Hosts become a fundamentally altered niche following infection. Any plant pathology textbook offers many examples of how pathogens can modify the morphology of hosts with the level of modification ranging from intracellular to the whole organism (Agrios, 2005). While these changes are well-documented, it remains unknown how changes to the host create new and preferred niches for organisms beyond the infecting pathogen. We and others have demonstrated that infected plants increase the incidence of rare members of the plant microbiota, namely bacterial human pathogens, such as Salmonella enterica (Cowles et al., 2022; Dixon et al., 2022; Potnis et al., 2014, 2015; Barak et al., 2008; Kwan et al., 2013; Wells and Butterfield, 1997; Yang et al., 2020; Ginnan et al., 2020; Gao et al., 2021). This increase in incidence and population growth results from the conversion of the rarely accessible, inhabitable interior space of the leaf, the apoplast, to an obtainable, habitable niche following infection.
Epiphytic bacteria, those found on the surface of plants, tolerate a harsh environment with rapidly fluctuating conditions. Survival of recent bacterial immigrants on a leaf surface is mostly due to luck on arrival near or in an oasis of nutrient and water availability, the base of glandular trichomes or in the grooves between cells (Monier and Lindow, 2003, 2004; Barak et al., 2011). Flagellar motility and the capacity to form aggregates, either inter- or intra-species, increases an immigrant’s probability of survival (Haefele and Lindow, 1987; Lindow et al., 1993). Success requires adaptation to changes in water or nutrient availability, temperature, and UV irradiation levels, among others. Bacterial phytopathogens lacking cell wall degrading enzymes abandon the leaf surface and choose the apoplast for their infection court. The leaf apoplast has several obvious advantages over the leaf surface: protection from most UV irradiation, little to no cuticular wax encasing plant cell surfaces, and reduced fluctuation in free water. Although the existence of bacteria in the apoplast is well documented by microscopy (Boureau et al., 2002), along with many bacterial factors that are necessary for disease, in general, little is known about the dynamic colonization of an infected apoplast during disease progression and distinct changes in the infected host.
One of most widely studied bacterial-plant interactions is Arabidopsis thaliana as a model host for Pseudomonas syringae pv. tomato (Pst). Pst uses a jasmonic acid mimic coronatine (COR) to open stomata for access to the leaf apoplast (Melotto et al., 2006). Pst then further manipulates the host immune system using Type III effectors HopM1 and AvrE to induce the abscisic acid pathway and to close stomata to increase water potential in the apoplast (Melotto et al., 2017; Roussin-Léveillée et al., 2022; Hu et al., 2022). These findings are the foundational understanding of the disease Pst causes in tomato, bacterial speck. A macroscopically similar disease, bacterial spot of tomato, is caused by four lineages of Xanthomonas: X. hortorum pv. gardneri (hereafter referred to as Xhg), X. euvesicatoria pv. euvesicatoria, X. euvesicatoria pv. perforans, and X. vesicatoria (Jones et al., 1998; Osdaghi et al., 2021; Timilsina et al., 2020). Tomato infection with either Pst or Xhg is characterized by water-soaked lesions and abundant phytobacterial growth.
Multiple works from our lab have demonstrated that Xhg infection alters the tomato host in ways that benefit non-phytopathogenic bacteria inhabiting the leaf surface (Cowles et al., 2022; Dixon et al., 2022; Potnis et al., 2014, 2015). Although the primary purpose of altering the plant environment during infection is likely for the benefit of the pathogen, sweeping changes in physical and biochemical characteristics of the host reshape the composition of the bacterial community in an infection court as shown in both human (Manos, 2022) and plant infections (Griffiths et al., 2020; Hu et al., 2020). Bacterial communities in multiple plant systems are impacted by the changes that occur during disease progression (Gao et al., 2021; Li et al., 2022; Huang et al., 2023). In tomato, we have found that the dramatic change to the apoplast as a result of Xhg infection creates an available and habitable niche for bacteria that are usually precluded from stomatal entry and exiled to the leaf surface, such as S. enterica serovar Typhimurium (S. Typhimurium) (Cowles et al., 2022; Dixon et al., 2022; Potnis et al., 2014, 2015). Xhg infection does two things: (1) permits S. Typhimurium access to the apoplast, a niche that the human pathogen cannot access on its own and (2) transforms the apoplast into a habitable niche for S. Typhimurium, altering the apoplast in ways that promote bacterial replication (Cowles et al., 2022; Dixon et al., 2022; Potnis et al., 2014, 2015). However, the mechanisms driving changes to the host that create new and preferred niches for organisms beyond the infecting pathogen and that influence bacterial dynamics in the apoplast remain unknown.
Here, we expanded our investigation of how leaf infection impacts the host and examined whether bacteria that create a water-soaked apoplast during infection, in general, permit leaf surface bacteria entry to this altered niche. We discovered that, unlike Xhg infection which promotes S. Typhimurium success, Pst infection disrupts S. Typhimurium colonization of the apoplast. In addition, we found that this effect is not specific to S. Typhimurium, but that immigrating populations of Xhg or Pst also struggled to join the infecting Pst population established in the apoplast. These results support several possible mechanisms that we began to investigate in this work: (1) changes to a Pst-infected plant result in a barrier to apoplast entry, (2) Pst infection creates an inhospitable niche, or (3) macroscopically imperceptible distinctions between immigrating bacteria and established Pst populations impact bacterial survival. By comparing and contrasting strategies used by Pst and Xhg, this study provides fundamental information about bacterial disease of leaves and reveals mechanisms used to reshape the host environment resulting in either a conducive or restrictive niche in the diseased host.
Results Pst infection delays successful S. Typhimurium colonization of tomato leavesPreviously, we had shown that an established Xhg infection promotes the growth of newly arriving S. Typhimurium on tomato leaves (Dixon et al., 2022). To determine if another phytopathogen that causes water-soaking, Pst, also enhances S. Typhimurium persistence, we examined the impact of Pst infection on S. Typhimurium populations over time. As done previously, tomato leaves were infiltrated with phytopathogen, infection was allowed to proceed for 48 h post-infiltration (HPI), and S. Typhimurium was applied to the infected leaf surface as a droplet (Dixon et al., 2022). UV irradiation was used to distinguish surface localized S. Typhimurium from S. Typhimurium found within the apoplast (Dixon et al., 2022). Here, UV treatment was not a significant factor in bacterial populations (p > 0.01), and data from UV-treated and non-UV-treated samples were collapsed to create Figure 1. At the arrival site (Figure 1A), S. Typhimurium suspensions were absorbed equally amongst the three treatments, and no significant differences in S. Typhimurium populations were observed at 3 h post-arrival (HPA) between treatments or from the starting inoculum. Xhg infection resulted in 2–3 log higher apoplastic S. Typhimurium populations compared to the initial arriving population and 1–2 log higher populations compared to plants treated with Pst or water infiltration at 24, 48, and 72 HPA (Figure 1A). Contrastingly, S. Typhimurium populations did not significantly increase from starting inoculum levels in Pst-infected leaves until 48 HPA and remained lower than those in Xhg-infected leaves through 72 HPA (Figure 1A). Similar to, but to a lesser extent than, Xhg infection, water congestion in healthy leaves also resulted in increased S. Typhimurium populations compared to the initial arriving population starting at 24 HPA (1–2 log; Figure 1A). However, as with Pst-infected leaves, water congested healthy leaves supported lower overall levels of S. Typhimurium compared to Xhg-infected leaves through 72 HPA (Figure 1A).
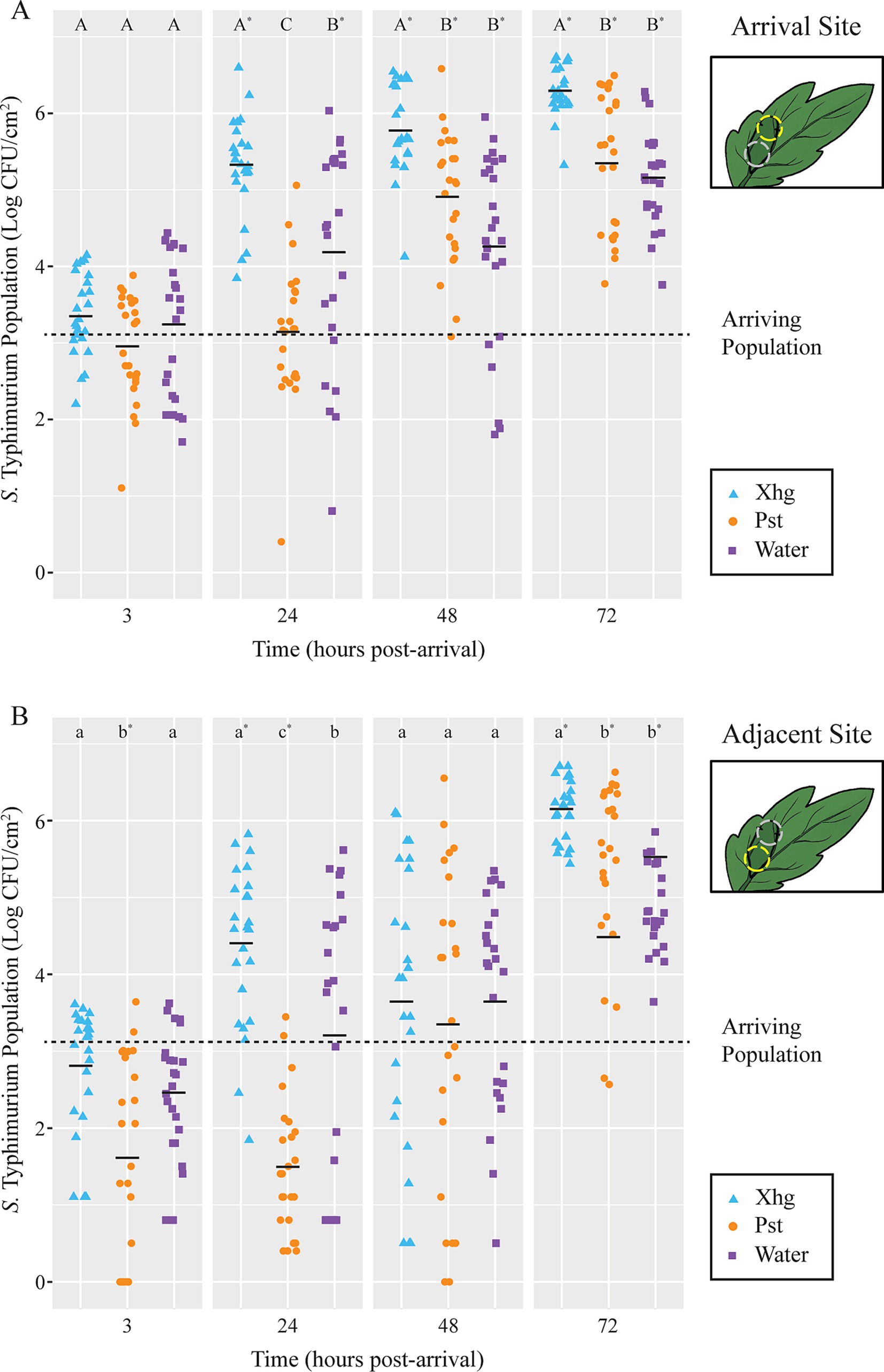
Figure 1. Immigrating S. Typhimurium have a delayed benefit from established Pst infection. S. Typhimurium populations were monitored 3, 24, 48, or 72 h after arrival on tomato leaves previously infiltrated with Xhg (48 HPI; cyan triangles), Pst (48 HPI; orange circles), or water (0 HPI; purple squares). Leaves were sampled at the S. Typhimurium arrival site (A) and a distinct adjacent site within the infiltrated area (B). Data from three independent experiments are presented as log CFU/cm2, and each symbol represents bacterial populations from one tomato leaf. Half of the leaves from each treatment and time point were treated with UV irradiation but data were collapsed as there was no significant difference between UV-treated and non-UV-treated samples (p > 0.01). The dashed line indicates the arriving S. Typhimurium population (3.1 log CFU/ cm2). Means for each treatment at each time point are depicted with horizontal black lines. Letters denote significant differences between treatments within a single time point and leaf site, and asterisks indicate significant differences from the initial arriving population (p < 0.05). Combining three independent experiments, n = 24 leaves per treatment per time point.
To examine migration of arriving bacteria, we monitored bacterial populations at an additional site within the infiltrated area, which we termed the adjacent site (Figure 1B). Bacterial populations at the adjacent site showed similar patterns as the arrival site with several notable differences. First, unlike at the arrival site, S. Typhimurium populations at the adjacent site in Pst-infected leaves are ~1.5 log smaller compared to the arriving population at 3 and 24 HPA (Figure 1B). Second, S. Typhimurium populations at the adjacent site on healthy water congested leaves did not grow from arriving population levels until 72 HPA (Figure 1B), instead of at 24 HPA as seen at the arrival site (Figure 1A). Third, although these experiments tend to show a relatively large amount of variation between samples and individual plants, all treatments displayed a wider range in S. Typhimurium population sizes at the adjacent site, when compared to the arrival site, at 48 HPA (Figures 1A,B). This 6-log range of bacterial population size at 48 HPA at the adjacent site resulted in no significant differences amongst treatments or compared to the arriving population at this timepoint. Phytopathogen populations were also monitored during these experiments and were not significantly different from one another at any time point at either the arrival site or the adjacent site (p > 0.05; Supplementary Figure S1).
Xhg- and Pst-infected leaves have similar macroscopic and microscopic symptomsTo identify differences in Xhg and Pst infection that could explain the delayed colonization of S. Typhimurium, we characterized multiple quantifiable phenotypes from Xhg- and Pst-infected leaves. To simulate a natural infection, tomato plants were dip-inoculated with Xhg and Pst suspensions and resulting disease symptoms were photographed at 1–4 days post-inoculation (DPI). To quantify disease symptoms, we developed the Leaf Lesion Detector application to quantify lesion numbers, size, and the percent infection observed over time in Xhg- and Pst-infected leaves (Figure 2). Representative images from 2 DPI leaves are shown at multiple stages of the application analysis to demonstrate functionality of this new software for potential use with other plant diseases. The details for image analysis are described in the methods below. The percentage of infected area is calculated as a ratio of lesion and leaf pixel counts. Conversion from pixel count to mm2 is handled by multiplying a segment’s pixel count by the ratio of known reference area to known reference pixel count. No significant differences in Xhg or Pst infection characteristics were measured over the four-day time course using the Leaf Lesion Detector app (Figure 2B).
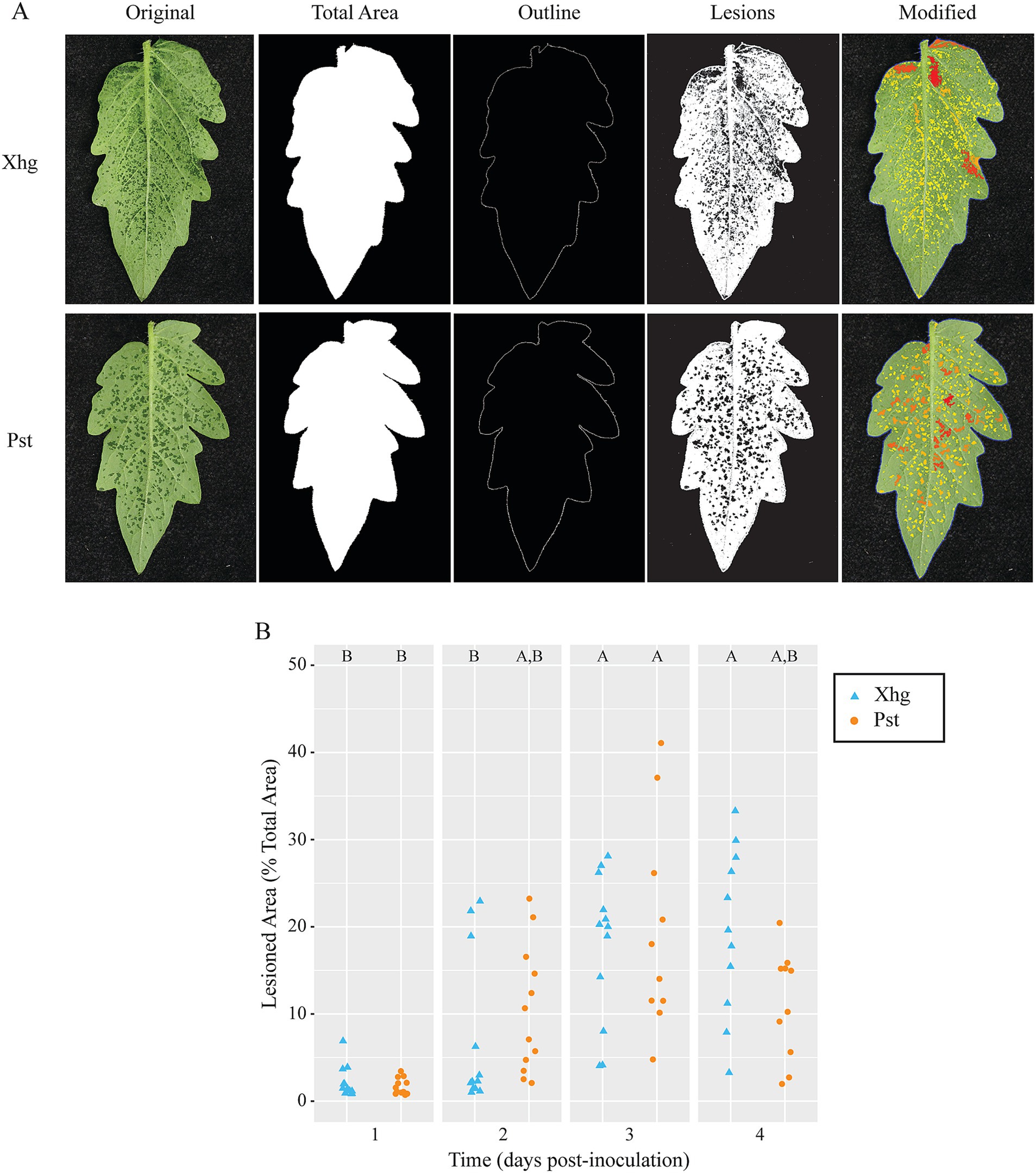
Figure 2. Water-soaking symptoms in Xhg- and Pst-infected leaves are macroscopically similar. (A) Representative images from Xhg- and Pst-infected leaves at 2 DPI are shown at multiple stages of the Leaf Lesion Detector application analysis. Each original leaf image is processed to identify total area, leaf outline, and lesions. The modified image is presented as a color map based on lesion size. (B) Lesions are quantified as the percentage of the total leaf area of Xhg-infected (cyan triangles) or Pst-infected (orange circles) leaves at 1–4 DPI. Letters denote significant differences between treatments within a single time point (p < 0.05). Combining three independent experiments, n = 12 leaves per treatment per time point.
S. Typhimurium persistence in Figure 1 was quantified in infiltrated plants. To qualitatively examine plant disease under those conditions, infiltrated plants were imaged over time, and representative images from infection of each pathogen are shown in Figure 3A. No qualitative differences were observed when comparing infected leaves at each time point. Both Xhg- and Pst-infiltrated leaves showed patchy water soaking at 1DPI, complete water soaking of the infiltrated area by 2 DPI, a combination of water soaking and the beginnings of necrosis at 3 DPI, and drier, more necrotic tissue by 4 DPI (Figure 3A).
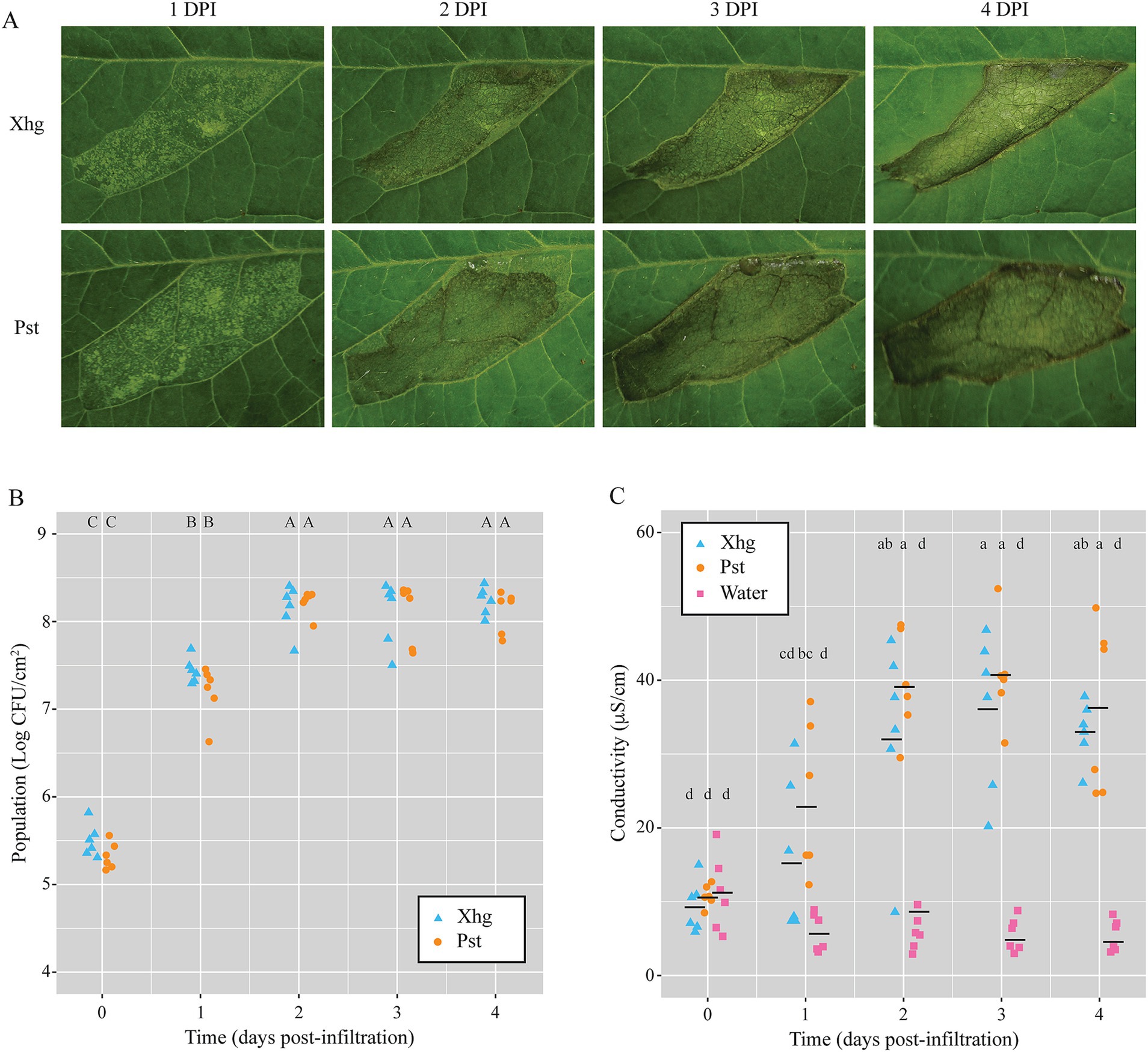
Figure 3. Leaves infiltrated with Xhg or Pst have indistinguishable symptomology, bacterial populations, and cellular damage. (A) Representative images from Xhg- and Pst-infiltrated leaves from 1 to 4 DPI. Images were taken of the same leaves on consecutive days. (B) Bacterial populations were monitored 0–4 DPI from tomato leaves infiltrated with Xhg (cyan triangles) or Pst (orange circles). Leaves were sampled within the infiltrated areas, and Xhg and Pst populations are presented as log CFU/cm2. (C) Electrolyte leakage was measured by conductivity levels (μS/cm) from 0–4 DPI in leaves infiltrated with Xhg (cyan triangles), Pst (orange circles), or water (pink squares). Each symbol represents bacterial populations or conductivity readings from one tomato leaf. Means for each treatment at each time point are depicted with horizontal black lines. Letters denote significant differences between treatments over time (p < 0.05). Combining three independent experiments, n = 6 leaves per treatment per time point.
In addition to visible symptoms following infection, we measured both bacterial populations and cellular damage in infiltrated areas. As with symptomology, no differences were detected between Xhg- and Pst-infected leaves. Infiltrated pathogens reached a carrying capacity of 8.0–8.5 log CFU/cm2 by 2 DPI, and bacterial populations were statistically equivalent at each time point examined (Figure 3B). Electrolyte leakage measured by conductivity was used as a proxy for cellular damage (Stall and Hall, 1984) throughout disease progression compared to leaves infiltrated with water. In parallel with lesion development, infection with both pathogens resulted in increasing levels of conductivity, and no differences were detected between Xhg- and Pst-infected leaves (Figure 3C).
Stomatal aperture patterns differ between Xhg- and Pst-infected leavesPrevious work has shown that Pst opens and closes stomata of Arabidopsis thaliana or tomato leaves depending on the stage of infection (Roussin-Léveillée et al., 2022; Hu et al., 2022). To determine if stomatal aperture patterns could play a role in the different impacts of Xhg and Pst on S. Typhimurium success, we infiltrated leaves with phytopathogen or water and used a dental resin method to capture impressions of stomata over time. Stomatal apertures were measured using ImageJ and indicated by a ratio of width to length. The outline of stomata results in some measurement value for both length and width, making it impossible to get a ratio of 0.0. Thus, we considered a stomatal aperture ratio of 0.25 to be closed. Larger stomatal aperture ratios indicate more “open” stomata. Analysis of the resulting impression images demonstrated that while some temporal differences in stomatal apertures exist between Xhg- and Pst-infected leaves, infection with either pathogen results in open stomata at 48 HPI (Figure 4A), the point in time when S. Typhimurium cells were applied in our earlier experiments (Figure 1). All treatments had relatively closed stomata at 1 and 4 HPI (~ 0.25 aperture ratio; Figure 4A). Pst-infected leaves had open stomata by 24 HPI while Xhg-infected leaves had open stomata at 48 and 72 HPI (> 0.35 aperture ratio; Figure 4A). Water infiltration resulted in open stomata at 24 and 48 HPI (> 0.35 aperture ratio), but stomata were closed at 72 HPI (~ 0.25 aperture ratio; Figure 4A).
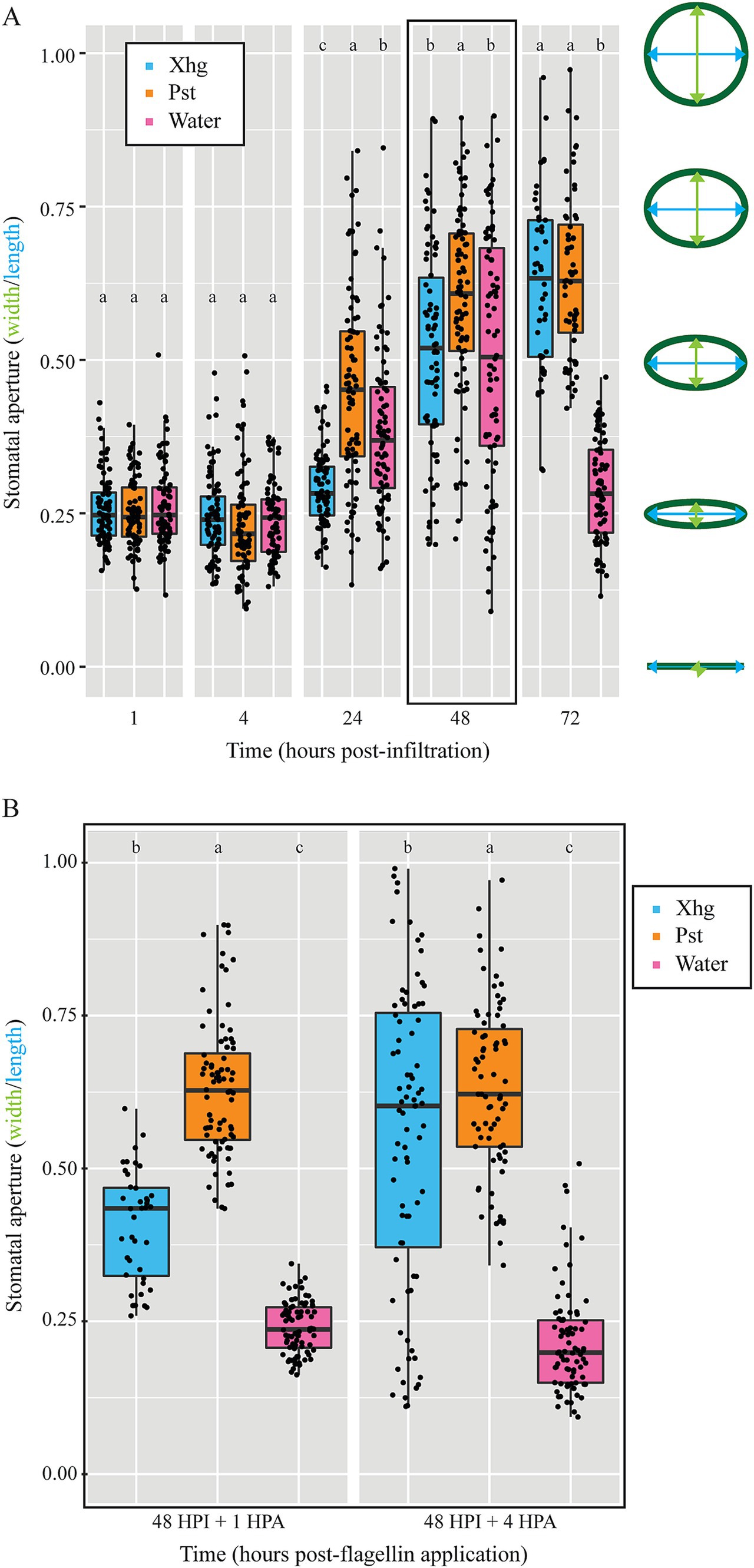
Figure 4. Both Xhg- and Pst-infiltrated leaves have open stomata upon arrival of immigrating S. Typhimurium at 48 HPI. (A) Stomatal apertures were monitored at 1, 4, 24, 48, and 72 HPI in tomato leaves infiltrated with Xhg (cyan), Pst (orange), or water (pink). Apertures were measured using ImageJ and indicated by a ratio of width (green) to length (blue) as depicted on the right. The 48 HPI data are outlined in black to highlight the point when S. Typhimurium arrives at the infected area in other experiments. (B) Stomatal apertures were measured at 1 and 4 HPA of flagellin from leaves that had been previously infiltrated with Xhg (cyan), Pst (orange), or water (pink) 48 h earlier (48 HPI + 1 or + 4). Data from three independent replicates of each experiment are represented as boxplots with each symbol corresponding to one stomatal aperture (n > 50 per treatment per time point). Letters denote significant differences between treatments within a single time point (p < 0.05).
In the S. Typhimurium survival experiments above (Figure 1), both S. Typhimurium and either phytopathogen were present on leaves at the same time while S. Typhimurium was not present in the stomatal aperture experiments in Figure 4A. We hypothesized that stomatal aperture regulation could be influenced by S. Typhimurium, and we repeated the stomata experiments with the addition of S. Typhimurium flagellin, a known signal for stomatal aperture movement (Melotto et al., 2017). As done above, leaves were infiltrated with a phytobacterial pathogen or water, and infection was allowed to proceed for 48 HPI. Then, flagellin was spotted on the surface of infiltrated areas, similar to S. Typhimurium arrival in persistence experiments, and resin impressions were taken 1 or 4 h later (48 HPI + 1 and 48 HPI + 4). In persistence experiments (Figure 1), droplets of S. Typhimurium suspensions were absorbed into leaves by 3–4 h post-arrival, so these time points represent stomatal apertures at the time of S. Typhimurium arrival and absorption. As stated above, at 48 HPI, water-treated leaves had open stomata in the absence of flagellin (Figure 4A). However, in the presence of flagellin, water-treated leaves had closed stomata at both 48 HPI + 1 and 48 HPI + 4 (< 0.25 aperture ratios; Figure 4B). In contrast, both Xhg- and Pst-infected leaves had comparably open stomata in both the presence and absence of flagellin (> 0.35 aperture ratio; Figures 4A,B). Xhg-infected leaves had smaller stomatal apertures compared to Pst-infected leaves at both time points post-flagellin treatment (Figure 4B).
Phytopathogens target potential competing bacteria in an in vitro growth inhibition assayOur data demonstrates that Xhg infection creates a more conducive environment for S. Typhimurium persistence (Cowles et al., 2022; Dixon et al., 2022; Potnis et al., 2014, 2015) (Figure 1). To test the hypothesis that Pst could be directly inhibiting growth of other bacteria, we performed assays to study bacterial competition (Hood et al., 2010). In these experiments, in planta grown Xhg or Pst were mixed with target bacterial strains to assess growth inhibition. These assay conditions mimic the arrival of naïve bacteria to an established phytopathogen infection in leaves; bacterial strains are grown under the same conditions and mixed at the same ratios (1:1000; target strain to killer strain). Homogenized, healthy leaf tissue was used as a control for potential effects of plant factors. These data demonstrate that incubation with homogenized healthy leaf tissue had no impact on S. Typhimurium levels while Xhg- or Pst-infected tissue samples negatively affected S. Typhimurium populations (Figure 5A). Incubation with Xhg-infected tissue reduced S. Typhimurium populations by ~0.25 log while incubation with Pst-infected tissue resulted in ~1.25 log reduction in S. Typhimurium (Figure 5A).
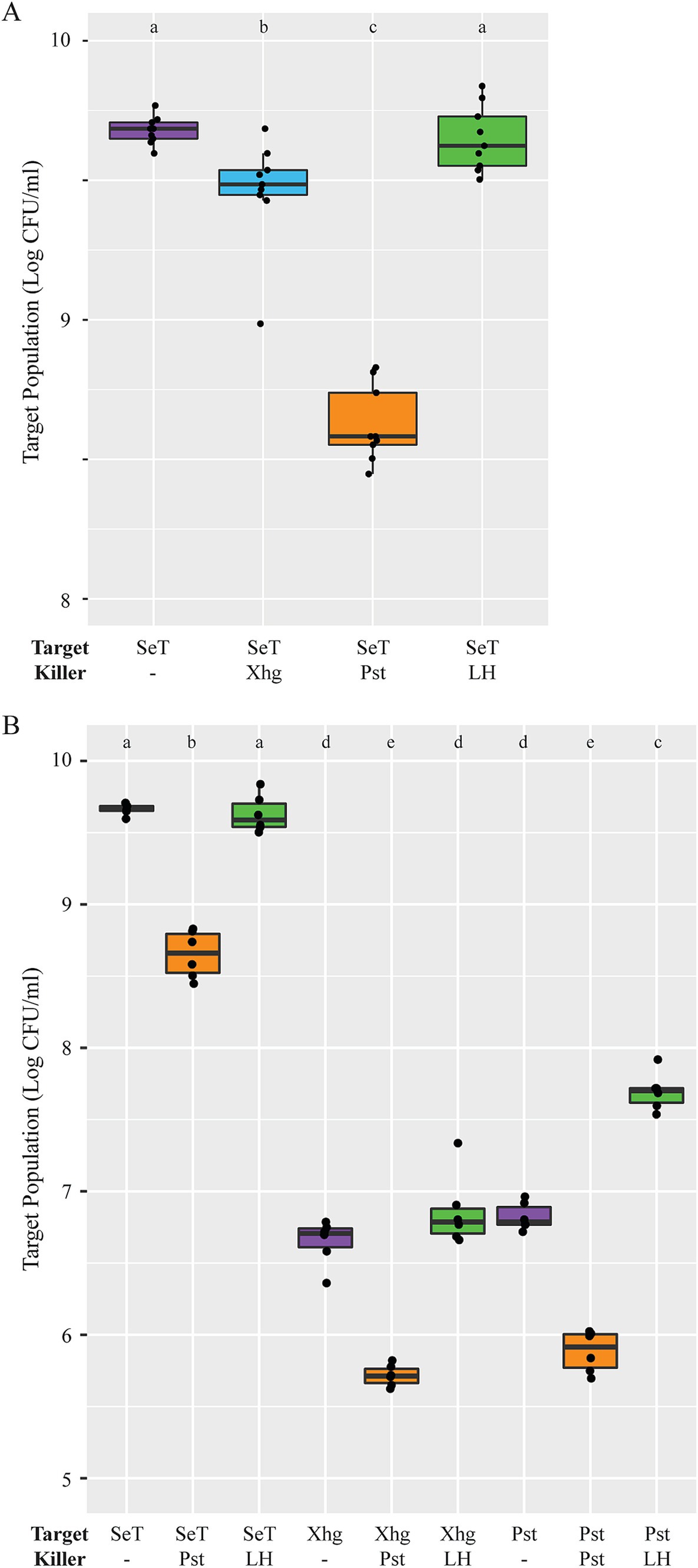
Figure 5. In planta grown Pst inhibits in vitro grown S. Typhimurium. (A) Using a bacterial competition assay, S. Typhimurium populations were measured after 24-h incubation with water (purple), in planta grown Xhg (cyan), in planta grown Pst (orange), or healthy leaf homogenate (green). (B) Assays were repeated with three target bacterial strains (S. Typhimurium, Xhg, and Pst) and three treatment conditions (water, purple; in planta grown Pst, orange; healthy leaf homogenate, green). Target bacterial population data from two independent experiments are presented as log CFU/mL in boxplots (n = 6). Letters denote significant differences between treatments (p < 0.05).
To determine if in planta grown Pst affects other target bacteria, we performed additional competition assays with in vitro grown S. Typhimurium, Xhg, and Pst as the target strains. As seen above, Pst-infected tissue lowered S. Typhimurium populations by ~1.25 log while homogenized tissues from healthy plants had no effect (Figure 5B). As with the S. Typhimurium target, incubation of Pst-infected tissue with Xhg or Pst targets reduced populations by ~1 log (Figure 5B). While incubation with homogenized healthy leaf tissue had no impact on S. Typhimurium or Xhg populations, Pst target populations increased ~1 log when compared to incubation with water (Figure 5B).
A naïve, water-congested environment promotes phytopathogen success over pre-colonized tissuesWith the knowledge that in planta grown Pst inhibits S. Typhimurium, Xhg, and Pst growth, we monitored the fate of newly arriving Xhg or Pst (immigrants) on leaf tissue with an established Xhg or Pst infection. As above for S. Typhimurium, leaves were infiltrated with Xhg or Pst, incubated for 48 HPI, and immigrant Xhg or Pst cells were applied as droplets to the infection site. One day after arrival on water-congested, healthy leaf tissue, both Xhg (Figures 6A,B) and Pst (Figures 6C,D) immigrant populations were 1–3 log larger compared to the initial arriving population, suggesting growth in healthy water-congested apoplasts that lacked established bacterial populations. UV irradiation to remove surface bacteria (Dixon et al., 2022) reduced both Xhg and Pst immigrant populations ~1.0 log compared to non-UV treated samples in water-congested leaves (Figure 6). However, UV-treated samples, at the arrival site, still had higher populations when compared to the initial arrival population, suggesting that the phytobacterial pathogen immigrants migrated to the UV-protected apoplast and grew (Figures 6A,C). UV-treated samples from the adjacent site were not significantly different from the arriving population (Figures 6B,D).
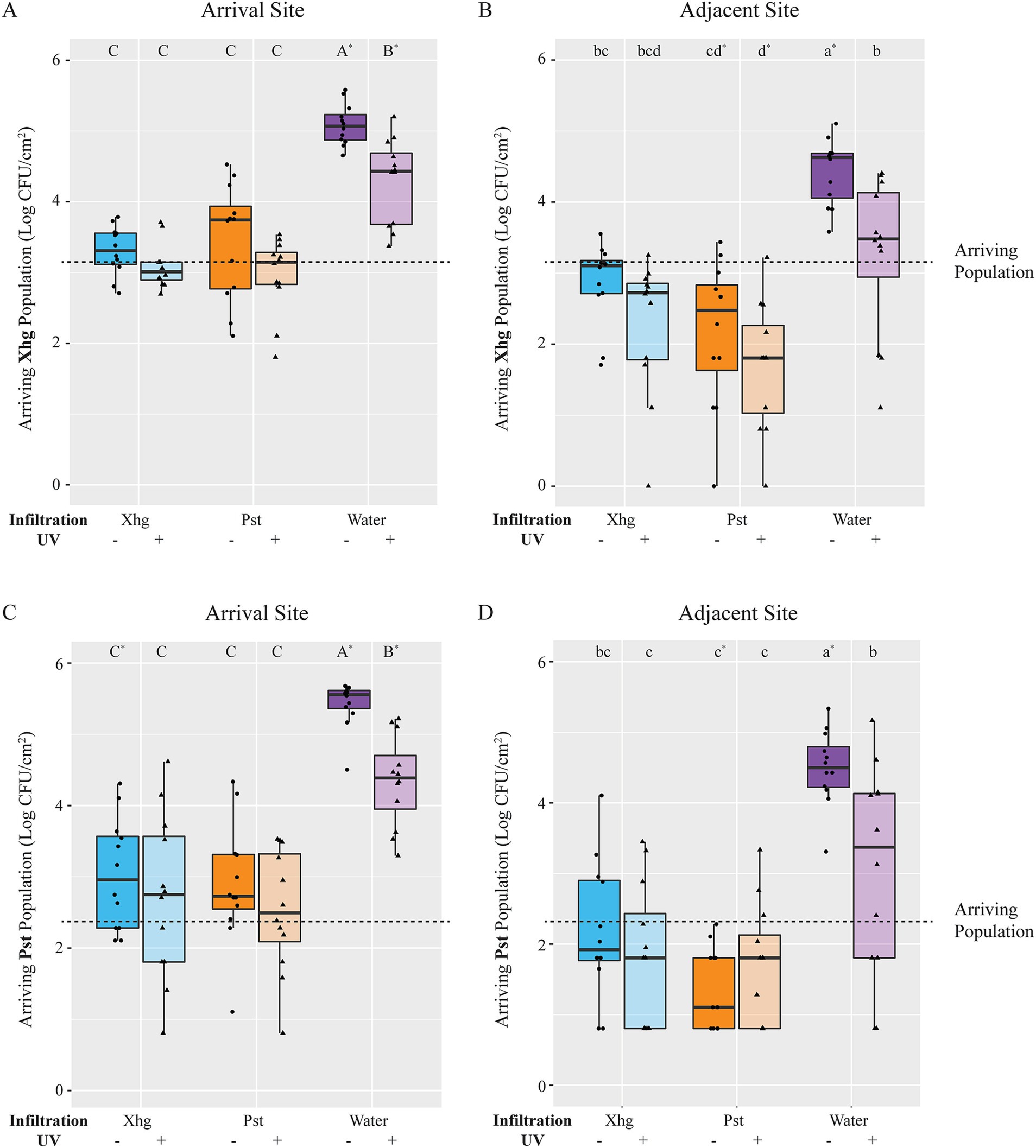
Figure 6. The fate of immigrating bacteria depends on the identity of the established infection. Xhg (A,B) and Pst (C,D) populations were monitored 24 h after arrival on tomato leaves previously infiltrated with Xhg (48 HPI; cyan), Pst (48 HPI; orange), or water (0 HPI; purple). Leaves were sampled at the arrival site (A,C) and a distinct adjacent site within the infiltrated area (B,D). Data from three independent experiments are presented as log CFU/cm2 in boxplots, and each symbol represents bacterial populations from one tomato leaf (n = 12 leaves per treatment per time point). Half of the leaves from each treatment and time point were treated with UV irradiation and distinguished here with color shading. The dashed line indicates the arriving bacterial population (3.1 log CFU/cm2). Letters denote significant differences between treatments within a single leaf site for each bacterium separately, and asterisks indicate significant differences from the initial arriving population (p < 0.05).
In contrast to increased immigrant populations on water-congested, healthy leaves, leaves with established Xhg or Pst infection, for the most part, either inhibited or reduced immigrant bacterial populations. In addition, unlike populations on water-congested, healthy leaves, UV irradiation had no impact on immigrant Xhg and Pst populations on infected leaf tissue (Figure 6), suggesting that the bulk of these bacteria had migrated into the UV-protected apoplast. Regardless of UV treatment, Xhg immigrants arriving on Xhg-infected leaves were not significantly different from the initial arriving populations (Figures 6A,B), indicating a lack of growth. Contrastingly, Pst immigrants arriving on Xhg-infected leaves were either the same as the arriving population or ~ 0.5 log higher (Figures 6C,D). While some Pst immigrants displayed growth from the initial arriving population, Pst populations were 1.5–2.5 log below levels seen in water congested, healthy tissue (Figures 6C,D), suggesting that Pst immigrants have some success on Xhg-infected leaves but replicate to greater numbers on naïve water congested tissue. Commonly, the Pst-infected environment had a more negative impact on immigrant bacteria, especially S. Typhimurium (Figure 1) and Xhg (Figures 6A,B), than the Xhg-infected leaf. For example, 1 day after arrival on Pst-infected leaves, Xhg immigrant populations at the adjacent site were reduced ~0.5–1.5 log compared to the initial arriving populations (Figure 6B). Comparably, 1 day after arrival on Pst-infected leaves, Pst immigrant populations at the adjacent site either remained at arriving population levels or were reduced by 1.0 log (Figure 6D).
DiscussionThe leaf surface is a highly heterogenous environment and can be characterized as a fragmented habitat driving the development of microbial communities (Doan et al., 2020; Fahrig, 2003; Schlechter et al., 2019). Factors that determine the maximum number of individuals on the leaf surface are host-driven (plant species, water pooling, or nutrient leaching through the cuticle) or bacteria-driven (environmental stress response, motility, or aggregate formation). An immigrant’s fate on the leaf surface is determined by the luck of arriving at or near an oasis (Remus-Emsermann and Leveau, 2010) or with others (Monier and Lindow, 2003; Remus-Emsermann et al., 2012). One avenue used by phytopathogenic bacteria to infect leaves is entry to the leaf interior through stomata, the gas exchange portal. Early in infection, both Xhg and Pst transform the air-filled apoplast to an aqueous environment (reviewed in Xin et al., 2016). Based on our results, bacteria that arrive on a leaf as immigrants and attempt to establish themselves in the apoplast appear to encounter fundamentally different niches upon arrival to a naive host compared with a plant hosting a previously established infecting population. Furthermore, our data show that the identity of the established population also influences the fitness outcomes for the immigrating bacteria.
Nutrient availability in the apoplastUpon arrival to a healthy, water-congested apoplast, all three examined bacteria, whether phytopathogenic or not, demonstrated a two-log increase in growth from the initial inoculum population (Figures 1, 6). The similar fate for bacteria on a healthy, yet water-congested, host suggests that uptake of the droplet containing bacteria concurrently allows bacterial entry to the apoplast. In addition, bacteria that reach the apoplast have access to some degree of available nutrients, even S. Typhimurium which does not manipulate the host niche. A “dry” healthy apoplast is mainly air-filled with a thin water film over cell surfaces. Apoplastic nutrients are thought to be in a bound state, attached to cell surfaces, or within surrounding cells, and relatively unavailable to invading bacteria (Roussin-Leveillee et al., 2024; Melotto et al., 2008). It is hypothesized that an influx of water in the “wet” healthy apoplast may disrupt metabolite uptake and result in a dysregulation of metabolite partitioning with higher than normal water-soluble metabolite concentrations in the apoplast (Roussin-Leveillee et al., 2024; Gentzel et al., 2022). This access to nutrients could lead to the observed increases in S. Typhimurium, Xhg, and Pst growth (Figures 1, 6). The temporary relief from nutrient restriction appears to be nondiscriminatory and available to any bacterium lucky enough to arrive in a water droplet on the water-congested leaf.
Unlike the transient nature of abiotic water congestion described above, pathogen-induced water soaking is maintained over days and represents a more complex system of pathogen manipulation of the host and the host response. As with abiotically water-congested leaves, S. Typhimurium replicates within the protected niche of the Xhg-infected apoplast within the first 24 h of arrival on infected tissue (Figure 1). Contrastingly, S. Typhimurium does not demonstrate growth in Pst-infected tissue until 48 HPI and does not reach the same level as S. Typhimurium populations in Xhg-infected tissue at any examined time point (Figure 1). There are also differences in the fates of immigrant phytopathogens on infected tissue, compared with abiotically water-congested leaves. Neither Xhg nor Pst immigrants increase from arriving population levels on Xhg- or Pst-infected leaves (Figure 6). The static nature of these populations could represent lack of replication, slow replication (that is not detectable within 24 HPI), or an equal rate of replication to death, resulting in no net increase in population levels. We hypothesize that the differential impact of established phytopathogens and/or infections on immigrant success results from differences in available resources within the apoplast. In support of the idea that competition plays an important role in this niche, we showed that in planta grown Pst inhibits growth of multiple bacteria, including S. Typhimurium, Xhg, and even Pst itself (Figure 5). Monopolization of resources within the infection court by established bacteria could lead to direct competition for space and/or nutrients.
The formation of distinct niches based on the identity of the infecting phytopathogen may be the result of the available nutrient profile. As mentioned above, water congestion itself leads to a misregulation of metabolite partitioning (Roussin-Leveillee et al., 2024; Gentzel et al., 2022) and growth of S. Typhimurium (Figure 1). Water soaking due to infection can also influence plant metabolism directly to the benefit of the resident organism. Phytobacterial pathogens such as Pst and Ralstonia solanacearum use secreted effectors to manipulate their host to create a more nutritionally favorable environment or increase specific nutrient availability (Ward et al., 2010; Xian et al., 2020). Multiple additional phytopathogens also hijack plant metabolism to shift source leaves into sink leaves, providing the pathogens with access to nutrients (McIntyre et al., 2021; Rodenburg et al., 2019; Doehlemann et al., 2008; Gohlke and Deeken, 2014). Alternatively, established Xhg populations may have distinct nutritional requirements from S. Typhimurium, allowing both bacteria to thrive, while established Pst populations have overlapping nutritional needs, resulting in competition for resources. Future experiments examining the metabolite profiles of the infected apoplasts could reveal details of this mechanism.
Stomatal response and bacterial entryWithout cell wall degrading enzymes, bacteria like S. Typhimurium have restricted access to the leaf interior through natural openings such as stomata, hydathodes, and wounds (Golberg et al., 2011; Chahar et al., 2021). Although the primary function of stomata is the regulation of gas exchange and water retention, stomatal cells (guard cells) can recognize conserved microbe associated molecular patterns (MAMPs) and close the opening to reduce or prevent bacterial entry (Melotto et al., 2006; Melotto et al., 2017; Melotto et al., 2008; Daszkowska-Golec and Szarejko, 2013). We see this closure response in our experiments when S. Typhimurium flagellin is added to the surface of tissue infiltrated with Xhg or water (Figure 4). Some bacterial phytopathogens that infect the apoplast have evolved toxins that open stomata, increasing access to the leaf interior (reviewed in Melotto et al., 2017; Melotto et al., 2008). Published works describe a model where Pst uses stomatal apertures as an entryway; using T3SS effectors to open stomata for bacterial entry and close the door behind them to create a water-soaked environment and reduce evaporation (Melotto et al., 2006; Roussin-Léveillée et al., 2022; Hu et al., 2022). We hypothesized that the closed stomata in Pst-infected tissue may prevent S. Typhimurium entry when it arrives at 48 HPI, thus delaying S. Typhimurium success in this environment. However, unlike published works in A. thaliana (Roussin-Léveillée et al., 2022; Hu et al., 2022), our data demonstrate that from 24 HPI to 72 HPI, Pst-infected tomato leaves maintain open stomata (Figure 4). We predict that differences between our results here and published results (Roussin-Léveillée et al., 2022; Hu et al., 2022) are due to multiple differences in the investigated plant—microbe interactions. We anticipate that the primary driver of these disparities come from differences in the Pst strains used in the respective experiments. Pst DC3000 is pathogenic towards some tomato cultivars and A. thaliana while the Pst NY15125 strain used here is nonpathogenic towards A. thaliana (Kraus et al., 2017). There are also known differences between these two bacterial strains in both T3SS effector profiles as well as virulence towards tomatoes, depending on the tomato host (Kraus et al., 2017; Mazo-Molina et al., 2019). As T3SS effectors are vital for manipulating stomatal immunity, it is not surprising that profile differences could result in differential effects on stomata. While these results point to subtle differences between the two phytopathogens in terms of plant response to environmental cues, S. Typhimurium has access to open stomata in both Xhg- and Pst-infected leaves. Thus, differential regulation of stomata does not explain why Pst fails to support S. Typhimurium colonization as quickly as Xhg.
Dispersal within host tissueFor more than a century, plant pathologists have used disease severity indices to rate gross aspects of infection that are generally based on the percent area with visible symptoms (for review Bock et al., 2022). Yet, this disease quantification is a crude characterization of changes to the infected host and reveals nothing of the infected population’s current activities and dispersal since the bacterial pathogen is rarely isolated from diseased tissue. To quantify changes to the host following infection, we created a new software application, Leaf Lesion Detector (LLD). This application is publicly available and provides a new tool for researchers examining plant disease. Here, LLD was used to quantify disease progression in Xhg- and Pst-infected leaves (Figure 2) and demonstrated that both phytopathogens produced quantitatively and qualitatively similar lesions, in abundance and size, over the course of infection. While no differences were noted that would explain the differential success of S. Typhimurium in either dip-inoculated or infiltrated plants, heterogenous patches of water soaking developed in both Xhg- and Pst-infiltrated leaves at 1 DPI (Figure 3A). The process of infiltration immediately produces a homogenously water-congested area that quickly dissipates within several hours. The resulting heterogeneity of apoplast water soaking just 1 day later suggests that the phytopathogens may form microcolonies or that infection of host cells is not in synchronicity before water-soaking floods the entire infected area by 2 DPI (Figure 3A). The formation of distinct subpopulations occurs in P. syringae pv. phaseolicola in the apoplast of bean leaves where bacteria are found clustered in microcolonies after infiltration (Rufian et al., 2018). Multispecies interactions within these subpopulations even support the success and dispersal of non-pathogenic strains (Rufian et al., 2018). Thus, although symptomology of Xhg- and Pst-infected leaves appears macroscopically similar, the infected and colonized apoplast reflects a more complex environment that requires further exploration to identify phytopathogen-specific mechanisms of niche establishment and host manipulation.
In terms of space, the carrying capacity of bacteria that colonize leaf surfaces have been well documented, at least for the phytopathogen Pst and the non-pathogenic Pantoea agglomerans (Remus-Emsermann et al., 2012; Woody et al., 2007; Wilson and Lindow, 1994a,b, 1995; Nix et al., 2009; Knief et al., 2010; Kinkel et al., 2000). Despite this wealth of information, the carrying capacity within the apoplast remains poorly understood. We predict that the carrying capacity of the apoplast is dynamic and changes as the state of the host fundamentally transforms following infection. Our results indicate that tomato leaves have similar carrying capacities for Pst and Xhg as both phytopathogens reach plateaus at approximately the same level and time point in infection (8.0–8.5 log CFU/cm2; Figure 3). Despite this similarity, Xhg-infection enhances persistence of immigrating bacteria, such as S. Typhimurium, while Pst-infection does not (Figures 1, 6). While these results indicate that space restriction does not appear to be a primary mechanism for impacting immigrant success, it again suggests that infection or established status with the different genera results in unique niches within the same host.
Water congestion may also impact bacterial migration and dispersal within host tissues. Bacteria utilize swimming and other forms of motility to reach preferential niches, responding to environmental cues to reach essential nutrients (Dixon et al., 2022; Haefele and Lindow, 1987; Lindow et al., 1993; Leveau and Lindow, 2001; Berger et al., 2009). Here, we show that, unlike S. Typhimurium (Figure 1), both Xhg and Pst remain susceptible to UV (Figure 6), indicating that the phytopathogens spend more time on the leaf surface than S. Typhimurium, even once water soaking has begun. Bacterial phytopathogens use motility as a mode of dispersal within and between plants (Monier and Lindow, 2003; Haefele and Lindow, 1987; Remus-Emsermann et al., 2012; Kinkel, 1997), and Xhg and Pst may be transitioning in and out of the apoplast via stomata or through damaged tissue once lesions have developed. Thus, the observed UV susceptibility could reflect increased attempts of the phytopathogens to migrate to distal sites within or on the infected host. In contrast, our data suggest that, once within the apoplast, few S. Typhimurium cells, if any, migrate back to the leaf surface. We also found that Pst infection appears to inhibit migration to the adjacent site for all arriving bacteria (S. Typhimurium, Xhg, or Pst; Figures 1, 6). Previous results have suggested that Pst infection transforms the apoplast, either building physical barriers through biofilm production or manipulating the host response to prevent migration once inside the leaf tissue. For example, Pst infection in A. thaliana leads to restricted vascular flow to the infection site, which could also limit bacterial migration (Freeman and Beattie, 2009). Similarly, the ROS response to phytobacterial infection may link bacterial cells to cell wall components within the apoplast (Soylu et al., 2005), inhibiting bacterial movement. A phytopathogen-specific mechanism during infection would explain why movement of immigrating bacteria is restricted in Pst-infected tissue while Xhg-infected tissue allows bacteria to move more freely.
To summarize, the leaf surface and apoplast present a complex and dynamic environment for bacterial communities, influenced by both host and bacterial factors. Previous studies have demonstrated that the fate of immigrant bacteria is shaped by arrival site conditions (Monier and Lindow, 2003, 2004; Barak et al., 2011). Here, we have added that the presence of established bacterial populations can create distinct niches and competition for resources among bacterial community members. We demonstrate that abiotic water congestion, pathogen-induced water soaking, and interbacterial competition play crucial roles in bacterial colonization and survival. Further research is needed to dissect the molecular mechanisms underlying these processes and develop targeted strategies for disease control. A more comprehensive understanding of the bacterial infection and colonization mechanisms in plant tissues could ultimately inform crop protection strategies, enhance agricultural sustainability, and improve food safety.
Materials and methods Bacterial strains, media, and culture conditionsStrains used in this study are shown in Table 1. Kanamycin resistant Xhg was created by transforming Xhg 444 wildtype strain with pKTKan (Miller et al., 2000). Bacterial cultures were grown in lysogeny broth (LB) for S. Typhimurium, nutrient broth (NB) for Xhg, and nutrient yeast extract dextrose broth (NYD) for Pst. All bacterial strains were incubated at 28°C with shaking at 200 rpm. The antibiotics nalidixic acid (Nal), kanamycin (Kan), and gentamicin (Gent) were used at concentrations of 20, 50, and 10 μg mL−1, respectively.
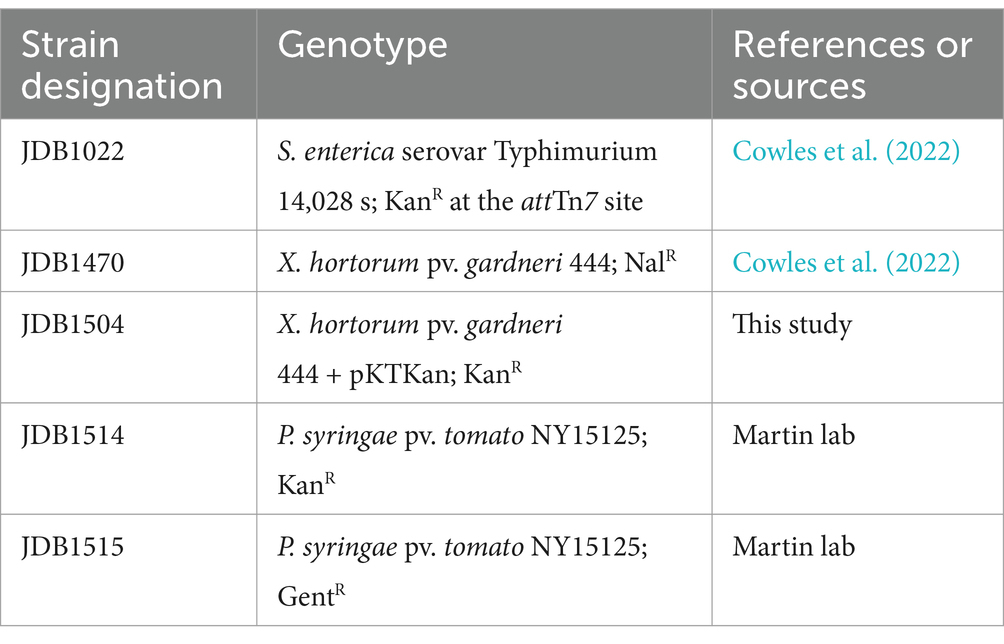
Table 1. List of strains.
Plant inoculationSolanum lycopersicum (tomato cultivar MoneyMaker) seeds were purchased commercially (Eden Brothers). This study complies with the relevant institutional, national, and international guidelines and legislation for experimental research on plants. Seedlings were cultivated in Professional Growing Mix (Jolly Gardener Pro Line, Carlin Sales) with a 16 h photoperiod at 24°C for 5 weeks. For colonization assays, Xhg and Pst bacterial cultures were grown for 2 days in NB or NYD, respectively, at 28°C, and S. Typhimurium cultures were grown overnight in LB at 28°C. Bacterial strains were normalized to an optical density at 600 nm (OD600) of 0.2 (for S. Typhimurium and Pst strains) and 0.3 (for Xhg) in sterile water. These OD600 values correspond to a bacterial population level of ~108 CFU/mL for the respective strains. For dip inoculation, normalized Xhg and Pst cultures were diluted 1:200 in sterile water for an inoculum level of ~5 × 105 CFU/mL. Prior to dip inoculation, 0.025% Sil-Wett was added to the bacterial inoculum. Tomato plants were dip-inoculated by inverting plants in the bacterial inoculum for 30 s with gentle agitation to prevent bacterial cell settlement. Plants were incubated at high humidity for 48 h in lidded, plastic bins under grow lights with a 12 h photoperiod at room temperature (~26°C). After 48 h, plants were exposed to low humidity conditions (bin lids were removed) during the day and high humidity conditions (bin lids were replaced) during the night. For infiltration experiments, two leaflets on the third true leaf of MoneyMaker tomato plants were infiltrated with Xhg or Pst at ~1 × 107 CFU/mL, prepared as above for dip inoculation experiments, following published protocols (Cowles et al., 2018, 2022). Bacterial solutions were infiltrated into the abaxial leaf surface using a plastic, disposable 3 mL, needleless syringe (Fisher Scientific), and, for some experiments, infiltrated zones were delineated with permanent marker. Infiltrated plants were incubated in lidded, plastic bins as described above. For disease comparison of infiltrated plants, non-destructive images of leaflets were taken at multiple days post-infiltration using a Canon PowerShot ELPH 1901S camera.
Image collection and processingAt multiple days post-dip inoculation, individual leaflets were removed, submerged in water for 10 s (to increase lesion visibility), gentle patted dry with kimwipes, and imaged for lesion quantification on a black velvet background using a Canon PowerShot ELPH 1901S camera. Images were cropped to center the leaflet and remove background. Leaf area and lesion analysis was performed with the custom image processing software: Leaf Lesion Detector v2023.2.0 (LLD), developed for this project. LLD automates manual image segmentation procedures (Pride et al., 2020). The Leaf Lesion Detector application segments images using a series of hue, saturation, value (HSV) thresholding operations, and contour finding (van der Walt et al., 2014) on greyscale-converted images, both of which use empirically predetermined, but configurable pixel-value limits. Each leaf and reference image are processed as follows: (1) Segment reference area by thresholding, applying noise reduction, then counting resulting pixels (not shown). (2) Segment leaf area by thresholding and contour finding (Figure 2A, Total Area and Outline). (3) Segment lesion area by thresholding, contour finding, labelling all lesions and summing the area for all lesions above the configured minimum lesion size threshold pixel count (Figure 2A, Lesions). All the segmentations are combined and a color map applied to the lesions by size to create the final image (Figure 2A, Modified). If the total lesion area of a leaf exceeds 3.5%, the image is reprocessed with a more stringent lower bound (supplied in the configuration) for lesion size. After testing several different cutoff values on a range of test images, 3.5% was chosen as it appeared to give the best trade-off between maximizing detection and minimizing false positives. Results from LLD visually correspond to leaf lesions and correlate with manually produced results (R2 = 0.95). The application is publicly available at this url: https://leaf-lesion-detector.streamlit.app/. The code and the configuration settings used in this is work are also publicly available on GitHub (pending publication): https://github.com/UW-Madison-DSI/plant-pathology-image-processor.
Immigrant arrivalS. Typhimurium, Xhg, and Pst cultures were grown as described above, normalized to OD600 = 0.1, and diluted in sterile water for a final concentration of ~106 CFU/mL. Normalized and diluted immigrant cultures were applied as 15 μL droplets to the adaxial surface of infiltrated leaf tissue, which included leaflets infected with Xhg or Pst for 2 DPI, as well as leaflets freshly infiltrated with sterile water, as done previously (Dixon et al., 2022). The droplet “arrival site” was denoted with permanent marker and is depicted in Figure 1 for clarity. Inoculated plants were incubated at room temperature for 3 h to allow for droplet absorption and then moved under grow lights until sampling. Bacterial inoculums were diluted and plated for population counts.
Bacterial population samplingBacterial populations on leaves were determined as described (Cowles et al., 2018, 2022; Dixon et al., 2022). Briefly, at indicated times, two individual leaflets were removed from each plant, and the adaxial surface of one leaflet per plant was treated with 254 nm UV radiation (Stratalinker UV Crosslinker 1800) at 150,000 μJ/cm2 (for S. Typhimurium) or 300,000 μJ/cm2 (for Xhg or Pst). Irradiated leaflets were chosen
留言 (0)