Glutathione peroxidases (GPXs) are a group of enzymes that can detoxify hydrogen peroxide to produce other free radicals (Handy and Loscalzo, 2022). Glutathione peroxidase-3 (GPX-3) is one of the 8 isozymes of GPX and particularly, as plasma GPX because it is mainly distributed extracellularly unlike other GPX isozymes (Rush and Sandiford, 2003). Several studies were measuring serum GPX-3 concentrations and investigating the clinical significance in patients with various inflammatory or cancerous diseases. Low serum GPX-3 concentration is considered a biomarker to assess a high degree of inflammation or poor prognosis (Bierl et al., 2004; Manzanares et al., 2009; Agnani et al., 2011), which might be attributed to be due to the clinical properties of GPX-3 as an antioxidant enzyme (Chang et al., 2020). Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a systemic vasculitis that invades the smallest units of arteries, veins, and capillaries and may potentially affect almost all major organs (Jennette et al., 2013; Watts et al., 2007). According to the clinical, laboratory, radiological, and histological features, AAV is divided into three subtypes: microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA), and eosinophilic GPA (EGPA) (Suppiah et al., 2022; Robson et al., 2022; Grayson et al., 2022). Although three subtypes are essentially characterized by each subtype’s predominant features, given the affected vessel system, they may share the common clinical manifestations related to the three typical major organs with the largest surface area of capillaries such as the kidneys, lungs, and skin (Kronbichler et al., 2024). On the other hand, the pathogenesis of AAV involves many immunological processes and responses, and particularly, the role of reactive oxygen species (ROS) is known to contribute significantly to vascular inflammation and damage (Kitching et al., 2020; Choi et al., 2019). Therefore, it can be reasonably assumed that if the expression of GPX isozymes with a peroxidase function to remove ROS is reduced, the degree of vascular inflammation and damage may increase. Moreover, it could be speculated that serum GPX-3 concentration may be inversely associated with the current degree of vascular inflammation and damage in patients with AAV. However, no study has elucidated the clinical role of serum GPX-3 concentration in assessing the current degree of vascular inflammation and damage in patients with AAV. Hence, in this study, we investigated whether serum GPX-3 concentration at diagnosis could be used to assess vasculitis activity and damage at diagnosis in immunosuppressive drug-naïve patients with AAV.
2 Materials and methods2.1 PatientsWe randomly selected 71 immunosuppressive drug-naïve patients newly diagnosed with AAV from an observational single-centre cohort of Korean patients with AAV at the University Tertiary Hospital and included them in this study. The inclusion criteria were i) fulfilment of the 2007 European Medicine Agency algorithm for AAV, the 2022 revised Chapel Hill Consensus Conference nomenclature of vasculitides, and the 2022 American College of Rheumatology and European Alliance of Associations for Rheumatology classification criteria for MPA, GPA, and EGPA (Handy and Loscalzo, 2022; Rush and Sandiford, 2003; Suppiah et al., 2022; Robson et al., 2022; Grayson et al., 2022); ii) first diagnosis of AAV by the specialised Rheumatologists in this tertiary hospital; iii) sufficiently documented medical records for collecting clinical, laboratory, radiological, and histological data; iv) well-prepared sera obtained on patients’ consent and stored at diagnosis; v) follow-up duration for 6 months or greater after diagnosis; vii) no serious concomitant medical conditions mimicking AAV such as malignancies, severe infectious diseases requiring hospitalization, and autoantibody-medicated autoimmune diseases, or inducing ANCA false positive at diagnosis; vii) no exposure to immunosuppressive drugs for AAV treatment at least within 4 weeks before diagnosis. This study was approved by the Institutional Review Board (IRB) of Severance Hospital, Seoul, Republic of Korea (IRB number 4-2016-0901) and, when required, written informed consent was obtained from patients at the time of blood sampling. The IRB waived the need for written informed consent when it was previously obtained at entry into the SHAVE cohort.
2.2 Blood sampling and consent formOn the day of AAV diagnosis, informed consent was obtained, AAV-specific indices were assessed, and whole blood was collected from patients with AAV. Sera were immediately isolated from whole blood and stored at −80°C on the day of AAV diagnosis.
2.3 Clinical data at diagnosisAge, sex, smoking history, and body mass index (BMI) were recorded. Data on AAV-subtype, ANCA type and positivity, and AAV-specific indices were also collected. In the present study, myeloperoxidase (MPO)-ANCA and proteinase 3 (PR3)-ANCA measured using an immunoassay as well as perinuclear (P)-ANCA and cytoplasmic (C)-ANCA detected using an indirect immunofluorescence assay with ethanol fixation were all recognised as ANCA test results according to the 2022 criteria (Suppiah et al., 2022; Robson et al., 2022; Grayson et al., 2022). AAV-specific indices included the Birmingham vasculitis activity score (BVAS), the five-factor score (FFS), the 36-Item Short Form Survey (SF-36) physical and mental component summaries (PCS and MCS), and the vasculitis damage index (VDI) (Mukhtyar et al., 2009; Guillevin et al., 2011; Flossmann et al., 2007; Han et al., 2004). In this study, the degrees of vascular activity and the extent of damage were expressed using BVAS and VDI, respectively. Routinely performed laboratory test results, including those of acute-phase reactants such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) tests, were recorded.
2.4 Measurement of serum GPX-3 concentration at diagnosisSerum GPX-3 concentration was assessed from stored sera at diagnosis using enzyme-linked immunosorbent assay kits (Mybiosource, San Diego, CA, United States) according to the manufacturer’s instructions. In this study, a continuous variable of serum GPX-3 concentration was used for statistical analyses.
2.5 Clinical data during follow-upAll-cause mortality, end-stage kidney disease (ESKD), cerebrovascular accident (CVA), and acute coronary syndrome (ACS) were considered poor AAV outcomes during follow-up. The follow-up duration based on each poor outcome was defined as the period from diagnosis to its occurrence in patients with a corresponding poor outcome, whereas the duration from diagnosis to the last visit was defined for those without. Medications administered after AAV diagnosis and during the disease course, including glucocorticoids and immunosuppressive drugs, were also assessed. Accordingly, ESKD, CVA, and ACS that occurred before diagnosis were not considered poor AAV outcomes in the present study.
2.6 Statistical analysesAll statistical analyses were performed using the SPSS version 26 (IBM Corporation, Armonk, NY, United States) for Windows (Microsoft Corporation, Redmond, WA, United States). Continuous and categorical variables are expressed as medians (interquartile range, Q1−Q3), and numbers (percentages). The Mann-Whitney U test was used to compare significant differences between continuous variables. The correlation coefficients (r) between the two variables were determined using Pearson’s correlation analysis. Cox proportional hazards model analysis was performed to obtain the hazard ratio (HR) of serum GPX-3 concentration at diagnosis for each poor outcome during follow-up. A significant area under the curve (AUC) was determined using receiver operating characteristic (ROC) curve analysis. A comparison of the cumulative survival rates between the two groups was performed using Kaplan Meier survival analysis with the log-rank test. Statistical significance was set at P < 0.05.
3 Results3.1 Characteristics of patients with AAV at diagnosisThe median age of the study subjects was 63.0 (51.0–74.0) years, and 26 and 45 patients were males and females, respectively. Two patients were ex-smokers and the median BMI was 22.4 (21.1–24.8) kg/m2. Of these 71 patients, 35, 23, and 13 were diagnosed with MPA, GPA, and EGPA, respectively. The median BVAS, FFS, SF-36 PCS and MCS, and VDI values were 5.0 (3.0–17.0), 0 (0–1.0), 52.5 (34.4–68.1), 58.4 (40.0–73.4), and 3.0 (2.0–4.0), respectively. The median ESR and CRP were 21.0 (7.0–85.3) mm/h and 3.8 (0.9–29.1) mg/L, respectively. The median GPX-3 concentration was measured as 82.8 (44.3–156.0) ng/mL (Table 1).
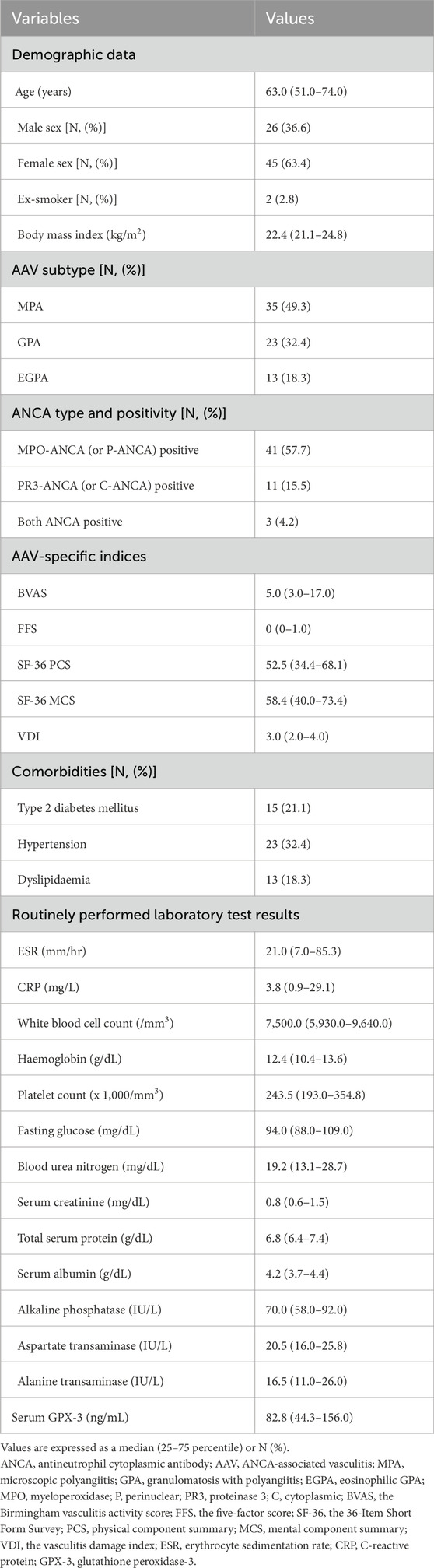
Table 1. Characteristics of patients with AAV at diagnosis (N = 71).
3.2 Correlation analysis of serum GPX-3 concentration and continuous variables at diagnosis in patients with AAVSerum GPX-3 concentration at diagnosis was inversely correlated with BVAS (r = −0.280, P = 0.018), VDI (r = −0.263, P = 0.029), and CRP (r = −0.261, P = 0.028) at diagnosis, whereas, it was positively correlated with haemoglobin (r = 0.255, P = 0.032), and serum albumin (r = 0.240, P = 0.045) at diagnosis, respectively. Additionally, both SF-36 PCS and ESR tended to correlate with serum GPX-3 concentration simultaneously but the correlation was not statistically significant (Table 2).
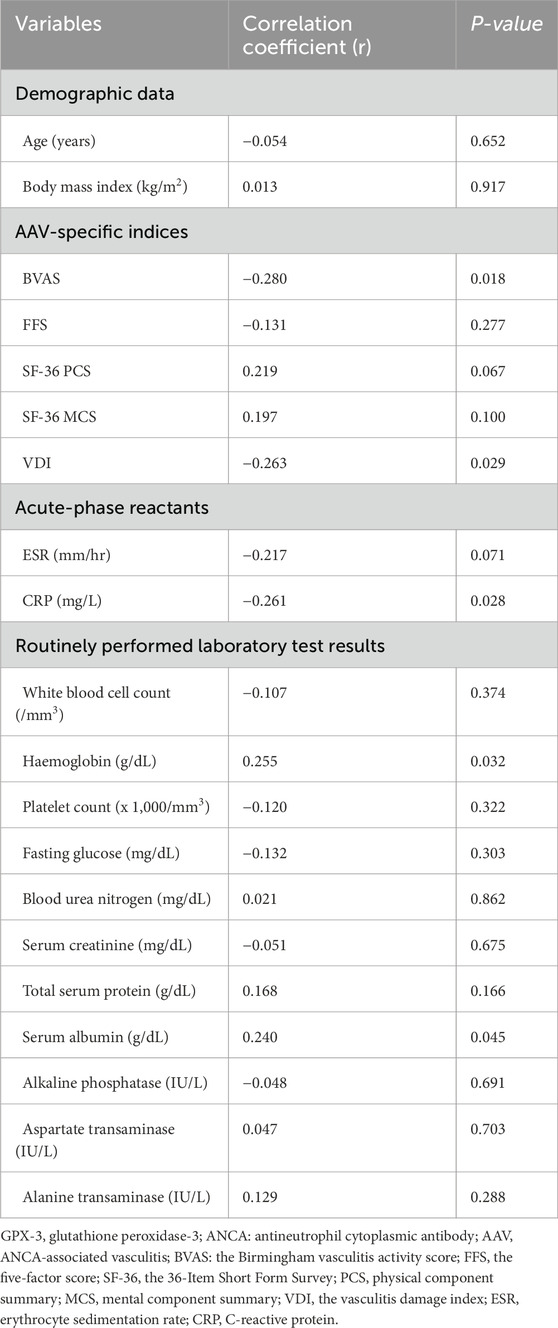
Table 2. Correlation analysis of serum GPX-3 concentration and continuous variables at diagnosis in patients with AAV.
3.3 Comparison of serum GPX-3 concentration between the two groupsAt the time of diagnosis, serum GPX-3 concentrations according to sex, AAV subtype, MPO-ANCA (or P-ANCA) positivity, PR3-ANCA (or C-ANCA) positivity, and comorbidities were compared but no significant differences between the two groups were observed (Figure 1). Additionally, since serum GPX-3 concentration was inversely correlated with BVAS, we divided the study participants into two groups according to each of the nine systemic items of BVAS and compared serum GPX-3 concentrations between the two groups (Mukhtyar et al., 2009). We found that only serum GPX-3 concentrations, according to general manifestation, were significantly different between the two groups (55.8 ng/mL for patients with general manifestation vs. 84.8 ng/mL for those without, P = 0.021) (Table 3).
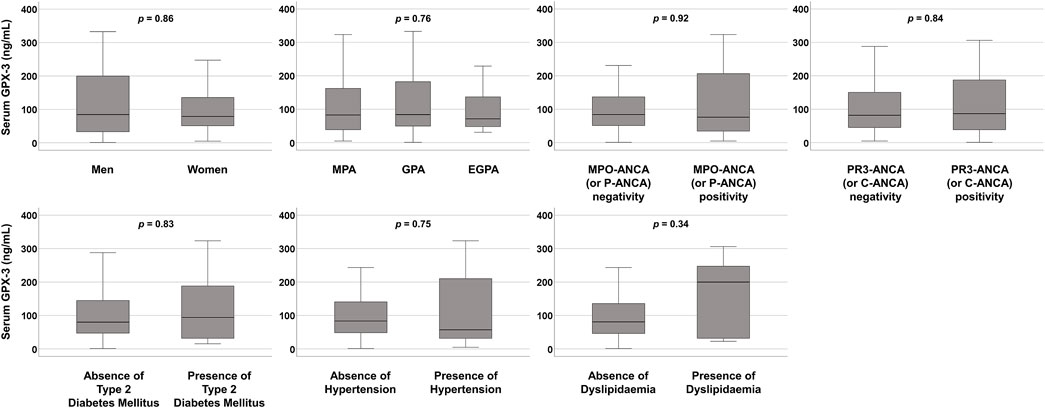
Figure 1. Comparison of serum GPX-3 concentrations. No significant differences between the two groups were observed. GPX-3, glutathione peroxidase-3; MPA, microscopic polyangiitis; GPA, granulomatosis with polyangiitis; EGPA, eosinophilic GPA; MPO, myeloperoxidase; ANCA, antineutrophil cytoplasmic antibody; P, perinuclear; PR3, proteinase 3: C, cytoplasmic.
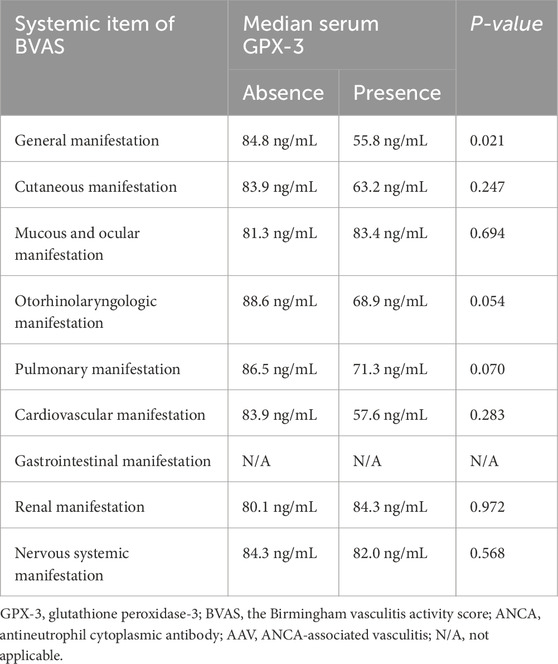
Table 3. Comparative analysis of serum GPX-3 according to the presence of each systemic item of BVAS in patients with AAV.
3.4 Poor outcomes and immunosuppressive drugs administered during follow-up in patients with AAVDuring follow-up after diagnosis, among the 71 patients, 6 (8.5%) died, and 17, four, and one had ever experienced and suffered from ESKD, CVA, and ACS, respectively. Glucocorticoids were administered to 70 (98.6%) patients. Among the intravenous induction therapeutic regimens, cyclophosphamide, and rituximab were administered to 47 (66.2%), and 13 (18.3%) patients, respectively. Among oral immunosuppressive drugs, the most frequently administered was azathioprine (57.7%), followed by mycophenolate mofetil (26.8%) (Table 4).
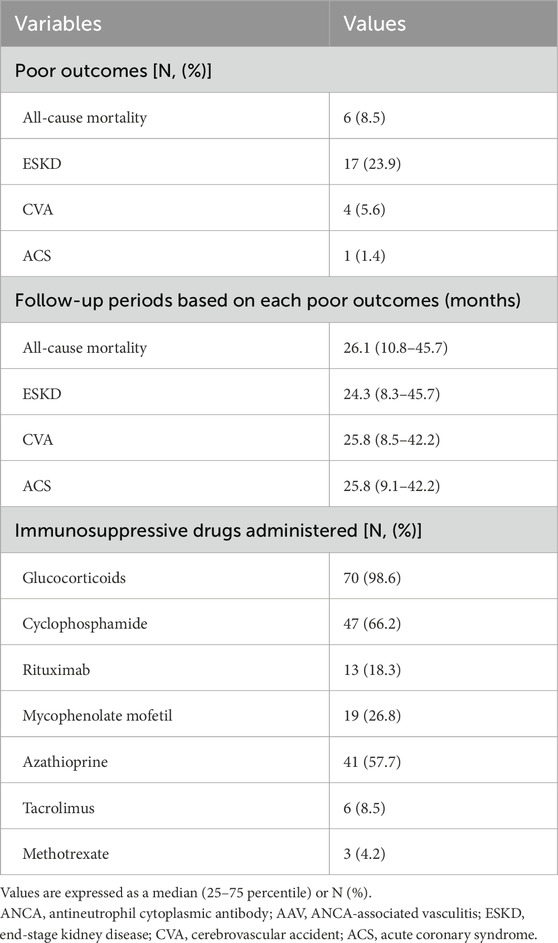
Table 4. Poor outcomes and immunosuppressive drugs administered during follow-up in patients with AAV (N = 71).
3.5 Cox analysis of serum GPX-3 concentration for each poor outcomeSerum GPX-3 concentration at diagnosis was not significantly associated with all-cause mortality, ESKD, CVA, or ACS during follow-up in patients with AAV (Table 5).
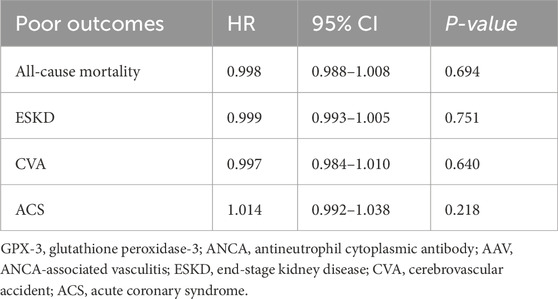
Table 5. Univariable Cox proportional hazards analysis of serum GPX-3 concentration at diagnosis for each poor outcome during follow-up in patients with AAV.
4 DiscussionConsidering the clinical utility of low serum GPX-3 concentration (or GPX-3 deficiency) in various diseases, in this study we investigated whether serum GPX-3 concentration at diagnosis could be used to assess vasculitis activity and damage at diagnosis in immunosuppressive drug-naive patients with AAV, and obtained some interesting findings. First, significant correlations were observed between serum GPX-3 concentration at diagnosis and both BVAS and VDI at diagnosis, indicating that serum GPX-3 can be a potential biomarker to assess vasculitis activity and damage at diagnosis in patients newly diagnosed with AAV. Additionally, serum GPX-3 had another potential to reflect the inflammatory burden as much as traditional acute-phase reactants such as CRP and serum albumin. However, serum GPX-3 did not predict poor AAV outcomes during follow-up. Therefore, in the present study, we demonstrated that serum GPX-3 concentration at diagnosis has the potential to be a novel biomarker that can assess and estimate activity and damage caused by AAV, although it is confined to the time of diagnosis.
ROS are well-known mediators of the pathophysiological signal transduction of inflammation processes (Forrester et al., 2018). At the site of inflammation, the increased ROS production by polymorphonuclear cells may induce endothelial cell dysfunction and tissue damage. Furthermore, the combination of endothelial impairment and oxidative stress may loosen the endothelial junction of the blood-tissue barrier and accelerate the extravascular migration of inflammatory immune cells to the inflamed tissue (Mittal et al., 2014). Similarly, in the pathophysiology of AAV, ROS is also known to play an important role in the development and exacerbation of AAV (Kitching et al., 2020; Choi et al., 2019). GPX-3 can reduce oxidative stress by scavenging ROS, which may reduce the nuclear translocation of transcription factors such as the p65 unit of nuclear factor kappa-light-chain-enhancer of activated B cells, ultimately suppressing the expression of inflammatory mediators located downstream of the promoter sites (An et al., 2018; Nirgude and Choudhary, 2021). Based on these findings, it is reasonable to infer that decreased GPX-3 expression and production may increase vascular activity and damage owing to a diminished effect in suppressing the transcription of inflammatory mediators. This inference supports the key finding of this study that serum GPX-3 concentration at diagnosis was inversely correlated with BVAS, VDI, and CRP at diagnosis.
We investigated which item among the nine systemic items of BVAS based on major organ involvement contributed to the inverse correlation with serum GPX-3 concentration. We divided the study participants into two groups according to each of the nine systemic items of BVAS and compared serum GPX-3 concentrations between the two groups (Mukhtyar et al., 2009). Only serum GPX-3 concentrations, according to general manifestation, was significantly different between the two groups. Additionally, otorhinolaryngologic (68.9 ng/mL vs. 88.6 ng/mL, P = 0.054) and pulmonary (71.3 ng/mL vs. 86.5 ng/mL, P = 0.070) manifestations showed significant differences between the two groups but they did not reach statistical significance. Although GPX-3 has been reported to be associated with renal pathological findings (Burk et al., 2011), this study was not able to reveal an association between serum GPX-3 concentration and kidney involvement of AAV (Table 3). As per the general manifestation, since its minor items were closer to constitutional symptoms, such as myalgia, arthralgia/arthritis, fever, and weight loss, rather than those limited to a specific organ (Mukhtyar et al., 2009), this subgroup analysis was not sufficient to explain the inverse correlation between serum GPX-3 concentration and BVAS. Therefore, we conclude that the correlation between serum GPX-3 concentration and BVAS may be based on the sum of the cumulative contributions of several organ involvements, such as the ear, nose, throat or lungs, rather than that of specific organ invasion by AAV.
In the present study, serum GPX-3 concentration at diagnosis could not predict poor outcomes during follow-up in patients with AAV. Given that the risk factors for all-cause mortality among the four poor outcomes were relatively well established, to verify the methodological error of the Cox analysis for the results in Table 4 results, we performed univariable and multivariable Cox proportional hazards analyses including traditional, AAV-specific, and inflammation-related risk factors along with GPX-3 concentration at diagnosis for all-cause mortality during follow-up (Murray et al., 2013; Tan et al., 2017; Mukhtyar et al., 2008). In univariable Cox analysis, dyslipidaemia, VDI, white blood cell count, haemoglobin, and serum albumin were significantly associated with all-cause mortality in patients with AAV; however, serum GPX-3 concentration was not. Nevertheless, to investigate the predictive and independent potential of serum GPX-3 concentration for all-cause mortality, we included serum GPX-3 concentration in multivariable Cox analysis. However, in multivariable Cox analysis, only dyslipidaemia was independently associated with all-cause mortality (Supplementary Table S1). Therefore, we verified that there was no methodological error in the Cox analysis, and demonstrated that the independent predictive potential of serum GPX-3 concentration for all-cause mortality was not notable.
Additionally, although a continuous value of serum GPX-3 concentration did not show significant predictive potential for poor outcomes, we wondered whether a categorical value of serum GPX-3 concentration according to its cut-offs of poor outcomes such as all-cause mortality, ESKD, CVA, and ACS could be a significant predictor of those poor outcomes. We attempted to obtain each optimal cut-off of serum GPX-3 concentration for each poor outcome using the receiver operating characteristic ROC curve analyses; however, we found no significant areas under the curve (AUC) of serum GPX-3 concentration for poor outcomes. Therefore, we failed to obtain the optimal cut-offs of serum GPX-3 concentration for all-cause mortality, ESKD, CVA, or ACS in this study (Supplementary Figure S1). Meanwhile, among the four poor outcomes, despite no statistical significance, all-cause mortality showed a relatively higher AUC compared to the remaining ESKD, CVA, and ACS. When the patients were divided into two groups such as the higher and lower groups according to the median value of GPX-3 concentration of 82.8 ng/mL, in Kaplan Meier survival analysis, however, no significant difference in cumulative patients’ survival rates between patients in the higher and lower groups was observed (Supplementary Figure S2). We concluded that serum GPX-3 can be useful in estimating the current activity and damage of vasculitis but not anticipating future poor outcomes in patients with AAV. This study has the advantage that this is the first to demonstrate the significant correlations between serum GPX-3 concentration with activity (BVAS) and damage (VDI) at diagnosis in patients with AAV.
Antiphospholipid syndrome (APS) is a systemic autoimmune disease characterized by thrombotic, non-thrombotic, and obstetric manifestations in individuals with persistent antiphospholipid antibodies (aPL). These antibodies are commonly detected through immunoassays for anticardiolipin (aCL) and anti-β2-glycoprotein-I (aβ2GPI) antibodies, or lupus anticoagulant tests. The interaction between aPL and targets like β2GPI triggers the activation of endothelial cells, platelets, monocytes, and neutrophils, as well as the complement and coagulation systems, forming a key molecular mechanism underlying APS pathogenesis (Knight and Erkan, 2024). This interaction occurs at specific microdomains of the cell plasma membrane known as lipid rafts, which are rich in glycosphingolipids and cholesterol (Capozzi et al., 2023). Recent reviews highlight that Mitogen-Activated Protein Kinases (MAPKs) play a crucial role in transmitting signals from the cell surface to the nucleus, regulating gene activity. Among the four main branches of the MAPK pathway, p38MAPK is a key regulator in APS. Additionally, aPL stimulates the production of ROS in endothelial cells. These ROS act as secondary messengers, activating the p38MAPK pathway, which is involved in the pathogenesis of APS (Feng et al., 2024). GPX-3 is an antioxidant enzyme that plays a critical role in removing ROS, and thus, APS patients may exhibit a decrease in serum GPX-3 concentration. However, this study did not include patients with aPL antibodies, and therefore, subgroup analysis related to this aspect could not be performed.
5 LimitationsThis study has several limitations. The most critical limitation was that the number of participating patients was not sufficiently large to generalize these results and apply them to real clinical practice and that clinical data and sera samples from the time of diagnosis were used retrospectively. Additionally, although this study focused on the clinical implications of serum GPX-3 concentration among patients with AAV only, this study could not provide the results of the comparative analysis of serum GPX-3 concentration between AAV patients and age- and sex-matched healthy controls. However, given that this is the first pilot study to explore the clinical utility of serum GPX-3 concentration in patients with AAV, we believe that this study has clinical implications in that this identified a novel biomarker that can assess and estimate activity and damage caused by AAV at diagnosis, although confined to the time of diagnosis. A prospective future study enrolling more patients and equipping serial clinical data and paired serum samples will provide more reliable and dynamic information on the clinical significance of serum GPX-3 concentration for not only assessing activity and damage at diagnosis but also predicting and monitoring the prognosis during follow-up in patients with AAV.
6 ConclusionIn this study, we demonstrated for the first time that serum GPX-3 concentration at diagnosis correlates with vasculitis activity and damage at diagnosis in patients with AAV, suggesting a possible role of serum GPX-3 as a complementary biomarker for assessing AAV activity in real clinical practice.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThis study was approved by the Institutional Review Board (IRB) of Severance Hospital, Seoul, Republic of Korea (IRB number 4-2016-0901) and, when required, written informed consent was obtained from patients at the time of blood sampling. The IRB waived the need for written informed consent when it was previously obtained at entry into the SHAVE cohort.
Author contributionsJC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Visualization, Writing–original draft, Writing–review and editing. JH: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Visualization, Writing–original draft, Writing–review and editing. Y-BP: Conceptualization, Data curation, Methodology, Project administration, Validation, Writing–original draft, Writing–review and editing. S-WL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Visualization, Writing–original draft, Writing–review and editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by a faculty research grant of Yonsei University College of Medicine for (6-2023-0155), CELLTRION PHARM, Inc. Chungcheongbuk-do, Republic of Korea (NCR 2019-6), and Chong Kun Dang Pharmaceutical Corp, Seoul, Republic of Korea. The funder was not involved in the study design, collection, analysis, interpretation of data, writing of this article or the decision to submit it for publication.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmolb.2025.1549454/full#supplementary-material
ReferencesAgnani, D., Camacho-Vanegas, O., Camacho, C., Lele, S., Odunsi, K., Cohen, S., et al. (2011). Decreased levels of serum glutathione peroxidase 3 are associated with papillary serous ovarian cancer and disease progression. J. Ovarian Res. 4, 18. doi:10.1186/1757-2215-4-18
PubMed Abstract | CrossRef Full Text | Google Scholar
An, B. C., Choi, Y. D., Oh, I. J., Kim, J. H., Park, J. I., and Lee, S. W. (2018). GPx3-mediated redox signaling arrests the cell cycle and acts as a tumor suppressor in lung cancer cell lines. PLoS One 13, e0204170. doi:10.1371/journal.pone.0204170
PubMed Abstract | CrossRef Full Text | Google Scholar
Bierl, C., Voetsch, B., Jin, R. C., Handy, D. E., and Loscalzo, J. (2004). Determinants of human plasma glutathione peroxidase (GPx-3) expression. J. Biol. Chem. 279, 26839–26845. doi:10.1074/jbc.M401907200
PubMed Abstract | CrossRef Full Text | Google Scholar
Burk, R. F., Olson, G. E., Winfrey, V. P., Hill, K. E., and Yin, D. (2011). Glutathione peroxidase-3 produced by the kidney binds to a population of basement membranes in the gastrointestinal tract and in other tissues. Am. J. Physiol. Gastrointest. Liver Physiol. 301, G32–G38. doi:10.1152/ajpgi.00064.2011
PubMed Abstract | CrossRef Full Text | Google Scholar
Capozzi, A., Manganelli, V., Riitano, G., Caissutti, D., Longo, A., Garofalo, T., et al. (2023). Advances in the pathophysiology of thrombosis in antiphospholipid syndrome: molecular mechanisms and signaling through lipid rafts. J. Clin. Med. 12, 891. doi:10.3390/jcm12030891
PubMed Abstract | CrossRef Full Text | Google Scholar
Chang, C., Worley, B. L., Phaëton, R., and Hempel, N. (2020). Extracellular glutathione peroxidase GPx3 and its role in cancer. Cancers (Basel) 12, 2197. doi:10.3390/cancers12082197
PubMed Abstract | CrossRef Full Text | Google Scholar
Choi, C. B., Park, Y. B., and Lee, S. W. (2019). Antineutrophil cytoplasmic antibody-associated vasculitis in Korea: a narrative review. Yonsei Med. J. 60, 10–21. doi:10.3349/ymj.2019.60.1.10
PubMed Abstract | CrossRef Full Text | Google Scholar
Feng, W., Qiao, J., Tan, Y., Liu, Q., Wang, Q., Yang, B., et al. (2024). Interaction of antiphospholipid antibodies with endothelial cells in antiphospholipid syndrome. Front. Immunol. 15, 1361519. doi:10.3389/fimmu.2024.1361519
PubMed Abstract | CrossRef Full Text | Google Scholar
Flossmann, O., Bacon, P., de Groot, K., Jayne, D., Rasmussen, N., Seo, P., et al. (2007). Development of comprehensive disease assessment in systemic vasculitis. Ann. Rheum. Dis. 66, 143–152. doi:10.1136/ard.2005.051078
PubMed Abstract | CrossRef Full Text | Google Scholar
Forrester, S. J., Kikuchi, D. S., Hernandes, M. S., Xu, Q., and Griendling, K. K. (2018). Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 122, 877–902. doi:10.1161/CIRCRESAHA.117.311401
PubMed Abstract | CrossRef Full Text | Google Scholar
Grayson, P. C., Ponte, C., Suppiah, R., Robson, J. C., Craven, A., Judge, A., et al. (2022). 2022 American College of Rheumatology/European alliance of associations for Rheumatology classification criteria for eosinophilic granulomatosis with polyangiitis. Ann. Rheum. Dis. 81, 309–314. doi:10.1136/annrheumdis-2021-221794
PubMed Abstract | CrossRef Full Text | Google Scholar
Guillevin, L., Pagnoux, C., Seror, R., Mahr, A., Mouthon, L., and Toumelin, P. L. (2011). The Five-Factor Score revisited: assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Med. Baltim. 90, 19–27. doi:10.1097/MD.0b013e318205a4c6
PubMed Abstract | CrossRef Full Text | Google Scholar
Han, C. W., Lee, E. J., Iwaya, T., Kataoka, H., and Kohzuki, M. (2004). Development of the Korean version of Short-Form 36-Item Health Survey: health related QOL of healthy elderly people and elderly patients in Korea. Tohoku J. Exp. Med. 203, 189–194. doi:10.1620/tjem.203.189
PubMed Abstract | CrossRef Full Text | Google Scholar
Jennette, J. C., Falk, R. J., Bacon, P. A., Basu, N., Cid, M. C., Ferrario, F., et al. (2013). 2012 revised international Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 65, 1–11. doi:10.1002/art.37715
PubMed Abstract | CrossRef Full Text | Google Scholar
Kitching, A. R., Anders, H. J., Basu, N., Brouwer, E., Gordon, J., Jayne, D. R., et al. (2020). ANCA-associated vasculitis. Nat. Rev. Dis. Prim. 6, 71. doi:10.1038/s41572-020-0204-y
PubMed Abstract | CrossRef Full Text | Google Scholar
Kronbichler, A., Bajema, I. M., Bruchfeld, A., Mastroianni Kirsztajn, G., and Stone, J. H. (2024). Diagnosis and management of ANCA-associated vasculitis. Lancet 403, 683–698. doi:10.1016/S0140-6736(23)01736-1
PubMed Abstract | CrossRef Full Text | Google Scholar
Manzanares, W., Biestro, A., Galusso, F., Torre, M. H., Mañay, N., Pittini, G., et al. (2009). Serum selenium and glutathione peroxidase-3 activity: biomarkers of systemic inflammation in the critically ill? Intensive Care Med. 35, 882–889. doi:10.1007/s00134-008-1356-5
PubMed Abstract | CrossRef Full Text | Google Scholar
Mittal, M., Siddiqui, M. R., Tran, K., Reddy, S. P., and Malik, A. B. (2014). Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal 20, 1126–1167. doi:10.1089/ars.2012.5149
PubMed Abstract | CrossRef Full Text | Google Scholar
Mukhtyar, C., Flossmann, O., Hellmich, B., Bacon, P., Cid, M., Cohen-Tervaert, J. W., et al. (2008). Outcomes from studies of antineutrophil cytoplasm antibody associated vasculitis: a systematic review by the European League against Rheumatism systemic vasculitis task force. Ann. Rheum. Dis. 67, 1004–1010. doi:10.1136/ard.2007.071936
PubMed Abstract | CrossRef Full Text | Google Scholar
Mukhtyar, C., Lee, R., Brown, D., Carruthers, D., Dasgupta, B., Dubey, S., et al. (2009). Modification and validation of the Birmingham vasculitis activity score (version 3). Ann. Rheum. Dis. 68, 1827–1832. doi:10.1136/ard.2008.101279
PubMed Abstract | CrossRef Full Text | Google Scholar
Murray, C. J., Atkinson, C., Bhalla, K., Birbeck, G., Burstein, R., Chou, D., et al. (2013). The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA 310, 591–608. doi:10.1001/jama.2013.13805
PubMed Abstract | CrossRef Full Text | Google Scholar
Nirgude, S., and Choudhary, B. (2021). Insights into the role of GPX3, a highly efficient plasma antioxidant, in cancer. Biochem. Pharmacol. 184, 114365. doi:10.1016/j.bcp.2020.114365
PubMed Abstract | CrossRef Full Text | Google Scholar
Robson, J. C., Grayson, P. C., Ponte, C., Suppiah, R., Craven, A., Judge, A., et al. (2022). 2022 American College of Rheumatology/European alliance of associations for Rheumatology classification criteria for granulomatosis with polyangiitis. Ann. Rheum. Dis. 81, 315–320. doi:10.1136/annrheumdis-2021-221795
PubMed Abstract | CrossRef Full Text | Google Scholar
Rush, J. W., and Sandiford, S. D. (2003). Plasma glutathione peroxidase in healthy young adults: influence of gender and physical activity. Clin. Biochem. 36, 345–351. doi:10.1016/s0009-9120(03)00039-0
PubMed Abstract | CrossRef Full Text | Google Scholar
Suppiah, R., Robson, J. C., Grayson, P. C., Ponte, C., Craven, A., Khalid, S., et al. (2022). 2022 American College of Rheumatology/European alliance of associations for Rheumatology classification criteria for microscopic polyangiitis. Ann. Rheum. Dis. 81, 321–326. doi:10.1136/annrheumdis-2021-221796
PubMed Abstract | CrossRef Full Text | Google Scholar
Tan, J. A., Dehghan, N., Chen, W., Xie, H., Esdaile, J. M., and Avina-Zubieta, J. A. (2017). Mortality in ANCA-associated vasculitis: a meta-analysis of observational studies. Ann. Rheum. Dis. 76, 1566–1574. doi:10.1136/annrheumdis-2016-210942
PubMed Abstract | CrossRef Full Text | Google Scholar
Watts, R., Lane, S., Hanslik, T., Hauser, T., Hellmich, B., Koldingsnes, W., et al. (2007). Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann. Rheum. Dis. 66, 222–227. doi:10.1136/ard.2006.054593
留言 (0)