Hepatoid adenocarcinoma of the lung (HAL) is an extremely rare tumor type, which was first reported as an Alpha-fetoprotein (AFP)-producing lung cancer by Ysunami (1). The pathological features of HAL were fully defined by Ishikura in 1990 (2) and were modified by Haninger in 2014 (3). Since AFP-producing HAL has scarcely been reported, the clinical features and molecular mechanism of this type of lung cancer are still unclear, with no standard treatment regime being recommended. When concomitant idiopathic pulmonary fibrosis (IPF) was diagnosed, the prognosis of lung cancer maybe much poorer (4–6).
Interestingly, we encountered a case of AFP-producing HAL in a patient with IPF, which benefit from the systematic treatment and achieved a long-term survival for this rare type of lung cancer. To the best of our knowledge, this is the first report of AFP-producing HAL in a patient with IPF.
Case presentationA 66-year-old man was admitted to our hospital with the chief complaint of cough in March 2018. Chest computed tomography (CT) revealed multiple nodules measuring from 8mm to 20mm in diameter located in bilateral lung, along with an enlarged #11L lymph node (Figures 1A–D). The patient had been diagnosed of IPF four years prior. Of the tumor markers, most were within the normal range, except for a markedly elevated serum AFP level of 6753ng/ml. A CT-guided percutaneous lung biopsy was performed in the largest pulmonary nodule located in the S6 segment of the left lung.
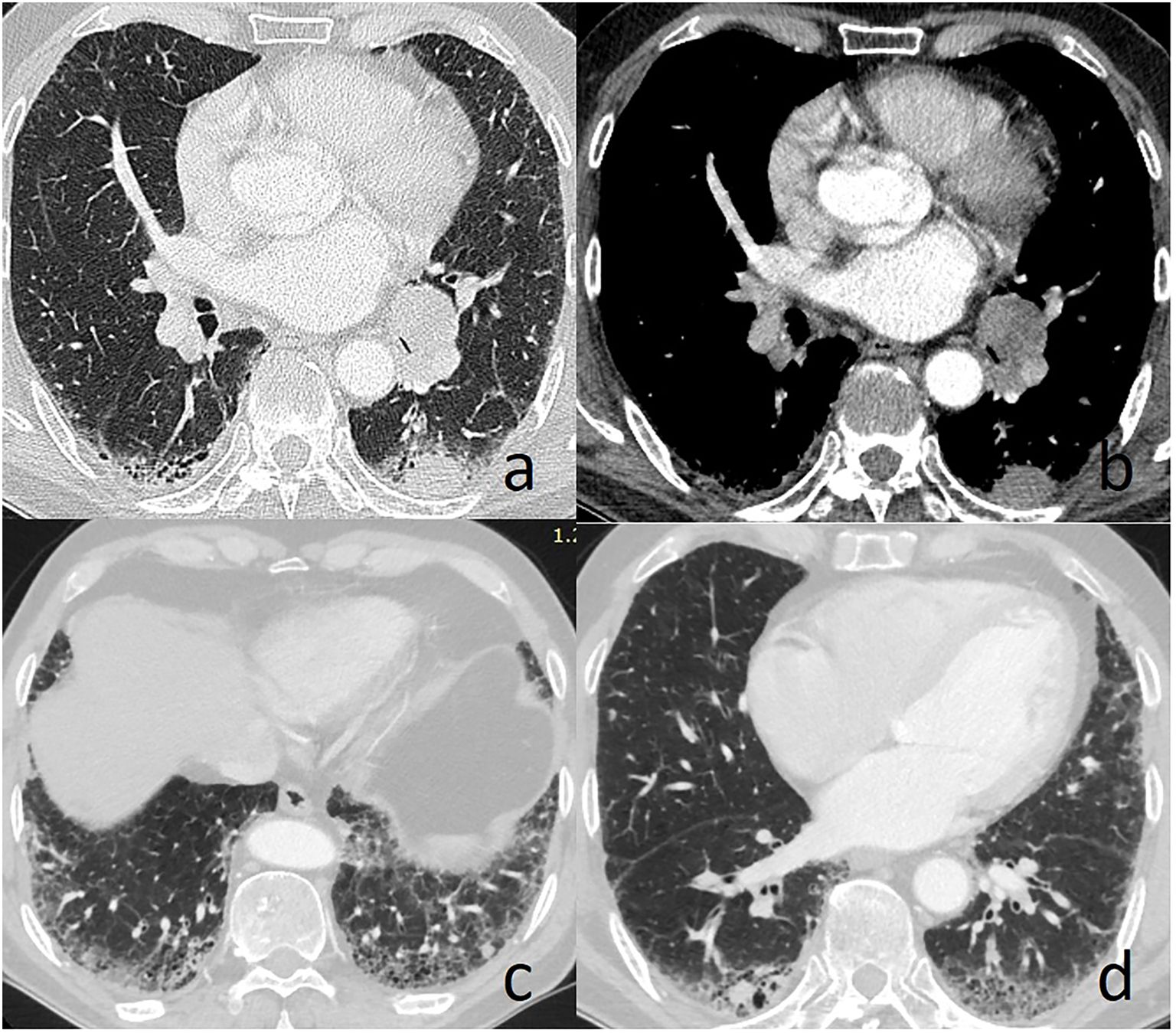
Figure 1. Chest computed tomography reveals left lung nodule and enlarged #11L lymph node (A, B), and multiple lung nodules in the area with obvious fibrotic changes (C, D).
Hematoxylin and eosin staining indicated the poorly differentiated cancer cells resembling hepatocellular carcinoma (Figures 2A, B). Immunohistochemical staining showed that the tumor cells were negative for TTF-1 (Figure 2C), but positive for Hepatocyte (Figure 2D) and CK8/18 (Figure 2E), with moderate positivity for Ki-67 (40%, Figure 2F). Abdominal magnetic resonance imaging (MRI) and CT examination showed no evidence of hepatic or other digestive system tumors. Positron emission tomography/computed tomography (PET-CT) revealed increased standard uptake value (SUV) in the lung nodules (SUV max: 8.1) and in the enlarged #11L lymph node (SUV max: 4.1). No abnormal hypermetabolic lesions were observed in any other organs. Serum biochemical tests did not reveal any evidence of hepatic dysfunction or hepatitis B or C infection. Based on these findings, the patient was diagnosed with AFP-producing primary HAL, clinical stage IV (T4N1M1a). Molecular studies were negative for EGFR, ROS-1, ALK, RET, MET, PIK-3CA, ERBB-2, KRAS, and BRAF mutations.
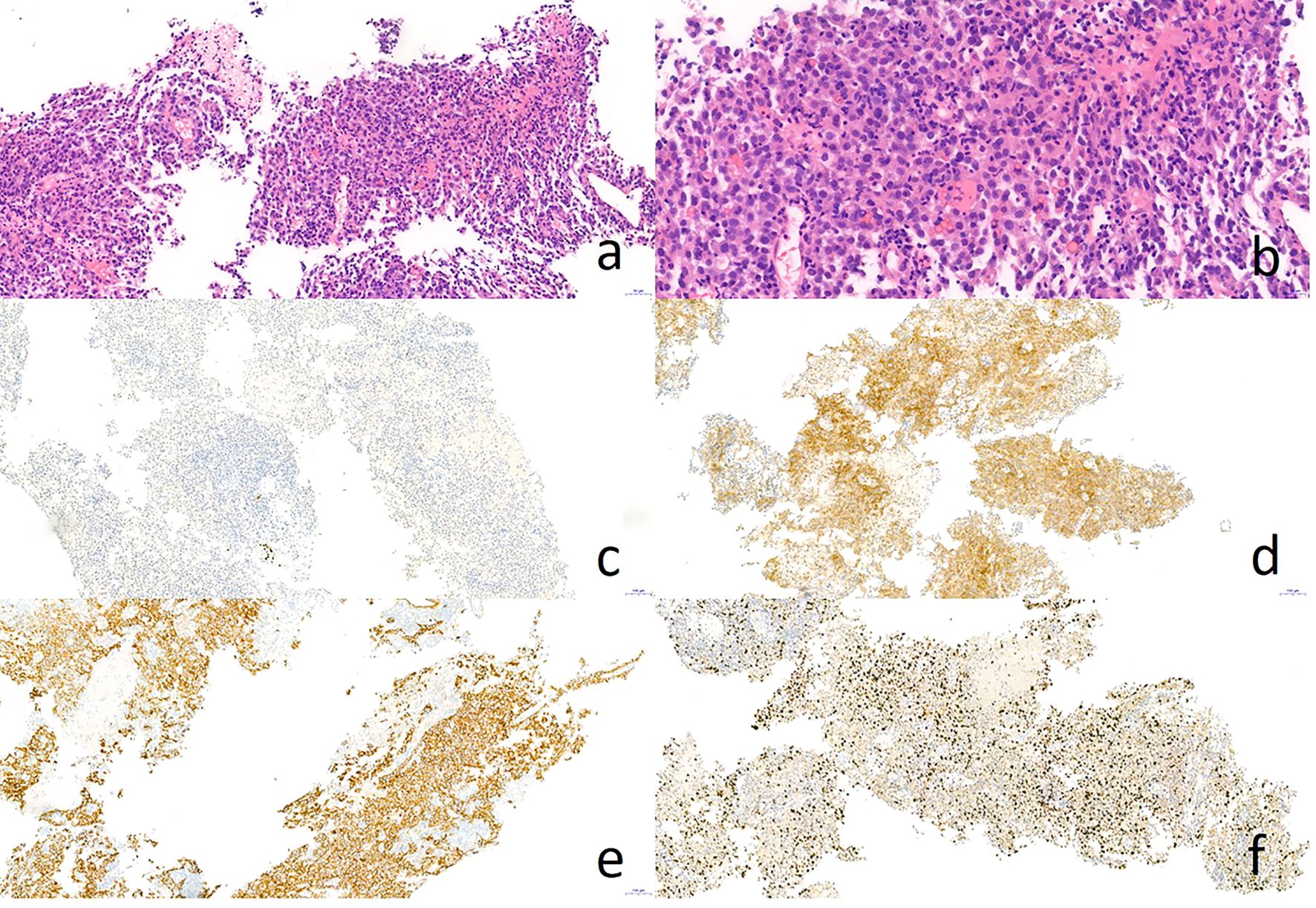
Figure 2. Pathology result of CT-guided percutaneous lung biopsy. Hematoxylin-eosin staining showed poorly differentiated adenocarcinoma like hepatocellular carcinoma (A, B). Immunohistochemical staining showed negative for TTF-1 (C) and positive for Hepatocyte (D) and CK8/18 (E), as well as Ki-67 40% (F).
Subsequently, the patient received six cycles of pemetrexed plus cisplatin chemotherapy, with bevacizumab administered every 21 days concurrently. There was a slight decrease in the AFP serum level (4679 ng/ml), as well as a reduction in the diameter of nodule in left lower lobar. However, due to the side effects, the patient refused further chemotherapy and was instead treated with oral Nintedanib for both lung cancer and IPF. After five months, an increase in the AFP serum level and enlargement of lung nodule and lymph node were noted. Consequently, the patient continued to receive docetaxel as second-line therapy, resulting in partial regression of the pulmonary lesions. Upon subsequent progression, Anlotinib was administrated for 16 months. Since March 2021, the patient has been treated with pembrolizumab, leading to the partial shrinkage of the pulmonary lesion and lymph node. After 2 years of treatment with pembrolizumab, the primary lesion progressed again and pemetrexed plus anlotinib was given for 2 cycles. Due to side effects, chemotherapy was stopped and anlotinib was taken orally intermittently, and the efficacy was evaluated to be stable. The primary lesion developed again 6 months later, and tracheoscopic tissue biopsy was performed, and the pathological results indicated hepatoid adenocarcinoma. The treatment with sintilimab combined with anlotinib lasted for 2 cycles, and the progress of efficacy was evaluated, followed by bronchial artery embolization. The patient is currently being followed up. The patient survived for 75 months following the diagnosis of AFP-producing IPF-HAL.
No acute exacerbation of IPF or immune-related injuries associated with chemotherapy or immune checkpoint inhibitors occurred throughout the treatment process. Figure 3 illustrate the diagnostic and treatment timeline.
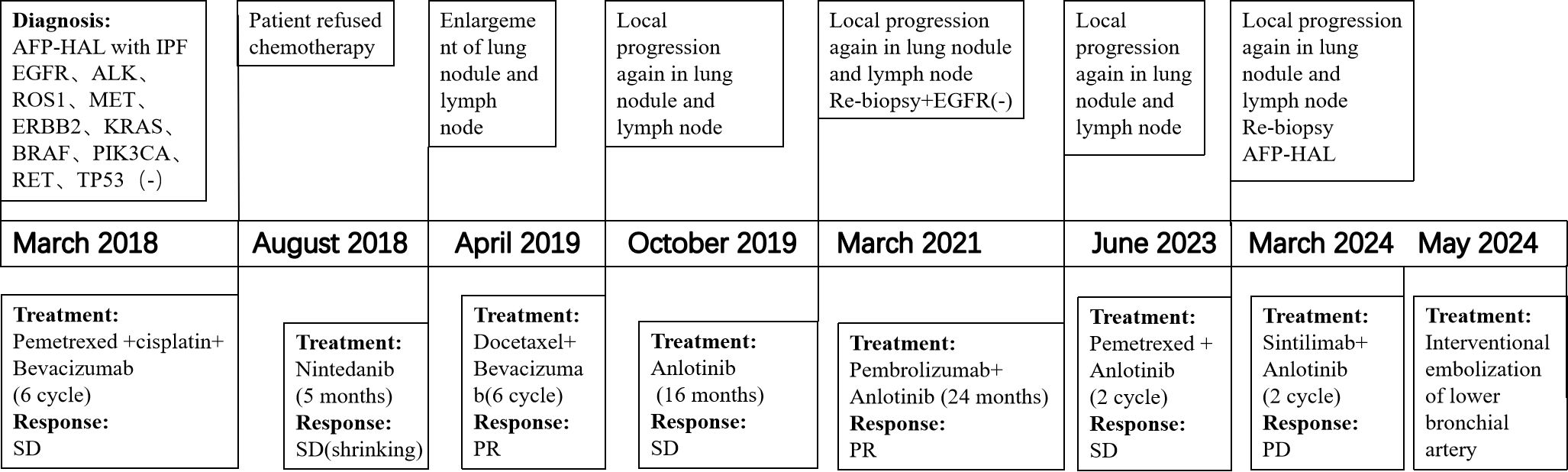
Figure 3. Flow chart of diagnosis and treatment process.
Discussion and conclusionsThe incidence of lung cancer in patients with IPF increases with each year following an IPF diagnosis (4–6). Squamous cell carcinoma and adenocarcinoma are the most frequent types of lung cancer in IPF patients, with isolated cases of large cell carcinoma and small cell lung cancer also reported (4). However, to our knowledge, AFP-producing HAL has not been previously reported. Here, we report a case of this rarely type of lung cancer in an IPF patient to raise awareness for clinicians.
Hepatoid adenocarcinoma of the lung is a relatively rare primary malignant tumor of the lung, with an incidence of 0.014/100000 people (7). Clinically, patients with HAL typically presented with nonspecific symptoms. Grossman et al. found that 96% of the tumors occur in men with elevated serum AFP levels and a history of tobacco use. HAL usually presents as a bulky mass in an upper lobe with metastasis and follows an aggressive clinical course (8). HAL closely mimics hepatocellular carcinoma (HCC) and can be misdiagnosed by both pathologists and clinicians, especially when serum AFP level is elevated (9). PET-CT can be used to comprehensively examine patients, aiding in confirm the origin of tumor when the serum AFP levels are elevated (10). Although it is not necessary for the diagnosis of HAL according to recent criteria, serum AFP level is still an important predictive factor in this condition (8, 11).
HAL is an extremely heterogeneous tumor type, and currently, no standard treatment is available. According to the guidelines for diagnosis and treatment of lung cancer, the common treatments for HAL patients include surgical resection, chemotherapy and radiotherapy. Recently, new treatments such as sorafenib, immunotherapy (Anti-PD-L1, Durvalumab), and radiofrequency ablation have been prescribed for HAL (12–16). However, when HAL is combined with IPF, many risk factors must be considered in treatment decisions. Lung status and postoperative complications should be fully evaluated before lung resection. The early and long-term outcomes of surgery in lung cancer patients with IPF are poor due to the high risk of acute exacerbation (AE) of IPF and lung cancer recurrence (17, 18). Radiation and chemotherapy are also known risk factors for AE-IPF (19, 20). With a deeper understanding of IPF and lung cancer, more treatment modalities are being explored (21–23). In our study, surgery and radiation were not performed; instead, long-term survival of 75 months was achieved through sequential chemotherapy (pemetrexed plus cisplatin, Docetaxel), anti-angiogenesis therapy (Bevacizumab, Anlotinib), antifibrotic therapy (Nintedanib), immunotherapy (Pembrolizumab, Sintilimab) and local treatment (Interventional embolization of lower bronchial artery).
In conclusion, this case report is the first to describe the occurrence of AFP-producing HAL in an IPF patient. The disease courses of both IPF and HAL are variable and somewhat unpredictable, potentially altered by the co-occurrence. Currently, there is no consensus on the treatment of patients with both diseases. The long-terms survival achieved in our case may provide prognostic value for this rare condition. Further understanding of the pathogenic overlap between lung cancer and IPF could guide the development of specific diagnostic modalities and targeted treatments for both conditions in the future.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statementThe studies involving humans were approved by Ethics Committee of Henan Provincial People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributionsQQZ: Conceptualization, Formal analysis, Writing – review & editing. XLW: Conceptualization, Writing – original draft. NW: Conceptualization, Writing – review & editing. HZY: Conceptualization, Writing – original draft. XYW: Formal analysis, Writing – original draft. XJZ: Conceptualization, Formal analysis, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
AcknowledgmentsWe are very grateful to the staff of the Department of Pathology of Henan Provincial People’s Hospital for their help and their efforts.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Yasunami R, Hashimoto Z, Ogura T, Hirao F, Yamamura Y. Primary lung cancer producing alpha-fetoprotein: a case report. Cancer-am Cancer Soc. (1981) 47:926–9. doi: 10.1002/1097-0142(19810301)47:5<926::AID-CNCR2820470518>3.0.CO;2-O
PubMed Abstract | Crossref Full Text | Google Scholar
2. Ishikura H, Kanda M, Ito M, Nosaka K, Mizuno K. Hepatoid adenocarcinoma: a distinctive histological subtype of alpha-fetoprotein-producing lung carcinoma. Virchows Arch A Pathol Anat Histopathol. (1990) 417:73–80. doi: 10.1007/BF01600112
PubMed Abstract | Crossref Full Text | Google Scholar
3. Haninger DM, Kloecker GH, Bousamra Ii M, Nowacki MR, Slone SP. Hepatoid adenocarcinoma of the lung: report of five cases and review of the literature. Mod Pathol. (2014) 27:535–42. doi: 10.1038/modpathol.2013.170
PubMed Abstract | Crossref Full Text | Google Scholar
5. Xiaohong X, Liqiang W, Na L, Xinqing L, Yinyin Q, Ming L, et al. Management and prognosis of interstitial lung disease with lung cancer (ILD-LC): A real-world cohort from three medical centers in China. Front Mol Biosci. (2021) 8:660800. doi: 10.3389/fmolb.2021.660800
PubMed Abstract | Crossref Full Text | Google Scholar
9. Chen Z, Ding C, Zhang T, He Y, Jiang G. Primary hepatoid adenocarcinoma of the lung: A systematic literature review. Onco Targets Ther. (2022) 15:609–27. doi: 10.2147/OTT.S364465
PubMed Abstract | Crossref Full Text | Google Scholar
11. Tonyali O, Gonullu O, Ozturk MA, Kosif A, Civi OG. Hepatoid adenocarcinoma of the lung and the review of the literature. J Oncol Pharm Pract. (2020) 26:1505–10. doi: 10.1177/1078155220903360
PubMed Abstract | Crossref Full Text | Google Scholar
12. Gavrancic T, Park YH. A novel approach using sorafenib in alpha fetoprotein-producing hepatoid adenocarcinoma of the lung. J Natl Compr Canc Netw. (2015) 13:387–91. doi: 10.6004/jnccn.2015.0054
PubMed Abstract | Crossref Full Text | Google Scholar
13. Wang C, Xu G, Wu G, Chen Z, Sun Z, Zheng P, et al. Hepatoid adenocarcinoma of the lung metastasizing to the gingiva. Onco Targets Ther. (2019) 12:8765–8. doi: 10.2147/OTT.S222974
PubMed Abstract | Crossref Full Text | Google Scholar
14. Chen L, Han X, Gao Y, Zhao Q, Wang Y, Jiang Y, et al. Anti-PD-1 therapy achieved disease control after multiline chemotherapy in unresectable KRAS-positive hepatoid lung adenocarcinoma: A case report and literature review. Onco Targets Ther. (2020) 13:4359–64. doi: 10.2147/OTT.S248226
PubMed Abstract | Crossref Full Text | Google Scholar
15. Basse V, Schick U, Guéguen P, Le Maréchal C, Quintin-Roué I, Descourt R, et al. A mismatch repair-deficient hepatoid adenocarcinoma of the lung responding to anti-PD-L1 durvalumab therapy despite no PD-L1 expression. J Thorac Oncol. (2018) 13:e120–2. doi: 10.1016/j.jtho.2018.03.004
PubMed Abstract | Crossref Full Text | Google Scholar
16. Li J, Qi H, Xu B, Zhao J, Gao H, Ma X, et al. Genomic profiles of a patient of pulmonary hepatoid adenocarcinoma with high AFP level: A case report. Front Oncol. (2019) 9:1360. doi: 10.3389/fonc.2019.01360
PubMed Abstract | Crossref Full Text | Google Scholar
17. Tane S, Ando Y, Matsumoto G, Ueda S, Uchino K. Basal segment deep wedge resection for lung cancer with pulmonary fibrosis. Gen Thorac Cardiovasc Surg. (2022) 70:413–5. doi: 10.1007/s11748-021-01764-5
PubMed Abstract | Crossref Full Text | Google Scholar
18. Sato S, Shimizu Y, Goto T, Kitahara A, Koike T, Ishikawa H, et al. Survival after repeated surgery for lung cancer with idiopathic pulmonary fibrosis: a retrospective study. BMC Pulm Med. (2018) 18:134. doi: 10.1186/s12890-018-0703-8
PubMed Abstract | Crossref Full Text | Google Scholar
19. Wang H, Yang R, Jin J, Wang Z, Li W. Impact of concomitant idiopathic pulmonary fibrosis on prognosis in lung cancer patients: A meta-analysis. PLoS One. (2021) 16:e0259784. doi: 10.1371/journal.pone.0259784
PubMed Abstract | Crossref Full Text | Google Scholar
20. Saha A, Dickinson P, Shrimali RK, Salem A, Agarwal S. Is thoracic radiotherapy an absolute contraindication for treatment of lung cancer patients with interstitial lung disease? A systematic review. Clin Oncol (R Coll Radiol). (2022) 34:e493–504. doi: 10.1016/j.clon.2022.01.043
PubMed Abstract | Crossref Full Text | Google Scholar
21. Otsubo K, Kishimoto J, Ando M, Kenmotsu H, Minegishi Y, Horinouchi H, et al. Nintedanib plus chemotherapy for nonsmall cell lung cancer with idiopathic pulmonary fibrosis: a randomised phase 3 trial. Eur Respir J. (2022) 60(6):2200380. doi: 10.1183/13993003.00380-2022
PubMed Abstract | Crossref Full Text | Google Scholar
22. Naoi H, Suzuki Y, Mori K, Aono Y, Kono M, Hasegawa H, et al. Impact of antifibrotic therapy on lung cancer development in idiopathic pulmonary fibrosis. Thorax. (2022) 77:727–30. doi: 10.1136/thoraxjnl-2021-218281
PubMed Abstract | Crossref Full Text | Google Scholar
23. Tomassetti S, Gurioli C, Ryu JH, Decker PA, Ravaglia C, Tantalocco P, et al. The impact of lung cancer on survival of idiopathic pulmonary fibrosis. Chest. (2015) 147:157–64. doi: 10.1378/chest.14-0359
留言 (0)