With the improvement of individuals’ living standards, there has been a significant increase in the prevalence of dyslipidemia. Genetic defects and unhealthy lifestyle are two risk factors of hyperlipidemia (Lorenzatti and Toth, 2020), especially, the change in people’s dietary structure towards high fat, high sugar and high calorific value, as well as ultra-processed foods, has led to a sharp increase in the prevalence of dyslipidemia (Arnett et al., 2019; Juul et al., 2021). Hypercholesterolemia stands as the primary contributor to cardiovascular diseases, which is one of the main reasons for adult death in the United States and causes huge economic losses every year (Dawber et al., 2015; Virani et al., 2020). Numerous guidelines advocate for statin usage to mitigate the morbidity and mortality associated with such conditions (Lloyd-Jones et al., 2017; Mach et al., 2020; Stone et al., 2014). Low density lipoprotein cholesterol (LDL-C) serves as a pivotal target for intervention in reducing atherosclerotic cardiovascular disease (ASCVD). Patients with hypertriglyceridemia exhibit elevated levels of residual lipoproteins that are likely to exert atherogenic effects. Consequently, non-high-density lipoprotein cholesterol (HDL-C) is also employed as an auxiliary intervention target. Although statins effectively lower LDL-C levels, the achievement of comprehensive lipid regulation necessitates the concomitant use of other lipid-modulating agents.
3- Hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors (statins) have been widely recommended to reduce the incidence rate and mortality of cardiovascular diseases (Heart Protection Study Collaborative Group, 2002; Lloyd-Jones et al., 2017; Mach et al., 2020; Pedersen et al., 2004; Stone et al., 2014). Statins primarily function by inhibiting HMG-CoA reductase, thereby impeding cholesterol synthesis within the body. Fibrates can augment lipoprotein lipase activity and diminish triglyceride levels. The 2019 ESC Lipid Guidelines suggest that a combination of statins and fibrates may be considered when a patient’s TG > 2.3 mmol/L (Mach et al., 2020). According to the 2023 Chinese Lipid Management Guidelines, individuals with ASCVD or at high risk should receive moderate dose statin therapy if their TG > 2.3 mmol/L, and fibrates can be administered to further mitigate the risk of ASCVD (Joint Committee on the Chinese Guidelines for Lipid Management, 2023). Even so, the cardiovascular benefits of statins in combination with fibrates remain a subject of debate and controversy within the scientific community. The safety of combining statins and fibrates in the Chinese population is deemed acceptable; however, further verification is required to establish the long-term safety of this combination (Joint Committee on the Chinese Guidelines for Lipid Management, 2023). Nevertheless, due to the similar metabolic pathways of statins and fibrates, their combination has the potential to cause liver injury and increase the risk of myositis and myopathy (Joint committee for guideline, 2018), greatly increasing the occurrence rate of adverse events (AEs). It is well-established that the concurrent administration of statins and fibrates can give rise to significant adverse reactions. In 2001, cerivastatin, a promising statin, was introduced to the market; however, Bayer Pharmaceutical, its manufacturer, subsequently contraindicated the combination of cerivastatin and gemfibrozil due to frequent and severe reports of rhabdomyolysis-related deaths (Staffa et al., 2002). Consequently, Bayer withdrew cerivastatin from the international drug market that same year (Wooltorton, 2001). Overall, the drug labels of AEs after the combination therapy of these two drugs is still deficient, which is not conducive to actual clinical applications. The safety of combining statins and fibrates should be given significant attention.
The real-world data could provide post-marketing drug safety information, which is beneficial for clinicians to weigh risks and benefits. The US Food and Drug Adiministration (via the FDA Adverse Event Reporting System, FAERS), the World Health Organisation (via VigiBase) and the European Medicines Agency (via Eudra Vigilance) are the most widely used databases for reporting spontaneous adverse drug reactions abroad. FAERS Data files are provided in ASCII or SGML format to ensure consistency in compiling drug and adverse event data. Information transfer between databases is carried out directly using standardized data formats, as FDA only accepts electronic submissions of ICSRs in XML format. Herein, this study is aimed to analyze the AEs reports of FAERS, so as to provide references for rational clinical application through detecting safety signals and identifying potential drug risk signals.
MethodsData sourceIn this study, we obtained data from the OpenFDA, a public data open project in the United States, and the original data of AEs were imported by FAERS (Joint committee for guideline, 2018). FAERS collects spontaneous safety reports and post-marketing clinical research reports related to drugs used in the United States and abroad. All AEs were coded using preferred terms (PT) from the Medical Dictionary for Regulatory Activities (MedDRA) (Ma et al., 2021).
We used Research AE as the analysis tool to extract AEs reports from the FAERS database which covered the period from 1 January 2004 to 19 March 2020. Research AE is a research AE analysis tool, which can directly extract AEs from the FAERS database through the interface of application programming (API). The generic names of rosuvastatin and fenofibrate were used as the keywords to perform searches, and the AEs reports were included when rosuvastatin and fenofibrate were the first suspect drugs. Reports pertaining to diseases, which related to drug indications, or concomitant disease were excluded from the analysis, other reports from the top 250 AE cases were left for signal detection in order to assess the association between drugs and AEs.
Signal detection methodDisproportionality analysis is a commonly used analytic method for AEs signal mining, which could be divided into two categories: frequentist and Bayesian methods. No “gold standard” is available, each of the above methods has its own shortages (van Puijenbroek et al., 2003). Both proportional reporting ratio (PRR) (Evans et al., 2001) and reporting odds ratio (ROR) (van Puijenbroek et al., 2002) are frequency methods. They are easy for calculation and can lead to a more sensitive output than bayesian approaches. The bayesian confidence propagation neural network (BCPNN) (Noguchi et al., 2019) is always applicable and large numbers of calculations can be made efficiently. Both approaches entailed inherent drawbacks, including: the limitation of frequentist statistical method mainly includes: i): false positive signals might be detected and ii): measured values are sensitive to small fluctuations. Correspondingly, the restriction of Bayesian Confidence Propagation Neural Network (BCPNN) mainly including: i): false-negative signals might be detected. ii): measured values are not specific and iii): signal value is difficult to be calculated (Bate et al., 2002; Noguchi et al., 2021). No one algorithm is universally better than others. In the present investigation, we used PRR, ROR, and BCPNN for safety signals detection. The two-by-two frequency table of disproportionality analysis is shown in Table 1.
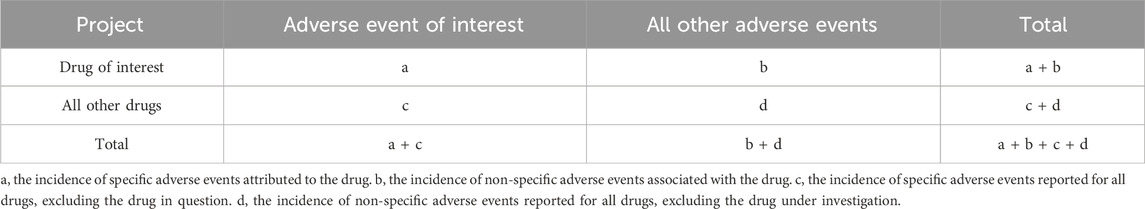
Table 1. Two-by-two frequency table.
Herein, the criteria of PRR and ROR were: a ≥ 3, the lower bound of 95% two-sided confidence interval (CI) > 1, and the criteria of BCPNN were: IC-2SD > 0 (Shen et al., 2019) and the algorithm was showed in Equation 1. The higher the scores of PRR, ROR, and BCPNN, the stronger the association between the drugs and AEs. In addition, to identify the impact of gender differences on AEs, we analyzed 10 AEs most frequently reported and performed ROR analysis (ROR > 1 means a higher likelihood of AEs occurring in females).
α1=β1=1; α=β=2; γ11=1; C=a+b+c+d; Cx=a+b; Cy=a+c; Cxy=a;γ=γ11C+αC+βCx+α1Cy+β1; EIC=log2Cxy+γ11C+αC+βC+γCx+α1Cy+β1;VIC=1ln22;IC−2SD=EIC−2VIC(1)ResultsAEs reports and demographic characteristics of patientsIn this study, a total of 3,587 AEs were reported with rosuvastatin and fenofibrate as the first suspect drugs. As shown in Table 2, doctors (21.60%), pharmacists (6.08%), and other medical staff (14.75%) were main reporters. The highest proportion of reports was by consumers and non-medical staff. In addition, there are more male patients (54.47%) than female patients (41.43%) and the patients aged 45∼64 are counted the most percent (31.28%). Notably, the percentage of serious AEs was 43.41% after combined therapy, of which 1,110 (30.95%) reported cases were hospitalization or prolonged hospitalization.
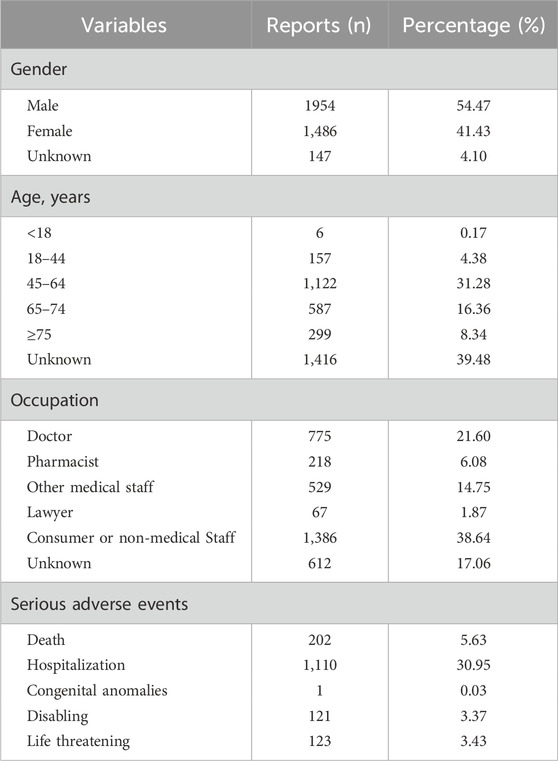
Table 2. Demographic characteristics of patients and composition of serious adverse events.
Signal detection of AEsAs defined in MedDRA, the safety signals were classified according to System Organ Class (SOC). Herein, a total of 68 safety signals were detected from the top 250 AEs in 3,587 events. As shown in Tables 3 40 (58.82%) safety signals, involving 12 SOC, were listed in the drug labels, of which the top 5 AEs were gastrointestinal diseases (468 reports, 13.05%), musculoskeletal and connective tissue diseases (400 reports, 11.15%), general diseases (292 reports, 18.14%), investigations (272 reports, 7.58%) and nervous system diseases (186 reports, 5.19%), respectively. In addition, 28 (41.18%) signals were not included in the drug labels, which mainly including blushing, back pain, weight loss, poor appetite and so on.
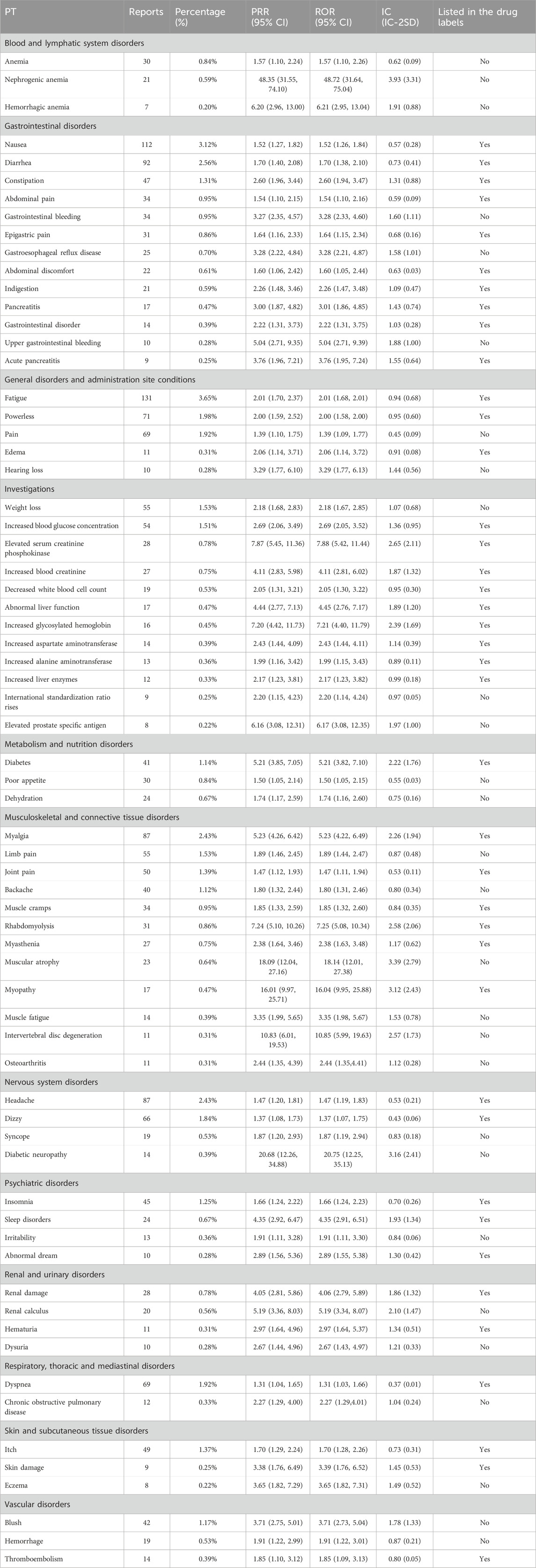
Table 3. Significant disproportionality results displayed according to SOC and PT.
Furthermore, according to the analysis of AEs in Table 4, we found that females exhibit a higher susceptibility to experiencing AEs, including pain, nausea, fatigue, myalgia, diarrhea, dyspnea, headache, weakness, and dizziness. Correspondingly, men are more likely to experience weight loss.
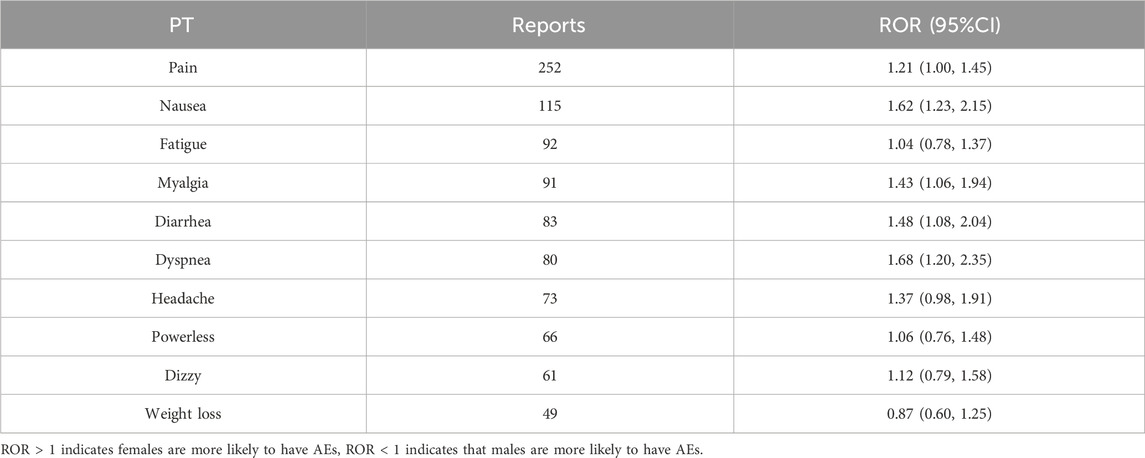
Table 4. Gender differences in adverse event reactions.
DiscussionAccording to the signal screening results, during the combined treatment of rosuvastatin and fenofibrate, the risks of gastrointestinal system disorders, musculoskeletal and connective tissue disorders, general disorders, medical tests as well as neurological disorders were increased when rosuvastatin and fenofibrate were applied in combination, which were consistent with previous researches (Ferdinand et al., 2012; Pepine et al., 2010; Roth et al., 2010). And the most commonly reported AEs were in the gastrointestinal system, mainly manifested as nausea, diarrhea and other discomfort, which would affect patients’ appetite and sleep quality, thus further increasing their discomfort and even cause discontinuation of treatment in severe cases, greatly limits the therapeutic effect of patients. To improve patients’ medication compliance, medication guide and health education could be strengthened, enabling them fully understand disease and drugs, reducing psychological burden and adjusting the diet as needed.
In addition, since both statins and fibrates have the potential to cause liver injury, myositis and myopathy, their combination is more likely to cause liver and kidney damage as well as muscle aches (Cranmer et al., 2021; Shen et al., 2019; van Puijenbroek et al., 2002). Therefore, it is recommended to closely monitor the indices of creatine kinase and liver enzymes, as well as reporting all unexplained muscle aches and pains. Besides, for special populations, such as the elderly and children, the overweight or slim people and patients simultaneously using several drugs, the dose could be adjusted according to the patient’s tolerance to avoid serious AEs (Alomar, 2014; Han et al., 2022; Zhang et al., 2023).
Furthermore, basing on the signal screening of FAERS database, we found that 28 signals were not included in the drug label, mainly including flushing, back pain, weight loss and loss of appetite, which suggests possible AEs outside instructions during the actual application of rosuvastatin and fenofibrate. Hence, our research is expected to provide data support beyond the instructions for rapid clinical evaluation of combined drugs.
However, this study still remains some deficiencies. On account of the detection of signal was based on the spontaneous reporting database, it was prone to have missed, duplicate, incomplete and irregular reports. While consumers or non-medical staff reports constituted the largest proportion, this subset of reports showed a greater tendency for incompleteness and irregularity, which consequently affected the accuracy of data analysis. In addition, the disproportionality analysis was focused on the number of reports, which failed to take the time-to-onset distribution into account (Noguchi et al., 2021). It also did not take into account patients’ basic diseases and other combined medication issues as well as reports that one drug was regarded as a suspicious drug and another drug was regarded as an accompanying drug. Besides, because it is difficult to identify which patient was prescribed with these drugs and for what reason, many heterogeneous patients were also included in our analyzation. Additionally, since PT was fixed, we counted the AEs and checked them with the drug labels objectively, which might bias the judgment of whether the adverse event was expected or not. Moreover, the safety signals detected in the study only indicated a statistical correlation between drugs and AEs, specific methods to investigate drug-drug interaction are still need to be considered in further studies.
ConclusionBased on the FDA adverse event database, this study identified a total of 68 positive signals. When rosuvastatin was combined with fenofibrate, the most prevalent AEs observed were related to gastrointestinal system diseases, musculoskeletal and connective tissue diseases, general diseases, investigations and nervous system diseases. Additionally, analysis of FAERS database data revealed 28 signals primarily associated with blushing, back pain, weight loss, poor appetite and so on, were not included in the drug labels. Therefore, more attention needs to be paid to the combined therapy of statin and fibrate. And we believe our real-world data analysis could be expected to provide helpful reference for rapid clinical assessment and further promote rational clinical medication.
Data availability statementThe datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Author contributionsQL: Data curation, Formal Analysis, Funding acquisition, Investigation, Writing–original draft. WS: Data curation, Formal Analysis, Investigation, Writing–original draft. SW: Conceptualization, Funding acquisition, Supervision, Writing–review and editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Administration of Traditional Chinese Medicine of Zhejiang Province, China (2018ZQ048), Foundation of Zhejiang Provincial Education Department (Y202148364) and Science and Technology program of Yiwu Science and Technology Bureau (Grant No. 18-3-29, 18-3-28).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ReferencesArnett, D. K., Blumenthal, R. S., Albert, M. A., Buroker, A. B., Goldberger, Z. D., Hahn, E. J., et al. (2019). 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation 140, e596–e646. doi:10.1161/CIR.0000000000000678
PubMed Abstract | CrossRef Full Text | Google Scholar
Cranmer, M., Tamayo, D., Rein, H., Battaglia, P., Hadden, S., Armitage, P. J., et al. (2021). A Bayesian neural network predicts the dissolution of compact planetary systems. Proc. Natl. Acad. Sci. U. S. A. 118, e2026053118. doi:10.1073/pnas.2026053118
PubMed Abstract | CrossRef Full Text | Google Scholar
Evans, S. J., Waller, P. C., and Davis, S. (2001). Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf. 10, 483–486. doi:10.1002/pds.677
PubMed Abstract | CrossRef Full Text | Google Scholar
Ferdinand, K. C., Davidson, M. H., Kelly, M. T., and Setze, C. M. (2012). One-year efficacy and safety of rosuvastatin + fenofibric acid combination therapy in patients with mixed dyslipidemia: evaluation of dose response. Am. J. Cardiovasc Drugs 12, 117–125. doi:10.2165/11597940-000000000-00000
PubMed Abstract | CrossRef Full Text | Google Scholar
Han, Y. Z., Guo, Y. M., Xiong, P., Ge, F. L., Jing, J., Niu, M., et al. (2022). Age-associated risk of liver-related adverse drug reactions. Front. Med. (Lausanne) 9, 832557. doi:10.3389/fmed.2022.832557
PubMed Abstract | CrossRef Full Text | Google Scholar
Heart Protection Study Collaborative Group (2002). MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 360, 7–22. doi:10.1016/S0140-6736(02)09327-3
PubMed Abstract | CrossRef Full Text | Google Scholar
Joint committee for guideline (2018). 2016 Chinese guidelines for the management of dyslipidemia in adults. J. Geriatr. Cardiol. 15, 1–29. doi:10.11909/j.issn.1671-5411.2018.01.011
PubMed Abstract | CrossRef Full Text | Google Scholar
Joint Committee on the Chinese Guidelines for Lipid Management (2023). Chinese guidelines for lipid management. Chin. Circ. J. 38, 237–271. doi:10.3760/cma.j.cn112148-20230119-00038
CrossRef Full Text | Google Scholar
Juul, F., Vaidean, G., Lin, Y., Deierlein, A. L., and Parekh, N. (2021). Ultra-processed foods and incident cardiovascular disease in the framingham offspring study. J. Am. Coll. Cardiol. 77, 1520–1531. doi:10.1016/j.jacc.2021.01.047
PubMed Abstract | CrossRef Full Text | Google Scholar
Lloyd-Jones, D. M., Morris, P. B., Ballantyne, C. M., Birtcher, K. K., Daly, D. D., Depalma, S. M., et al. (2017). 2017 focused update of the 2016 acc expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American college of cardiology task force on expert consensus decision pathways. J. Am. Coll. Cardiol. 70, 1785–1822. doi:10.1016/j.jacc.2017.07.745
PubMed Abstract | CrossRef Full Text | Google Scholar
Mach, F., Baigent, C., Catapano, A. L., Koskinas, K. C., Casula, M., Badimon, L., et al. (2020). 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur. Heart J. 41, 111–188. doi:10.1093/eurheartj/ehz455
PubMed Abstract | CrossRef Full Text | Google Scholar
Ma, R., Wang, Q., Meng, D., Li, K., and Zhang, Y. (2021). Immune checkpoint inhibitors-related myocarditis in patients with cancer: an analysis of international spontaneous reporting systems. BMC Cancer 21, 38. doi:10.1186/s12885-020-07741-0
PubMed Abstract | CrossRef Full Text | Google Scholar
Noguchi, Y., Nagasawa, H., Tachi, T., Tsuchiya, T., and Teramachi, H. (2019). Signal detection of oral drug-induced dementia in chronic kidney disease patients using association rule mining and Bayesian confidence propagation neural network. Pharmazie 74, 570–574. doi:10.1691/ph.2019.9426
PubMed Abstract | CrossRef Full Text | Google Scholar
Noguchi, Y., Tachi, T., and Teramachi, H. (2021). Detection algorithms and attentive points of safety signal using spontaneous reporting systems as a clinical data source. Brief. Bioinform 22, bbab347. doi:10.1093/bib/bbab347
PubMed Abstract | CrossRef Full Text | Google Scholar
Pedersen, T. R., Kjekshus, J., Berg, K., Haghfelt, T., Faergeman, O., Faergeman, G., et al. (2004). Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Atheroscler. Suppl. 5, 81–87. doi:10.1016/j.atherosclerosissup.2004.08.027
PubMed Abstract | CrossRef Full Text | Google Scholar
Pepine, C. J., Jacobson, T. A., Carlson, D. M., Kelly, M. T., Setze, C. M., Gold, A., et al. (2010). Combination rosuvastatin plus fenofibric acid in a cohort of patients 65 years or older with mixed dyslipidemia: subanalysis of two randomized, controlled studies. Clin. Cardiol. 33, 609–619. doi:10.1002/clc.20830
PubMed Abstract | CrossRef Full Text | Google Scholar
Roth, E. M., Rosenson, R. S., Carlson, D. M., Fukumoto, S. M., Setze, C. M., Blasetto, J. W., et al. (2010). Efficacy and safety of rosuvastatin 5 mg in combination with fenofibric acid 135 mg in patients with mixed dyslipidemia - a phase 3 study. Cardiovasc Drugs Ther. 24, 421–428. doi:10.1007/s10557-010-6266-4
PubMed Abstract | CrossRef Full Text | Google Scholar
Shen, J., Yang, J., and Zhao, B. (2019). A survey of the FDA’s adverse event reporting system database concerning urogenital tract infections and sodium glucose cotransporter-2 inhibitor use. Diabetes Ther. 10, 1043–1050. doi:10.1007/s13300-019-0611-9
PubMed Abstract | CrossRef Full Text | Google Scholar
Stone, N. J., Robinson, J. G., Lichtenstein, A. H., Bairey Merz, C. N., Blum, C. B., Eckel, R. H., et al. (2014). 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 63, 2889–2934. doi:10.1016/j.jacc.2013.11.002
PubMed Abstract | CrossRef Full Text | Google Scholar
VAN Puijenbroek, E. P., Bate, A., Leufkens, H. G., Lindquist, M., Orre, R., and Egberts, A. C. (2002). A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf. 11, 3–10. doi:10.1002/pds.668
PubMed Abstract | CrossRef Full Text | Google Scholar
Van Puijenbroek, E., Diemont, W., and VAN Grootheest, K. (2003). Application of quantitative signal detection in the Dutch spontaneous reporting system for adverse drug reactions. Drug Saf. 26, 293–301. doi:10.2165/00002018-200326050-00001
PubMed Abstract | CrossRef Full Text | Google Scholar
Virani, S. S., Alonso, A., Benjamin, E. J., Bittencourt, M. S., Callaway, C. W., Carson, A. P., et al. (2020). Heart disease and stroke statistics-2020 update: a report from the American heart association. Circulation 141, e139–e596. doi:10.1161/CIR.0000000000000757
PubMed Abstract | CrossRef Full Text | Google Scholar
Zhang, M., Lv, H., Yang, H., Zhang, H., Bai, X., and Qian, J. (2023). Elderly patients with moderate-to-severe ulcerative colitis are more likely to have treatment failure and adverse outcome. Gerontology 69, 119–129. doi:10.1159/000522569
留言 (0)