Leptomeningeal disease (LMD) of solid tumors is characterized by tumor cells invading the subarachnoid space and cerebrospinal fluid (CSF), leading to central metastasis of malignant tumors spreading throughout the central nervous system (CNS). It is a rare but fatal complication of malignant tumors. LMD can manifest across all types of malignant tumors. It is most prevalently observed in lung cancer, breast cancer, and melanoma (Thakkar et al., 2020). Approximately 5%–8% of patients with solid tumors can be associated with meningeal carcinomatosis (Carausu et al., 2021). The autopsy report shows that the incidence rate of many solid tumors exceeds 20% (Beauchesne, 2010; Carausu et al., 2021; Nguyen et al., 2021), indicating that the number of patients diagnosed with LMD is far lower than the actual incidence. LMD is more common in middle-aged and elderly people, with no significant gender differences. The median time from the primary tumor to the diagnosis of LMD is approximately 1–2 years, and the median overall survival (OS) is only 3–4 months, with a high mortality rate (Glantz et al., 1999a; Glantz et al., 1999b; Le Rhun et al., 2019). Hematological metastasis, lymphatic system metastasis, and CSF dissemination are its transmission pathways. The diagnosis of LMD requires the combination of the patient’s physical signs and symptoms with auxiliary examinations. In addition, the clinical manifestations are diverse and non-specific, with persistent headache as the main symptom of treatment (Wang et al., 2018). The overarching goals of treating LMD are twofold: first, to extend the survival of patients while ensuring a tolerable quality of life; second, to forestall or at least defer the progression of neurological deterioration. The LMD treatment plan encompasses supportive therapy, radiotherapy, chemotherapy, surgery, targeted therapy, immunotherapy, and intrathecal (IT) therapy (Pellerino et al., 2018). The specific treatment methods hinge on the histological characteristics, molecular expression, systemic disease progression, neurological function, prognosis, and other factors of the malignant tumor (Le Rhun et al., 2019; Thakkar et al., 2020).
The central nervous system is frequently regarded as a “pharmacological sanctuary” since the majority of anti-cancer drugs are incapable of effectively penetrating the blood–brain barrier (BBB). IT therapy is a key treatment strategy for patients with LMD, offering the advantage of directly bypassing the blood–cerebrospinal fluid barrier to effectively target meningeal lesions. Additionally, given the significantly smaller volume of the CSF than that of plasma, low-dose administration achieves higher drug concentrations in the CSF, resulting in enhanced anti-tumor efficacy with reduced systemic toxicity. The European Society for Medical Oncology (ESMO) (Le Rhun et al., 2023) expert consensus recommends that IT therapy is suitable for most CSF-positive nodular or linear nodules (IA/C type) and patients with LMD with a high tumor cell load in the CSF. IT therapy can be directly administered into the lateral ventricle via subcutaneous fluid storage sacs and ventricular catheters (using an implanted Ommaya reservoir) or into the lumbar dural sac by lumbar puncture (Figure 1). Researchers suggest that drugs should be directly injected into the lateral ventricle through subcutaneous fluid storage sacs and ventricular catheters for IT therapy. Pharmacokinetic studies have shown that drugs can be evenly distributed across various parts of the CNS through CSF circulation, and the concentration can reach up to 10 times that of the same dose administered through lumbar puncture (Roguski et al., 2015; Le Rhun et al., 2017), avoiding the risk of failing to inject drugs into the CSF cavity (approximately 10%) (Shapiro et al., 1975; Glantz et al., 2010). The use of Ommaya intraventricular therapy resulted in significantly longer OS than that of lumbar puncture (9.2 vs. 4 months, p = 0.0006) (Montes de Oca Delgado et al., 2018). In addition, in patients with solid tumor LMD, the incidence of complications in the intraventricular fluid reservoir is lower (Wang et al., 2016). However, the Ommaya sac needs to be surgically inserted, and common complications include intracranial hemorrhage and catheter translocation and blockage. Overall, IT therapy with Ommaya is recommended as a priority for patients with LMD.
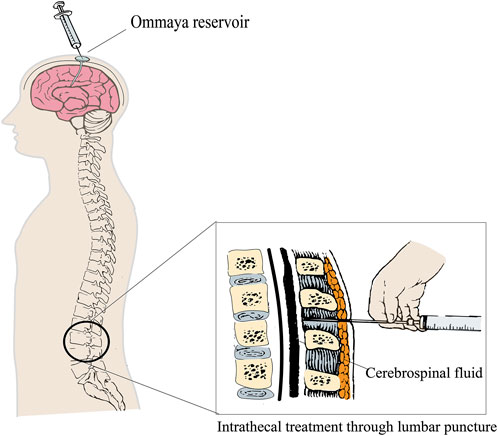
Figure 1. IT via ventricular catheter (Ommaya device) or lumbar puncture.
The most commonly used IT chemotherapy drugs for solid tumor LMD are methotrexate, thiotepa, and cytarabine (Le Rhun et al., 2023). However, no randomized clinical trials comparing any of these drugs with the optimal treatment for systemic diseases and the best supportive care have provided unequivocal evidence of a survival benefit in the treatment of solid tumor LMD (Boogerd et al., 2004). Nevertheless, occasional anecdotal responders and long-term survivors have been observed with IT chemotherapy. With continuous research and new explorations of diverse IT therapies for LMD, apart from chemotherapy drugs, targeted drugs and immunotherapy drugs also represent potential and effective pharmaceutical options. An increasing number of anti-tumor drugs are being recommended for the treatment of LMD. This article reviews the current research landscape and progress of IT anti-tumor drugs for solid tumors with LMD.
2 Standard IT therapeutic drugs2.1 Cytotoxic drugs2.1.1 MethotrexateAs a classic anti-metabolic anti-tumor drug, methotrexate can effectively combat breast cancer and hematological malignancies. As early as 1977, researchers (Bleyer and Dedrick, 1977) recommended methotrexate to treat patients with LMD, extending the survival period from 4 to 9 months. This study also investigated the pharmacokinetics of IT methotrexate, measuring the concentration of methotrexate in the CSF of 76 patients. The concentration of methotrexate in the lumbar CSF decreased in a biphasic metabolic manner with half-lives of 4.5 and 8 h. It is not metabolized within the CSF. The drug is slowly absorbed through the choroid plexus, released into the systemic circulation, partially bound to serum albumin, and then excreted through the kidneys (Rubin et al., 1968).
Several small-scale prospective studies focused on IT methotrexate in patients with solid tumor LMD. These studies included participants ranging from several dozen to one hundred, and the primary tumors were predominantly lung and breast cancer, with CSF cytological remission rates reaching 20%–61%. These studies can validate the therapeutic efficacy of IT methotrexate (Grossman et al., 1993; Siegal et al., 1994; Glantz et al., 1998). IT methotrexate combined with radiotherapy emerges as a promising approach in clinical practice. Multiple studies included patients with solid tumor LMD who underwent IT methotrexate treatment combined with radiotherapy. Such a retrospective study (Wasserstrom et al., 1982) included 90 patients. Approximately half of the patients exhibited improvement in neurological symptoms, with a median OS of 5.8 months (1–29 months). In another retrospective study that enrolled 98 patients who underwent radiotherapy in combination with the standard-dose IT methotrexate, 41 patients were evaluated as having achieved a CR, while 12 patients attained a PR. The median OS ranged from 3 months among breast cancer patients to 8 months for lymphoma patients. Notably, the survival duration of patients who responded to methotrexate was significantly prolonged (Pfeffer et al., 1988). Similarly, a phase-II prospective single-arm clinical study included 59 patients (42 with lung cancer, 11 with breast cancer, and 6 with other) with adverse prognostic factors. The treatment consisted of IT methotrexate (12.5–15 mg) and dexamethasone (5 mg) administered once a week, combined with brain radiotherapy at a dose of 40Gy/20fr. The clinical effective rate was 86.4% (51/59), and the median OS was 6.5 months (0.4–36.7 months). This study is the first to preliminarily explore, through prospective clinical research, that the treatment regimen of IT methotrexate combined with regional radiotherapy is highly effective for solid tumor leptomeningeal carcinomatosis, which can improve the quality of life of patients, alleviate neurological symptoms, and prolong survival (Pan et al., 2016). The abovementioned studies indicate that the treatment mode of IT methotrexate and synchronous regional radiotherapy has significant benefits for patients with LMD.
A common dosage regimen for IT methotrexate therapy involves induction treatment with two injections per week for 4 weeks, followed by consolidation treatment with one injection per week for 4 weeks, and maintenance therapy with one injection per month until disease progression (Shapiro et al., 1975; Le Rhun et al., 2023). However, the dosages of IT methotrexate in the current literature are not consistent, with most doses varying between 10 and 15 mg. Hou et al. (2021) conducted a prospective single-arm clinical trial to investigate the clinical efficacy and safety of different doses of IT methotrexate in the treatment of cancerous meningitis. The study encompassed 53 patients, who were randomly assigned to a 15-mg dose group or a 10-mg dose group. The results showed that the median OS of the 15-mg dose group was 15.7 weeks, while that of the 10-mg dose group was 27.1 weeks, with no statistically significant difference (p = 0.116). Increasing the dosage of IT methotrexate appropriately did not prolong OS, but it was more effective in alleviating clinical symptoms and did not increase the incidence of adverse events. Continuous exposure to low concentrations of methotrexate throughout the body can lead to severe bone marrow suppression. Patients with renal insufficiency, pleural effusion or ascites, and abnormal CSF flow are at an increased risk of severe bone marrow suppression. IT methotrexate treatment can cause various neurological complications, including chemical meningitis, delayed white matter encephalopathy, acute encephalopathy, and transverse myelopathy (Boogerd et al., 2004; Stemmler et al., 2008; Pan et al., 2016). In order to minimize the risk of systemic methotrexate toxicity, all patients should be given oral folic acid (10 mg once, twice a day, for 3 days) simultaneously as a routine to reduce the systemic toxic reactions caused by methotrexate leaving the CSF and entering the bloodstream. Methotrexate, one of the classic IT chemotherapy drugs, has high efficacy in patients with solid tumor LMD. IT or combined radiation therapy can improve the quality of life of patients, prolong survival, and have good safety.
2.1.2 ThiotepaThiotepa is an ethyleneimine alkylating agent that forms unstable ethylimine groups under physiological conditions and has strong cytotoxic effects. As a multifunctional alkylating agent, it inhibits nucleic acid synthesis, crosslinks with DNA, and is effective against ovarian, breast, bladder, and digestive tract cancers (Maanen et al., 2000). Thiotepa is a small-molecule, non-polar, highly lipid-soluble drug with good tissue infiltration. It has the shortest half-life among IT chemotherapy drugs. After IT therapy, it can quickly diffuse out of the CSF within 4 hours without being distributed to the CSF space or brain tissue, and it can be cleared through cerebral capillaries. A patient received IT 10 mg of thiotepa. The peak concentration of the CSF in the ventricles was >100 μg/mL, approximately 10 μg/mL after 2 hours, and approximately 1 μg/mL after 8 hours. The average clearance rate of the CSF was 1.8 mL/min, and the average systemic clearance rate was 518 mL/min (Strong et al., 1986).
The efficacy of IT thiotepa is not as clear as that of methotrexate. The typical dosage is 10 mg. The induction regimen entails two injections per week for a period of 4 weeks. Subsequently, the consolidation regimen involves one injection per week for 4 weeks. Thereafter, the maintenance regimen entails one injection per month until the disease progresses. If required, one injection per week can be implemented (Le Rhun et al., 2023). In most studies, thiotepa was administered at a dose of 10–15 mg per dose twice a week or continuously for 3 days per month (Chamberlain and Kormanik, 1997; Witham et al., 1999; Schrappe et al., 2000; Cho et al., 2015). As early as 1976, a phase I–II study on IT thiotepa was conducted in LMD patients with malignant tumors. The study involved 11 patients with meningeal leukemia, lymphoma, and ependymoma who received IT thiotepa at a dose of 1–10 mg/m2. Three patients achieved CR of the CNS symptoms, while five patients achieved PR. No hematological toxicity related to thiotepa was observed in this study, and neurotoxicity was limited to mild transient lower limb toxicity. This study preliminarily confirms the effectiveness and safety of IT thiotepa in patients with LMD (Gutin et al., 1976). Another multi-center randomized trial compared IT thiotepa and methotrexate in 59 patients with LMD, including breast cancer (48%), lung cancer (23%), lymphoma (19%), and other tumors (10%). It is important to mention that in this trial, the results showed that approximately one-third of patients in both groups achieved complete cytological clearance, and the median OS was similar between the groups (14 vs. 16 weeks) (Grossman et al., 1993). In order to further verify whether IT thiotepa has efficacy in breast cancer patients, a single-center retrospective study included 66 breast cancer LMD patients who were given IT thiotepa (10 mg) and methylprednisolone (40 mg) once every 2 weeks. This study reported comparable results; 16 out of the 36 evaluable patients had cytological remission, and the median OS was 4.5 months (Comte et al., 2013). This study confirmed that LMD patients with breast cancer had a good response to thiotepa. The selection of IT drugs following the failure of methotrexate treatment has always been a research focus. IT thiotepa appears to have good efficacy in LMD cases after methotrexate treatment failure. Cho et al. (2015) evaluated 398 patients with LMD of non-small-cell lung cancer (NSCLC) and breast cancer who were treated with IT therapy based on methotrexate. According to the standard treatment scheme, they received 10 mg of thiotepa twice a week for the rescue treatment of patients after the failure of methotrexate. Among the 30 patients who could be evaluated, 14 (47%) had cytological reactions to thiotepa in the CSF, and the median OS after the treatment with thiotepa was 19.4 weeks. This study confirmed that patients who failed methotrexate treatment could choose to use thiotepa for rescue treatment with good effectiveness.
IT administration of thiotepa is often well-tolerated and may cause systemic bone marrow suppression (Grossman et al., 1993). Neurotoxicity is limited to mild, transient lower-limb sensory abnormalities, and there have been occasional reports of spinal cord disease. As a classic IT chemotherapy drug such as methotrexate, thiotepa has good safety and effectiveness for LMD. At the same time, it is also a meaningful salvage treatment for patients with LMD who have progressed after methotrexate treatment.
2.1.3 Cytarabine and cytarabine liposomeThe structure of cytarabine is very similar to that of deoxycytidine, one of the components of DNA synthesis, which can interfere with DNA replication and kill tumor cells (Pace and Fabi, 2006). Cytarabine is mainly used for induction remission and maintenance therapy of acute non-lymphocytic leukemia in adults and children, and it is only effective for a small number of solid tumor patients. For solid tumors, such as lung and breast cancer, if the tumor has invaded the CSF or meninges, cytarabine can be used for IT therapy. The results were reported by Esteva et al. (2000) in seven patients with LMD from breast cancer, who were administered IT cytarabine three times in a single dose of 100 mg at 48-h intervals. The authors reported a mean (±SD) peak cytarabine concentration of 16.7 ± 6.3 mmol/L and a mean concentration of 0.77 ± 0.53 mmol/L after 6 h. The cytarabine concentration in the CSF decreased in a monophasic pattern with a concentration half-life of 1.45 ± 0.61 h. The accumulation of cytarabine in the CSF between the repeated injections was not observed, and cytarabine was not detectable in the plasma. The half-life of cytarabine in the CSF is less than 4 h, and it is completely cleared within 1–2 days (Fulton et al., 1982).
Cytarabine dosage is 25–100 mg, with an induction phase of two injections per week for 4 weeks, a consolidation phase of one injection per week for 4 weeks, and a maintenance phase of one injection per month until disease progression (Le Rhun et al., 2023). The researchers tested IT cytarabine in 32 patients with neoplastic meningitis from malignant gliomas and solid tumors (Fulton et al., 1982). The researchers administered cytarabine in two doses within just 1 week to the first group, which consisted of 20 patients, and 14 of these patients also received concurrent chemotherapy. All four patients exhibited clinical improvement. Among them, two patients remained alive for 8 weeks and 16 weeks, respectively, after the commencement of weekly cytarabine treatment. In another case, five patients demonstrated clinical improvement, and four of them were still alive without tumor recurrence at 26 weeks after the initiation of therapy. Although no distinct difference was observed between the two groups, it should be noted that these groups were not comparable in terms of prognostically significant clinical characteristics during diagnosis without recurrence. In the second group, three out of twelve patients were administered three consecutive doses. Shapiro et al. (2006) reported a series of 67 patients suffering from neoplastic meningitis caused by solid tumors, leukemia, and lymphomas. These patients received methotrexate or cytarabine alone or a combination of methotrexate and cytarabine through an intraventricular reservoir with or without CNS irradiation. Among these patients, 11 individuals underwent combination therapy with both drugs. Clinical improvement was observed in 58% of the patients with solid-tumor-related neoplastic meningitis. The median survival time for patients with breast cancer was 5 months. The above-mentioned research indicates that acute toxic effects associated with IT cytarabine included meningismus, nausea, vomiting, and myelosuppression.
Cytarabine liposome is a multi-vesicular system sustained-release liposome composition of cytarabine prepared using the sustained-release liposome platform. This nanostructure can encapsulate a large amount of drug and prolong the drug release time. It has been proven that the use of cytarabine liposome can delay the release of cytarabine, with a duration ranging from several days to several weeks and a longer half-life of over 80 h. Cytarabine liposome (50 mg) is administered intravenously every 2 weeks for LMD, with the number of IT does being only a quarter of that in conventional treatment (Jaeckle et al., 2001; Cole et al., 2003; Fusco et al., 2013). An open-label study (Phuphanich et al., 2007) investigated IT (50 mg) cytarabine liposome twice with a 14-day interval, analyzing the levels of cytarabine and metabolites in CSF and plasma samples. The results showed that the concentration range of free and encapsulated cytarabine in ventricular and lumbar CSF was 0.01–1,500 μg/mL, and it could be detected within 14 days. Occasionally, free cytarabine was detected in the plasma, and the concentrations of cytarabine metabolites were low in all samples.
To compare the efficacy of cytarabine liposome and methotrexate in patients with LMD, a randomized controlled trial was conducted, enrolling 61 patients with histologically confirmed cancer and positive CSF cytology. They were randomly divided into an IT cytarabine liposome group (31 patients) or a methotrexate group (30 patients). Patients received up to six doses of 50 mg cytarabine liposome or up to 16 doses of 10 mg methotrexate within 3 months. The trial results showed that in patients with LMD, cytarabine liposome was as effective as methotrexate and significantly increased the time of no progression in the nervous system while reducing the number of doses administered, providing convenience for patients (Glantz et al., 1999a). Another multi-center, randomized, controlled study also compared the benefits and safety of IT cytarabine liposome, methotrexate, and cytarabine in patients with solid tumor LMD (n = 103). The results showed that the benefits of cytarabine liposome and methotrexate in treating solid tumor LMD were comparable (Shapiro et al., 2006). These clinical trials encompassed a variety of cancer types, and the majority of the patients were non-primary cases. DEPOSEIN (NCT01645839) (Le Rhun et al., 2020) is a randomized open-label phase-III study that discusses the effectiveness and safety of IT cytarabine liposome combined with systemic therapy (n = 36) versus systemic therapy (n = 37) for patients with LMD from breast cancer at initial diagnosis. The study results indicate that the cytarabine liposome group significantly improved median PFS (3.8 vs. 2.2 months) and median OS (7.3 vs. 4 months) in patients with LMD. This study confirmed the role of IT cytarabine liposome in LMD patients with breast cancer. Cytarabine liposome, as a sustained-release liposome composition with a polycystic vesicle system, can encapsulate more drugs in its nanostructure, thereby reducing the administration time and increasing patient convenience. Clinical trials have also confirmed that for patients with solid tumor LMD, cytarabine liposome is as effective as methotrexate, which may be a good choice. Multiple studies have shown that the main adverse events of IT cytarabine liposome are headache and arachnoiditis. To prevent chemical meningitis caused by IT chemotherapy, each patient needs to be pretreated with dexamethasone.
2.1.4 EtoposideEtoposide acts on DNA topoisomerase II, forming a stable and reversible drug–enzyme–DNA complex that impedes DNA repair. Etoposide is mainly used for treating small-cell lung cancer (SCLC), malignant lymphoma, and malignant germ cell tumors (Issell and Crooke, 1979). The systemic administration of etoposide is effective in treating metastatic, recurrent, or refractory brain tumors, but its permeability to CSF is extremely low, almost not entering the CSF (<3%). Even after high-dose intravenous injection of etoposide, the CSF concentration does not exceed 0.1–0.5 μg/mL (Postmus et al., 1984; Kiya et al., 1992). The in vitro experimental results show that etoposide at concentrations ranging from 0.1 to 10 μg/mL exhibits cytotoxicity (Henwood and Brogden, 1990). IT etoposide can directly penetrate the BBB, and low-dose medication can lead to higher drug concentrations in the CSF. In a published case report (van der Gaast et al., 1992), IT (0.5 mg) etoposide was administered twice daily, with a 2-hour interval, for 5 days, followed by a second course of treatment 21 days later. Two hours after daily administration, the peak concentrations of the CSF in the ventricles were 3.6–5.2 μg/mL, and the trough concentrations of the CSF in the ventricles were 0.2–0.6 μg/mL. Another study reported that the peak concentration of etoposide in the CSF after IT administration exceeded that of intravenous infusion by more than 100 times. The steady-state distribution volume and total clearance rate showed significant individual differences, with a double exponential decrease. Etoposide was not detected in the plasma (Fleischhack et al., 2001).
Slavc et al. (2003) evaluated the long-term feasibility of IT etoposide in 11 children with disseminated non-hematological metastatic brain tumors. Two patients received IT mafosfamide before switching to intraventricular etoposide treatment, and nine patients received alternating IT etoposide and mafosfamide treatment. The dose of etoposide was 0.5 mg × 5 days, once every 3–6 weeks, for a total of 122 courses (1–29 courses/patient) until disease progression. The median time of etoposide treatment was 12 months (1–31 months), and the disease remission rate among the 11 patients was 54.5% (6/11). No long-term toxicity that could be attributed to IT therapy was observed. This study confirms that IT etoposide is both safe and effective for patients over 2 years old with LMD. In order to further validate the efficacy of IT etoposide in LMD of adult patients, another phase-II clinical trial (Chamberlain et al., 2006) was carried out, which included 27 adult patients (the median age was 55 years old); IT etoposide 0.5 mg per day was administered once every 5 days for 8 weeks to induce treatment. At the end of induction treatment, patients were evaluated through CSF cytology and neurological examination. Patients who had responded continued to receive etoposide treatment (every 4 weeks for 5 days) and undergo monthly evaluations. Among the 27 patients receiving etoposide treatment, 7 (26%) had stable or improved neurological status and 12 (44%) had no response to treatment. Among the patients who responded to treatment, the median PFS was 20 weeks (ranging from 8 to 20 weeks), and the 6-month PFS rate for neurological disease was 11%. For newly diagnosed LMD patients, the median OS is 10 weeks (4–52 weeks), and the survival probabilities at 3 months, 6 months, and 12 months are 30% (95% CI, 12%–47%), 11% (95% CI, 1%–21%), and 4% (95% CI, 0%–11%), respectively. During the treatment, five patients experienced transient chemical arachnoiditis, and no hematological toxicity related to etoposide was observed. The abovementioned report evaluated the efficacy of IT etoposide in the treatment of LMD, and patients demonstrated good tolerance to this treatment (Fleischhack et al., 2001; Slavc et al., 2003; Chamberlain et al., 2006). Based on the results of previous clinical trials on the efficacy of IT etoposide, Park (2015) first used IT etoposide to rescue a patient with NSCLC who failed first-line methotrexate treatment. The patient received 1 mg of etoposide intravenously once a week, and after 2 weeks of treatment, the brain lesion and clinical symptoms improved. However, the patient ultimately died due to liver metastasis. Unlike other studies, this study designed a weekly regimen of etoposide administration to rescue patients who failed methotrexate treatment, providing preliminary evidence for the feasibility of etoposide rescue therapy. In recent years, there has been relatively little research on IT etoposide. The safety of IT etoposide in children and young adults with refractory or recurrent malignant brain tumors was investigated (Pajtler et al., 2016). The research results showed that IT etoposide was well-tolerated, and the potential treatment-related adverse reactions included transient headache, epileptic seizures, nausea, and neuropsychological symptoms. No hematological side effects were observed.
2.1.5 TopotecanTopotecan is a topoisomerase I inhibitor with good therapeutic effects on various solid tumors in adults and children. The hydrolysis of topotecan lactone is pH-dependent, and the open carboxylate form as topoisomerase-I inhibitor has no activity. When topotecan is administered intravenously, approximately 30% reaches the CNS. After IT topotecan (0.4 mg) administration, the peak concentration of cerebroventricular CSF was 28 ± 11 μmol/L, and it was not detected in the plasma. The clearance of topotecan is mainly achieved through its conversion to inactive forms and CSF circulation, with only approximately 10% cleared through microvasculature (Stewart et al., 1994; Blaney et al., 2003; Glaberman et al., 2005).
A phase-I clinical trial (Blaney et al., 2003) enrolled 23 patients aged 3 years and above with tumor-associated LMD, who received IT topotecan to determine the optimal dose and dose-limiting toxicity. The study determined a maximum tolerable dose of 0.4 mg through a dose ramp-up test, and chemical subarachnoid inflammation was the dose-limiting toxicity that occurred within 24 h of administration, which was characterized by fever, nausea, vomiting, headache, and back pain. Among 23 assessable patients, six patients had stable disease, providing the optimal dosage for phase-II clinical trials. Another phase-II, open-label, non-randomized single-arm trial (Groves et al., 2008) included 62 patients with malignant LMD who received IT topotecan (0.4 mg) twice a week for 6 weeks. Primary cancers included breast cancer (n = 19), lung cancer (n = 13), CNS cancer (n = 14), and other cancers (n = 16). The estimated PFS rates at weeks 13 and 26 were 30% (20%–45%) and 19% (11%–34%), respectively. The median OS is 15 weeks (13–24 weeks). The most common side effect was chemical meningitis, which occurred in 32% of the patients (5% grade 3). IT topotecan was well-tolerated and had no obvious side effects. However, topotecan does not have significant advantages over other IT chemotherapy options. It can be combined with radiotherapy or systemic treatment to maximize the benefits for patients. Researchers treated two patients with recurrent or progressive CNS cancer with LMD using IT topotecan (0.2 mg/day) for 7 days in order to increase the exposure of topotecan in the CSF as much as possible (Tran et al., 2014). After administration, neither patient showed hematological toxicity or arachnoiditis that was grade 3 or above. Although this study is a case report, it offers a novel approach to individualized treatment options in clinical practice. In the latest study by Yamada et al. (2023), high-dose chemotherapy combined with stem cell rescue, followed by IT topotecan maintenance therapy, was used to treat six children with atypical rhabdomyoma. This treatment model successfully avoided whole-brain radiation therapy and prolonged their survival. IT topotecan maintenance therapy can improve CNS symptoms. The sample size of this study is small, and further large-scale research is needed to confirm this treatment model. IT topotecan is safe, with the most common adverse reactions being nausea, vomiting, headache, anorexia, and fever. Arachnoiditis is dose-limited toxicity, and no hematological toxicity was observed. Although topotecan does not have significant advantages over other IT chemotherapies, it can also be used as one of the drug choices for solid tumor LMD patients.
2.1.6 PemetrexedPemetrexed, as an anti-metabolic antitumor drug, is a first-line chemotherapy drug for NSCLC and exhibits good cytotoxicity. The incidence of NSCLC-LMD reaches as high as 5.0%–15.8% (Kwon et al., 2020). The pharmacokinetics of IT pemetrexed was investigated (Li et al., 2023). After IT 30 mg of pemetrexed was administered, a series of CSF samples were collected from eight patients, with 62.5% (5/8) reaching peak concentrations within 2–4 h, and the effective concentration level of pemetrexed in the CSF remained at an average of 93.44 μg/mL after 24 h of IT pemetrexed administration. The research results showed that pemetrexed exhibited a biphasic elimination mode in the CSF, while its concentration in the blood was extremely low.
To determine the efficacy of IT pemetrexed in lung adenocarcinoma patients with LMD who had undergone at least two previous treatments, a prospective phase-I study enrolled 23 lung adenocarcinoma LMD patients with failed multi-line treatment. According to the dose ramp-up test, IT pemetrexed at a dose of 30 mg was considered the optimal dose, with a median PFS of 6.3 months (ranging from 0.8 months to data not available (NA)) and a median OS of 9.5 months (ranging from 2.9 months to NA), respectively. In 2019, a study was reported on the use of IT pemetrexed for the salvage treatment of NSCLC-LMD, which was the world’s first phase-I clinical trial. The research results showed that the clinical effective rate of IT 10 mg pemetrexed was 31% (4/13), the disease control rate was 54% (7/13), and it had good tolerability. Subsequently, Pan et al. (2020) conducted a phase-I/II clinical study of IT pemetrexed and concurrent radiotherapy for solid tumor LMD. Based on the combination of affected field radiotherapy, the clinical effective rate of 10 mg pemetrexed was 68% (23/34). The median OS is 5.5 months (0.3–16.6 months). The research results suggest that the combination of IT pemetrexed and radiotherapy is effective for the treatment of LMD. In another prospective, single-center phase-II study conducted in the latest report (Fan et al., 2021), 30 patients with an epithelial growth factor receptor (EGFR) mutation and NSCLC-LMD were included and evaluated with IT pemetrexed and dexamethasone. The results showed a clinical efficacy rate of 84.6% and a median OS of 9 months, confirming that IT 50 mg pemetrexed has a high response rate and few adverse reactions in the treatment. These data are encouraging and offer new effective treatment methods for NSCLC-LMD patients. Multiple studies have demonstrated that pemetrexed exhibits good anti-tumor activity in the CSF without significant accumulation in the body. Bone marrow suppression and hepatotoxicity are the most prevalent adverse reactions (Pan et al., 2016; Geng et al., 2022). To reduce adverse reactions to pemetrexed, pre-treatment with oral folate, intramuscular injection of vitamin B12, and IT dexamethasone are equally important. Most studies use the classic dose escalation model to observe the tolerated dose and the relevant dose-limiting toxicity of IT pemetrexed. However, the sample size in these studies is relatively small. The dosages of pemetrexed vary significantly among different studies, and further larger sample studies are needed to further verify safety and effectiveness. Perhaps, after more clinical trials validating the safety and efficacy of IT pemetrexed in lung adenocarcinoma LMD patients, pemetrexed can replace methotrexate as a first-line treatment.
2.2 Targeted drugs2.2.1 TrastuzumabPrevious studies have shown that trastuzumab, whether as a single drug or in combination with chemotherapy, can delay the disease progression and improve the OS of breast cancer patients with human epidermal growth factor receptor 2 (HER-2) positivity (Kak et al., 2015; Malani et al., 2020). However, HER-2-positive breast cancer patients receiving systemic trastuzumab treatment often develop CNS metastasis, with approximately 20% of these cases occurring in LMD (Bendell et al., 2003). One of the reasons for stable or relieved CNS recurrence of systemic diseases may be the high molecular weight (145 kDa) of trastuzumab, which leads to its low CNS penetration rate. Research reported that when trastuzumab was administered intravenously on a weekly basis, there was a 300-fold difference in drug concentration between the patient’s serum and CSF (Pestalozzi and Brignoli, 2000). IT trastuzumab is a new treatment approach for HER-2-positive cancer, especially for patients with LMD from breast cancer. Multiple small-scale studies have shown that trastuzumab 25–150 mg has good stability when dissolved in physiological saline without preservatives (Laufman and Forsthoefel, 2001; Platini et al., 2006; Stemmler et al., 2006; Stemmler et al., 2008; Oliveira et al., 2011; Zagouri et al., 2013). The latest investigation shows that after IT (80 mg) trastuzumab administration, the distribution volume is 73 ± 48 mL, the CSF clearance rate is 14 ± 5 mL/h, and the apparent CSF half-life is 4.1 ± 3.0 h. The CSF half-life is much shorter than the 18–30 days half-life of trastuzumab intravenous administration. After repeated administration, the CSF concentration stabilizes, indicating that trastuzumab will not accumulate to toxic concentrations in the CSF (Kumthekar et al., 2023).
Trastuzumab was initially injected intrathecally for HER-2-overexpressing breast cancer patients. In these reports, patients who had received systemic treatment, IT chemotherapy, whole-brain radiotherapy, neurosurgery, and other treatment methods still did not experience relief. IT trastuzumab was used for rescue treatment. The literature has reported that the dose of IT trastuzumab ranges from 5–160 mg, either twice a week or once every 3 weeks. However, in most studies, the dose range is from 20 to 30 mg per week. Patients’ headaches, mental state, hemiplegia, ataxia, and other disorders were significantly improved. The duration of disease control with IT trastuzumab ranged from 39 days to over 72 months, and researchers observed no serious adverse reactions associated with trastuzumab (Laufman and Forsthoefel, 2001; Platini et al., 2006; Stemmler et al., 2006; Mir et al., 2008; Stemmler et al., 2008; Colozza et al., 2009; Ferrario et al., 2009; Oliveira et al., 2011; Smith et al., 2022). Some researchers believe that the effectiveness of IT trastuzumab seems to be dose-dependent. In the case that had been reported (Ferrario et al., 2009), patients who received IT trastuzumab 30 mg had better clinical benefits than those who received 20 mg. In another report (Mir et al., 2008), the IT dose of trastuzumab was increased to 50 mg in combination with 12 mg of thiotepa, which showed better efficacy than 20–30 mg. In a phase-I clinical trial (Bonneau et al., 2018), based on IT trastuzumab with a CSF concentration close to the conventional treatment plasma concentration (30 mg/L), a dose ramp-up test was conducted, and the maximum tolerable dose was determined to be 150 mg per week. In a large-scale phase-I/II multi-center study (Kumthekar et al., 2023), HER-2-positive histological cancer patients were included in the phase-I study, and the maximum tolerable dose for IT trastuzumab was determined to be 80 mg, twice a week, while ensuring both treatment effectiveness and safety. These studies indicate that the maximum tolerable overall dose per week for IT trastuzumab does not exceed 160 mg, rather than the traditional administration of 20–30 mg.
For patients with HER-2 overexpression in LMD associated with breast cancer, IT trastuzumab provides good benefits. Zagouri et al. (2013) retrospectively summarized and analyzed the effectiveness and safety of IT trastuzumab. A total of 13 articles were included, involving 17 patients with an average age of 48.2 years (38–66 years), and the average total dose was 399.8 mg (35–1,110 mg). With trastuzumab alone or in combination treatment, 88.2% of patients did not report serious adverse events, 68.8% of patients observed significant improvement in clinical symptoms, 31.2% of patients were stable or progressive, and the median OS was 13.5 months. The CNS-PFS is 7.5 months, and the longer CNS-PFS seems to be related to the improvement of clinical symptoms and CSF response. This retrospective study demonstrates the effectiveness and safety of IT trastuzumab. Based on a phase-I dose escalation test (Oberkampf et al., 2023), the study established the recommended dose of IT trastuzumab as 150 mg per week for the phase-II study. A total of 19 patients with LMD and HER-2-overexpressing breast cancer were included. All patients received at least one systemic anti-HER-2 treatment. After 8 weeks of IT trastuzumab administration, 74% (14/19) of patients had no neurological progress, the median LMD-related PFS was 5.9 months, and the median OS was 7.9 months. No adverse reactions of grade 3 or above were observed in all patients. Furthermore, a large-scale phase-I/II multi-center study (Kumthekar et al., 2023) included 23 HER-2-positive cancer patients who received IT trastuzumab 80 mg (twice a week for 4 weeks, then once a week for 4 weeks, followed by maintenance therapy every 1–2 weeks) for treatment. The partial response rate, disease stability rate, and disease progression rate were 19%, 50%, and 30%, respectively. At a median follow-up of 10.5 months, the median PFS and OS were 2.8 months and 10.5 months, respectively, indicating longer survival than that of the recognized historical control (median OS of approximately 3–4 months). In patients receiving treatment with IT trastuzumab, the most common side effects are headache, nausea, vomiting, and meningeal spasms, with only one case of grade-4 toxic reaction (arachnoiditis) found. However, in another meta-analysis (Lazaratos et al., 2023), the total survival period and PFS of IT trastuzumab (92 patients) and oral or intravenous HER-2 targeted therapy alternative route of administration (183 patients) in HER-2-positive breast cancer patients with LMD were compared. In univariate and multivariate analysis, the research results showed that there was no significant difference in OS and CNS-specific PFS between IT trastuzumab and oral or intravenous HER-2 targeted therapy. The study suggests that the reason for this result can be explained as follows: intravenous trastuzumab may reach the subarachnoid space at a sufficient concentration, effectively treating LMD in patients with trastuzumab-sensitive diseases. The patient had already received an intravenous injection of trastuzumab before IT therapy, which may have led to drug resistance. Even for patients receiving whole-brain radiotherapy, the concentration of trastuzumab in CSF is still one order of magnitude lower than the serum concentration (Stemmler et al., 2007; Steeg, 2021). Although this meta-analysis suggests that IT trastuzumab and HER-2 targeted therapy do not offer obvious advantages as an alternative route of administration, IT therapy can quickly alleviate patients’ neurological symptoms, and many clinical trials have shown that it has considerable efficacy and good tolerability for HER-2-positive breast cancer LMD patients. However, most of the enrolled clinical trials involve breast cancer with HER-2 positivity, which may not be applicable to the overall LMD population. Follow-up studies can include more HER-2-overexpressing solid tumor LMD patients. The most common adverse reactions of IT trastuzumab are headache, nausea, vomiting, and meningeal spasms.
2.3 Checkpoint inhibitors2.3.1 NivolumabNivolumab is a programmed cell death protein 1 (PD-1) inhibitor approved for the treatment of NSCLC, head and neck squamous cell carcinoma, and gastric cancer, among others. Nivolumab specifically binds to PD-1 receptors on T cells, preventing signals from reaching T cells from malignant tumor cells and allowing T cells to function normally, thereby exerting anti-tumor effects. In multiple prospective clinical trials, it has been demonstrated to bring long-lasting benefits to patients with metastatic melanoma (Kluger et al., 2019; Tawbi et al., 2021; Wolchok et al., 2022). The intracranial objective response rate (ORR) of nivolumab was 20% in asymptomatic encephalomyelitis patients, 55% in the checkmate-204 trial (n = 101) (Long et al., 2018), and 51% in the ABC trial (n = 35) (Tawbi et al., 2021). In all these studies, the 3-year survival rate of responsive patients exceeded 90% (Tawbi et al., 2021). Therefore, nivolumab may have a significant benefit for patients with melanoma LMD.
For symptomatic patients with melanoma LMD, rapid relief of neurological symptoms is necessary. Compared with interleukin-2, anti PD-1 therapy has improved efficacy and safety. Therefore, experts hypothesized that IT anti PD-1 therapy for melanoma is safe and feasible, and a phase-I clinical study was conducted (Glitza Oliva et al., 2023). First, the toxicity of IT administration of anti-PD-1 was evaluated in an immunocompetent mouse model. Then, a first-ever human dose exploration study was designed using both IT and intravenous administration of anti-PD-1. A total of 25 melanoma LMD patients were included and treated with a dual-pronged approach, injecting nivolumab into two sites: IT 50 mg every 2 weeks to treat meningeal metastases and intravenous 240 mg to treat extra brain lesions. The median OS of these patients was 4.9 months, with 44% and 26% of patients surviving over 26 and 52 weeks, respectively. Notably, four patients survived for 74 weeks, 115 weeks, 136 weeks, and 143 weeks after receiving the first IT nivolumab dose, which far exceeded the expected survival period for patients with LMD after multiple treatments. The most common grade-1 or grade-2 adverse events in the study were nausea (36%), diarrhea (24%), decreased lymphocyte count (24%), elevated aspartate aminotransferase and/or alanine aminotransferase (24%), and rashes (24%). Patients who used IT nivolumab alone did not show grade 3 or higher toxicity. This is the first clinical study that has utilized two different routes of administration for nivolumab therapy in patients with LMD secondary to melanoma. This study provides a detailed explanation of the dose escalation, recommended IT dosage, safety profile, and preliminary efficacy of synchronous IT and intravenous administration of nivolumab in melanoma LMD patients, supporting the feasibility of prospective clinical trials in LMD melanoma patients. At the same time, this trial is the largest prospective clinical trial of IT immunotherapy among all types of cancers, and it is also the first systematic evaluation of the application of IT anti-PD-1 drugs. Although this study is focused on melanoma patients, it also holds reference significance for other types of tumors. This study represents an important step forward and a new treatment method for patients with LMD. Perhaps, in the future, IT therapy will no longer be limited to chemotherapy drugs such as methotrexate but could also be combined with PD-1 inhibitors.
3 Potential IT drugs3.1 Targeted drugs3.1.1 NimotuzumabEGFR is a transmembrane glycoprotein with a molecular weight of 170 kD, and its intracellular region has special tyrosine kinase activity. In vivo and in vitro studies have shown that nimotuzumab can block the binding of EGFR and its ligand, and it has anti-angiogenic, anti-proliferative, and pro-apoptotic effects on tumors overexpressing EGFR (Crombet-Ramos et al., 2002). Nimotuzumab is suitable for combined treatment with radiotherapy for stage-III/IV nasopharyngeal carcinoma with positive EGFR expression. The efficacy of IT nimotuzumab in the treatment of solid tumor LMD may be related to the antibody-dependent cell-mediated cytotoxicity (ADCC) induced by nimotuzumab, and the effect of ADCC is usually observed 1–3 months after IT treatment. There are relatively few studies on IT nimotuzumab, with only two relevant reports. One of the reports (Ju et al., 2016a) included 20 patients with NSCLC-LMD who received IT nimotuzumab 50 mg per week. The average number of IT therapy sessions was 8.9 (range: 1–35), and the median OS after treatment was 5 months (2.4–7.6 months). The average CSF open pressure decreased from 270 mmH2O (202–338 mmH2O) before treatment to 140 mmH2O (127–153 mmH2O) (p < 0.001). All patients experienced LMD-related symptoms, especially headaches, in the nimotuzumab group. After IT nimotuzumab, all patients were relieved, and their quality of life improved. In the same year, another case report was published by Ju et al. (2016b), in which two patients were diagnosed with NSCLC-LMD. The first patient received IT nimotuzumab weekly at the dose of 25 mg, with an OS of 35 months after the diagnosis of LMD. The second patient received 50 mg, with an OS of 12.5 months. Both patients received systemic treatment before IT therapy. After 2–3 sessions of IT treatment, the neurological symptoms of both patients were significantly improved, and the opening pressure of CSF decreased. Neither of these reports mentioned the adverse reactions of IT nimotuzumab. There are relatively few studies on IT nimotuzumab, but it can also provide preliminary evidence of its effectiveness and safety. In the future, more research and larger sample sizes are needed for further verification.
3.1.2 BevacizumabBevacizumab is the first anti-tumor angiogenesis-targeting drug approved for marketing. It plays an anti-tumor role by specifically targeting vascular endothelial growth factors (VEGF), and it has definite efficacy in a variety of malignant tumors. At present, it has been approved for a variety of indications, including metastatic colorectal cancer and advanced/metastatic or recurrent NSCLC (Cao, 2008). Angiogenesis in solid tumor brain metastases occurs through multiple pathways, among which the VEGF-mediated pathway is the most important and extensively studied one. Research has confirmed that brain metastasis relies on VEGF, and the high expression of VEGF in brain metastases is associated with poor prognosis (Hanibuchi et al., 2014; Ebben and Ming, 2016). Basic studies have shown that bevacizumab can reach high concentrations in brain metastases, exerting its anti-angiogenic effect and significantly inhibiting the proliferation of brain metastases (Ilhan-Mutlu et al., 2016). Numerous studies have confirmed that bevacizumab can effectively inhibit the development of LMD in lung cancer patients without increasing the risk of cerebral hemorrhage (Besse et al., 2015; Gubens et al., 2018). The meta-analysis showed that in NSCLC patients with LMD, the ORR and disease control rate (DCR) of the anti-tumor treatment regimen with bevacizumab were significantly better than those of the current therapy. Patients treated with bevacizumab have better intracranial DCR and PFS than those treated extracranially (Liang et al., 2020). Bevacizumab can reduce the risk of new-onset LMD by 70%, indicating its potential to prevent brain metastasis, and the risk of intracranial hemorrhage caused by bevacizumab treatment is lower. The abovementioned research provides a theoretical basis for IT bevacizumab in the treatment of solid tumor LMD patients. There is relatively scant research on IT bevacizumab. In the experimental group, four New Zealand white rabbits were randomly selected to receive IT bevacizumab at a dose of 1.5 mg per week, while four rabbits in the control group received IT physiological saline for 4 weeks, aiming to explore the safety of IT bevacizumab. The experimental results indicate that all rabbits in the experimental group can tolerate repeated IT bevacizumab, and there is no alteration in the baseline neurological function state of the control group. In addition, no adverse reactions were observed. IT bevacizumab has no clinical or pathological neurotoxicity, and this neurotoxicity study provides safety data for IT bevacizumab (Brastianos et al., 2012). The first use of IT bevacizumab in humans was for the treatment of LMD in a glioblastoma patient (Holdaway et al., 2023). The 19-year-old female patient had a recurrence of glioblastoma in the thalamus and received two arterial infusions of bevacizumab. The disease remained relatively stable until it progressed in 2022. Magnetic resonance imaging then showed glioblastoma with LMD. Subsequently, five doses of IT bevacizumab at 25 mg, 37.5 mg, 50 mg, 50 mg, and 37.8 mg were administered, with a two-week interval between the first and fourth doses. As the patient had good tolerance and requested discharge, the interval between the fifth and fourth doses was only 1 day, thus reducing the dosage of the fifth dose. Throughout the treatment period, the patient had good tolerance and did not experience any serious adverse reactions or dose-limiting toxicity. After the first IT bevacizumab, the patient survived for 110 days. Due to the rapid progression of glioblastoma with LMD, the patient’s PFS could not be determined. Although the survival benefits of the patient could not be evaluated, the research team preliminarily verified the effectiveness and safety of IT bevacizumab through dose escalation experiments. IT bevacizumab may have good application prospects in solid tumor LMD.
3.2 Checkpoint inhibitors3.2.1 PembrolizumabPembrolizumab, a humanized macromolecular monoclonal antibody, can selectively bind to PD-1 immune checkpoints on the surfaces of immune cells. It can accurately block the interaction between PD-1 and programmed death ligand 1 (PD-L1), relieve immune suppression mediated by the PD-1 pathway, promote T-cell proliferation and cytokine generation, and exert anti-tumor effects. It is approved for use in various solid tumors such as melanoma, NSCLC, esophageal cancer, and head and neck squamous cell carcinoma. Multiple studies have shown that systemic administration of pembrolizumab has good efficacy and safety for solid tumor LMD (Hendriks et al., 2019; Brastianos et al., 2020; Naidoo et al., 2021; Zheng et al., 2021). A phase-II clinical study included 13 patients with solid tumor LMD who received intravenous 200 mg pembrolizumab once every 3 weeks (Naidoo et al., 2021). The results showed that at 12 weeks, 38% of patients exhibited a CNS response (95% CI: 13.9%–68.4%). The median CNS-PFS and OS were 2.9 months (95% CI: 1.3-NA) and 4.9 months (95% CI: 3.7-NA), respectively. Only 15% of patients experienced treatment-related adverse events of grade 3 or higher.
Pembrolizumab has a limited amount of drug reaching the CSF through the BBB during systemic administration. A case report described a patient with triple-negative breast cancer who developed LMD following systemic administration of pembrolizumab and subsequently received IT pembrolizumab (Ensign et al., 2021). The patient was diagnosed with triple-negative breast cancer. After 2 years of systemic treatment, brain metastases occurred. Pembrolizumab was administered intravenously and combined with local radiotherapy for the brain. One year later, the patient developed LMD accompanied by cerebral edema, and two cycles of high-dose systemic methotrexate were given to control the neurological symptoms. However, imaging examinations indicated the progression of the CNS disease. Subsequently, 5 mg of pembrolizumab was injected intrathecally once every 3 weeks. During the first IT therapy, the patient did not have any adverse reactions. However, after the second administration, the patient experienced pain, weakness, nausea, and diplopia. Eventually, the patient died 9 weeks after receiving IT therapy. Coincidentally, the latest published study also reported the effects of IT pembrolizumab on a melanoma LMD patient (Dan et al., 2024). The patient was diagnosed with cutaneous melanoma, and genetic testing showed BRAF V600E mutation, PD-L1-positive expression, and a combined positive score of 10. After being diagnosed with LMD, the patient received whole-brain radiation therapy combined with targeted therapy and immunotherapy for 2 months, and the patient experienced disease progression. In May of the same year, the patient developed paraplegia, spinal hyperalgesia, nausea, vomiting, and headache, but the CSF cell pathology results were negative. The research group adjusted the anti-tumor regimen to 25 mg of pembrolizumab via IT injection and 175 mg via intravenous injection and continued with targeted therapy. No adverse events were reported after the first IT administration. The patient’s symptoms of nausea, vomiting, and headache were relieved, and muscle strength in both lower limbs improved. The patient then received the second IT pembrolizumab, but the symptoms did not improve significantly, and intermittent breathing and cardiac arrest occurred. Ultimately, the patient died of respiratory arrest 2 weeks after receiving IT pembrolizumab. The abovementioned reports suggest that it is not possible to draw a conclusion from case studies that IT pembrolizumab benefits patients with LMD, but it may help temporarily alleviate neurological symptoms. The findings of the abovementioned studies provide a new exploration direction for the administration of pembrolizumab; further studies using IT pembrolizumab are required before any therapeutic treatment decisions can be made concerning its efficacy and safety in the treatment of LMD.
3.3 Other immunotherapeutic drugs3.3.1 Chimeric antigen receptor natural killerNumerous studies have shown that depletion of the natural killer (NK) cell population before tumor transplantation induces a more aggressive phenotype in metastatic tumors. The presence of a large number of tumor-infiltrating NK cells is associated with a good prognosis. NK cells play an important role in brain metastases and meningiomas, which have become the basis for the development of NK cell-based brain tumor therapies (Avril et al., 2012; Poli et al., 2013; Blaylock, 2015). Chimeric antigen receptor natural killer (CAR-NK) cell therapy is an innovative adoptive cell therapy that, through genetic engineering modification, connects antibodies or receptors that recognize target cell surface antigens with signaling molecules required to activate immune cells, thereby activating NK cells and allowing them to specifically attack tumor cells (Pan et al., 2022). As early as 2004, clinical trials were conducted using NK cells to treat recurrent malignant gliomas, and four out of nine patients who received treatment experienced tumor regression (Ishikawa et al., 2004). At present, there are no large-scale clinical trials reporting the effectiveness of NK cells in treating brain tumors or solid tumor LMD. Researchers first injected CAR-NK cells derived from umbilical cord blood intrathecally into T-cell acute lymphoblastic leukemia patients who experienced symptoms of CNS after receiving stem cell transplantation (Yuan et al., 2023). After a short period of infusion, the patient developed neurological symptoms including high fever, headache, nausea, vomiting, and spinal cord transection incontinence. However, the symptoms of the patient, including upper eyelid ptosis and blurred vision, were completely improved. After 9 months of IT CAR-NK cells, the patient’s bone marrow was completely chimeric with the donor. Although IT CAR-NK cells have certain neurotoxicity, adverse reactions can be reduced by increasing the dose of CAR-NK cells administered, increasing the number of IT, and improving the preparation process of CAR-NK cells. A phase-I clinical trial based on CAR-NK (NCT03383978) is currently underway, using IT NK-92/5.28.z CAR-NK to treat leptomeningeal spread caused by recurrent HER-2-positive glioblastoma. The abovementioned evidence suggests that IT CAR-NK cells may be a potentially feasible and effective option for treating tumor LMD in the future. For patients with solid tumor LMD, the BBB is a challenge faced by CAR-NK, including all macromolecular drugs. At present, changing the administration method, such as local delivery of NK cell therapeutic drugs through intraventricular and/or IT pathways, can increase drug concentration in the ventricular CSF, adjust NK cell distribution, and further kill tumor cells. However, further research and clinical trials are needed to verify the efficacy of IT NK cells.
4 Conclusions and future perspectivesIT anti-tumor drugs are a common method for treating LMD. IT chemotherapy drugs and targeted and immunotherapy drugs are summarized in Tables 1, 2, respectively. Based on current clinical data of antineoplastic drugs in patients with solid tumor LMD, methotrexate, thiotepa, cytarabine, and cytarabine liposomes are recommended by the ESMO (L
留言 (0)