Hashimoto’s thyroiditis (HT), also known as chronic lymphocytic thyroiditis or autoimmune thyroiditis, is a chronic inflammatory disease of the thyroid with an etiology that remains incompletely understood. According to a meta-analysis (1), the global prevalence of HT among adults is 7.5%, with women being approximately four times more likely to be affected than men.
In HT, the immune system mistakenly targets the thyroid gland (2), producing antibodies such as anti-thyroid peroxidase antibody (TPOAb) and thyroid globulin antibody (TgAb), leading to persistent lymphocytic infiltration and chronic inflammation of the thyroid tissue, which results in specific thyroid dysfunction (3). As an autoimmune disease, the immune attack and antibody production are ongoing and often coexist with other autoimmune disorders (3), such as type 1 diabetes (3) and systemic lupus erythematosus (4).
Long-term immune activation may promote abnormal proliferation of lymphocytes, making HT a significant risk factor for primary thyroid lymphoma (5) and thyroid carcinoma (6), being associated with over 90% of thyroid lymphoma cases. Furthermore, persistent autoimmune responses can lead to severe central nervous system complications, such as Hashimoto’s encephalopathy (7). If not treated promptly, these complications may result in irreversible neurological damage (8). Therefore, early treatment of HT plays a crucial role in preventing complications and improving patient outcomes.
The late-stage glycation end products (AGEs) formed under chronic hyperglycemic conditions are evaluated as potential new biomarkers of oxidative stress (9). Elevated levels of oxidative stress-mediated by AGEs can induce damage to thyroid follicular cells, trigger thyroid inflammation and ultimately promote the development of HT (10). Research indicates that (11) the expression of IL-23 in thyroid follicular cells of HT patients is increased, contributing to autophagy suppression and the accumulation of reactive oxygen species (ROS). Additionally, elevated levels of TPOAb (12) in HT patients are also believed to be associated with increased concentrations of interleukin-6 and tumor necrosis factor-alpha, which play significant roles in the pathogenesis of insulin resistance (13). Therefore, some researchers suggest that TPOAb may exacerbate insulin resistance by promoting chronic inflammation, thereby interfering with normal glucose metabolism.
HT patients often experience dyslipidemia (14). Liu et al. (15)found a significant positive correlation between TPOAb levels and total cholesterol, triglycerides, insulin resistance and high-sensitivity C-reactive protein concentrations. Several studies (16–18) also suggest that TPOAb may serve as a potential link between HT and dyslipidemia. Previous research (19, 20) indicates that both hypothyroid and hyperthyroid patients may have an increased risk of developing hyperuricemia(HUA). The relationship between HT and metabolic disorders extends beyond the direct effects of hypothyroidism (21); autoimmune responses and chronic inflammation may play a more active role in the metabolic disturbances observed in HT patients (22). There is growing evidence that (23) thyroid hormones and the immune system interact in a complex bidirectional manner. As modulators of immune responses, thyroid hormones may ultimately lead to functional abnormalities through immune-mediated mechanisms.
Diabetic patients are often accompanied by metabolic disorders, including dyslipidemia (24). In addition, a strong correlation between diabetes and its related complications with elevated uric acid levels has been well established (25). To further investigate the relationship between HT and metabolic disturbances, we decided to select a non-diabetic population as the study group. Previous studies have shown that the relationship between metabolic-related indicators and non-diabetic populations with HT remains unclear. This study aims to explore the relationship between HT and metabolic products, providing a stronger theoretical basis for the long-term management of HT patients.
2 Materials and methods2.1 Data collectionThe data used in this study were collected from adult participants who underwent health examinations at Guangdong Provincial Hospital of Chinese Medicine from January 2023 to December 2023, with complete datasets. After evaluating the availability of laboratory data, including age, gender, glycated hemoglobin (HbA1c), fasting plasma glucose (FPG), lipid profiles (triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), non-HDL cholesterol (nonHDL-C) and low-density lipoprotein cholesterol (LDL-C)), serum creatinine (SCr) and serum uric acid (SUA), participants meeting the screening criteria for non-diabetic adults were included (specifically, those aged over 18 years, with HbA1c levels below 6.0% and FPG below 6.1 mmol/L). A total of 2,015 participants were included in the study (1,164 males and 851 females).
According to the 2008 Guidelines for the Diagnosis and Treatment of Thyroid Diseases in China and the American Thyroid Association’s handbook on Hashimoto’s thyroiditis (26), participants with positive TPOAb and TgAb were defined as the Hashimoto’s thyroiditis group (HT group). Participants with negative serum TPOAb and TgAb were defined as the non-Hashimoto’s thyroiditis group (Non-HT group).
2.2 Statistical analysisWe employed univariate and multivariate logistic regression models to analyze the association between metabolic-related indicators and HT in a non-diabetic adult population. In evaluating the goodness of fit for the multivariable logistic regression model, we used the Hosmer-Lemeshow test. The results showed that the model fits well. To further assess the performance of the model, we introduced the concordance index as an evaluation metric. In this study, the concordance index of the constructed multivariable logistic regression model was 0.64 (95% confidence interval: 0.59-0.69). To further assess the impact of these indicators on the risk of HT, we constructed a multivariate logistic regression model based on restricted cubic splines (RCS). RCS is an effective strategy for analyzing the relationship between the risk of disease occurrence and independent variables, particularly suitable for exploring non-linear associations. Restricted cubic splines utilize smooth connections of polynomial functions to avoid assuming a linear relationship between covariates and the response variable. To avoid overfitting, the model was selected based on the minimum Akaike Information Criterion (AIC). The AIC value was smallest when the number of nodes was set to 4. Therefore, we used a restricted cubic spline function with 4 nodes at the 5th, 35th, 65th, and 95th percentiles to flexibly model the association between metabolic markers and HT. A P-value for non-linearity < 0.05 was defined as evidence of a non-linear relationship between the two. Additionally, the RCS model can identify risk inflection points (thresholds), defined as the values that minimize the odds ratio (OR). Once the thresholds were established, we conducted piecewise logistic regression analyses to explore the relationships between relevant indicators and HT across different intervals. The chi-squared test was used for categorical variables, while non-parametric tests were employed for continuous variables. All statistical tests were two-sided, with P < 0.05 considered statistically significant. All statistical analyses were performed using SPSS 26.0, R (version 4.2.1), and the “Zstats” package.
3 Results3.1 Participant characteristicsA total of 2,015 non-diabetic adult participants were included. Among them, 138 participants were in the HT group, while 1,877 were in the Non-HT group. There were no significant differences in age and sex between the two groups. Notable differences with statistical significance were observed in metabolic-related indicators such as SUA, SCr, ALB and HDL-C between the two groups. In terms of thyroid-related indicators, the HT group showed significantly higher levels of TSH, TPOAb and TgAb compared to the Non-HT group, with these differences also being statistically significant (see Table 1).
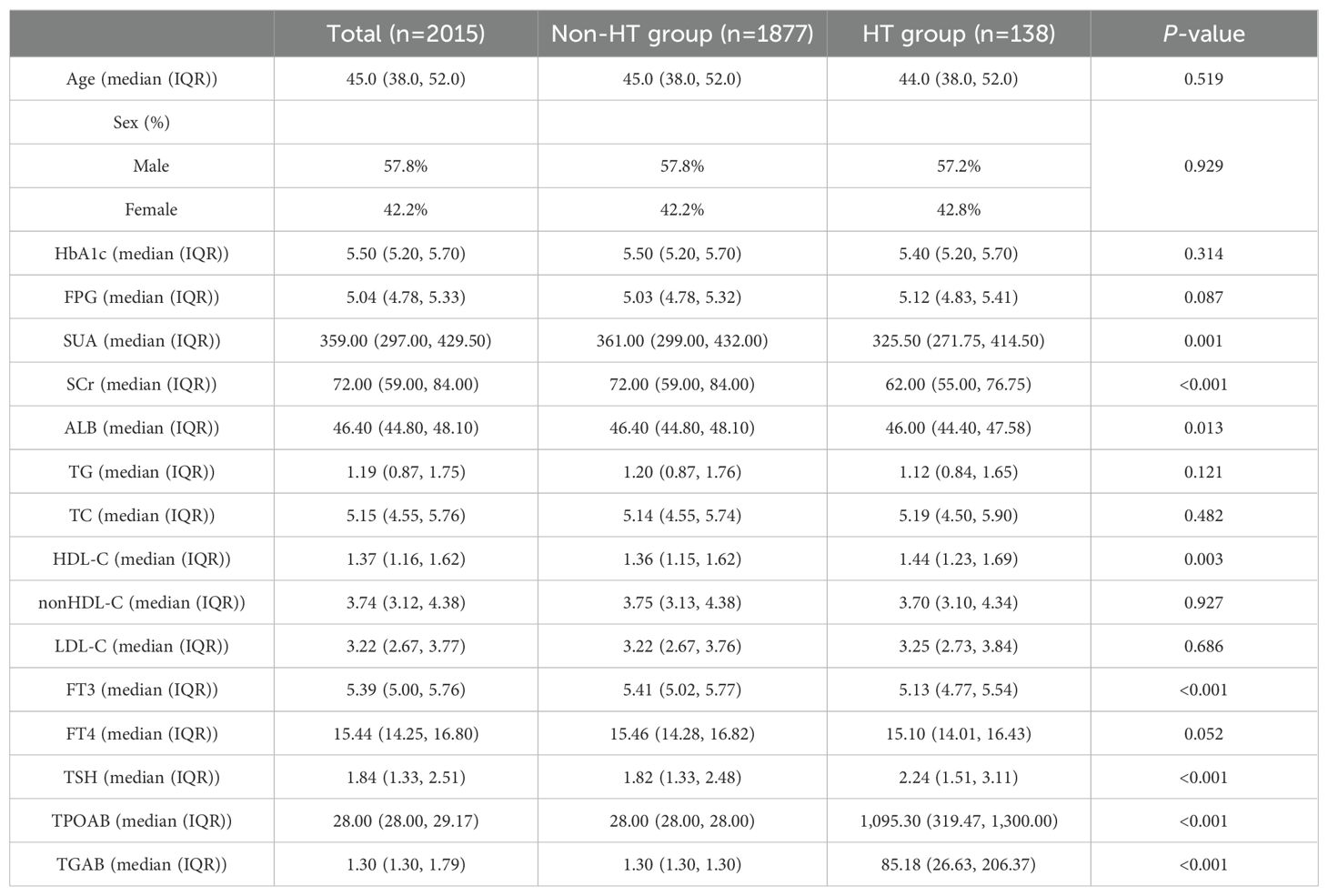
Table 1. Comparison of baseline characteristics in the non-diabetic population.
3.2 Univariate and multivariate logistic regression analysisWe established a binary logistic regression model, incorporating variables with P < 0.05 into both univariate and multivariate logistic regression analyses (see Table 2). The influence of SCr was significant in both the univariate and multivariate analyses (univariate analysis: OR 0.976; 95% CI 0.965–0.987; multivariate analysis: OR 0.979; 95% CI 0.966–0.993; both P < 0.01). The impacts of SUA, ALB and HDL-C were not significant in the multivariate analysis (P > 0.05), indicating that their associations with the outcome diminished after controlling for other variables.
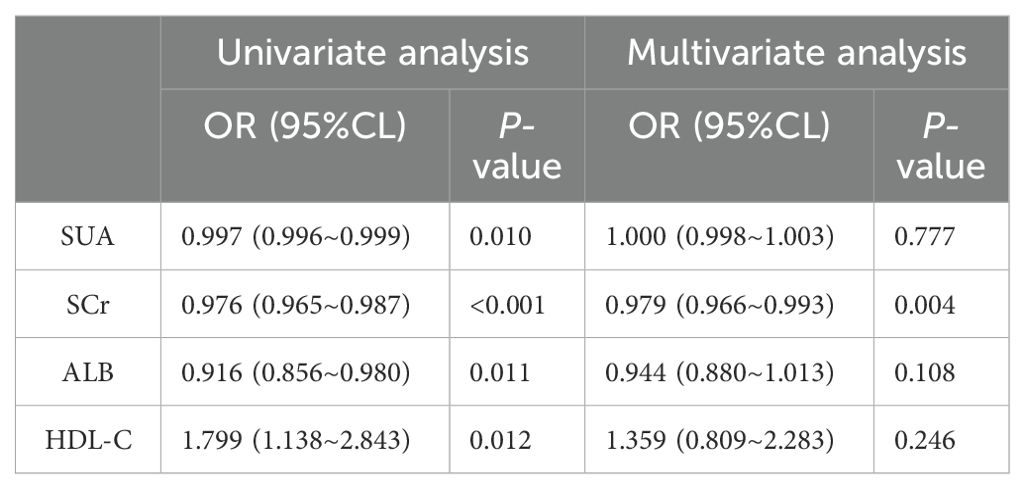
Table 2. Binary logistic regression analysis of the relationship between metabolic-related indicators and HT in the non-diabetic population.
3.3 Restrictive cubic spline identification of uric acid ranges related to minimum riskTo further investigate the relationship between metabolic-related indicators and HT, we conducted a RCS analysis (see Figures 1, 2). The results showed that neither SCr, ALB, nor HDL-C demonstrated a significant non-linear relationship with HT in both univariate analyses and after adjusting for age and gender (all P > 0.05). In contrast, SUA exhibited a significant non-linear relationship with HT before and after adjustment (all P < 0.01). Therefore, based on the inflection points derived from the RCS, we further analyzed the “U” shaped relationship between SUA and HT. The reference range was set at 359.0-540.0 μmol/L, where the lower subgroup consisted of participants with SUA levels below 359.0 μmol/L, and the higher subgroup comprised those with SUA levels above 540.0 μmol/L. By establishing a segmented logistic regression model, we analyzed the relationship between SUA levels in different ranges and HT. Compared to the reference range, the adjusted logistic model indicated that both lower and higher SUA levels were significantly associated with HT (Lower OR: 2.043; 95% CI: 1.405-3.019; P < 0.001; Higher OR: 2.369; 95% CI: 0.998-4.999; P = 0.034) (see Table 3).
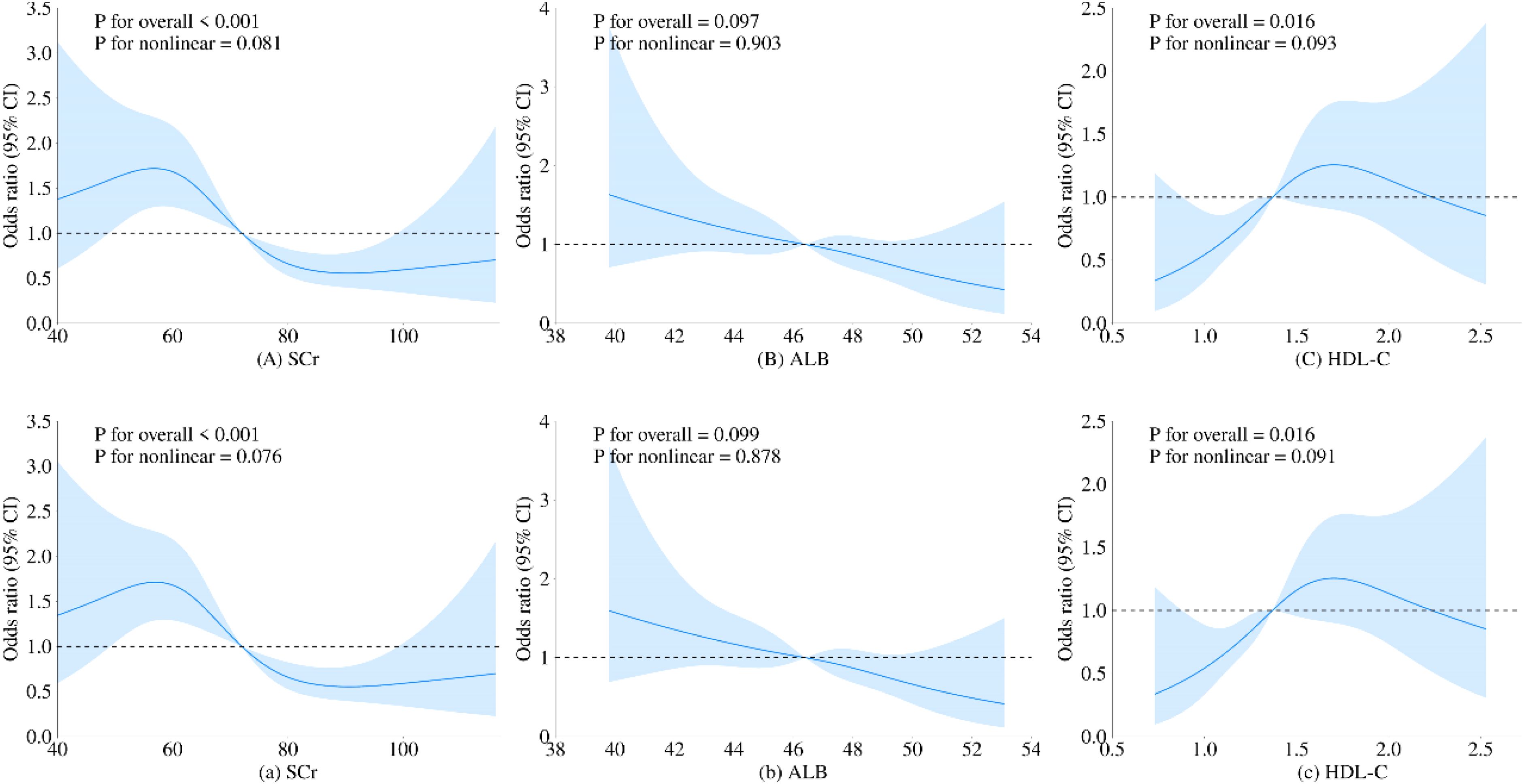
Figure 1. Restricted cubic spline regression analysis of the relationship between SCr, ALB, and HDL-C as continuous variables and HT. The blue solid line represents the estimated adjusted OR, while the shaded area indicates the 95% confidence interval. The dashed line represents an odds ratio or risk ratio of 1.0. Panels (A–C) show the spline curves for univariate regression, while subpanels (a-c) present the spline curves adjusted for age and gender.
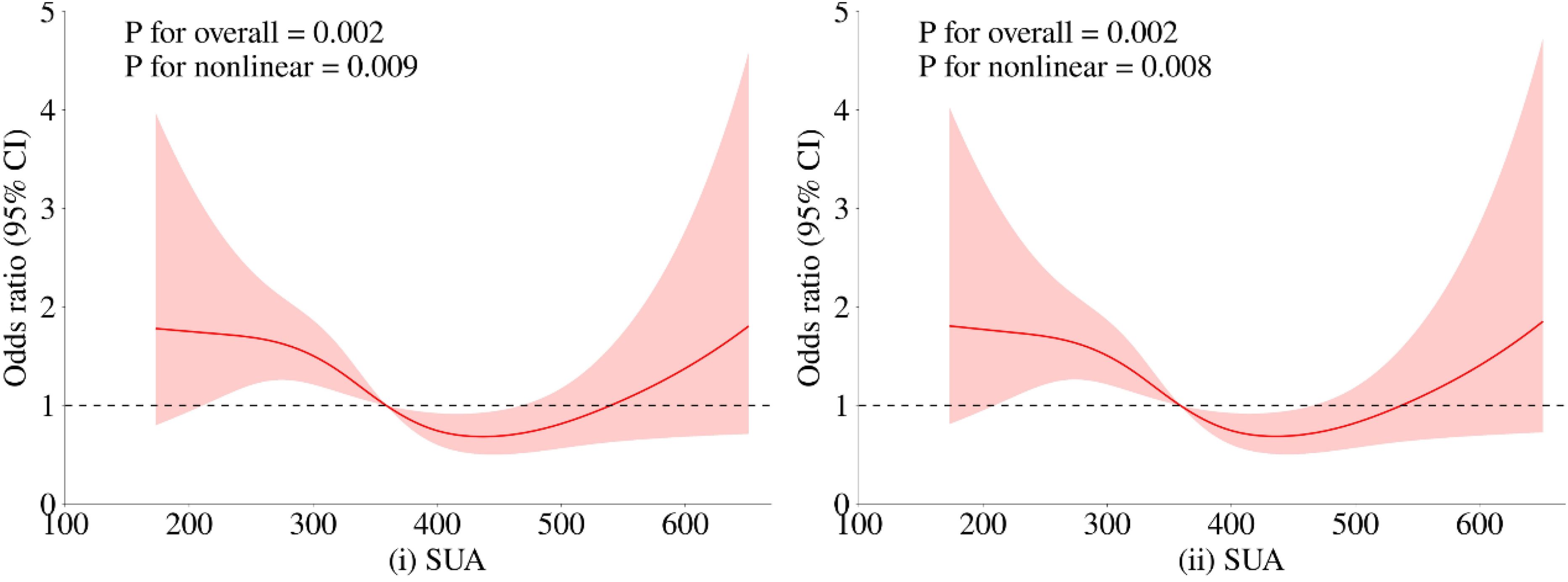
Figure 2. Restricted cubic spline regression analysis of the relationship between SUA as a continuous variable and HT. The red solid line represents the estimated adjusted OR, while the shaded area indicates the 95% confidence interval. The dashed line represents an odds ratio or risk ratio of 1.0. Panel (i) shows the spline curve for univariate regression, while panel (ii) presents the spline curve adjusted for age and gender.
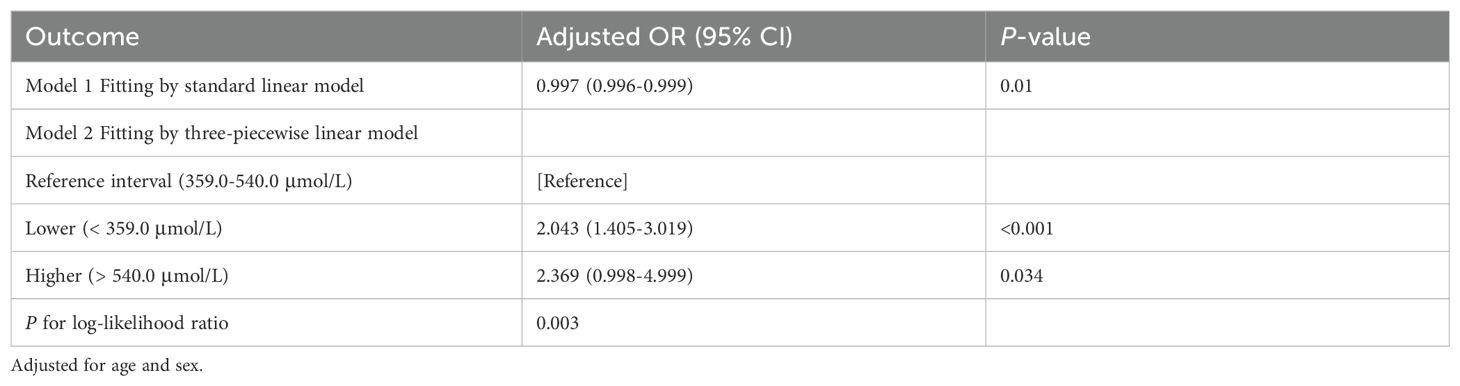
Table 3. Threshold effect analysis of SUA and HT in the non-diabetic population.
4 DiscussionTo our knowledge, this is the first cross-sectional study investigating the relationship between metabolic-related indicators and HT in a non-diabetic population. Our findings reveal a significant non-linear U-shaped relationship between SUA and HT in this cohort.
UA (27) is primarily synthesized in the liver, intestines and vascular endothelium. It is produced endogenously through purine metabolism, catalyzed by enzymes, from damaged, dying and dead cells. Additionally, UA levels are influenced by the purine content in dietary intake (28).
The human body maintains homeostasis of UA concentration through a dynamic balance of production and excretion (29). However, when this balance is disrupted, it often results in elevated UA levels in the blood, leading to HUA.
UA is known to promote the elimination of reactive oxygen species (ROS), which contributes to its antioxidant properties (30). However, recent studies (31) have shown that the formation of UA is also accompanied by the generation of ROS. UA can stimulate the activity of NADPH oxidase while inhibiting the activity of endothelial nitric oxide synthase, consequently reducing the metabolism of nitric oxide (NO). Furthermore, UA enhances the affinity of arginase for L-arginine, which further promotes ROS production. Although ROS (32) plays a crucial role in regulating cellular signaling and physiological homeostasis, excessive production of ROS (32, 33) can lead to pathological states of oxidative stress. This overproduction has been shown to irreversibly alter cellular structure and function. Overall, the generation of ROS is vital for regulating appropriate immune responses.
The human gut (34) is the only site that continuously activates the immune system through direct contact with the microbiome. The production of ROS in the gut is considered a double-edged sword (34): it is an indispensable mechanism for defending against pathogens and facilitating mucosal healing, but excessive ROS production can adversely affect mucosal integrity and epithelial barrier function, and is even closely associated with the occurrence and progression of multisystem diseases.
HT is characterized by the infiltration of inflammatory cells into the thyroid gland and the production of antibodies against thyroid-specific antigens, which triggers a persistent state of immune inflammation (26). Chronic inflammation leads to progressive destruction and fibrosis of follicular cells, ultimately resulting in hypothyroidism. Recent studies suggest (35) that oxidative stress is a primary molecular driver of tissue damage and has garnered significant attention in the pathogenesis of autoimmune and inflammatory diseases. Under the influence of environmental and genetic factors, lymphocytes (36) participate in the pathogenesis of autoimmune diseases by producing autoantibodies and ROS.
Research by Virili et al. (37) has indicated that in patients with HT, transmission electron microscopy reveals changes in microvillus thickness and increased spacing between adjacent microvilli, along with ultrastructural morphological changes in the epithelial cells of the distal duodenum. Several studies (37, 38) have also found a close relationship between gut microbiota and thyroid function, as well as the risk of HT.
Approximately 30-40% (39) of SUA is excreted via the gut. Recent studies have suggested that gut clearance defects (40) are one of the significant causes of HUA, highlighting the important role of gut microbiota (41) in regulating UA metabolism. Research by Li, Tianhe et al. (42) has confirmed the feasibility of alleviating endocrine diseases by improving gut microbiota to reduce gut oxidative stress. UA is closely related to nutrition, immune inflammation, oxidative stress and gut microbiota, and these critical biological characteristics position UA as a key player in the pathogenesis of various diseases (43, 44).
In our study, we observed that SUA levels within a specific range (359-540 μmol/L) were significantly associated with a lower risk of HT. Therefore, maintaining SUA levels within this reference range may help reduce the incidence of HT, which is potentially linked to mechanisms involving oxidative stress and gut microbiota. This finding could provide important clinical recommendations for the prevention and management of HT.
5 LimitationsThis study is a large-sample, single-center cross-sectional investigation; however, it does have some limitations. For instance, the sample size is relatively small, and the study population is drawn from a specific region and ethnic group, which may limit the generalizability of the findings. Additionally, the study did not consider individual-specific disease-related information, which could introduce bias into the results. Another limitation is that thyroid function was not categorized or analyzed in detail, as we considered immune dysregulation to be the primary driver of metabolic abnormalities. However, thyroid dysfunction could potentially amplify the effects of immune factors, or abnormal thyroid function itself may serve as an indicator of immune factor severity. This is an important aspect worth further discussion, which could lead to a more comprehensive understanding of the relationship between thyroid function, immune factors and metabolic disturbances.
6 ConclusionThis study is the first to explore the relationship between metabolic-related indicators and HT in a non-diabetic population. We found a significant non-linear U-shaped relationship between UA levels and the risk of HT. Specifically, lower risk of HT was significantly associated with UA levels within the range of 359-540 μmol/L. Our results suggest that maintaining UA levels within the reference range may help reduce the incidence of HT, potentially linked to mechanisms involving oxidative stress and gut microbiota regulation.
Despite limitations such as the relatively small sample size and the specificity of the regional and ethnic population, these findings provide important clinical insights for the prevention and management of HT. They emphasize the potential role of SUA in endocrine diseases, warranting further research and validation.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statementThe studies involving humans were approved by the Ethics Committee of Guangdong Provincial Hospital of Chinese Medicine (Approval No. ZE2024-411). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributionsMY: Data curation, Writing – original draft, Writing – review & editing. WS: Data curation, Writing – original draft, Writing – review & editing. PG: Data curation, Writing – original draft, Writing – review & editing. YX: Data curation, Writing – original draft, Writing – review & editing. KZ: Data curation, Writing – original draft. XL: Writing – review & editing. HW: Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from the Guangdong Famous Chinese Medicine Workshop: Shusen Li Workshop Foundation Program, the Guangdong Famous Chinese Medicine Workshop: Zhizheng Lu Workshop Foundation Program (E43710), State Key Laboratory of Dampness Syndrome of Chinese Medicine (SZ2021ZZ3203) and Lin Dingkun Guangdong Famous Traditional Chinese Medicine Inheritance Workshop (0103030912).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Hu X, Chen Y, Shen Y, Tian R, Sheng Y, Que H. Global prevalence and epidemiological trends of Hashimoto's thyroiditis in adults: A systematic review and meta-analysis. Front Public Health. (2022) 10. doi: 10.3389/fpubh.2022.1020709
PubMed Abstract | Crossref Full Text | Google Scholar
4. Ralli M, Angeletti D, Fiore M, D'Aguanno V, Lambiase A, Artico M, et al. Hashimoto's thyroiditis: An update on pathogenic mechanisms, diagnostic protocols, therapeutic strategies, and potential Malignant transformation. Autoimmun Rev. (2020) 19. doi: 10.1016/j.autrev.2020.102649
PubMed Abstract | Crossref Full Text | Google Scholar
5. Iskra I, Tomaš MI, Crnčić TB, Kukić E, Hadžisejdić I, Avirović M, et al. Two lymphoma histotypes and papillary thyroid carcinoma coexisting on Hashimoto ground: a case report and review of the literature. Diagn Pathol. (2024) 19. doi: 10.1186/s13000-024-01472-7
PubMed Abstract | Crossref Full Text | Google Scholar
6. Klubo-Gwiezdzinska J, Wartofsky L. Hashimoto thyroiditis: an evidence-based guide: etiology, diagnosis and treatment. Polish Arch Internal Med. (2022) 132(3). doi: 10.20452/pamw.16222
PubMed Abstract | Crossref Full Text | Google Scholar
9. Ruggeri RM, Vicchio TM, Cristani M, Certo R, Caccamo D, Alibrandi A, et al. Oxidative stress and advanced glycation end products in hashimoto's thyroiditis. Thyroid. (2016) 26:504–11. doi: 10.1089/thy.2015.0592
PubMed Abstract | Crossref Full Text | Google Scholar
11. Zheng T, Xu C, Mao C, Mou X, Wu F, Wang X, et al. Increased interleukin-23 in hashimoto’s thyroiditis disease induces autophagy suppression and reactive oxygen species accumulation. Front Immunol. (2018) 9. doi: 10.3389/fimmu.2018.00096
PubMed Abstract | Crossref Full Text | Google Scholar
12. Rodríguez-Muñoz A, Vitales-Noyola M, Ramos-Levi A, Serrano-Somavilla A, González-Amaro R, Marazuela M. Levels of regulatory T cells CD69+NKG2D+IL-10+ are increased in patients with autoimmune thyroid disorders. Endocrine. (2015) 51:478–89. doi: 10.1007/s12020-015-0662-2
PubMed Abstract | Crossref Full Text | Google Scholar
13. Duvnjak L, Blaslov K, Perković MN, Ćuća JK. Dipeptidyl peptidase-4 activity might be a link between tumour necrosis factor alpha and insulin resistance in type 1 diabetes. Endocrine. (2016) 53:453–8. doi: 10.1007/s12020-016-0899-4
PubMed Abstract | Crossref Full Text | Google Scholar
15. Liu J, Duan Y, Fu J, Wang G. Association between thyroid hormones, thyroid antibodies, and cardiometabolic factors in non-obese individuals with normal thyroid function. Front Endocrinol. (2018) 9. doi: 10.3389/fendo.2018.00130
PubMed Abstract | Crossref Full Text | Google Scholar
16. Wu Y, Shi X, Tang X, Li Y, Tong N, Wang G, et al. The correlation between metabolic disorders and tpoab/tgab: A cross-sectional population-based study. Endocrine Pract. (2020) 26:869–82. doi: 10.4158/EP-2020-0008
PubMed Abstract | Crossref Full Text | Google Scholar
17. Tamer GMM, Tamer I, Mesci B, Kilic D, Arik S. Effects of thyroid autoimmunity on abdominal obesity and hyperlipidaemia. Endokrynol Pol. (2011) 62:421–8.
PubMed Abstract | Google Scholar
18. Hu Y, Zheng J, Ye X, Song Y, Wu X. Association between elevated thyroid peroxidase antibody and abdominal fat distribution in patients with type 2 diabetes mellitus. Diabetes Metab Syndr Obes. (2022) 15:863–71. doi: 10.2147/DMSO.S345507
PubMed Abstract | Crossref Full Text | Google Scholar
19. Lu Y, Wang J, An Y, Liu J, Wang Y, Wang G, et al. Impaired sensitivity to thyroid hormones is associated with hyperuricemia in a Chinese euthyroid population. Front Endocrinol (Lausanne). (2023) 14:1132543. doi: 10.3389/fendo.2023.1132543
PubMed Abstract | Crossref Full Text | Google Scholar
20. Wu Z, Jiang Y, Li P, Wang Y, Zhang H, Li Z, et al. Association of impaired sensitivity to thyroid hormones with hyperuricemia through obesity in the euthyroid population. J Trans Med. (2023) 21:436. doi: 10.1186/s12967-023-04276-3
PubMed Abstract | Crossref Full Text | Google Scholar
21. Mikulska AA, Karaźniewicz-Łada M, Filipowicz D, Ruchała M, Główka FK. Metabolic characteristics of hashimoto’s thyroiditis patients and the role of microelements and diet in the disease management—An overview. Int J Mol Sci. (2022) 23. doi: 10.3390/ijms23126580
PubMed Abstract | Crossref Full Text | Google Scholar
22. Rajarajeswari R, Sumathi S, Asmathulla S, Ar S, Girija S, Maithilikarpagaselvi N. Association of anti-TPO antibodies with insulin resistance and dyslipidemia in hashimoto’s thyroiditis: an observational study on South Indian population. Int J Curr Res Rev. (2021) 13:88–94. doi: 10.31782/IJCRR.2021.13933
Crossref Full Text | Google Scholar
24. Tomkin GH, Owens D. Diabetes and dyslipidemia: characterizing lipoprotein metabolism. Diabetes Metab syndrome obesity: Targets Ther. (2017) 10:333–43. doi: 10.2147/DMSO.S115855
PubMed Abstract | Crossref Full Text | Google Scholar
26. Yan M, Wu H, Zhang K, Gong P, Wang Y, Wei H. Analysis of the correlation between Hashimoto's thyroiditis and food intolerance. Front Nutr. (2024) 11:1452371. doi: 10.3389/fnut.2024.1452371
PubMed Abstract | Crossref Full Text | Google Scholar
27. Yanai H, Adachi H, Hakoshima M, Katsuyama H. Molecular biological and clinical understanding of the pathophysiology and treatments of hyperuricemia and its association with metabolic syndrome, cardiovascular diseases and chronic kidney disease. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22179221
PubMed Abstract | Crossref Full Text | Google Scholar
30. Wang Z, Zhang Y, Huang S, Liao Z, Huang M, Lei W, et al. UA influences the progression of breast cancer via the AhR/p27(Kip1)/cyclin E pathway. FASEB J. (2024) 38:e70058. doi: 10.1096/fj.202400938R
PubMed Abstract | Crossref Full Text | Google Scholar
31. Zhang S, Li D, Fan M, Yuan J, Xie C, Yuan H, et al. Mechanism of reactive oxygen species-guided immune responses in gouty arthritis and potential therapeutic targets. Biomolecules. (2024) 14. doi: 10.3390/biom14080978
PubMed Abstract | Crossref Full Text | Google Scholar
32. Chen Z, Su Z, Pang W, Huang Y, Lin J, Ding Z, et al. Antioxidant status of serum bilirubin and uric acid in patients with polymyositis and dermatomyositis. Int J Neurosci. (2017) 127:617–23. doi: 10.1080/00207454.2016.1220380
PubMed Abstract | Crossref Full Text | Google Scholar
33. Elhinnawi MA, Boushra MI, Hussien DM, Hussein FH, Abdelmawgood IA. Mitochondria's role in the maintenance of cancer stem cells in hepatocellular carcinoma. Stem Cell Rev Rep. (2024). doi: 10.1007/s12015-024-10797-1
PubMed Abstract | Crossref Full Text | Google Scholar
34. Kunst C, Schmid S, Michalski M, Tümen D, Buttenschön J, Müller M, et al. The influence of gut microbiota on oxidative stress and the immune system. Biomedicines. (2023) 11(5):1388. doi: 10.3390/biomedicines11051388
PubMed Abstract | Crossref Full Text | Google Scholar
35. Ates I, Arikan MF, Altay M, Yilmaz FM, Yilmaz N, Berker D, et al. The effect of oxidative stress on the progression of Hashimoto's thyroiditis. Arch Physiol Biochem. (2018) 124:351–6. doi: 10.1080/13813455.2017.1408660
PubMed Abstract | Crossref Full Text | Google Scholar
36. Ates I, Yilmaz FM, Altay M, Yilmaz N, Berker D, Güler S. The relationship between oxidative stress and autoimmunity in Hashimoto's thyroiditis. Eur J Endocrinol. (2015) 173:791–9. doi: 10.1530/EJE-15-0617
PubMed Abstract | Crossref Full Text | Google Scholar
37. Virili C, Fallahi P, Antonelli A, Benvenga S, Centanni M. Gut microbiota and Hashimoto's thyroiditis. Rev endocrine Metab Disord. (2018) 19:293–300. doi: 10.1007/s11154-018-9467-y
PubMed Abstract | Crossref Full Text | Google Scholar
39. Lv Q, Xu D, Zhang X, Yang X, Zhao P, Cui X, et al. Association of hyperuricemia with immune disorders and intestinal barrier dysfunction. Front Physiol. (2020) 11:524236. doi: 10.3389/fphys.2020
PubMed Abstract | Crossref Full Text | Google Scholar
40. Hosomi A, Nakanishi T, Fujita T, Tamai I. Extra-renal elimination of uric acid via intestinal efflux transporter BCRP/ABCG2. PLoS One. (2012) 7:e30456. doi: 10.1371/journal.pone.0030456
PubMed Abstract | Crossref Full Text | Google Scholar
41. Wang H, Zheng Y, Yang M, Wang L, Xu Y, You S, et al. Gut microecology: effective targets for natural products to modulate uric acid metabolism. Front Pharmacol. (2024) 15:1446776. doi: 10.3389/fphar.2024.1446776
PubMed Abstract | Crossref Full Text | Google Scholar
42. Li T, Zhang T, Gao H, Liu R, Gu M, Yang Y, et al. Tempol ameliorates polycystic ovary syndrome through attenuating intestinal oxidative stress and modulating of gut microbiota composition-serum metabolites interaction. Redox Biol. (2021) 41:101886. doi: 10.1016/j.redox.2021.101886
PubMed Abstract | Crossref Full Text | Google Scholar
43. Dos Santos M, Veronese FV, Moresco RN. Uric acid and kidney damage in systemic lupus erythematosus. Clinica chimica acta; Int J Clin Chem. (2020) 508:197–205. doi: 10.1016/j.cca.2020.05.034
PubMed Abstract | Crossref Full Text | Google Scholar
44. Liu T, Zuo R, Song J, Wang J, Zhu Z, Sun L, et al. Association of serum uric acid level with risk of abdominal aortic calcification: A large cross-sectional study. J Inflammation Res. (2023) 16:1825–36. doi: 10.2147/JIR.S404668
留言 (0)