Head and neck cancers (HNCs) are highly diverse and comprise nearly 4% of all cancers within the United States. While some subtypes of HNC are decreasing in overall incidence, others are either stable or becoming more common – for example, oropharyngeal cancer due to human papillomavirus infection, or salivary gland neoplasms for unclear reasons.[1,2] As a result, HNC patients are presenting acutely to the emergency department (ED) at increasing rates.[3] HNC patients can develop emergent symptoms/complications which can result in complex clinical and imaging presentations. Furthermore, acute presentations of HNC pathology can be a diagnostic radiological challenge in part due to the complexity of HNC anatomy combined with the advanced stage of presentation, and poor functional status, which can lead to disastrous consequences for missed diagnoses. In addition, the interval between symptom onset and clinical presentation is often prolonged in HNC compared to other cancers.[4] In fact, a retrospective review by Bannister et al. found that all patients presenting on an emergency basis (either initial or post-diagnosis), did so at higher cancer stages (level 3 or 4).[5] Because of this, a thorough knowledge of imaging features of HNC emergencies is critical for making both accurate and timely diagnoses for these patients. The purpose of this review is to describe radiographic findings in oncological emergencies in both undiagnosed and previously treated HNC patients.
AIRWAYStridor is the second most common presenting symptom for emergency room patients with HNC, second only to dysphagia.[5] In addition, tracheostomies are the most common emergent intervention required in this population.[6] Likewise, some studies have suggested that oropharyngeal cancers are the most common cause of acute airway obstruction.[5] It is thought that this may be a consequence of initial misdiagnosis; slowly growing oropharyngeal tumors might be incorrectly identified as recurrent infection/tonsillitis which results in continued tumor growth and risk of airway compromise. Alternatively, this delayed presentation may occur because these oropharyngeal cancers grow slowly, causing insidious symptoms that a patient may disregard before seeking treatment. Furthermore, given the anatomical location of oropharyngeal/glottic malignancies, the associated edema and/or hemorrhage can result in emergent airway obstruction [Figures 1 and 2]. Glottic tumors or metastatic cervical lymphadenopathy may also result in chronic vocal cord paralysis due to the involvement of the vocal folds or recurrent laryngeal nerve. It is important to note that rapid growth in the tumor (from submucosal edema or intratumoral hemorrhage) superimposed on chronic vocal cord paralysis can precipitate acute airway compromise, although this danger is much less common.
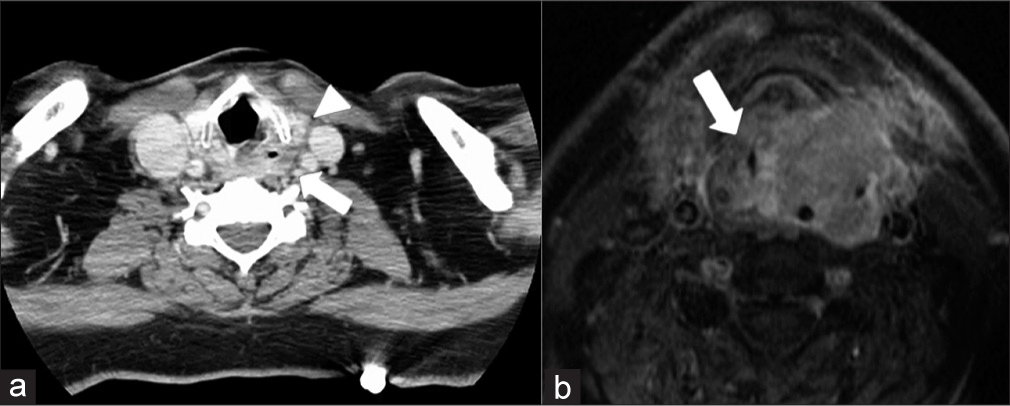
Export to PPT
Export to PPT
Special considerations in post-treatment patientsPost-treatment patients can have different etiologies of airway obstruction. Post-surgical airway compromise is not an uncommon phenomenon in the post-anesthesia care unit and relies heavily on clinical/endoscopic evaluation.[7] On the other hand, radiation injury tends to arise more insidiously, requiring imaging to play a critical role in identification and subsequent management.
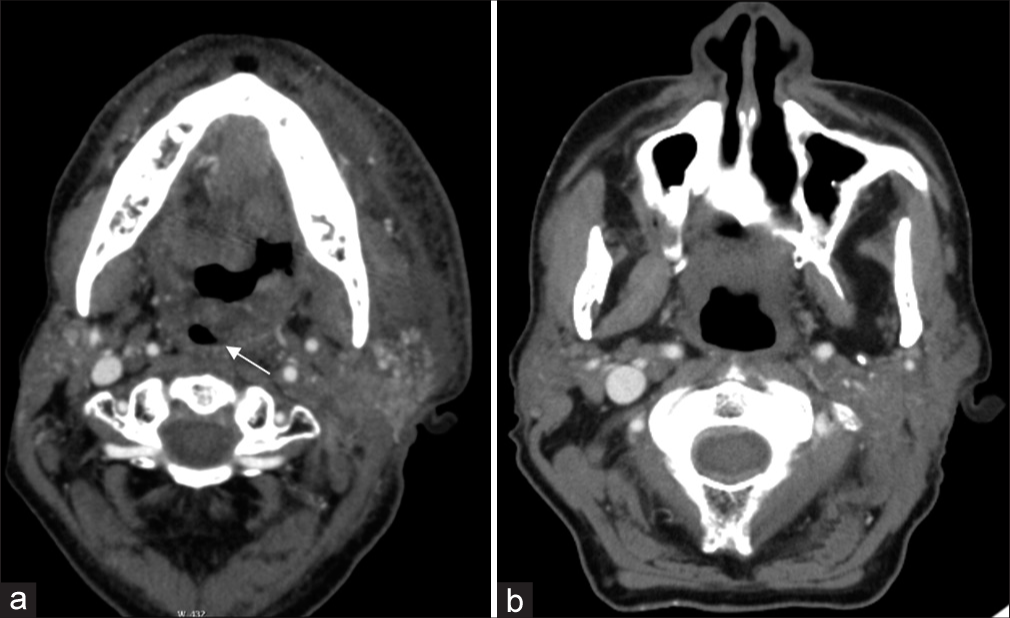
Radiation therapy, either alone or combined with chemotherapy or surgery, is utilized in the vast majority of squamous cell cancer (SCC) HNC patients. Radiation damages endothelial cells lining small blood vessels, leading to ischemia, edema, and ultimately fibrosis. In the early post-radiation period, this endothelial damage results in disruption of the venous and lymphatic drainage and results in significant interstitial edema, most commonly manifested on computed tomography (CT) as reticulations within the subcutaneous fat or thickening of the dermis along the port trajectory.[8] Consequently, an emergent presentation can occur when there is an accumulation of laryngeal or retropharyngeal edema-resulting in acute airway compromise. Key clinical information that can help clarify the clinical scenario includes radiation timing and dosage. There is a significant increase in the risk of edema with increased radiation dose, leading some authors to recommend 43.5 Gy or below at 2 Gy/fraction to reduce this risk.[9] Early edematous changes typically occur between 2 and 12 weeks of treatment [Figure 3] and chronic laryngeal edema has been found to have a median observation time of 36 weeks.[10] Additional sequelae, such as osteoradionecrosis peak at 1 year after treatment, and are further discussed below.
Export to PPT
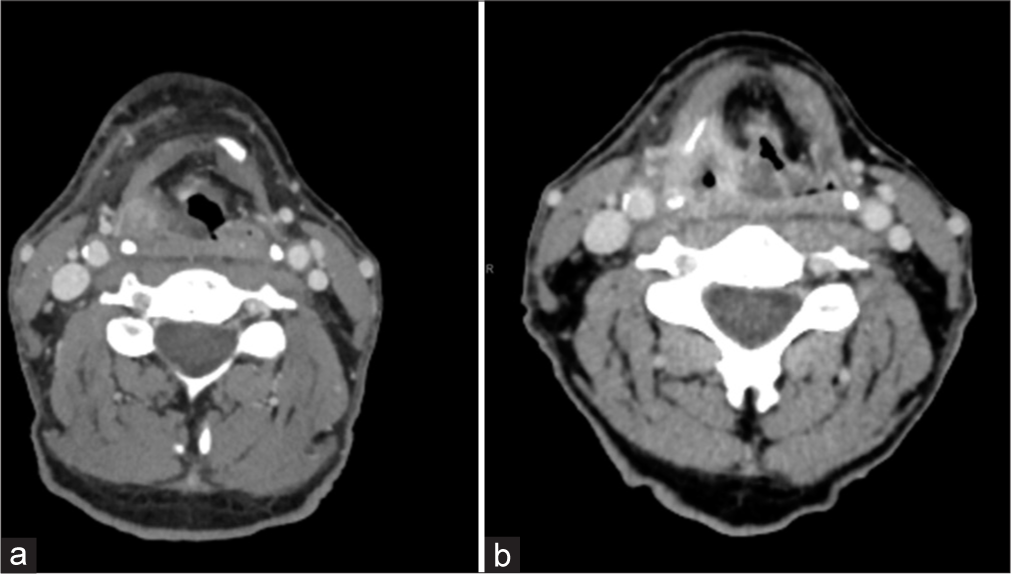
Temporal variability in symptom onsetAn important point must be considered when regarding the variability in the timing of the aforementioned post-radiation changes. For example, osteoradionecrosis has been reported in patients up to 50 years after treatment.[11] While the typical onset of chronic laryngeal edema is less than a year as described above, [Figure 4] demonstrates an emergent presentation of a 63-year-old man who developed acute symptoms of increased dyspnea and inability to speak in full sentences which occurred 3.5 years after chemoradiation treatment.
Export to PPT
VASCULAR Venous occlusion/thrombusAs with most malignancies, HNC tumors exhibit a propensity for thrombogenicity. However, in contrast to most thrombogenic cancers that result in distant phlebitis or thromboembolism, HNC more commonly exerts local effects.[12] While it is poorly understood why HNC is associated with local rather than distant thrombosis, this phenomenon is well demonstrated in clinical practice. For example, head and neck tumors more commonly result in venous obstruction secondary to external compression of the internal jugular vein (IJV) due to SCC metastatic lymph nodes [Figure 5]. Moreover, when venous thrombosis arises, particularly involving the lower IJV at the neck base, it becomes, especially important for the radiologist to follow the venous system down toward the superior vena cava (SVC) to look for evidence of SVC syndrome or mediastinal tumor involvement.
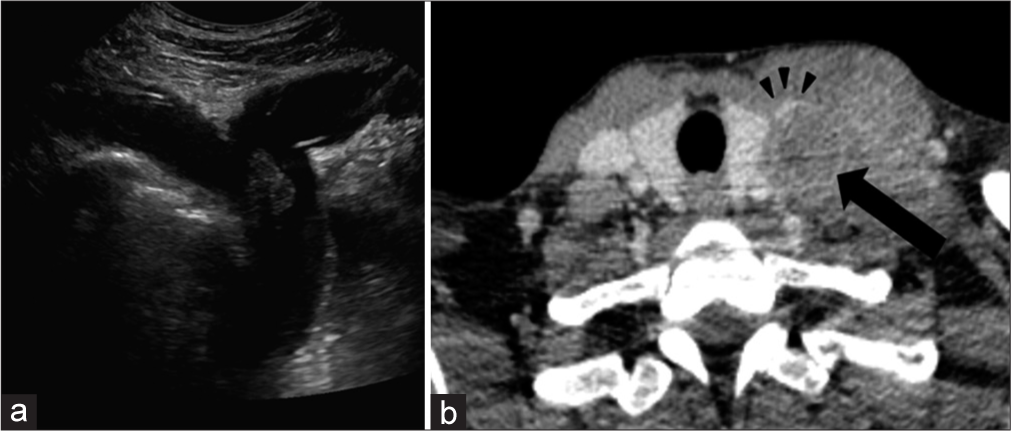
Export to PPT
Extratumoral extension into vascular structures can also result in a tumor thrombus precipitating vascular compromise, and this has been described in both the carotid artery and IJV.[13] Although infrequently seen with head and neck SCC, tumor thrombus has also been well described in the setting of differentiated thyroid cancer [Figure 6]. A key consideration in cases of suspected tumoral vascular invasion is utilizing the correct imaging modality. CT may provide high specificity, but there may be lower sensitivity when detecting vascular invasion, particularly when compared to other imaging modalities, such as ultrasound, which are readily available in the ED.[14]
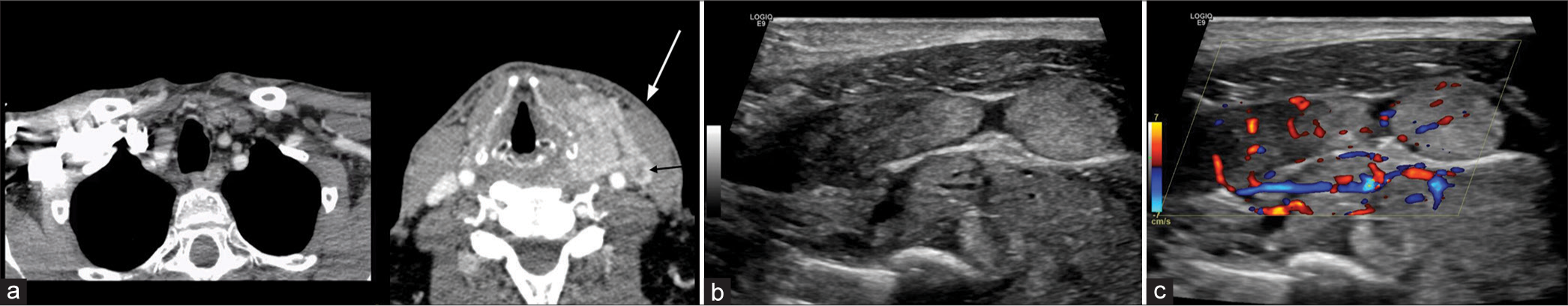
Export to PPT
Special considerations in post-treatment patients Carotid blowout (CBO)Common etiologies for CBO include radical resection, radiation necrosis, carotid exposure, wound infection, pharyngocutaneous fistula, and recurrent tumors. In addition, this can present after neck dissection (overall incidence of ~4.3%). While prior radiation has been shown to be an independent risk factor for CBO, overall risk has also been shown to increase substantially (7.6-fold) following neck dissection in patients with prior radiation.[15] CBO is classically divided into three categories: Threatened, impending, and acute.[16] Threatened CBO has imaging or clinical evidence of carotid at risk for rupture with the patient remaining asymptomatic. Common imaging findings include necrotic tissue/tumor adjacent to a vessel or the presence of a pharyngocutaneous fistula adjacent to a vessel. Impending CBO consists of transient bleeding that resolves spontaneously or with packing/pressure. Imaging may show a focal pseudoaneurysm without active bleeding or visible hematoma [Figure 7].[17] Acute CBO is the presence of uncontrollable hemorrhage requiring surgical/endovascular intervention. Imaging often shows active contrast extravasation at the site of vessel injury. Although the radiologist has a key role in the workup of all three types, identifying a threatened CBO can be the most beneficial as this diagnosis may preclude clinical suspicion.
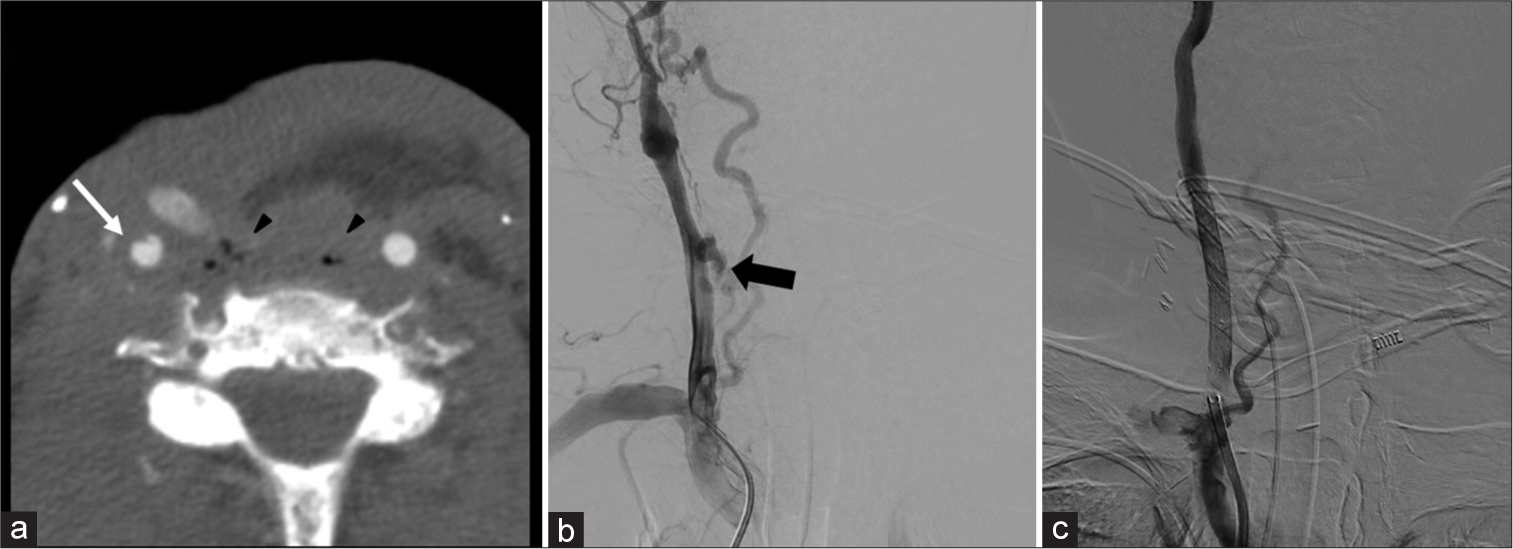
Export to PPT
Carotid stenosis/occlusionIn the delayed phase, radiation increases the intima-media thickness and rapidly accelerates atherosclerosis,[18] which can result in progressive stenosis or thrombotic occlusion. Occlusion has been reported up to 10 years after treatment,[19] and can be seen in patients with no other concurrent risk factors.[20] In addition, adjacent soft-tissue radiation changes such as perivascular circumferential soft-tissue formation may provide subtle clues of this particular etiology. Figure 8 demonstrates progressive carotid artery occlusion in a patient following chemoradiation. Fortunately, this patient demonstrated reconstitution of flow at the cavernous segment of the internal carotid artery due to the formation of collaterals.
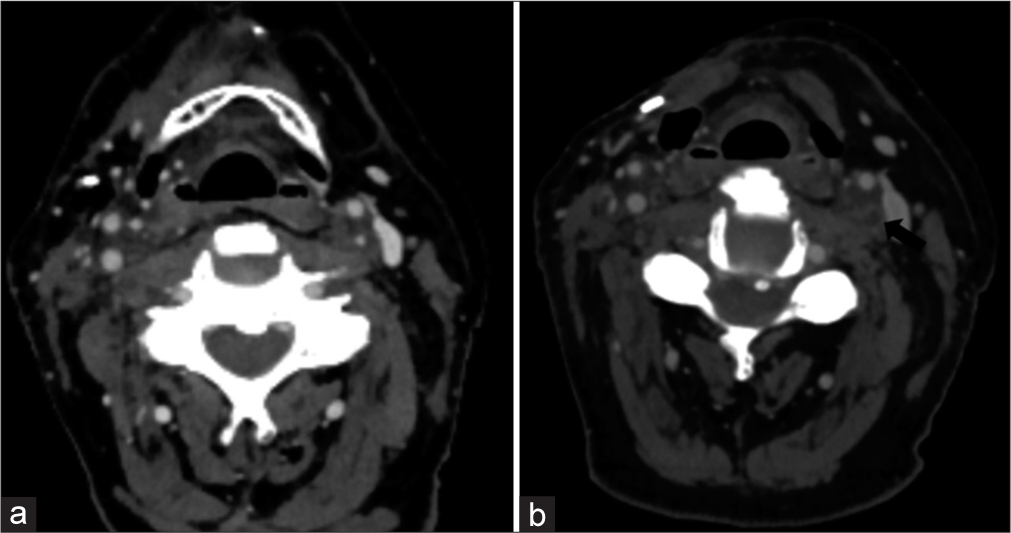
Export to PPT
ORBITALA commonly missed emergent complication of HNC on imaging is that of orbital compartment syndrome. This is a clinical diagnosis that occurs when there is a rise in orbital pressure (>20 mmHg) resulting in clinical signs of optic nerve ischemia. Symptoms such as enlarging blind spots, decreased color vision, and relative afferent pupillary defects are often clinically encountered. The low compliance of the rigid orbital walls results in low tolerance for optic nerve and retinal artery compression. The most common compressive etiologies include retrobulbar hemorrhage, infection, orbital emphysema, neoplasm, and recent surgery. Key radiographic findings include proptosis and tenting of the posterior globe resulting in a “guitar pick morphology”[21] [Figure 9]. Stretching of the optic nerve can also be a useful radiologic finding.[22]
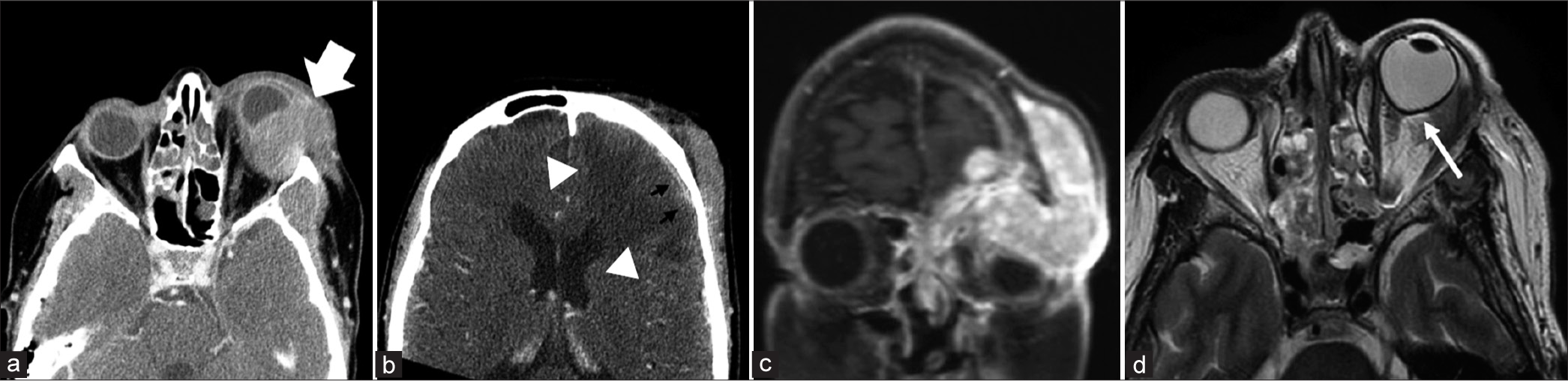
Export to PPT
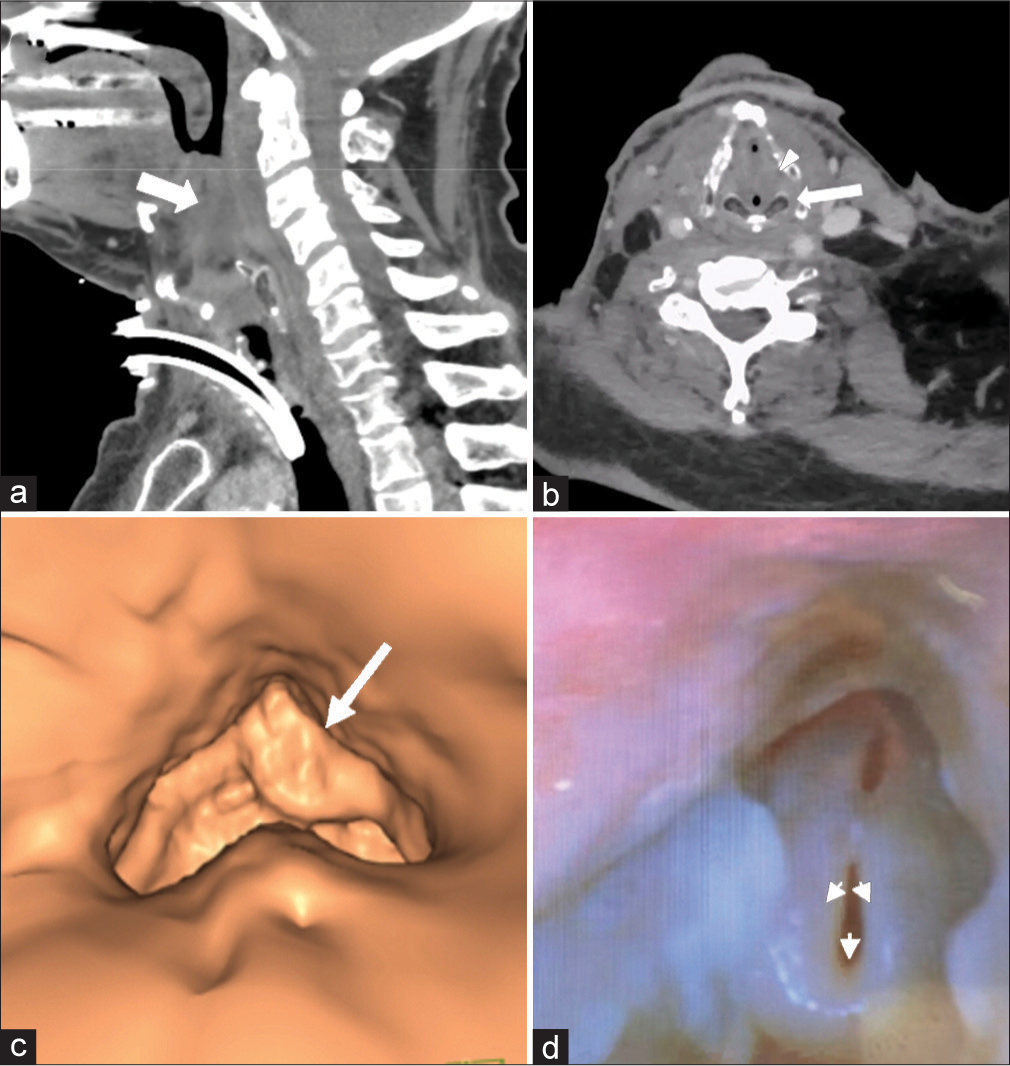
CERVICAL SPINEDiscitis/osteomyelitis can be seen with dehiscence/breakdown of the pharynx or neopharynx. This is often seen after esophageal dilatation (a treatment for post-radiation upper esophageal strictures), likely as a result of bacterial translocation (Goldman et al., 2014). Key imaging findings include disc space fluid and adjacent vertebral body endplate erosion. Prevertebral or epidural phlegmon/abscess may also be present [Figure 10]. Identifying a site of possible pharyngeal dehiscence/breakdown is critical to guide clinical management. Of note, infectious discitis/osteomyelitis has a predilection for the avascular disc space before involving the vertebral body (unlike metastasis, which primarily involves the highly vascularized vertebral body). Figure 10 demonstrates two destructive C6 lesions; one lesion demonstrates an infectious etiology originating from the disc space and extending to the vertebral bodies, and the other demonstrates metastatic etiology, primarily encompassing the vertebral body.
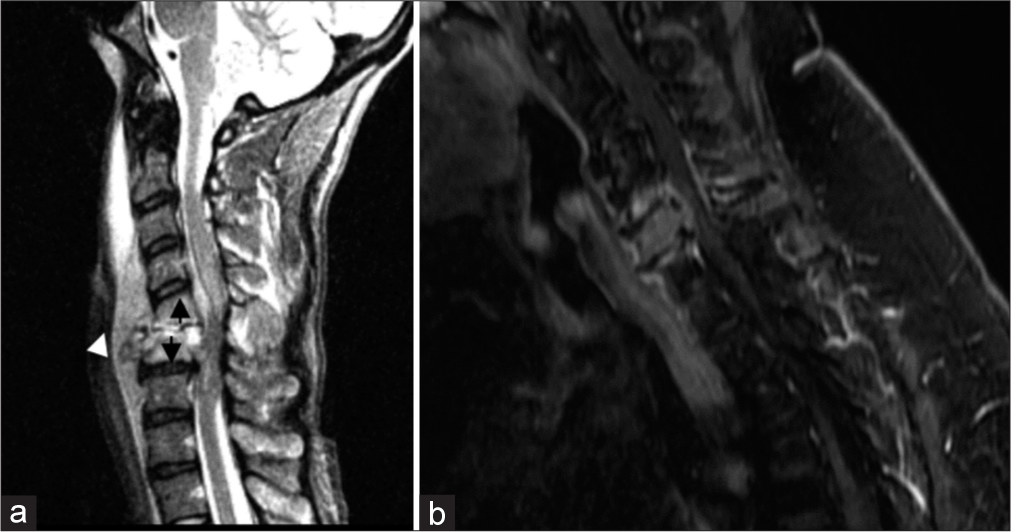
Export to PPT
Special considerations in post-treatment patientsOsteoradionecrosis has been reported in the cervical spine following radiotherapy for HNC, of which nasopharyngeal carcinoma is most commonly described.[23] Common presenting symptoms include neck pain, kyphosis, paresthesia, discitis/osteomyelitis, fracture, and cord compression. This often has a subacute or chronic course, occurring between 4 and 48 months after treatment. Key imaging findings are similar to discitis/osteomyelitis, with mixed sclerotic-lucent appearance and bone destruction. Often confounded by secondary discitis/osteomyelitis, a subtle differentiating imaging feature can be the absence of fluid in the disc spaces. However, tissue sampling is often needed to exclude the presence of infection as findings can be nearly identical. Figure 11 demonstrates progressive cervical destruction at the C5–6 level in subsequent outpatient images. This patient was noted to have negative blood cultures and even demonstrated no culture growth on biopsy. The patient was later taken for cervical decompression and fusion due to concern for spinal instability. This case emphasizes how unrecognized cervical spine osteoradionecrosis can result in instability necessitating surgical fixation. In addition, magnetic resonance imaging demonstrates no fluid in the intervertebral disc spaces, which is not a specific finding but can support the diagnosis of osteoradionecrosis over osteomyelitis.
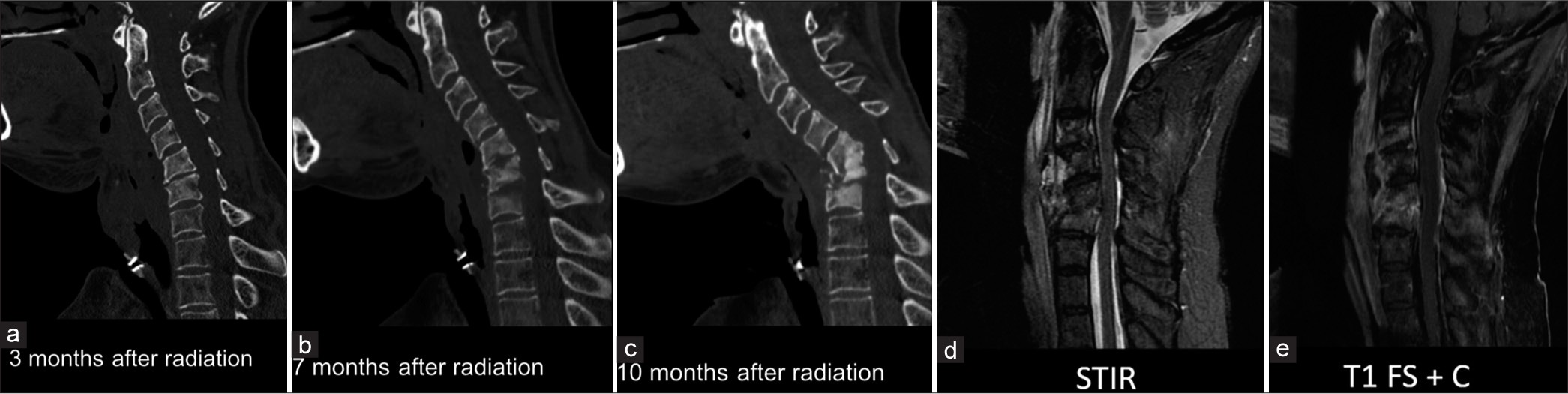
Export to PPT
CONCLUSIONPatients with HNC can present with uniquely challenging imaging features in the emergent setting. The difficulty in discerning the correct diagnosis arises from the complex head and neck anatomy, often compounded by advanced stage at presentation, poor overall functional status, and the potential for disastrous consequences in missed diagnoses. Radiologist familiarity with common HNC emergent presentations is essential for the accurate diagnosis of these patients; and radiologists are uniquely equipped to enable detection, ensuring prompt patient care.
留言 (0)