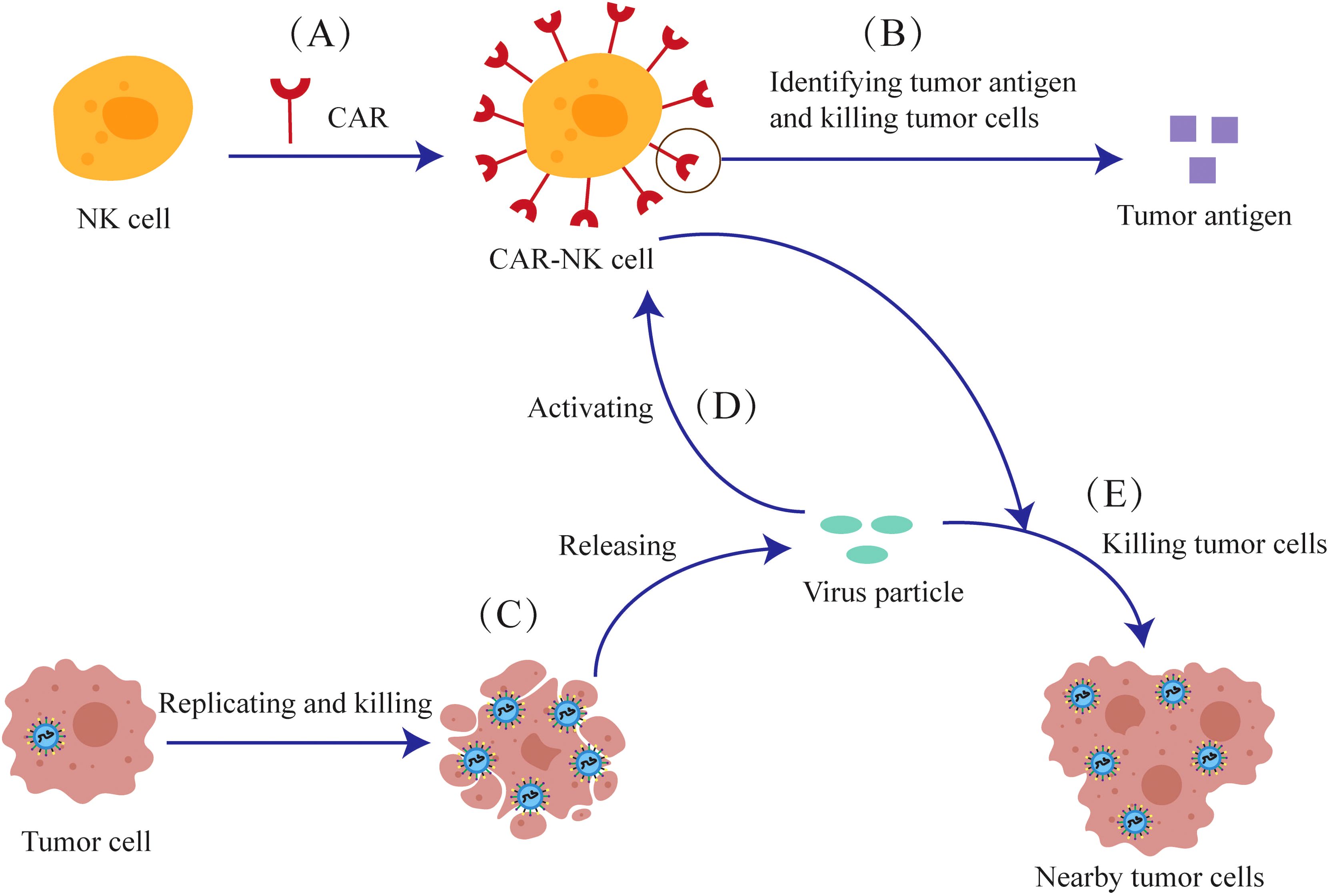
Figure 6. The anti-tumor mechanism of the combination of oncolytic viruses and CAR-NK cells. (A) NK cells are genetically modified to express CAR-Transgenes. (B) The CAR expressed on CAR-NK cells can recognize tumor antigens and kill tumor cells. (C) Oncolytic viruses can release viral particles after they replicate and lyse tumor cells (D) Viral particles can also activate CAR-NK cells (E) CAR-NK cells can kill nearby tumor cells together with viral particles.
CAR-NK cells are easier to construct than CAR-T cells and avoid issues such as alloreactivity, cytokine release syndrome, and graft-versus-host reactions. As long as NK cells are used, CAR-NK can be mass-produced. CAR-NK therapy is also significantly safer and does not induce strong neurotoxicity. At the same time, the survival cycle of NK cells is much shorter than that of T cells, and any off-target effects are rapidly cleared (91, 92).
As mentioned above, the shorter lifespan of NK cells necessitates multiple drug injections for CAR-NK therapy. But once the drug is injected multiple times, it will inevitably reduce safety. The continuous stimulation of immunity by oncolytic viruses helps extend the duration of CAR-NK cells without repeatedly injecting drugs.
At present, relevant preclinical studies have confirmed this view. EGFR-CAR constructs have been designed and transduced into NK cells to generate CAR-NK cells. The combination of second-generation EGFR-CAR NK cells and oncolytic herpes simplex virus to in treating breast cancer brain metastases in mouse models demonstrated significant tumor cell killing and improved survival (93). Another study showed that oncolytic viruses expressing IL15/IL15Rα, when combined with EGFR-CAR NK cells, induced a strong anti-tumor response in glioblastoma treatment (94).
In recent years, preclinical trials combining CAR-NK cells and oncolytic viruses have shown encouraging results. There are more and more clinical trials on CAR-NK cells, but the safety and efficacy of CAR-NK cells for clinical use remain subjects of debate. At present, no clinical trials have been conducted combining oncolytic viruses with CAR-NK cells.
5 ConclusionsIn recent years, oncolytic viruses have received widespread attention as a promising cancer therapy. Meanwhile, genetic modification of oncolytic viruses can substantially enhance their anti-tumor efficacy.
This article reviews the factors influencing the anti-tumor efficacy of oncolytic viruses and their application in combination with other therapies, while also listing ongoing or completed clinical trials. The results showed that the combination of oncolytic viruses with other treatments was more effective than monotherapy. In the future, the design of more oncolytic viruses and their use in combination with other therapeutic approaches are expected to further enhance clinical efficacy and safety. In the future, We expect that oncolytic viruses will increasingly be combined with CAR-T cells and CAR-NK cells in clinical trials.
For the genetically modified part of the oncolytic viruses, utilizing better-targeted nanomaterials as a carrier to deliver the virus may enhance its efficacy, representing an innovative and more effective treatment strategy when combined with nanomaterials. At present, a team has started similar experiments, but further studies are needed to evaluate their effects (95).
Author contributionsWD: Writing – original draft. JN: Writing – original draft. LZ: Writing – review & editing. PZ: Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Supported by the Scientific and Technological Innovation Major Base of Guangxi (No.2022-36-Z05).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References2. Gállego Pérez-Larraya J, Garcia-Moure M, Labiano S, Patiño-García A, Dobbs J, Gonzalez-Huarriz M, et al. Oncolytic DNX-2401 virus for pediatric diffuse intrinsic pontine glioma. N Engl J Med. (2022) 386:2471–81. doi: 10.1056/NEJMoa2202028
PubMed Abstract | Crossref Full Text | Google Scholar
3. Kawamura Y, Hua L, Gurtner A, Wong E, Kiyokawa J, Shah N, et al. Histone deacetylase inhibitors enhance oncolytic herpes simplex virus therapy for Malignant meningioma. BioMed Pharmacother. (2022) 155:113843. doi: 10.1016/j.biopha.2022.113843
PubMed Abstract | Crossref Full Text | Google Scholar
5. Seyed-Khorrami SM, Soleimanjahi H, Soudi S, Habibian A. MSCs loaded with oncolytic reovirus: migration and in vivo virus delivery potential for evaluating anti-cancer effect in tumor-bearing C57BL/6 mice. Cancer Cell Int. (2021) 21:244. doi: 10.1186/s12935-021-01848-5
PubMed Abstract | Crossref Full Text | Google Scholar
6. Ghajar-Rahimi G, Kang KD, Totsch SK, Gary S, Rocco A, Blitz S, et al. Clinical advances in oncolytic virotherapy for pediatric brain tumors. Pharmacol Ther. (2022) 239:108193. doi: 10.1016/j.pharmthera.2022.108193
PubMed Abstract | Crossref Full Text | Google Scholar
8. Chan LC, Kalyanasundram J, Leong SW, Masarudin MJ, Veerakumarasivam A, Yusoff K, et al. Persistent Newcastle disease virus infection in bladder cancer cells is associated with putative pro-survival and anti-viral transcriptomic changes. BMC Cancer. (2021) 21:625. doi: 10.1186/s12885-021-08345-y
PubMed Abstract | Crossref Full Text | Google Scholar
9. Sarwar A, Hashim L, Faisal MS, Haider MZ, Ahmed Z, Ahmed TF, et al. Advances in viral oncolytics for treatment of multiple myeloma - a focused review. Expert Rev Hematol. (2021) 14:1071–83. doi: 10.1080/17474086.2021.1972802
PubMed Abstract | Crossref Full Text | Google Scholar
10. Villa NY, Rahman MM, Mamola J, Sharik ME, de Matos AL, Kilbourne J, et al. Transplantation of autologous bone marrow pre-loaded ex vivo with oncolytic myxoma virus is efficacious against drug-resistant Vk*MYC mouse myeloma. Oncotarget. (2022) 13:490–504. doi: 10.18632/oncotarget.28205
PubMed Abstract | Crossref Full Text | Google Scholar
11. Kolyasnikova NM, Pestov NB, Sanchez-Pimentel JP, Barlev NA, Ishmukhametov AA. Anti-cancer virotherapy in Russia: lessons from the past, current challenges and prospects for the future. Curr Pharm Biotechnol. (2023) 24:266–78. doi: 10.2174/1389201023666220516121813
PubMed Abstract | Crossref Full Text | Google Scholar
13. Raja J, Ludwig JM, Gettinger SN, Schalper KA, Kim HS. Oncolytic virus immunotherapy: future prospects for oncology. J Immunother Cancer. (2018) 6:140. doi: 10.1186/s40425-018-0458-z
PubMed Abstract | Crossref Full Text | Google Scholar
14. Zhang S, Rabkin SD. The discovery and development of oncolytic viruses: are they the future of cancer immunotherapy? Expert Opin Drug Discovery. (2021) 16:391–410. doi: 10.1080/17460441.2021.1850689
PubMed Abstract | Crossref Full Text | Google Scholar
17. Buijs PR, Verhagen JH, van Eijck CH, van den Hoogen BG. Oncolytic viruses: From bench to bedside with a focus on safety. Hum Vaccin Immunother. (2015) 11:1573–84. doi: 10.1080/21645515.2015.1037058
PubMed Abstract | Crossref Full Text | Google Scholar
21. Marelli G, Howells A, Lemoine NR, Wang Y. Oncolytic viral therapy and the immune system: A double-edged sword against cancer. Front Immunol. (2018) 9:866. doi: 10.3389/fimmu.2018.00866
PubMed Abstract | Crossref Full Text | Google Scholar
22. Jhawar SR, Thandoni A, Bommareddy PK, Hassan S, Kohlhapp FJ, Goyal S, et al. Oncolytic viruses-natural and genetically engineered cancer immunotherapies. Front Oncol. (2017)7:202. doi: 10.3389/fonc.2017.00202
PubMed Abstract | Crossref Full Text | Google Scholar
23. Groeneveldt C, van Hall T, van der Burg SH, Ten Dijke P, van Montfoort N. Immunotherapeutic potential of TGF-β Inhibition and oncolytic viruses. Trends Immunol. (2020) 41:406–20. doi: 10.1016/j.it.2020.03.003
PubMed Abstract | Crossref Full Text | Google Scholar
24. Heo J, Reid T, Ruo L, Breitbach CJ, Rose S, Bloomston M, et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med. (2013) 19:329–36. doi: 10.1038/nm.3089
PubMed Abstract | Crossref Full Text | Google Scholar
26. John LB, Howland LJ, Flynn JK, West AC, Devaud C, Duong CP, et al. Oncolytic virus and anti-4-1BB combination therapy elicits strong anti-tumor immunity against established cancer. Cancer Res. (2012) 72:1651–60. doi: 10.1158/0008-5472.CAN-11-2788
PubMed Abstract | Crossref Full Text | Google Scholar
27. Guo ZS, Naik A, O’Malley ME, Popovic P, Demarco R, Hu Y, et al. The enhanced tumor selectivity of an oncolytic vaccinia lacking the host range and antiapoptosis genes SPI-1 and SPI-2. Cancer Res. (2005) 65:9991–8. doi: 10.1158/0008-5472.CAN-05-1630
PubMed Abstract | Crossref Full Text | Google Scholar
28. Sinkovics JG, Horvath JC. Natural and genetically engineered viral agents for oncolysis and gene therapy of human cancers. Arch Immunol Ther Exp (Warsz). (2008) 56 Suppl 1:3s–59s. doi: 10.1007/s00005-008-0047-9
留言 (0)