Coronavirus disease 2019 (COVID-19), a highly contagious multisystem disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, has been reported as an unprecedented threat to the human population (1). As a global pandemic between February 2020 and May 2023, COVID-19 resulted in 776 million cases and 7.07 million deaths reported by the World Health Organization (WHO) to date, and the percentage of samples testing positive for SARS-CoV-2 is now approximately 9.85%(world, week 17 November 2024); meanwhile, the 28-day prevalence of various variants of SARS-CoV-2 ranged from 0.22% to 53.59% (2). SARS-CoV-2 has been confirmed to infect host cells predominantly through the binding of its spike (S) protein to the angiotensin-converting enzyme 2 (ACE2) receptor, which is expressed at high levels in many tissues (3–6).
Neuroinflammation, which is mediated by increases in cytokines and chemokines, reactive oxygen species (ROS) and second messenger lipids produced by astrocytes and microglia, and endothelial and peripheral immune cells (7), has been shown to significantly affect cognition and behaviour in animal models (8, 9). The persistent activation of microglia and other immune cells within the central nervous system (CNS) has a potentially damaging effect on chronic neuroinflammation (10, 11). In a few reports, this type of autoimmune response in patients with COVID-19 is considered to involve acute CNS diseases and conditions, such as encephalitis, meningitis, acute necrotizing myelitis, acute disseminated encephalomyelitis, acute ischaemic stroke, and chorea (12–19). Two years of pandemic experience have shown that approximately 13% of infected patients suffer from severe neurological complications (20). In a prospective tertiary centre cohort with a 3-month follow-up of 61 COVID-19 patients, more than forty central or peripheral nervous system complications were identified among 28 patients of them(45.9%), and the most common central nervous system complication was encephalopathy(n=19, 31.1%) (21). Some histological studies have shown that neuroinflammation, characterized by retinal glial cell proliferation and T-cell infiltration, parallels ongoing neurodegeneration in space and time (22–24). Neuroinflammation is considered an important pathogenic factor of neurodegenerative lesions (25).
Some patients have shown ocular symptoms as their primary symptoms or developed ocular complications, and there has been a significant increasing trend in the number of patients with ophthalmic diseases associated with SARS-CoV-2 infection. Approximately 11% of patients develop ocular disease after SARS-CoV-2 infection, and an average of 13.6% of patients with COVID-19 develop follicular conjunctivitis (26). Conjunctivitis is considered the main manifestation among the current reports, but other inflammatory reactive ocular diseases and conditions, such as optic neuritis, ocular vascular diseases, glaucoma, orbital neuropathy, xerophthalmia and muscae volitantes, have also been reported (27–42). A study revealed that patients who had COVID-19 were at greater risk (hazard ratio 1.18, 95% CI 1.03–1.34) of developing a new diagnosis of uveitis than were matched controls within multiple time periods between one-month and two-years (43). Among the 117 patients with severe COVID-19 examined, forty-two patients had ophthalmological manifestations (unilateral in 23 patients and bilateral in 19 patients), 10 of these patients had more than one ophthalmological manifestation, and the most frequent fundoscopic findings were optic nerve inflammation, microvasculature occlusion, and major vascular occlusions (44).
It has been widely confirmed that SARS-CoV-2 infection can induce an immunological response, followed by unique changes in the numbers of immune cells and the levels of chemokines, cytokines and inflammatory molecules, as well as some neurological symptoms (45, 46). Persistent inflammation, as evidenced by elevated levels of some inflammation-related factors within ocular tissue secondary to SARS-CoV-2 invasion, appears to contribute to long-standing ocular symptoms.
To date, although prior studies have suggested potential mechanisms underlying neuroinflammation secondary to COVID-19, reports on postinfection ocular manifestations and their associations with intraocular neuroinflammation are limited. However, these conditions present a challenge for ophthalmologists and researchers. Further investigations are needed to identify the persistent ocular effects after SARS-CoV-2 infection. Within this context, we analysed the associations among SARS-CoV-2 infection, ocular complications and intraocular neuroinflammation.
2 Ocular manifestations and neuroinflammatory response of COVID-19The fact that ocular manifestations can be the first presenting feature of COVID-19 should not be ignored (27). To our knowledge, the ocular manifestations of COVID-19 mainly involve the conjunctiva, cornea, sclera, anterior chamber, pupils, retina, optic nerve and visual cortex, extraocular muscles and their innervating cranial nerves, orbit and lacrimal system (47). Ocular complications have been reported in some patients with COVID-19 (Table 1). In addition, diplopia and blepharoptosis can also be found in patients with COVID-19 (48–51).
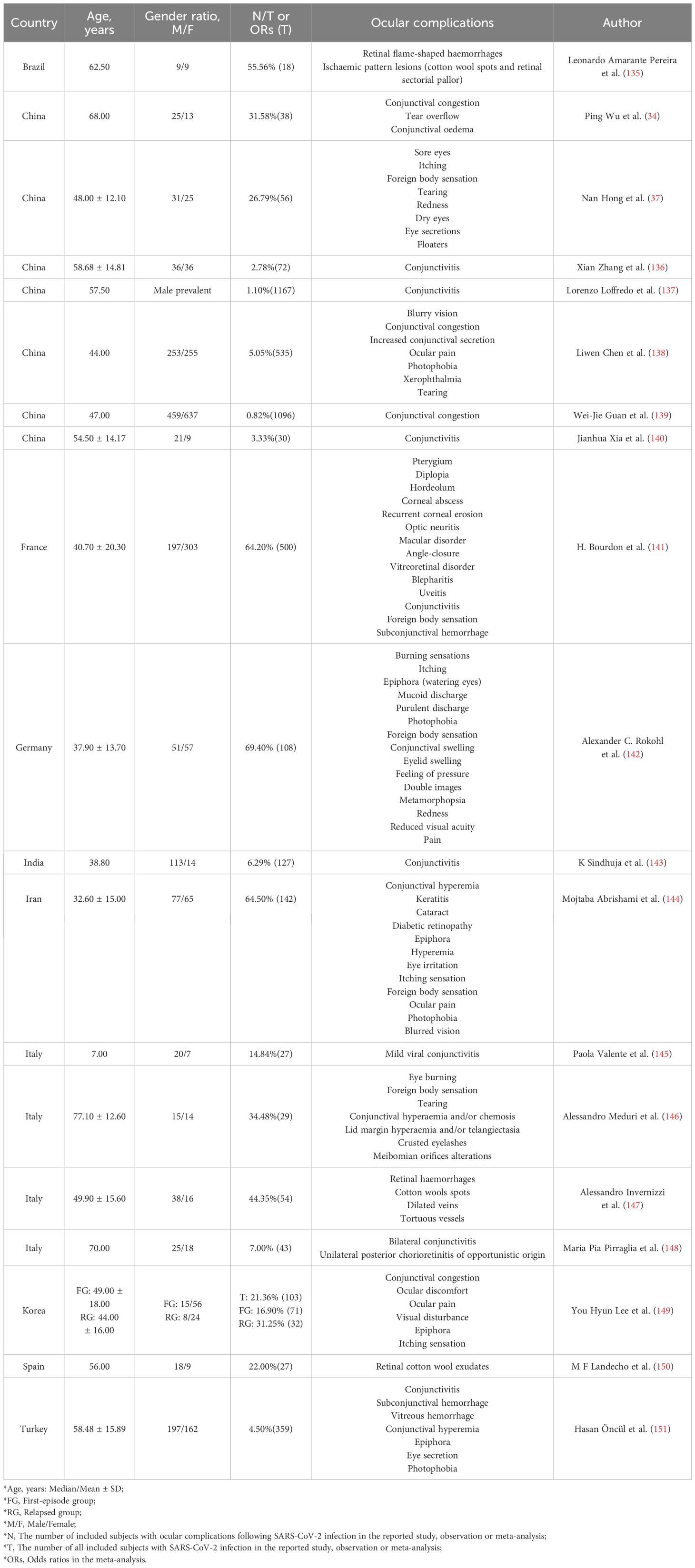
Table 1. Common ocular complications reported in SARS-CoV-2-infected individuals.
Previous studies have indicated that SARS-CoV-2 has ocular tropism and can infect the eyes directly or migrate from the respiratory tract to the brain and eye via the trigeminal and optic nerves and survive (45). Similar to the blood-brain barrier(BBB), the blood-retinal barrier(BRB), which consists of the outer barrier of the RPE and the inner barrier of the retinal vascular endothelium, is an important component of ocular health, regulating metabolic homeostasis while maintaining the immune privilege. Notably, the study of Monu Monu et al. provided the first evidence of SARS-CoV-2 ocular tropism via cells lining the BRB and that the virus can infect the retina via systemic permeation and induce retinal inflammation (52). This is comparable to the Ebola virus, which disrupts the BRB, leading to ocular complications. Ebola virus-like particles stimulate pericytes to secrete VEGF, leading to rupture of the inner BRB, but do not cause direct cytotoxic effects on retinal endothelial cells (53). SARS-CoV-2 is known to invade human cells through engagement with specific membrane cell receptors, which include the ACE2 transmembrane receptor and activation of the SARS-CoV-2 S protein by transmembrane serine protease 2 (TMPRSS2) cleavage (6, 54–56). CD147 plays an important role in facilitating SARS-CoV-2 invasion of host cells, and CD147 is expressed in tear fluid, aqueous humour and the vitreous humour (57). In addition, ACE2 has been shown to be expressed in the human conjunctiva, cornea, aqueous humour and retinal cells (58–60). These findings suggest that ocular tissues may also be target tissues for SARS-CoV-2 infection. In an animal model, SARS-CoV-2 infection promoted retinal inflammation, and immunofluorescence analysis of ocular tissues from infected mice suggested that intraocular T-cell and neutrophil counts were significantly elevated, whereas the production levels of proinflammatory cytokines and the chemokines G-CSF, IP-10, MCP-1, MIP-2, IL-6 and IL-2 were increased (61).
Through autopsies of eye tissues of COVID-19 patients, the study of H Nida Sen et al. have reported the localization of SARS-CoV-2 RNA in the ocular tissues demonstrated by in situ hybridization, including neuronal cells of the retinal inner and outer layers, ganglion cells, corneal epithelia, scleral fibroblasts and oligodendrocytes of the optic nerve (62). SARS-CoV-2 RNA and SARS-CoV-2 S and nucleocapsid (N) proteins were detected in the eyes of patients who had recovered from COVID-19 (27, 28, 41), whereas IFN-γ, TNF-α, IL-5, IL-8, and granulocyte−macrophage colony−stimulating factor (GM−CSF) expression levels were significantly elevated in the tear film of patients with SARS-CoV-2 RNA-positive conjunctival swabs (27). Cytokine expression levels in the eyes may be independent of plasma cytokine expression levels. Previous studies have shown greater expression levels of cytokines such as IL-7, IL-8, IL-10, IFN-γ, G-CSF, interferon-inducible protein 10 (IP-10) and IL-1α in the plasma of patients with cytomegalovirus retinitis (CMVR) than in those of patients with ocular syphilis, but in aqueous humour, the levels of IL-1α, IP-10 and GM-CSF were greater (63). Therefore, the immunological characteristics of the aqueous humour and plasma are independent of each other, and the cytokine levels in the aqueous humour cannot be inferred from the cytokine levels in the plasma of patients.
However, previous studies focused mostly on changes in intraocular cytokine expression after SARS-CoV-2 infection in animal models or autopsies. Therefore, further in vivo studies of human samples are needed.
2.1 Ocular surface diseases and manifestations associated with COVID-19Ocular surface complications often occur in patients with SARS-CoV-2 infection (64). For example, five cases of nonremitting conjunctivitis as the sole presenting sign and symptom of COVID-19 have been reported; these patients tested positive for SARS-CoV-2 infection via RT−PCR of nasopharyngeal swabs but developed no fever, malaise or respiratory symptoms throughout their infection period (65). In another report, a patient with COVID-19 initially presented with keratoconjunctivitis, with respiratory symptoms appearing four days later (66). Similarly, episcleritis has also been described as a possible presenting sign of COVID-19 (67).
A random-effects meta-analysis of 8219 patients with COVID-19 revealed an estimated prevalence of ocular manifestations of 11.03% (95% confidence interval [CI]: 5.71–17.72), and the most common ocular manifestations were dry eye or foreign body sensation (16%), redness (13.3%), tearing (12.8%), itching (12.6%), eye pain (9.6%) and discharge (8.8%) (68). Considering studies of certain children hospitalized with COVID-19, ocular surface manifestations, such as conjunctival discharge, eye rubbing and conjunctival congestion, were associated with systemic clinical symptoms or cough (69). The ocular surface symptoms eventually recovered or were ameliorated (69). Another meta-analysis involving 5717 patients with COVID-19 revealed ocular manifestations, including conjunctival hyperaemia (7.6%), conjunctival discharge (4.8%), epiphora (6.9%) and foreign body sensation (6.9%) (70). In addition, the positive rate of conjunctival swab tests was 3.9%, and severe cases of COVID-19 were associated with an increased risk of developing ocular complications (odds ratio [OR]=2.77, 95% CI 1.75–4.40) (70).
Viral conjunctivitis is the most common ocular manifestation of COVID-19, generally with symptoms such as conjunctival congestion, pain, foreign body sensation and increased secretion (40, 47). Moreover, alterations in ocular protection mechanisms promote the extreme susceptibility of the corneal surface to exposure keratopathy and microbial keratitis (64, 71). Patients with COVID-19 have been reported to have an increased risk of microbial keratitis with prolonged hospitalization in the intensive care unit (72, 73). The ocular surface serves not only as a direct connection between the eye and the outside environment but also as a connection to the respiratory tract through the nasolacrimal duct and nasal cavity. The positive viral nucleic acid test results confirmed in conjunctival sac swabs and tear samples from patients with COVID-19 combined with conjunctivitis provided objective evidence of SARS-CoV-2 infection on the ocular surface (27, 28, 30, 41, 74, 75).
However, persistent dry eye disease induces major central neuroinflammatory responses known to participate in chronic pain, and such central neuroinflammatory responses may participate in the development of the chronic ocular pain observed in patients with dry eye disease (76, 77). Several studies have shown that females and older patients are at increased risk of developing long COVID-19 (45, 78–82). Additionally, female sex and older age are associated with neurological manifestations of long COVID-19 (83). Female sex and older age are also susceptibility factors for some ocular diseases, such as xerophthalmia (84–86).
Additionally, patients with ocular surface symptoms (such as epiphora, conjunctival congestion or chemosis) are more likely to have higher white blood cell counts; neutrophil counts; and procalcitonin, C-reactive protein and lactate dehydrogenase levels in their blood test results than patients without these manifestations (34). Presumably, SARS-CoV-2 invasion of ocular cells and subsequent long-lasting local inflammation may lead to noticeable changes in these inflammatory indicators, which may be drivers of persistent ocular manifestations.
2.2 Ocular anterior segment diseases and neuroinflammatory response associated with COVID-19Anterior segment changes, such as acute atrial angle closure (64) and bilateral acute iris transillumination (87–90), have also been reported to occur after SARS-CoV-2 infection. The pathogenesis of ocular anterior segment diseases associated with SARS-CoV-2 infection needs to be further investigated but may be directly or indirectly induced by viral infection; alternatively, ocular complications may develop during the treatment of systemic symptoms of COVID-19.
Acute uveitis, including acute blurry vision, photophobia, redness, ocular hypertension, pain and iris atrophy discolouration, has been observed in many patients following SARS-CoV-2 infection (41, 87, 88, 90, 91). Evidence suggests that cough, sore throat, hyponatremia, prone positioning and high-dose steroid therapy can be triggers for ocular diseases secondary to COVID-19 (64, 92).
According to a few reports, several patients develop an acute attack of glaucoma after contracting COVID-19 (28, 41, 91). Patients with COVID-19 often present with symptoms such as fever, cough and sore throat, and the incidence of negative manifestations such as insomnia, anxiety and depression has also increased compared with that in the preepidemic period (93, 94). All these factors may contribute to elevated intraocular pressure. In addition, the treatment of patients with COVID-19 with high-dose glucocorticoid therapy, the consumption of large amounts of water and the use of some cough and antipyretic drugs are potential factors for inducing acute attacks in high-risk groups with local ocular anatomical features of closed-angle glaucoma. Sedat Özmen et al. reported the cases of three patients with COVID-19 and hyponatremia who also had acute angle-closure glaucoma (AACG) (95). Another case of AACG was shown to have occurred in a patient with severe COVID-19 treated with prone position ventilation (96). Guido Barosco et al. described a case in which bilateral primary angle closure (PAC) progressed to unilateral end-stage primary angle closure glaucoma (PACG) associated with treatment for COVID-19. The patient’s severe clinical condition and prolonged systemic therapy masked the symptoms and delayed the diagnosis, suggesting that COVID-19 treatment may pose an increased risk for PAC (97). The use of antiviral drugs has also been correlated with glaucoma. A study of a cohort of Australian individuals revealed an increased incidence of glaucoma medication usage in middle-aged Australian males taking antiretroviral medication (98). A few clinical case studies have revealed the development of bilateral AACG with the use of oseltamivir (99, 100). Although there is no clear evidence that antiviral drugs increase the incidence of glaucoma, it is reasonable to speculate that this may be related to drug-induced toxicity, high intraocular pressure or an idiosyncratic drug response (98).
In recent years, many studies have suggested that, similar to other neurodegenerative diseases of the CNS (such as Alzheimer’s disease, PD, and multiple sclerosis), glaucoma can be considered an autoimmune disease mediated by autoreactive T cells, and all of these diseases are characterized by impaired barrier function and chronic neuroinflammation triggered by autoantigens (101, 102). The level of ACE in the aqueous humour of patients with glaucoma is significantly greater than that in nonglaucoma patients; furthermore, the expression of ACE in the aqueous humour of patients without glaucoma is lower than that of ACE2, which controls intraocular pressure by interacting with ACE (103). Experiments in animals revealed that the hypotensive effect of ACE2 works through the ACE2–Ang(1-7)–MasR axis and that the activation of ACE2 contributes to decreasing the expression of caspase-3 and reducing the death of RGCs, thus protecting nerve fibres and RGCs (104). Furthermore, SARS-CoV-2 infection has been shown to decrease ACE2 expression in lung tissue and lead to the generation of proinflammatory cytokines such as IL-6 (105–107). Overall, the activation of endogenous ACE2 could be a strategy for the treatment of optic nerve damage.
Furthermore, intraocular neuroinflammation is most likely associated with the immune responses of T cells in patients with COVID-19. Increased TGF-β1 and TGF-β2 expression levels in aqueous humour during acute episodes of AACG have been reported in studies of individuals from the Asia–Pacific region, and cytokine levels are closely related to temporary ischaemic damage to the eyes (108). The dynamic balance of cytokines and chemokines is essential for maintaining intraocular homeostasis, and their changes also reflect the metabolic and immune status of intraocular tissues. IgG and plasma cells have been shown to be deposited in the retinas of patients with glaucoma and can maintain the proinflammatory environment via microglia (109). Moreover, in glaucoma, the activation of immunoreactive cells (microglia and macroglia) within the retina and the infiltration of peripheral immune cells (e.g., T cells, B cells, macrophages, and monocytes) are connected with the apoptosis of RGCs (109–111).
Some reports have shown that SARS-CoV-2 infection can cause secondary glaucoma (112, 113). Many studies have demonstrated the permissivity of the trabecular meshwork towards viruses with a concomitant increase in IOP in animal models (113). Virus-induced uveitis disrupts the blood–aqueous humour barrier, facilitating the entry of inflammatory cytokines and immune cells into the intraocular environment (114). Manoj Soman et al. reported the case of a male patient who developed neovascular glaucoma (NVG) 5 weeks after SARS-CoV-2 infection, suggesting that the COVID-19-associated prothrombotic state secondary to retinal vascular involvement has the potential to trigger this type of glaucoma (115).
Considering the ocular tropism of SARS-CoV-2 and the similar pathophysiologic mechanisms of glaucoma and uveitis in terms of oxidative stress, immune response-mediated inflammation, it is possible that SARS-CoV-2 infection can induce AACG and uveitis. Studies by Ron NeumannPrivate and Alejandra de-la-Torre et al. demonstrated that patients with herpes simplex virus or cytomegalovirus-infected uveitis presented with elevated intraocular pressure (IOP), iris atrophy and pupil dilation (116, 117). Some populations are susceptible to AACG because of their inherently dangerous anatomy and may need to experience a long period of time to develop in the absence of stressors. SARS-CoV-2 infection may be an intense stressor that accelerates the progression. Yue Ying et al. compared 171 patients with acute primary angle-closure (APAC) and found that there was a surge in the incidence of COVID-19-positive group in the Asia-Pacific region compared to the COVID-19-negative group on December 22, 2022, which coincided with an increase in the COVID-19 antigen-positive population, and that COVID-19-positive patients with APAC were younger, with a significantly greater depth of the anterior chamber, and with pupil dilation was higher (118).
2.3 Ocular posterior segment diseases and neuroinflammatory response associated with COVID-19Ocular diseases of the posterior segment, such as optic neuritis, retinal vascular occlusion, ischaemic optic neuropathy and orbital osteofascial compartment syndrome, are not infrequent among SARS-CoV-2 infection-associated ophthalmopathies (64, 119).
Optic nerve damage in patients with COVID-19 is characterized by subacute visual loss, visual field defects, relative afferent pupillary block, pain with movement, optic disk oedema and optic nerve thickening (47). Furthermore, SARS-CoV-2 infection causes widespread inflammation and cerebral venous thrombosis, which lead to intracranial pressure, decreased vision and optic disk oedema (120). Bosello F et al. described a patient who developed myelin oligodendrocyte glycoprotein (MOG) antibody-associated unilateral retrobulbar optic neuritis a few weeks after asymptomatic COVID-19 and subsequently experienced acute inflammatory demyelinating polyneuropathy after the resolution of optic neuritis (121).
Central retinal vein occlusion (CRVO) is a common vitreoretinal disease reported in patients with COVID-19 (33). Several cases of CRVO secondary to COVID-19 with visual loss, fundus macular oedema and retinal haemorrhage have been reported (122–124). Retinal circulation dysfunction should be considered a potential aetiology of ocular manifestations in patients with COVID-19. Several parallels, such as anterior ischaemic optic neuropathy and choroidal ischaemia, have been described in patients with COVID-19 (125). There was a significantly greater likelihood of retinal microangiopathy in 972 patients with COVID-19 than in controls; moreover, optical coherence tomography angiography (OCTA) revealed reduced vessel density and an enlarged foveal avascular zone in patients with COVID-19 (126). Compared with controls, holistic analysis of retinal markers revealed that the retinal microvasculature changed, inflammation increased and gliosis occurred in eyes afflicted by COVID-19; concurrently, analysis of the choroidal vasculature revealed localized changes in density and signs of increased inflammation in COVID-19 samples (127).
Many studies have suggested that COVID-19-related retinal microangiopathy is a significant ocular manifestation of COVID-19 that can be used to predict future retinal complications. In addition, these microvascular impairments regularly occur before clinically visible changes occur, and OCTA may be used for early detection.
It has been reported that functionally active autoantibodies against G protein-coupled receptors (GPCR-AAbs) are observed in patients after SARS-CoV-2 infection, and a study revealed that GPCR-Aab seropositivity can be linked to impaired retinal capillary microcirculation, potentially mirroring the systemic microcirculation with consecutive clinical symptoms (128). Another study revealed that neutralization of GPCR-AAbs can improve retinal capillary microcirculation in patients with glaucoma who have recovered from COVID-19 (129).
H Nida Sen et al. have found six eyes showed potential COVID-19-associated macroscopic lesions in the retina through postmortem human eyes from 25 patients with confirmed SARS-CoV-2 infection, the retinal lesions potentially associated with SARS-CoV-2 infection included small neovascularization, retinal sclerotic vessels, retinal vascular occlusion with fibrin/thrombi in retinal vessels and vitreous hemorrhages (62).
Owing to the damaging effects of neuroinflammation and viral invasion of the optic nerve, an abnormal neurological immunological response develops in the intraocular environment. Although this neuroinflammatory state has not been revealed to directly result in ocular manifestations, it may contribute to disease progression through chronic activation of specific inflammatory molecules and immune cells and dysfunction of the BRB, whereas immunocyte activation can promote excessive release of inflammatory mediators. With ongoing cytokine abnormalities and autoantibody generation, specific inflammatory molecules and immune cells can be chronically active, leading to impaired BRB integrity and neurodegeneration. In addition to autoantibody generation, this persistent inflammatory response results in T-cell dysregulation, which is also related to BRB dysfunction and microcirculation disorders.
2.4 Other ocular diseases and neuroinflammatory response associated with COVID-19Because the ACE2 receptor can be detected in many intraocular tissues, through SARS-CoV-2 infection, COVID-19 may contribute to persistent intraocular neuroinflammation, microcirculation dysfunction, ischaemic neuronal injury and ocular symptoms in addition to known multisystem disturbances.
During the pandemic, facial nerve palsy, ocular movement abnormalities and vestibular alterations were reported in the paediatric population (130, 131); moreover, in children, cases of acute acquired concomitant esotropia not caused primarily by neuritis were reported (132, 133).
Opsoclonus–myoclonus–ataxia syndrome (OMAS) is a rare neurological syndrome characterized by saccadomania or spontaneous conjugate multidirectional eye movements, myoclonus in the limbs, and ataxia (134). A 57-year-old man who presented with OMAS was positive for SARS-CoV-2 infection according to a PCR test; this is the first reported case of OMAS associated with COVID-19 (134). Darion L Heald et al. reported a case of opsoclonus after COVID-19 in an infant (14).
Taken together, these findings suggest that COVID-19 eye disease can be the first symptom, complication or the only manifestation in patients with SARS-CoV-2 infection. Differences in study participants, such as race, age, past medical history, chronic underlying medical conditions, and severity of COVID-19, could be the reason for the distinct incidence of various ocular complications across different cases or analyses.
2.5 Ocular adverse effects of COVID-19 vaccinationSimilar to the ocular manifestations of COVID-19. The Ocular adverse effects of COVID-19 vaccines include facial nerve palsy, abducens nerve palsy, acute macular neuroretinopathy, central serous retinopathy, thrombosis, uveitis, multiple evanescent white dot syndrome, Vogt-Koyanagi-Harada disease reactivation, and new-onset Graves’ disease (152).
A retrospective chart review among 25 patients(76% females, mean age 43.2 years) revealed that anterior uveitis was the most common type of uveitis (56%), 19 patients(76%) developed uveitis after COVID-19 vaccination, and 15.8% of patients needed increased systemic therapy during post-vaccine uveitis (153). The primary analysis of Zhenyu Zhong et al. included 438 non-COVID-19 participants(median age 41 years and 57.3% female), with 857 doses of COVID-19 vaccination in total, this study revealed that a total of 39 episodes of uveitis relapse events occurred in 34 patients after the receipt of a dose of COVID-19 vaccine within 30 days, and concomitant use of systemic glucocorticoids at the time of vaccination was independently associated with a decrease in the risk of relapse after vaccination (HR, 0.23 [95% CI, 0.07-0.74]; P value = 0.014) (154). Another retrospective study from December 11, 2020, to May 9, 2022, reported a total of 1094 cases of vaccine-associated uveitis (VAU) in 40 countries, with estimated crude reporting rates (per million doses) of 0.57, 0.44 and 0.35 for BNT162b2, mRNA-1273 and Ad26.COV2.S respectively, the mean age of patients with VAU was 46.24 ± 16.93 years, and 68.65% (n=751) were females; most cases occurred after the first dose (n=452, 41.32%) and within the first week (n=591, 54.02%) of vaccination (155).
As the rate of COVID-19 vaccination has gradually increased, related adverse events, including optic neuritis, have been reported. Christian García-Estrada et al. reported the case of a woman with unilateral optic neuritis one week after COVID-19 vaccination (156). W-J A Lee described a similar case of a woman with unilateral optic neuritis one week after COVID-19 vaccination (157). Hiroyasu Katayama et al. reported the case of a healthy man who was diagnosed with bilateral optic neuritis after COVID-19 vaccination (158). Khalid Sawalha et al. reported a case that elucidated an interesting and rare finding for the presentation of COVID-19 with optic neuritis, and this patient also suffered from myelin oligodendrocyte glycoprotein-IgG-associated disease (MOGAD) (159). Ayman G. Elnahry et al. reported the cases of two women who developed optic neuropathy after COVID-19 vaccination, with clinical manifestations of optic papillary oedema and progressive blurred vision (119), suggesting that neuroimaging and cerebrospinal fluid analysis may be helpful in determining the cause of vision loss in these patients. Although a clear connection cannot be confirmed because of insufficient evidence, these cases may help guide further research on the potential pathogenesis of optic neuritis related to SARS-CoV-2 infection.
Several case reports have shown that abnormal hypercoagulability and increased risk of thromboembolism are not uncommon during pandemics. Among the 26 patients who were vaccinated with either mRNA or adenoviral vector vaccines for COVID-19 and presened with retinal vascular occlusions, there were more retinal vein occlusions than retinal artery occlusions, and ocular symptoms mostly occurred within 3 weeks after vaccination (160).
Studies have shown that compared with the influenza vaccine, the COVID-19 vaccine has a greater relative risk of allergies, general cardiovascular events, coagulation, bleeding, ocular adverse effects(AstraZeneca RR39.28; Pfizer-BioNTech RR34.51), and especially thrombosis, compared to the influenza vaccine (161). There are more than 20 described cases of new-onset uveitis after vaccination with the ChAdOx1 nCoV-19 (Oxford/AstraZeneca) and BNT162b2 (Pfizer/BioNTech) vaccines, with the onset of symptoms varying from one to 30 days (162). Peixuan Zhang et al. reported a total of 9 cases of IgG4-related ophthalmic disease, of which 5 developed symptoms after COVID-19 vaccination and 4 developed symptoms after SARS-CoV-2 infection (163).
In a self-controlled case series study, COVID-19 vaccines were administered to 81 people, 25 (31%) of whom developed thyroid eye disease(TED) in the exposed period and 56 (69%) in the unexposed period, the results of the study revealed that there was an increased risk of TED after COVID-19 vaccination. In parallel, there were no differences in the clinical manifestations of TED in terms of disease duration(P=0.80), activity(P=0.48), severity(P=0.23), and treatment(P=0.80) in relation to COVID-19 vaccination status (164). Similar reports are available. A multicentre retrospective study suggested that COVID-19 vaccination may enhance the risk of non-arteritic anterior ischemic optic neuropathy (NAION); nevertheless, the overall clinical characteristics and follow-up visual condition of NAION patients vaccinated with COVID-19 were similar to those of typical NAION patients (165).
There is evidence that some retinal pigment epithelium surface proteins (MRP-4, MRP-5, RFC1, SNAT7, TAUT and MATE) can cross-react with the SARS-CoV-2 E protein and also induce autoimmune reactions after vaccination (166), which may be one of the mechanisms underlying the ocular manifestations of COVID-19 or the occurrence of ocular adverse reactions after vaccination. However, owing to the use of only a few retrospective studies or independent case reports alone, it is not possible to clarify the causality of the occurrence of ocular adverse reactions after COVID-19 vaccination, and determining each potential ocular effect of vaccination is a formidable challenge; however, it is only possible to hypothesize that it is related to the immune response to vaccination.
2.6 Ocular diseases and manifestations during post-COVID-19 or Long COVIDPersistent or late-onset neurological and neuropsychiatric symptoms affect a substantial fraction of people after COVID-19 and represent a major component of the post-acute COVID-19 syndrome, also known as long COVID (167). Ocular manifestations are similar. In a retrospective study of 12 COVID-19 patients without known risk factors, the median time from COVID-19 diagnosis to ophthalmic symptoms was 6.9 weeks (168). It can be hypothesized that the persistent body hypercoagulability and the inflammatory response due to SARS-CoV-2 infection may lead to an increased risk of ocular manifestations.
Notably, the cornea was susceptible to SARS-CoV-2 in the presence of SARS-CoV-2 receptors (CD147 and ACE2) and spike protein remnants (4 out of 58) in post-recovery corneal lenticules, SARS-CoV-2 infection triggered immune responses in the corneal stroma in recovered COVID-19 patients, with elevated IL-6 levels observed between 45 and 75 days post-recovery, which were then lower at approximately day 105 (169).
Yuzhu Gao et al. reported alterations in parameters of the foveal avascular zone (all p < 0.05) and hyper-reflective dots in the vitreous of 27 patients (54 eyes) (71.1% vs. pre-COVID-19, 34.2%, p=0.006) via swept-source optical coherence tomography and angiography (170). Compared with the pre-COVID-19 status, patients with 1- and 3-month post-COVID-19 statuses presented significant thinning of ganglion cells and the inner plexiform layer, thickening of the inner nuclear layer, a decrease in the vessel density of the superficial vascular complex, and an increase in the vessel density of the deep vascular complex (170).
Longer observations of ocular manifestations during post-COVID-19 have not been reported. Further longitudinal studies are needed to examine long-term changes during post-COVID-19 or Long COVID in the human eye.
3 Viral infection and neuroinflammation3.1 Other viral infections and neuroinflammationThe predominant types of neurotropic viruses that pose a threat to human populations include herpesvirus, Epstein–Barr virus (EBV), SARS coronavirus, neurotropic enteric viruses, flaviviruses, alphaviruses and togaviruses, and a wide array of neurological manifestations can occur (171, 172). Infection of the cells in the nervous system is an integral part of the survival strategy of neurotropic viruses. Virus reactivation contributes to neuroinflammation because of the involvement of the nervous system. To our knowledge, the immune response to EBV reactivation has been shown to reflect that of myalgic encephalomyelitis (ME) or chronic fatigue syndrome (CFS) (167, 173). EBV viremia is clearly a risk factor for the development of long COVID-19 symptoms (174).
Studies in animals have demonstrated that coronavirus infections lead to retinal damage, which is associated with ocular microvascular disease, retinitis, retinal degeneration and BRB disruption (175–177). Some studies have shown that coronavirus species can also cause serious ocular diseases and conditions, including conjunctivitis, anterior uveitis, retinitis, and optic neuritis, in animal models (29). In addition, it is suggested that viral infection increases the risk of secondary bacterial infection.
Common aetiologies of viral anterior uveitis (VAU) include herpes simplex, varicella-zoster, cytomegalovirus and rubella virus (178). Additionally, EBV and Varicella-zoster virus (VZV) can remain latent in host cells after primary infection until the emergence of a stressor, such as another acute viral infection, leading to the reactivation of these herpes viruses and the induction of inflammation and neurological symptoms (45).
Enteric viruses can be transported axonally, along the enteric nervous system, through the vagus nerve and prevertebral ganglion to connect with the central nervous system, and also via the fluidic system through the BBB and blood-cerebrospinal fluid barrier (179). The gut-brain axis is a major route for neurotropic viruses.
The induction and secretion of IFN, particularly IFN-α and IFN-β, are hallmarks of the innate immune response against viruses (180). Previous studies have shown that infection of dendritic cells and fibroblasts by alphaviruses triggers the activation of the innate immune system via pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs) and RIG-I type receptors (RLRs). This initiates a series of immune responses, including the recruitment of macrophages and other immune cells, and the production of pro-inflammatory cytokines (IL-6 and TNF-α), chemokines (CCL2, CXCL10), and interferons (IFN-α, IFN-β); and signals such as TNF-α and IL-1β, which activate the neuroglial cells, and the activated astrocytes release a variety of molecules such as IL -1β, TNF-α, IL-6, IFN-γ, TGF-β and CCL2, which modulate BBB permeability and promote neuroinflammation (181). BBB dysfunction occurs through cytokine storms increasing membrane permissibility (171). Neuroinflammation is closely associated with BBB destruction and neuroinvasive viral disease.
To the best of our knowledge, some of neuroviruses are able to invade the brain parenchyma via a “Trojan horse” mechanism, through the diapedesis of infected immune cells that either cross the BBB paracellularly or transcellularly (182). The role of immune cells in pathologic neuroinflammation and neurodegeneration in neurotropic viruses infections remains to be further investigated because of the diversity and uncertainty of the origin of immune cells in the CNS.
3.2 SARS-CoV-2 infection and neuroinflammationTheoretically, SARS-CoV-2 infection leads to cellular damage, immune cell activation, and inflammation, which together result in microvascular injury and BBB compromise. By studying the K18-hACE2 infection model, Haowen Qiao et al. reported clear evidence of microvascular damage and breakdown of the BBB, and substantial microglia activation occurred in all three major brain regions after SARS-CoV-2 infection, indicating the coexistence of neuroinflammation and vascular inflammation (183). Preliminary clinical data indicate that SARS-CoV-2 infection is associated with neurological and neuropsychiatric illness; additionally, SARS-CoV-2 infection has been suggested to induce the body to initiate a postinfection-mediated immune response mechanism that ultimately results in autoimmune diseases (184). According to several studies of patients with COVID-19, the CNS shows sustained trends of monocyte expansion and T-cell changes after SARS-CoV-2 infection, and the expansion of monocyte subsets with antiviral and antigen-presenting phenotypes, which are largely mediated by Th1 cytokines, may contribute to BBB disruption and neuroinflammation (185–187).
Laura Pellegrini et al. reported the expression of the viral receptor ACE2 in mature choroid plexus cells expressing abundant lipoproteins, infection with SARS-CoV-2 damages the choroid plexus epithelium, leading to leakage across this important barrier and resulting in pathogens, immune cells and cytokines entering the cerebrospinal fluid and brain (188). Disruption of the barrier may lead to abnormal entry of immune cells and cytokines, which can trigger harmful neuroinflammation. However, previous reports suggest that SARS-CoV-2 is usually not present in the CSF of patients with neurological symptoms arguing against frequent active CNS invasion of the virus (189). Nevertheless, as in other virus infections, considering sample quality, preservation, transportation, handling and technical issues, a negative PCR-test does not exclude the presence of the virus in tissue. Therefore, further studies on antibodies against SARS-CoV-2 would be useful. It’s different from other neurotropic viruses.
Previous evidence indicates that replicative infection of endothelial cells by SARS-CoV-2 has yet to be demonstrated both in vitro and in vivo, and the limitations of the current public database and available animal models constrain the progress of mechanistic studies revealing how SARS-CoV-2 infection triggers endothelial inflammation and the research on the cellular tropism of SARS-CoV-2 (190). Peng Wang et al. described a microphysiological system integrating alveolus and BBB tissue chips and suggested that systemic inflammation likely contributes to neuropathogenesis following SARS-CoV-2 infection, and that direct viral neural invasion might not be a prerequisite for this neuropathogenesis (191). In this study, compared with direct exposure of chips to SARS-CoV-2, infusion of media from infected chips resulted in more severe damage to the chips, including endothelial dysfunction, pericyte detachment and neuroinflammation (191).
The findings of Juan Prieto-Villalobos et al. indicate that a novel mechanism through which SARS-CoV-2 disrupts cell function, upon SARS-CoV-2 infection, the activation of Cx43 hemichannels by spike S1 occurs rapidly, and this activation leads to an increase in the release of ATP and enhances ATP-mediated [Ca2+]i dynamics, and the presence of the SARS-CoV-2 binding receptor ACE2 potentiates this effect (192). These findings provide a new target for identifying the molecular mechanism underlying the neuroinflammation and cytosolic damage caused by SARS-CoV-2 infection.
Cytokines play important roles as mediators of the inflammatory response during viral infections (27). Numerous lines of evidence suggest that a dysregulated immune response, with the release of large amounts of pro-inflammatory cytokines, is involved in the pathogenesis of the severe manifestations of COVID-19 (193). SARS-CoV-2 infection has been confirmed to cause immune cell disorders and cytokine storms in the body (194, 195). These findings suggest that in addition to being directly affected by SARS-CoV-2 infection, indirect mechanisms, such as neuroinflammation secondary to viral infection, may be key in causing neurologic symptoms. In one study, the levels of cytokines such as IL-6, IL-10 and TNF-α significantly increased during SARS-CoV-2 infection but decreased during the recovery period; thus, the expression levels of these cytokines may be directly dependent on active viral replication (196).
Considering the overlapping pathogenesis of different viruses, intraocular inflammation may also occur following SARS-CoV-2 infection (178). Additionally, SARS-CoV-2 can act as a stressor and reactivate other viruses in patients with COVID-19 and prolong COVID-19 symptoms (45).
3.3 Pathogenesis associated with neuroinflammation due to SARS-CoV-2 infection3.3.1 Direct neuronal damagePrevious studies have shown that ACE2 receptors are expressed in glial cells of the brain and spinal cord neurons (197); thus, SARS-CoV-2 can adhere to, replicate in, and directly damage neuronal tissue. Neuroinflammation is a feature of neurodegenerative diseases. Nerve infections are associated with neurodegenerative diseases and neurovascular remodelling, which can cause vascular endothelial dysfunction, leading to circulatory disturbances (198, 199).
A study revealed that patients with severe long COVID-19 exhibited high degrees of peripheral macrophage activation, which disrupted the BBB and damaged tissues (200). The permeability and microvascular injury of the BBB after the addition of extracted SARS-CoV-2 spike protein have been observed in microfluidic models, and it has been speculated that endothelial damage is due to a proinflammatory response (201).
3.3.2 Cytokine stormIn addition to causing critical pulmonary and systemic injuries, SARS-CoV-2 infection is also responsible for cytokine storms (202). There is no doubt that inflammatory cytokines play important roles in the development of viral infection and the amplification of virus invasion.
SARS-CoV-2 infection leads to the production of a variety of immune mediators, such as IL-1β, IL-6, CXCL10, TNFα and other cytokines, which exacerbate the immune response and dysregulate soluble immune mediators, referred to as a “cytokine storm” (195). Increased expression levels of proinflammatory cytokines may exacerbate neuroinflammation. COVID-19 induces the production of inflammatory cytokines that can activate the hypothalamic−pituitary−adrenal axis and indoleamine-2,3-dioxygenase enzymes, which can contribute to neuroglial activation, neuroinflammation, neurotoxicity, and neuronal cell death (203).
After the acute phase, the special immune and inflammatory responses to SARS-CoV-2 infection can continue for months, leading to a state of persistent inflammation with increased levels of chemokines, cytokines and inflammatory molecules. To our knowledge, this prolonged variation in inflammation-related factors has been linked to the activation of some immune cell populations. Owing to chronic stimulation by antigens, T cells express different cytokines, especially those that are more common in CD8+ T cells (204). Cytotoxic CD8+ T cells can accumulate near the vasculature and generate massive amounts of cytokines that disrupt the BBB, leading to vascular leakage and the propagation of inflammation (205, 206). However, persistent T-cell changes and neurological deficits are related to age (207). Furthermore, some macrophage-derived proteins have been confirmed to stimulate the axonal regeneration of retinal ganglion cells (RGCs) (208), and macrophage inflammatory protein 1β (MIP-1β) has been found to induce both the chemotaxis and adhesion of T cells (209).
Not surprisingly, individual features and the complex interactions between the virus and the patient’s immune system play notable roles in the diversity of clinical manifestations. However, effective immune responses mediated by both T and B lymphocytes are produced in the bodies of patients postinfection, regardless of whether they are asymptomatic or severely infected (210–213). In particular, the numbers of CD4+ T and CD8+ T lymphocytes are significantly increased, and these cells secrete the antiviral protein interferon-γ (IFN-γ) (213, 214). Similarly, many studies have shown that typical T-cell responses against SARS-CoV-2 increase with increasing viral load, as demonstrated by significant increases in the levels of IFN-γ-producing CD8+ T cells in the sera of patients with persistent SARS-CoV-2 PCR positivity (215, 216). One study revealed that increased severity of neurological symptoms was associated with a diminished CD4+ T-cell response against the SARS-CoV-2 spike protein (174). The T-cell response is clearly important for diminishing the severity of long-term neurological COVID-19; in addition, COVID-19 mRNA vaccination can increase the ability of T cells to respond (174).
BBB dysfunction is considered the key mechanism underlying long-term COVID-19 complications. With alterations in cytokine activity and glial cell overactivation, ezrin (EZR) levels are increased in patients with long COVID-19, whereas increased nuclear factor-κB (NF-κB) levels cause endothelial cell death and increase extracellular glutamate levels, resulting in BBB disruption and neurodegeneration (200, 217, 218). Moreover, a survey of novel brain organoid models revealed that at 72 hours postinfection, IFN-stimulated gene expression and microglial phagocytosis were upregulated, resulting in engulfment of nerve termini and elimination of synapses (219). The levels of tumour necrosis factor receptor superfamily member 11b (TNFRSF11B), which are also increased in patients with severe long COVID-19, have been confirmed to contribute to neuroinflammatory processes and microglial overstimulation (200). COVID-19 is also known to increase the risk of changes in the CNS, including haemorrhages, ischaemic infarcts and hypoxia, during the acute phase of infection (167, 220). Within this context, there may be intimate associations between ischaemia, hypoxia and neuroinflammation secondary to COVID-19. These changes may develop similarly in ocular tissue at the same time.
3.3.3 Oxidative stressThe level of ROS in organisms is significantly elevated during SARS-CoV-2 infection, and overactivation of oxidative stress and impairment of antioxidant defence mechanisms induce apoptosis (221), with unavoidable effects on viral replication and associated diseases. SARS-CoV-2 increases oxidative stress in neural tissues and promotes neuronal cell death (203, 222).
Thomas Ernst et al. evaluated 29 participants post-COVID-19 who had persistent neuropsychiatric symptom sequelae and reported that lower total N-acetylaspartate (tNAA) and glutamate + glutamine levels indicated neuronal injury, whereas lower myoinositol levels reflected glial cell dysfunction, possibly related to mitochondrial dysfunction and oxidative stress in patients (223).
Owing to its neuroinvasive potential, SARS-CoV-2 infection may increase susceptibility to the development of Parkinson’s disease (PD), as the pathological changes in PD involve oxidative stress, mitochondrial dysfunction, neuroinflammation, and neurodegeneration (224). Oxidative damage to DNA, RNA, proteins, and lipids, along with the depletion of antioxidant enzymes, suggests that oxidative stress plays central roles in the pathophysiology of COVID-19 and PD (225).
3.3.4 AutoantibodiesAutoantibodies play a role in the immune response induced by SARS-CoV-2 infection. Research has revealed that GPCR-AAbs are present in patients with SARS-CoV-2 infection and that GPCR-Aab seropositivity may be related to the loss of retinal capillary microcirculation (128), which may lead to persistent chronic neuroinflammation. Another study showed that neutralization of GPCR-AAbs can increase retinal capillary microcirculation in patients with glaucoma who have recovered from COVID-19 (129).
Anosmia, dysgeusia and headache are common neurological manifestations of acute COVID-19; however, fatigue, cognitive dysfunction (brain fog, memory issues, and attention disorders) and sleep disturbances, the most common neurological symptoms observed in patients with long COVID-19, are caused mostly by prolonged neuroinflammation secondary to innate immune activation (immune cell migration and chemokine release) and humoral activation (autoantibody generation) (226–228). To our knowledge, the autoimmune antibody reaction is suggested to be the product of specific immune and inflammatory reactions rather than being caused by the virus directly (229–233).
There is evidence that anti-ACE2 antibodies can cause an abnormal renin–angiotensin system response as well as ischaemia associated with malignant hypertension and upregulation of the expression levels of thrombo-inflammatory pathway components (233). It can be presumed that these antibodies are associated with neurological manifestations after SARS-CoV-2 infection, both in the acute postinfection phase and in the chronic persistent infection period. Research supporting autoantibody generation after SARS-CoV-2 infection has focused mostly on case reports and studies, which are limited by sample size, follow-up time constraints and generalizability. Although autoantibodies can drive inflammation, neuronal dysfunction, and neurodegeneration, which are observed in patients with COVID-19, the underlying mechanisms have not been fully identified, and further investigations are needed to elucidate the role of autoantibodies in the pathogenesis of SARS-CoV-2 infection. The possible pathogenesis of neuroinflammation due to SARS-CoV-2 infection is summarized in Figure 1.
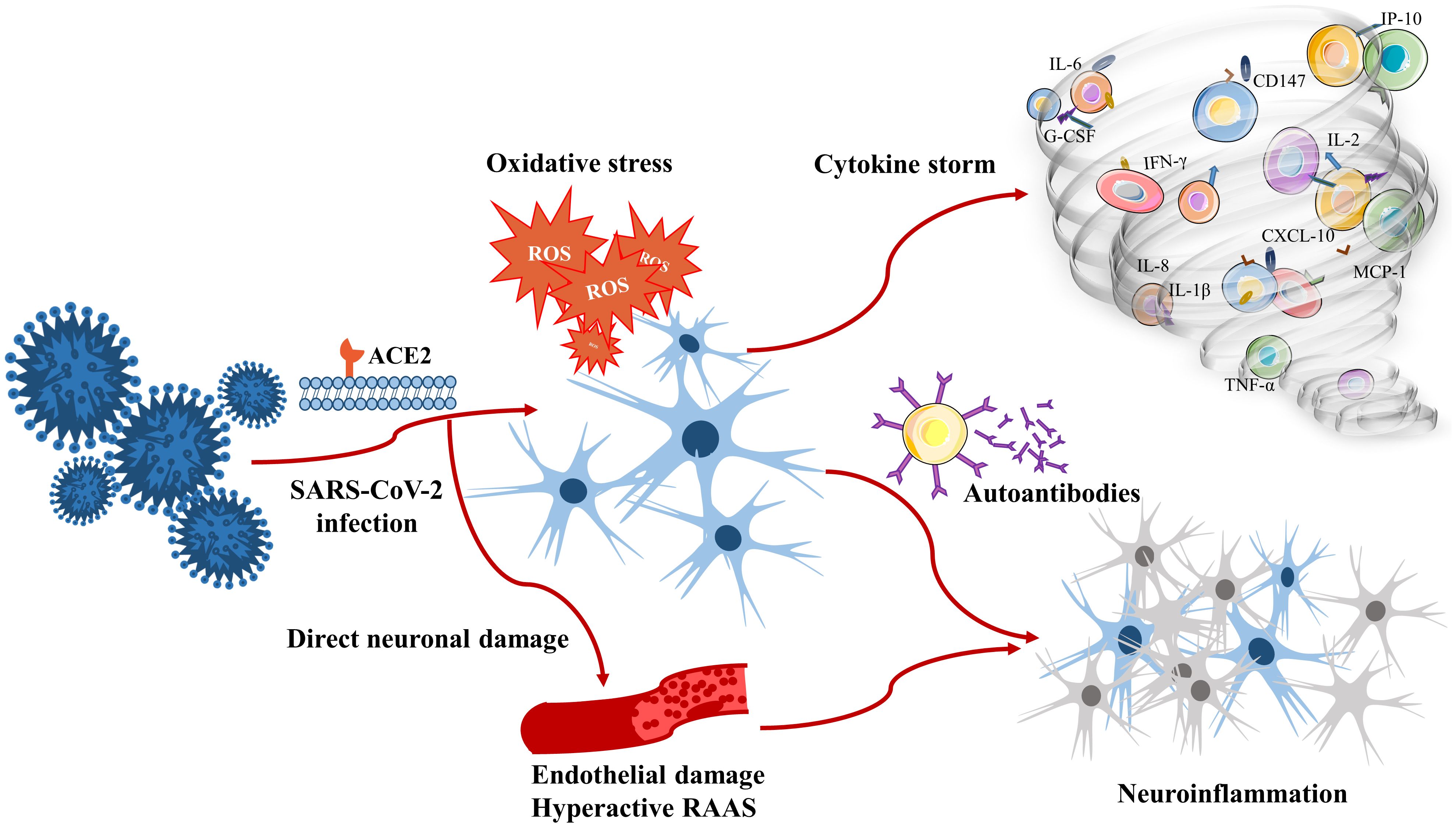
Figure 1. Pathogenesis associated with neuroinflammation due to SARS-CoV-2 infection.
4 Interventions4.1 Antiviral therapy and neuroprotective effectsThe main protease (Mprotease) of SARS-CoV-2 is a key target for antiviral drug development. Guilherme Schmitt Rieder et al. reported that the interaction of selenocompounds with Cys145 can contribute to the inhibition of Mprotease and viral replication in vitro (234), which provides new ideas for studying potential inhibitors of viral replication.
According to reports, antiviral drugs, specifically remdesivir, molnupiravir, fluvoxamine and the nirmatrelvir/ritonavir combination (Paxlovid), which are used to treat acute COVID-19, can substantially reduce mortality and hospitalization (235, 236). Cannabidiol has been confirmed to downregulate the key enzymes ACE2 and TMPRSS2, which are involved in SARS-CoV-2 invasion and the potential evolution process (237). Additionally, some of the available literature focuses on neuroprotective effects related to cannabidiol (238, 239), showing that cannabidiol may help alleviate the neurological symptoms of long COVID-19.
Remdesivir shows antiviral activity by binding to and blocking the action of more than one target in SARS-CoV-2, such as the membrane protein (Mprotein), the RNA-dependent RNA polymerase (RDRP), and the Mprotease (240). Bernal et al. suggested that antiviral drugs in combination with glucocorticoids (e.g., remdesivir plus dexamethasone) can reduce the risk of hospitalization and improve the clinical outcome of patients hospitalized with COVID-19 (241).
The authors of another report suggested that disulfiram inhibits viral replication, thereby suppressing acute inflammation and progressive fibrosis; therefore, it is the drug of choice for patients with COVID-19 with elevated early D-dimer concentrations and the development of microangiopathy (242).
4.2 Anti-inflammatory therapy and immunotherapyMany of the findings of available studies that focused on vaccination and medication for patients with COVID-19 broadly suggest that controlling inflammation postinfection can decrease the activation of immune cells and the significant neuroimmune response while reducing the release of subsequent inflammatory cytokines. Therefore, infection-related symptoms can be alleviated (243–247).
It has also been suggested that tetracyclines, including minocycline and doxycycline, have significant neuroprotective and anti-inflammatory properties and may be potential agents for the treatment of SARS-CoV-2-related neuroinflammation (248).
Immunomodulators have extensive anti-inflammatory effects. Among patients suffering from COVID-19 syndrome with dangerous hyperinflammatory conditions, to our knowledge, the findings of many reliable pathophysiological and pharmacological studies have supported the use of therapeutic strategies targeting IL-6 or its receptor (249). For example, the antagonistic blockade of IL-6R by tocilizumab appears to be a viable treatment for patients with severe COVID-19 (249). Furthermore, expectations are especially high for anakinra and baricitinib (214, 250). IL-10, an antifibrotic agent, has been confirmed to exert potent immunomodulatory, anti-inflammatory and antiviral effects (251). Thus, IL-10 is considered another possible treatment agent for COVID-19. In addition, lithium carbonate was shown to reduce the number of days of hospital and intensive care unit admission as well as the risk of death while decreasing inflammatory cytokine levels by preventing cytokine storms and reducing the duration of COVID-19 (252).
Omega-3 polyunsaturated fatty acids (PUFAs) have anti-inflammatory and neuroprotective effects. Ting-Hui Liu et al. reported that omega-3 supplementation significantly reduced the risk of developing psychiatric sequelae post-COVID-19 diagnosis (253), suggesting the potential efficacy of omega-3 PUFAs in alleviating psychiatric sequelae following COVID-19.
César A Zaa et al. reported that natural compounds such as flavonoids, alkaloids, terpenoids, curcumin, and resveratrol coul
留言 (0)