Due to immune dysfunction, patients with underlying conditions are at increased risk of serious complications and higher mortality when exposed to the novel coronavirus disease (COVID-19) (1–3). People with diabetes are twice as likely to develop COVID-19 pneumonia and have higher in-hospital mortality compared to people without diabetes (4, 5). In patients with chronic kidney disease (CKD), COVID-19 infection can significantly worsen kidney function and lead to complications such as acute kidney injury (AKI), which is common in critically ill COVID-19 patients and is associated with higher mortality rates (6). Patients with diabetes and CKD face even more complex health challenges when infected with COVID-19, which significantly increases disease severity and associated mortality. Evaluating the long-term impact of COVID-19 on this vulnerable population may help to develop targeted management strategies.
In December 2022, China experienced the peak of the COVID-19 epidemic, leading to a surge in hospitalizations for COVID-19 within a short period. Our previous study reported that among hospitalized patients with diabetes and CKD, the incidence of acute kidney injury (AKI) was 21%, sepsis was 4.7%, respiratory failure was 40%, the proportion receiving mechanical ventilation was 30.1%, and the initial hospital mortality was 26.3% (7). Despite our greater understanding of the clinical characteristics of hospitalized patients (8), we still know very little about their long-term survival and renal outcomes after discharge. Current follow-up studies have focused on symptoms and lung assessment (9, 10), and little is known about the progression of CKD after COVID-19 infection. In addition, the heterogeneity of COVID-19 follow-up studies has led to a lack of specific data on long-term mortality, which limits our in-depth understanding of long-term health outcomes for discharged patients. Therefore, we initiated a prospective observational study to assess the risk of death and changes in kidney function in patients within one year of discharge from the hospital, in order to better understand the long-term impact of COVID-19 and improve patient management.
MethodsPatientsThis was a single-center prospective observational cohort study. Data were collected from the inpatient electronic medical record system of West China Hospital, Sichuan University. A total of 338 patients with diabetes (including both type 1 and type 2 diabetes) and CKD combined with COVID-19 infection between December 7, 2022, and January 31, 2023, were included. Patients who died during hospitalization or had no visit history were excluded, as well as patients with end-stage renal disease (ESRD) who had already begun renal replacement therapy before COVID-19. Discharged patients were followed up in outpatient clinics or by telephone. The primary outcome was all-cause mortality after discharge, and the secondary outcomes included the proportion of patients with rapid progression of CKD and COVID-19-associated AKI.
DefinitionsAKI is defined as an increase in serum creatinine (Scr) of 0.3 mg/dL within 48 hours, or 50% over 7 days compared to baseline, according to KDIGO criteria. If there was a downward trend in Scr post-discharge, the baseline was recalculated using the value of Scr after discharge. Estimated glomerular filtration rate (eGFR) was calculated using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation. In patients with AKI, failure to recover renal function means a decrease in Scr of less than 50% of its peak or the need to continue hemodialysis. CKD staging was based on eGFR levels: Stage 1 (≥ 90 mL/min/1.73 m²), Stage 2 (60-89 mL/min/1.73 m²), Stage 3 (30-59 mL/min/1.73 m²), Stage 4 (15-29 mL/min/1.73 m²), and Stage 5 (< 15 mL/min/1.73 m² or dialysis). CKD rapid progression was defined as a decrease in eGFR of at least 5 mL/min/1.73 m² during the follow-up period (11). Mechanical ventilation during hospitalization included invasive mechanical ventilation and non-invasive mechanical ventilation.
The severity of COVID-19 infections is categorized according to the Chinese Clinical Guidance for COVID-19 Infection Diagnosis and Treatment (7th edition), published by the Chinese National Health Commission. The moderate type is characterized by fever, respiratory symptoms, and imaging findings indicative of pneumonia. Severe infections are marked by shortness of breath, low resting oxygen saturation, a PaO2/FiO2 ratio of ≤300 mmHg, a standard respiratory rate of ≥30 breaths per minute, and a resting oxygen saturation of ≤93%. Critically severe cases are identified by respiratory failure necessitating mechanical ventilation, shock, or the failure of other organs requiring intensive care.
CovariatesDemographic data were collected during hospitalization, including concomitant diseases, severity of COVID-19, major complications, medications, and more. Hypertension and other comorbidities were identified based on ICD-10 codes. Scr levels were recorded at the time of discharge and again at 6 to 12 months post-discharge.
Statistical analysisContinuous variables with a normal distribution were expressed as mean ± standard deviation and compared using the t-test; categorical variables were expressed as percentages and compared using the χ² test or Fisher’s exact test. Univariate and multivariate Cox regression analyses were used to evaluate risk factors for post-discharge mortality. In the construction of the Cox regression model, we used stepwise AIC-based selection, combining forward selection and backward elimination, to identify significant predictors while including clinically relevant variables. The proportional hazards assumption of the model was tested using Schoenfeld residuals, and time-dependent interaction analyses were performed for variables that might violate the proportional hazards assumption to assess their impact on the model.
Because some patients had inconsistent renal function follow-up times, making it difficult to determine the exact time of CKD progression, we chose to use univariate and multivariate logistic regression analyses to evaluate risk factors for rapid CKD progression. For missing covariates, multiple imputation was used to impute missing data. P values <0.05 are considered statistically significant. Statistical analysis was performed using RStudio software, and figures were generated using GraphPad Prism 10.
ResultClinical characteristicsBetween December 7, 2022, and January 31, 2023, a total of 338 patients diagnosed with COVID-19 who had diabetes and CKD were admitted. Of these patients, 29 patients with end-stage renal disease were excluded, 90 died during the initial period of hospitalization, and 219 were discharged. Discharged patients were followed up until December 2023. During the follow-up period, 32 patients were lost to follow-up. The final study cohort consisted of 187 patients (Figure 1).
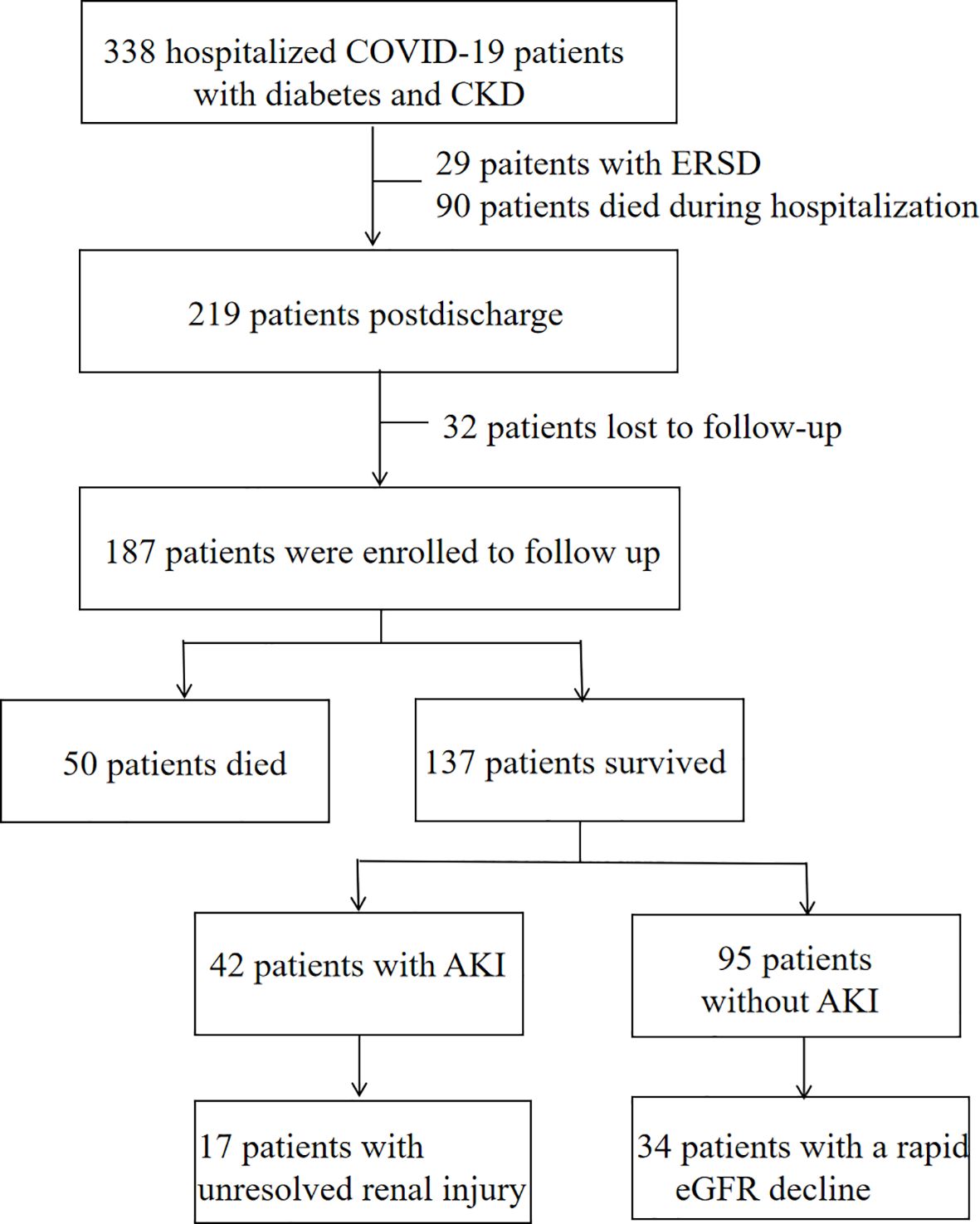
Figure 1. Flow chart of hospital survivors with COVID-19.
The mean age of patients in the study cohort was 68.7 ± 15.3 years, and 63.6% were male. The majority of patients (48.7%) were diagnosed with moderate COVID-19, with 20.9% and 30.5% of patients diagnosed with severe and critical infections, respectively. The distribution of CKD stages was as follows: 16.0% of patients were in CKD stage 1-2, 66.3% were in CKD stage 3-4, and 17.6% were in CKD stage 5. Compared with CKD stage 1-2 patients, CKD stage 3-5 patients were significantly older, had a lower proportion of severe COVID-19, higher NT-proBNP and uric acid, and lower hemoglobin and bilirubin (Table 1).
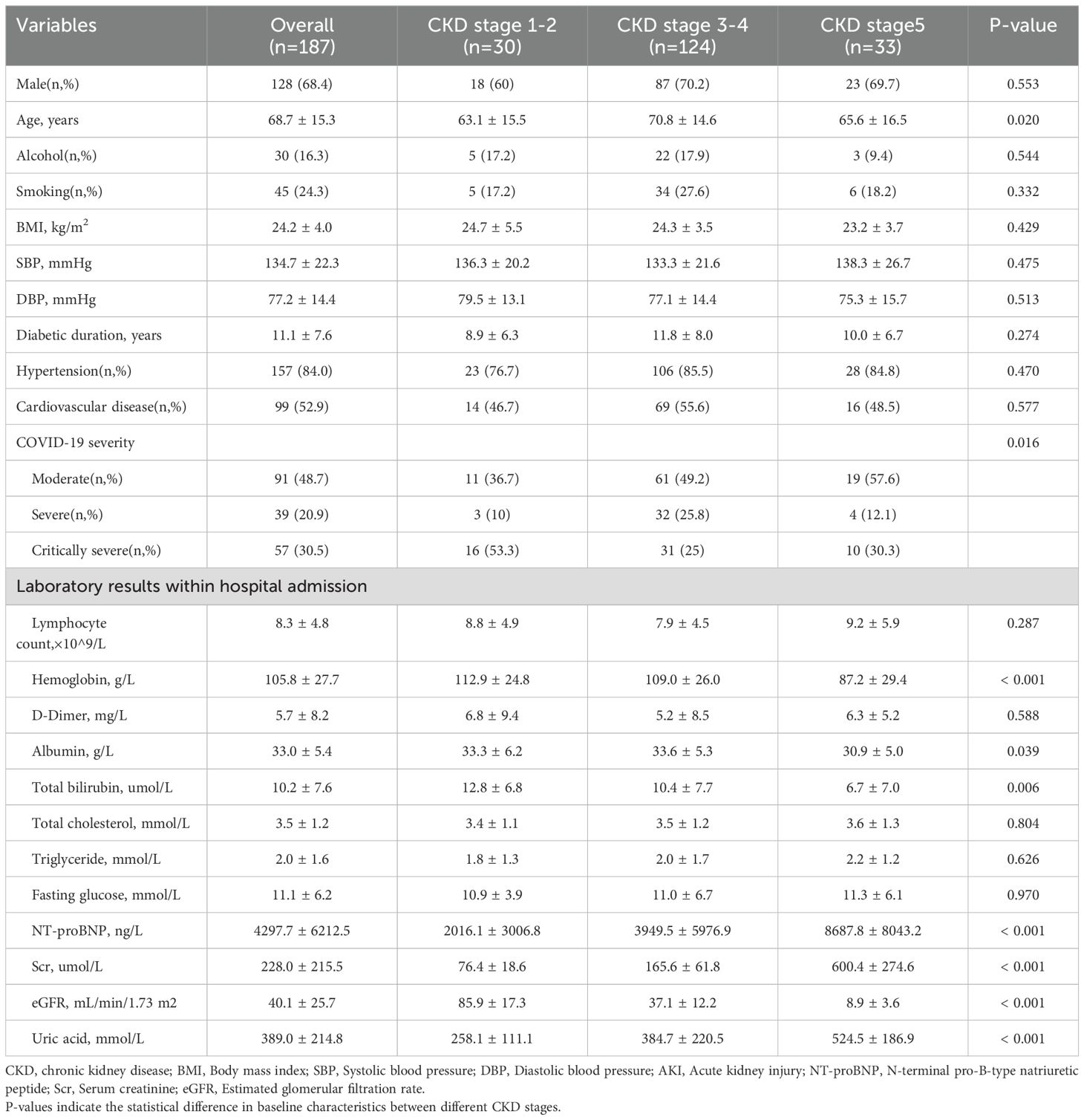
Table 1. Baseline characteristics of discharged patients during hospitalization.
MortalityDuring the follow-up period, the all-cause mortality rate of discharged patients was 26.7% (50/187). Compared with patients who survived after discharge, patients who died had a higher proportion of critically severe COVID-19, respiratory failure, stroke, and AKI when hospitalized during the peak period of COVID-19, and the levels of D-dimer and NT-proBNP were significantly increased. In terms of treatment, patients who died received more immunoglobulin and diuretic therapy, but the use of Angiotensin-Converting Enzyme Inhibitors (ACEI)/Angiotensin II Receptor Blockers (ARB) was relatively less, and there was no significant difference between glucocorticoid and antiviral drug use. Among discharged patients who had experienced sepsis while hospitalized, 46% died after discharge, and among those who received mechanical ventilation, the mortality rate was as high as 63% (Table 2).
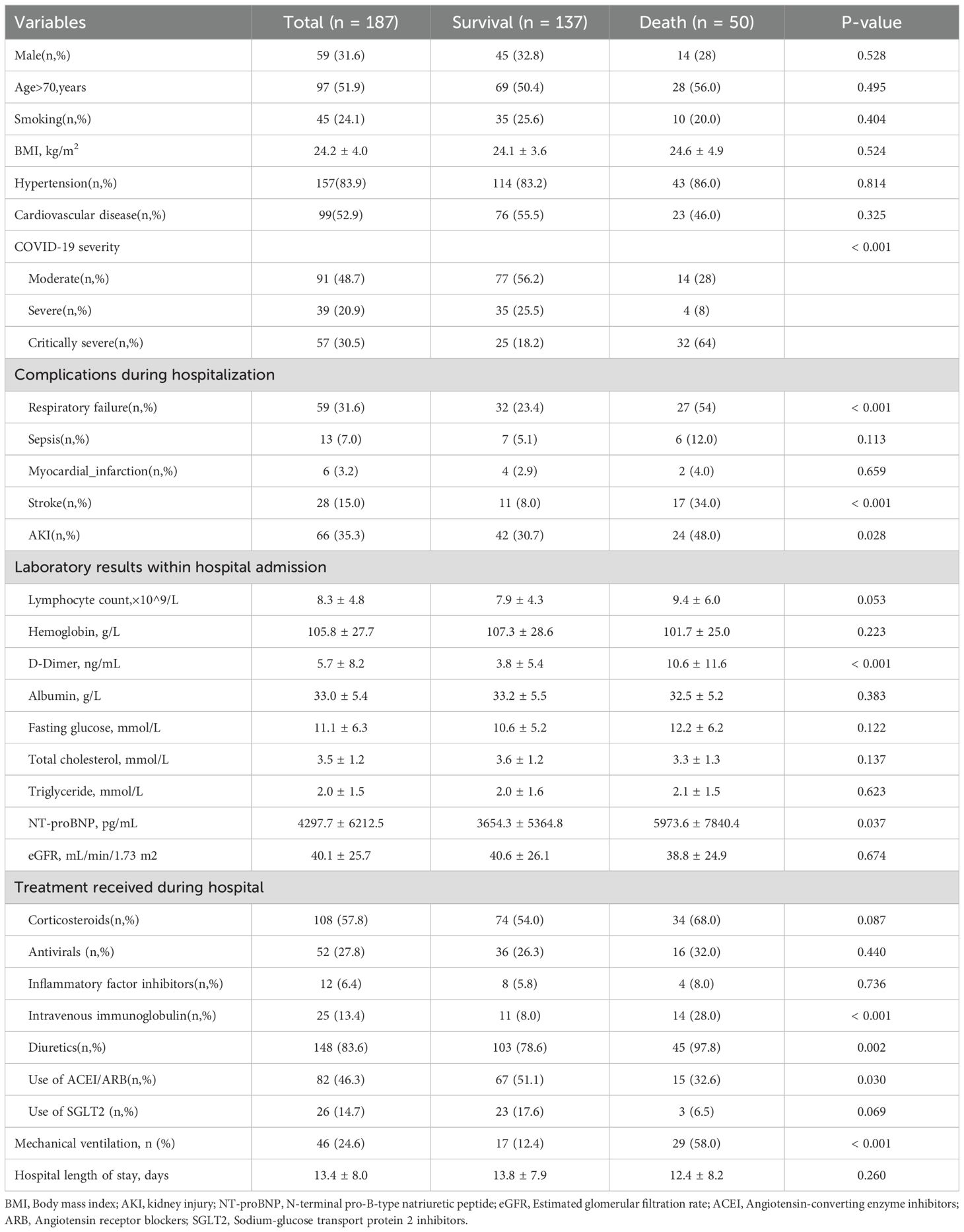
Table 2. Clinical characteristics during initial hospitalization of patients who died after discharge.
Kaplan-Meier curves (Figure 2) show that patients with critically severe COVID-19, combined with respiratory failure, and treated with diuretics, immunoglobulin, or mechanical ventilation have a higher mortality rate, while patients with oral ACEI/ARB have a relatively low risk of death. Further analysis indicated that patients’ all-cause mortality peaked in the first three months after discharge. A Cox proportional hazards regression model was used to analyze the potential relationship between COVID-19 severity, complications, treatment received during hospitalization, and all-cause mortality after discharge. Univariate analysis revealed that critically severe COVID-19, respiratory failure, stroke, and AKI during the hospital course were significantly associated with post-discharge all-cause mortality. Patients who received mechanical ventilation, intravenous immunoglobulin, and diuretics had a higher risk of death. Additionally, high D-dimer and NT-proBNP levels were associated with an increased risk of death. In a multifactor analysis, we adjusted for possible confounding factors; sepsis (HR 2.76, 95% CI 1.06-7.17) and receiving mechanical ventilation (HR 3.88, 95% CI 1.73-8.68) were the main risk factors for all-cause mortality after discharge (Table 3).
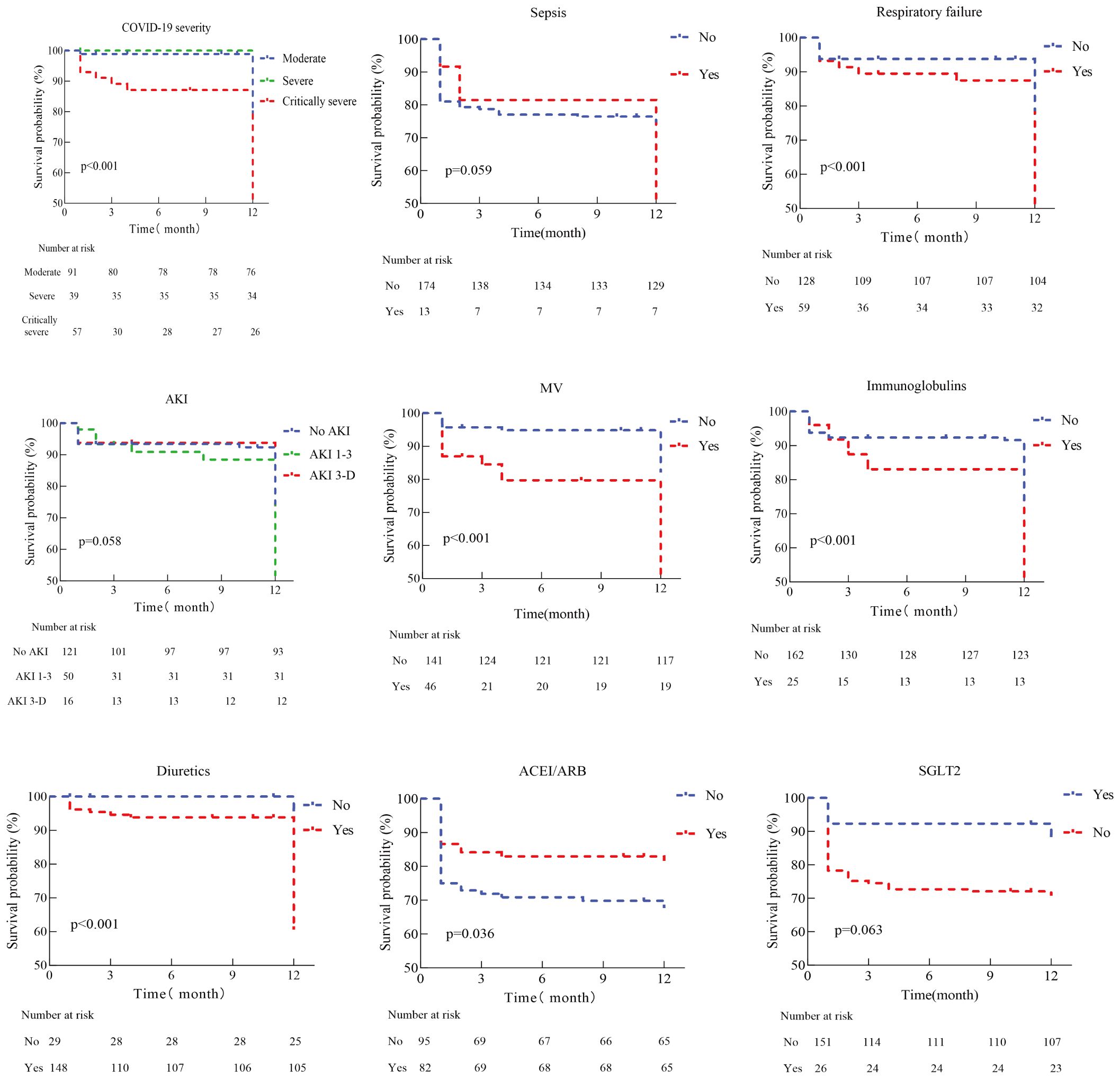
Figure 2. Kaplan-Meier analysis for death of discharge patients with diabetes and chronic kidney disease after COVID-19 infection. MV, Mechanical ventilation; AKI, Acute kidney injure; ACEI, Angiotensin-converting enzyme inhibitors; ARB, Angiotensin ii receptor blockers; SGLT2, Sodium-glucose transport protein 2 inhibitors.
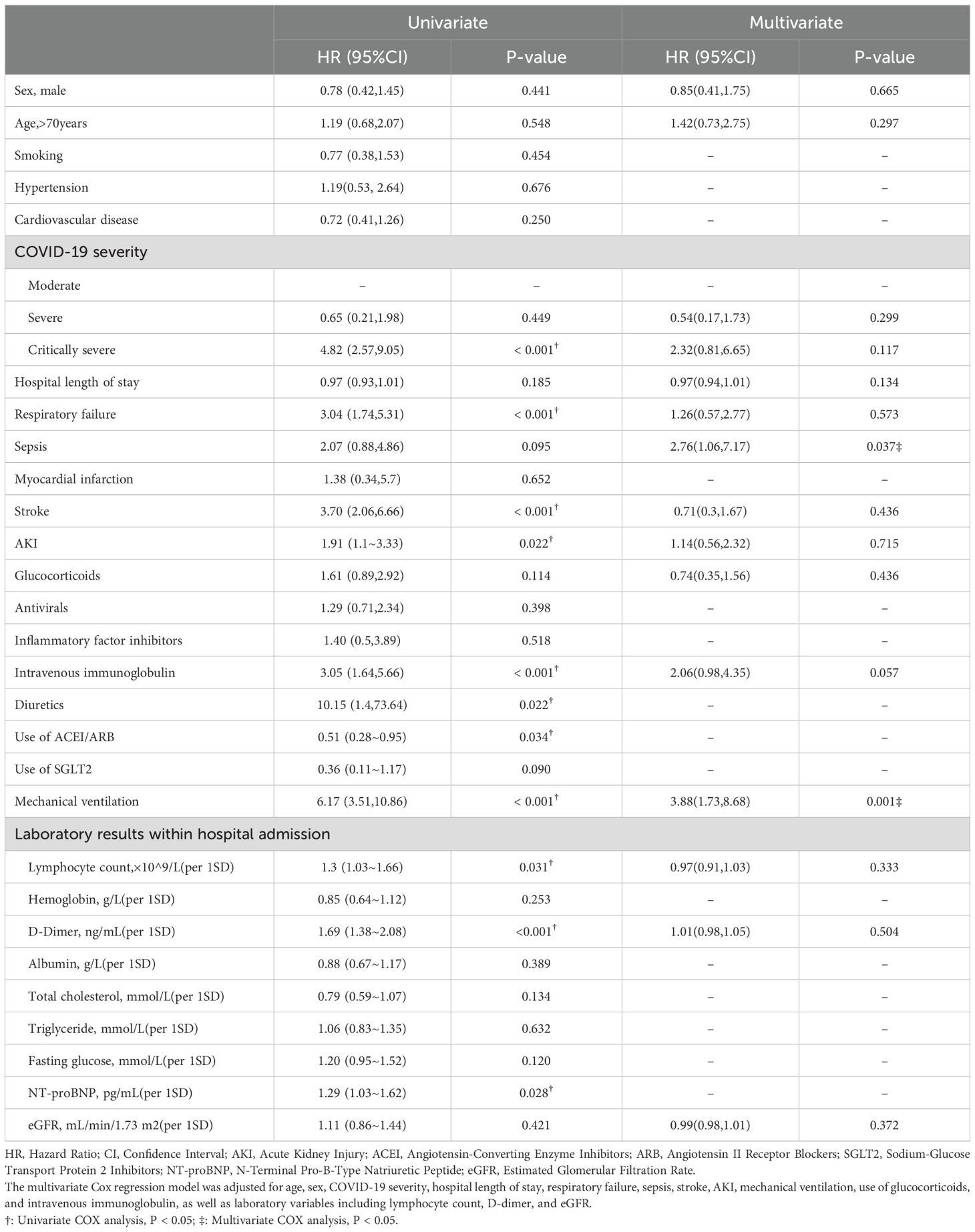
Table 3. Risk factors of post-discharge mortality based on cox proportional hazard regression.
CKD progressionOf the patients who survived after discharge, 95 did not develop AKI during hospitalization. However, during follow-up, 35.8% of them developed rapid progression of CKD (Figure 3). Patients with CKD progression had lower baseline eGFR levels compared to patients without CKD progression. There were no significant differences in gender, age, BMI, or severity of COVID-19 infection between the two groups. In addition, HbA1c levels decreased during follow-up compared to the acute phase of COVID-19 infection. Patients with CKD progression tended to have lower HbA1c levels (Table 4). Logistic regression univariate analysis (OR 3.69, 95% CI 1.27-10.71) and multivariate analysis (OR 7.14, 95% CI 1.13-45.15) showed that a baseline eGFR < 60 mL/min/1.73 m² was an independent risk factor for renal function progression (Table 5).
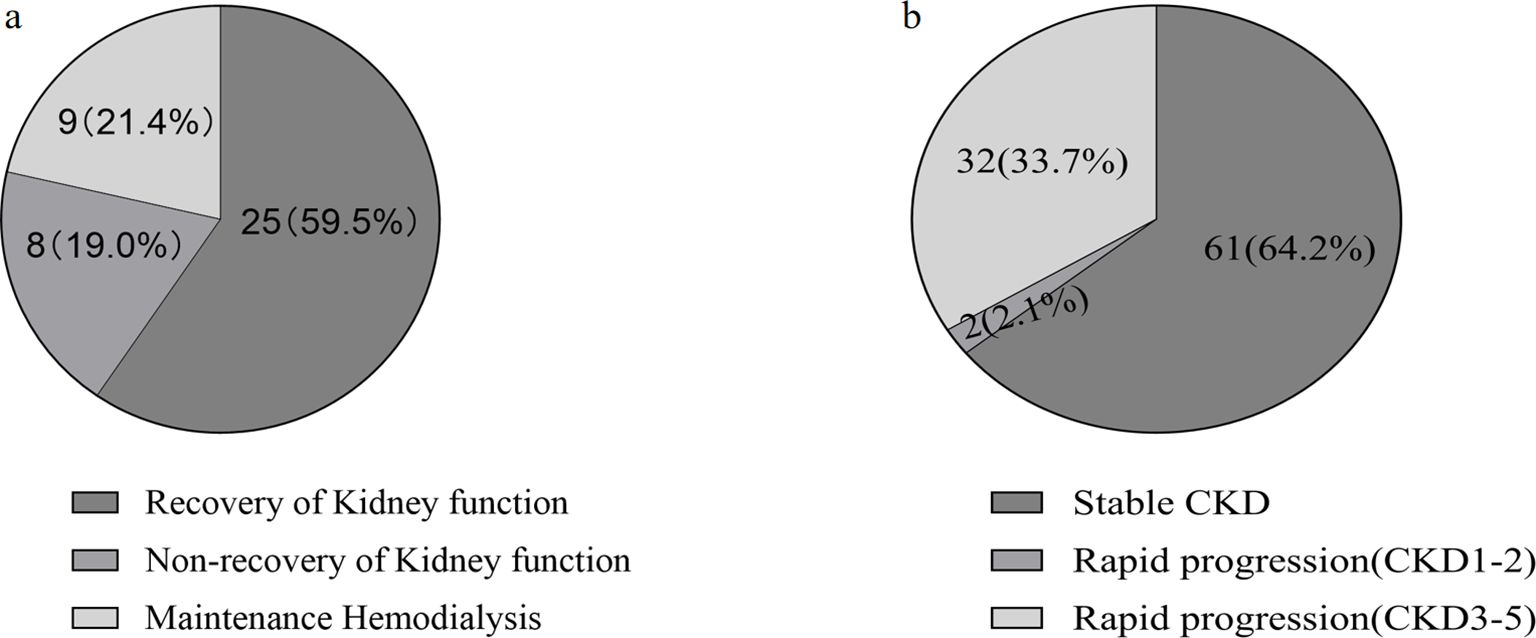
Figure 3. Analysis of renal outcomes in discharged COVID-19 patients with diabetes and chronic kidney disease (CKD) during one-year follow-up. (A) Patients developed aute kidney injure(AKI) during hospitalization. (B) Patients without AKI during hospitalization.
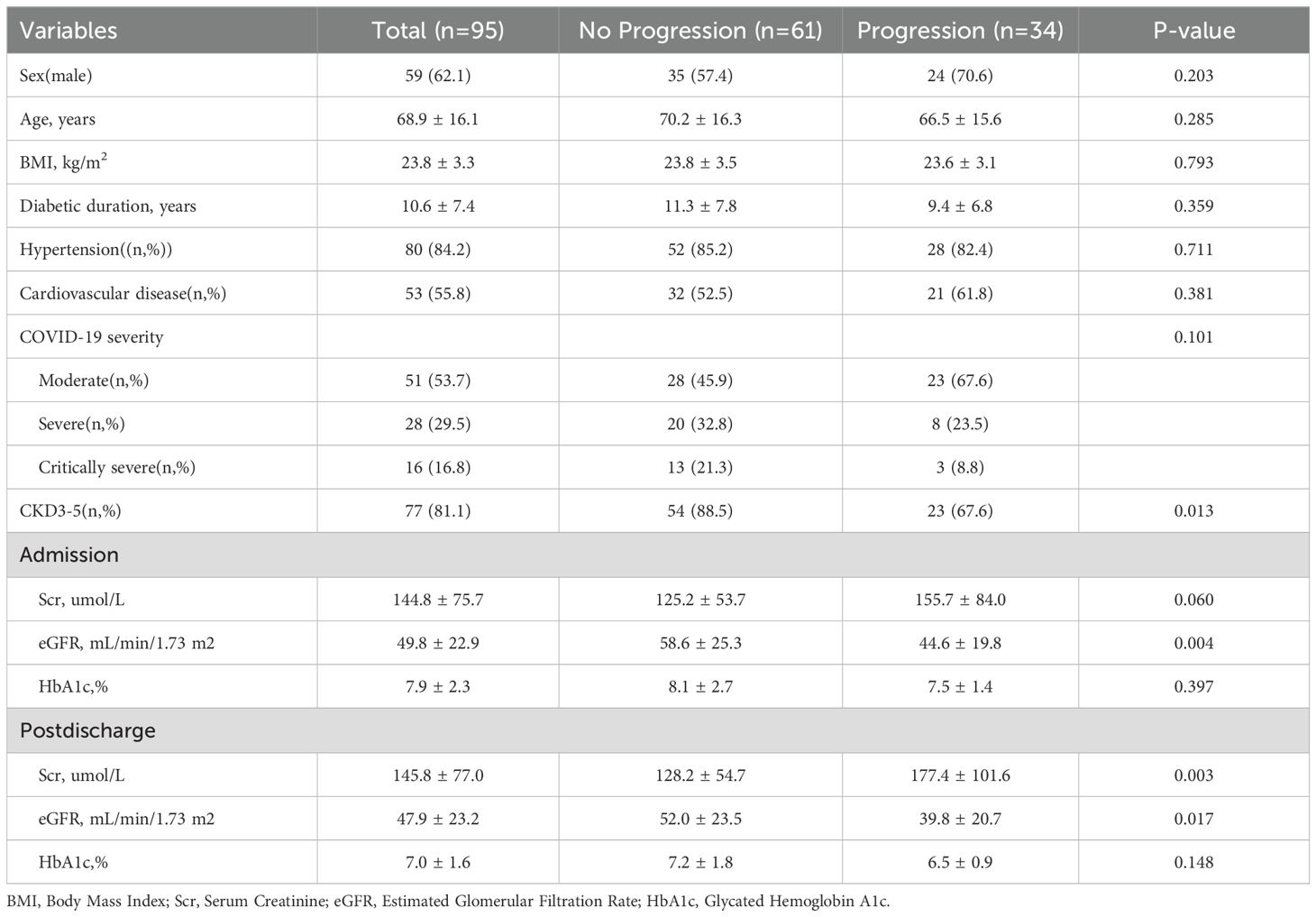
Table 4. Comparative analysis of Scr, eGFR, and Hb1Ac during follow-up: rapid progression of renal function vs. no progression.
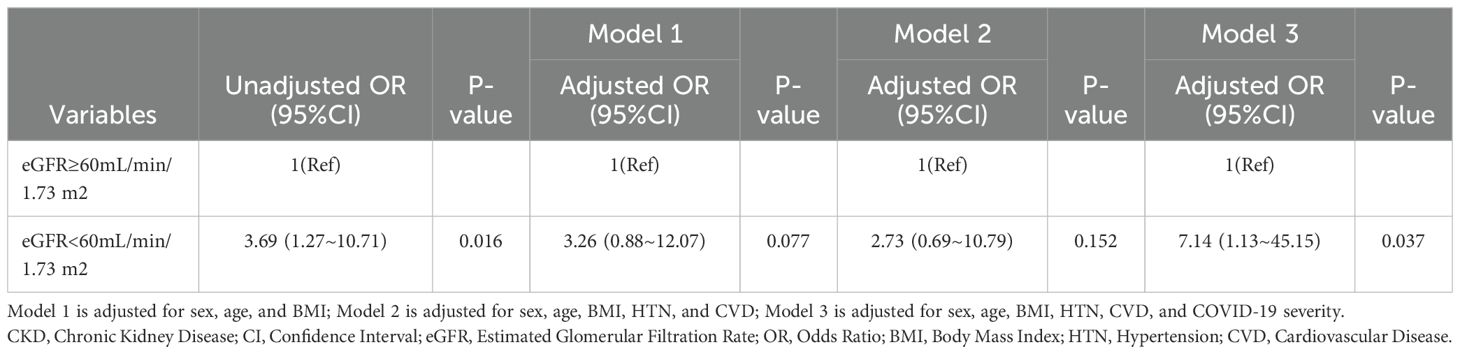
Table 5. Odds ratios for CKD rapid progression according to eGFR in patients without AKI during hospitalization.
Incidence of AKIThe incidence of AKI during hospitalization in discharged patients, as subsequently reassessed by renal function, was 35.3% (66/187) (Supplementary Material S1). There were no significant differences between the AKI and non-AKI groups in terms of gender, age, or duration of hospitalization. Regarding laboratory parameters, AKI patients had higher lymphocyte counts, D-dimer, fasting glucose, Scr, and NT-proBNP levels compared to non-AKI patients. Additionally, hemoglobin was significantly lower in AKI patients. Patients with AKI received more mechanical ventilation. During the follow-up period, 42 AKI patients survived after discharge, 40% (17/42) of AKI patients had unresolved renal injury, of which 9 patients received long-term maintenance hemodialysis (Figure 3A).
DiscussionRecent studies have shown that the mortality rate of discharged patients with COVID-19 is 4.2% to 6.5% (8, 9). Our research has shown that patients with diabetes and CKD who undergo the acute phase of COVID-19 remain at a high risk of all-cause mortality after discharge, with rates reaching up to 26.7%. This risk was particularly evident in patients with sepsis, and in severe COVID-19 patients receiving mechanical ventilation. Furthermore, our observations suggest that the incidence of COVID-19-related AKI may exceed the rates reported during hospitalization. Additionally, a significant proportion of patients with CKD stages 3 to 5 have been observed to experience rapid deterioration of renal function during the follow-up period.
People aged 65 years and older, those who have not received or have a poor response to the COVID-19 vaccine, and those with multiple comorbidities such as diabetes and CKD are at higher risk for severe COVID-19 (1). Severe COVID-19 can trigger an abnormal immune response characterized by elevated levels of pro-inflammatory and anti-inflammatory cytokines, which may lead to the occurrence of viral sepsis (12). Mechanical ventilation is a critical life-support intervention for patients with sepsis and septic shock. While its use is widespread in the treatment of severe COVID-19, ranging from 12.2% to 40% (9, 10), as our understanding of COVID-19 infection and the advancements in medical care, most patients with severe COVID-19 infection can safely survive the acute phase of the disease and be discharged from the hospital. However, after discharge, patients with sepsis, and patients who have received mechanical ventilation still face a higher risk of death, especially in the first 3 months after discharge, which deserves special attention. Studies have shown that only 50% of sepsis patients fully recover, and about 17% of sepsis survivors have post-sepsis syndrome, which leaves severe persistent physical disability, cognitive impairment, and other long-term effects, and cannot return to the physical state before the onset of sepsis (13). The reasons for the deterioration of health after discharge from sepsis are multifactorial, including the accelerated progression of pre-existing chronic diseases, residual organ damage, and impaired immune function (12). For these patients, the harm of sepsis to them has not ended with infection control and discharge from the hospital after improvement. Mechanical ventilation may be a life-support treatment for patients with severe COVID-19. However, mechanical ventilation is an invasive treatment and lung damage may occur if it is not carefully monitored. Butler MJ et al. (14) reported that among 17,652 patients hospitalized for COVID-19, the post-discharge all-cause mortality was 15.3% (173/1,131) in the mechanical ventilation group and 3.4% (562/16,431) in the non-mechanical ventilation group. It remains a challenge to determine the mechanism of the association between mechanical ventilation and adverse outcomes, whether due to differences in disease severity or consequences of mechanical ventilation itself. Vaccines have played a key role in reducing the severity of COVID-19 and reducing hospitalization rates. However, the effectiveness of vaccines may be reduced in individuals with certain comorbidities, such as obesity, type 2 diabetes, and hypertension (15). Likewise, the effectiveness of vaccines against COVID-19 may decline over time (16). Additionally, as viruses mutate, they may escape the effects of neutralizing antibodies and/or cellular immunity. These are factors that need to be taken into account.
Currently, there are varying reports on the proportion of patients with diabetes and CKD who experience a rapid decline in eGFR each year. A 3-year study found that 14% of patients experienced a significant decline in eGFR (17). Another study found that 61% of patients experienced a rapid decline in eGFR during 6.9 years of follow-up (18). In our study, 35.7% of patients with diabetes and CKD who recovered from COVID-19 experienced a rapid decline in eGFR within one year. Baseline eGFR < 60 mL/min/1.73 m² was identified as the main independent risk factor. Additionally, the progression of renal function may be influenced by concomitant medications, such as antivirals, steroids, and vasopressors, as well as by altered pathophysiological conditions, including acute or chronic kidney injury. Larger-scale, long-term follow-up studies are needed in the future to accurately assess the trajectory of changes in renal function. The higher incidence of hyperglycemia in COVID-19 patients may be related to the release of cytokines induced by COVID-19 infection, which may accelerate metabolic changes by interfering with glucose homeostasis (19, 20). In our study, a downward trend in HbA1c levels was observed after the acute phase of COVID-19 in patients with diabetes and CKD. This may be related to decreased kidney function, reduced insulin secretion, and relatively elevated serum insulin levels. A decrease in HbA1c levels may not represent an improvement in actual blood sugar control, but rather an indirect marker of changes in kidney function.
Although AKI is typically considered a benign, self-limiting, and reversible disease, it is noteworthy that each episode of AKI and increasing severity of AKI increase the risk of progression to CKD, ESRD, and mortality (21, 22). It is now recognized that AKI is a common complication in hospitalized patients with COVID-19. The etiology of COVID-19-associated AKI is likely multifactorial, involving viral sepsis, pneumonia, endothelial damage, hypercoagulability, myocardial dysfunction, drug nephrotoxicity, as well as the effects of general hypoxia and dehydration on renal perfusion (23–25). Patients with diabetes and CKD are more susceptible to AKI than are patients without diabetes or CKD (26), with one study reporting that 35% of patients with COVID-19-associated AKI had underlying CKD stage 3-5 (27). During follow-up, we dynamically monitored Scr values and found that the incidence of COVID-19-related AKI was 35.3%, which exceeded the incidence reported during hospitalization. Although other studies have reported AKI associated with COVID-19 during hospitalization, the patterns of AKI identification in these studies differed (28–31). This may impede the identification of potential AKI and pose a challenge to the reliable detection and staging of AKI. Although most AKI survivors have improved renal function, it is crucial to assess their long-term recovery to ensure appropriate allocation of renal resources (25).
This study has several limitations. Conducted during a particular phase of the COVID-19 pandemic, it faced constraints in data availability, resulting in a relatively small sample size. Due to limitations in the available data, it was not possible to collect information regarding patients’ histories of pulmonary diseases or to record medication use and other potential factors that could exacerbate kidney disease during the follow-up period. Consequently, the study was unable to comprehensively evaluate the impact of pulmonary diseases on the long-term survival of discharged patients or to thoroughly assess the effects of medication interventions. Additionally, the lack of detailed records on the causes of post-discharge mortality restricted further exploration of the mechanisms underlying patient fatalities. A further limitation is that this study was conducted at a single center, with participants primarily consisting of critically ill patients referred from community hospitals. This may have introduced a degree of selection bias, thereby limiting the generalizability of the findings. Given the severity of most patients’ conditions, the results may not fully represent the broader patient population across different healthcare settings, including community hospitals. To ensure the validity of the findings, future research should involve larger, multicenter studies that include a more diverse patient cohort. Such subsequent studies should include detailed analyses of medication use, baseline pulmonary conditions, and other potential factors. Furthermore, the accumulation of long-term follow-up data will facilitate a more comprehensive understanding of the long-term outcomes for patients with diabetes and CKD following COVID-19 infection, offering valuable evidence to guide clinical management.
In conclusion, we observed that patients with diabetes and CKD exhibited higher all-cause mortality and experienced rapid deterioration of kidney function after acute infection with COVID-19 during the one-year follow-up period. This underscores the importance of ongoing longitudinal follow-up to more accurately track the long-term health effects of COVID-19 on patients with diabetes and CKD.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statementThe studies involving humans were approved by the Ethics Committee of the West China Medical Center of Sichuan University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributionsFL: Conceptualization, Data curation, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review & editing. ER: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft. YZ: Writing – review & editing, Conceptualization, Data curation, Formal analysis, Investigation, Methodology. CZ: Writing – review & editing. KaiL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. JMY: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. WY: Formal analysis, Methodology, Software, Visualization, Writing – review & editing. LZ: Data curation, Investigation, Writing – review & editing. QY: Data curation, Investigation, Writing – review & editing. JY: Data curation, Investigation, Writing – review & editing. XJ: Data curation, Investigation, Writing – review & editing. LC: Data curation, Investigation, Writing – review & editing. YL: Data curation, Investigation, Writing – review & editing. XL: Data curation, Investigation, Writing – review & editing. KeL: Writing – review & editing, Data curation, Investigation.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Health Commission of Sichuan Province Program (No.21ZD001).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1519993/full#supplementary-material
References1. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. (2020) 8:475–81. doi: 10.1016/s2213-2600(20)30079-5
PubMed Abstract | Crossref Full Text | Google Scholar
3. Al-Muhanna FA, Albakr WIA, Subbarayalu AV, Cyrus C, Aljenaidi HA, Alayoobi LA, et al. Impact of COVID-19 on kidney of diabetic patients. Med (Kaunas). (2022) 58:644. doi: 10.3390/medicina58050644
PubMed Abstract | Crossref Full Text | Google Scholar
4. Leon-Abarca JA, Memon RS, Rehan B, Iftikhar M, Chatterjee A. The impact of COVID-19 in diabetic kidney disease and chronic kidney disease: a population-based study. Acta BioMed. (2020) 91:e2020161. doi: 10.23750/abm.v91i4.10380
PubMed Abstract | Crossref Full Text | Google Scholar
5. Shi Q, Zhang X, Jiang F, Zhang X, Hu N, Bimu C, et al. Clinical characteristics and risk factors for mortality of COVID-19 patients with diabetes in wuhan, China: A two-center, retrospective study. Diabetes Care. (2020) 43:1382–91. doi: 10.2337/dc20-0598
PubMed Abstract | Crossref Full Text | Google Scholar
6. Hadadi A, Farrokhpour H, Rashedi S, Kafan S, Sotoudehnia M, Rahimzadeh H, et al. The COVID-19 pandemic: consequences for nephrology. Nat Rev Nephrol. (2021) 17:81–2. doi: 10.1038/s41581-020-00381-4
PubMed Abstract | Crossref Full Text | Google Scholar
7. Zou Y, Qin C, Yang Q, Lang Y, Liu K, Yang F, et al. Clinical characteristics, outcomes and risk factors for mortality in hospitalized diabetes and chronic kidney disease patients after COVID-19 infection following widespread vaccination. J Endocrinol Invest. (2023) 47:619–31. doi: 10.1007/s40618-023-02180-7
PubMed Abstract | Crossref Full Text | Google Scholar
9. Huang L, Yao Q, Gu X, Wang Q, Ren L, Wang Y, et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. (2021) 398:747–58. doi: 10.1016/s0140-6736(21)01755-4
PubMed Abstract | Crossref Full Text | Google Scholar
10. Fortini A, Torrigiani A, Sbaragli S, Lo Forte A, Crociani A, Cecchini P, et al. COVID-19: persistence of symptoms and lung alterations after 3–6 months from hospital discharge. Infection. (2021) 49:1007–15. doi: 10.1007/s15010-021-01638-1
PubMed Abstract | Crossref Full Text | Google Scholar
13. Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. (2020) 395:200–11. doi: 10.1016/s0140-6736(19)32989-7
PubMed Abstract | Crossref Full Text | Google Scholar
14. Butler MJ, Best JH, Mohan SV, Jonas JA, Arader L, Yeh J. Mechanical ventilation for COVID-19: Outcomes following discharge from inpatient treatment. PloS One. (2023) 18:e0277498. doi: 10.1371/journal.pone.0277498
PubMed Abstract | Crossref Full Text | Google Scholar
15. Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, et al. BNT162b2 mRNA covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. (2021) 384:1412–23. doi: 10.1056/NEJMoa2101765
PubMed Abstract | Crossref Full Text | Google Scholar
16. Wu N, Joyal-Desmarais K, Ribeiro PAB, Vieira AM, Stojanovic J, Sanuade C, et al. Long-term effectiveness of COVID-19 vaccines against infections, hospitalisations, and mortality in adults: findings from a rapid living systematic evidence synthesis and meta-analysis up to December, 2022. Lancet Respir Med. (2023) 11:439–52. doi: 10.1016/s2213-2600(23)00015-2
PubMed Abstract | Crossref Full Text | Google Scholar
17. Yoshida Y, Kashiwabara K, Hirakawa Y, Tanaka T, Noso S, Ikegami H, et al. Conditions, pathogenesis, and progression of diabetic kidney disease and early decliner in Japan. BMJ Open Diabetes Res Care. (2020) 8:e000902. doi: 10.1136/bmjdrc-2019-000902
PubMed Abstract | Crossref Full Text | Google Scholar
18. Furuichi K, Shimizu M, Yamanouchi M, Hoshino J, Sakai N, Iwata Y, et al. Clinicopathological features of fast eGFR decliners among patients with diabetic nephropathy. BMJ Open Diabetes Res Care. (2020) 8:e001157. doi: 10.1136/bmjdrc-2019-001157
PubMed Abstract | Crossref Full Text | Google Scholar
19. Montefusco L, Nasr MB, D’Addio F, Loretelli C, Rossi A, Pastore I, et al. Acute and long-term disruption of glycometabolic control after SARS-CoV-2 infection. Nat Metab. (2021) 3:774–85. doi: 10.1038/s42255-021-00407-6
PubMed Abstract | Crossref Full Text | Google Scholar
21. Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. (2017) 13:241–57. doi: 10.1038/nrneph.2017.2
PubMed Abstract | Crossref Full Text | Google Scholar
22. Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, et al. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. (2009) 20:223–8. doi: 10.1681/asn.2007080837
PubMed Abstract | Crossref Full Text | Google Scholar
23. Copur S, Berkkan M, Basile C, Tuttle K, Kanbay M. Post-acute COVID-19 syndrome and kidney diseases: what do we know? J Nephrol. (2022) 35:795–805. doi: 10.1007/s40620-022-01296-y
PubMed Abstract | Crossref Full Text | Google Scholar
27. Mohamed MMB, Lukitsch I, Torres-Ortiz AE, Walker JB, Varghese V, Hernandez-Arroyo CF, et al. Acute kidney injury associated with coronavirus disease 2019 in urban new orleans. Kidney360. (2020) 1:614–22. doi: 10.34067/kid.0002652020
PubMed Abstract | Crossref Full Text | Google Scholar
28. Hadadi A, Farrokhpour H, Rashedi S, Kafan S, Sotoudehnia M, Rahimzadeh H, et al. Long-term impact of the COVID-19 associated AKI: the relationship between kidney recovery and mortality in a 10-month follow-up cohort study. Kidney Blood Press Res. (2022) 47:486–91. doi: 10.1159/000524451
PubMed Abstract | Crossref Full Text | Google Scholar
29. Ng JH, Hirsch JS, Hazzan A, Wanchoo R, Shah HH, Malieckal DA, et al. Outcomes among patients hospitalized with COVID-19 and acute kidney injury. Am J Kidney Dis. (2021) 77:204–215.e1. doi: 10.1053/j.ajkd.2020.09.002
PubMed Abstract | Crossref Full Text | Google Scholar
30. Kim SG, Han CH, Yu SB, Lee H, Kwon S, Kim Y, et al. Trajectory of AKI and hospital mortality among patients with COVID-19. Ren Fail. (2023) 45:2177086. doi: 10.1080/0886022x.2023.2177086
PubMed Abstract | Crossref Full Text | Google Scholar
31. Gabarre P, Dumas G, Dupont T, Darmon M, Azoulay E, Zafrani L. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med. (2020) 46:1339–48. doi: 10.1007/s00134-020-06153-9
留言 (0)