The World Health Organization (WHO) estimates that antimicrobial resistance (AMR) could cause up to 10 million deaths annually by 2050, with a severe impact on global healthcare costs and economic stability. Bacterial infections are among the most life-threatening healthcare challenges, accounting for approximately 13.6% of global mortality and affecting 1 in 8 individuals worldwide (Appanna, 2018). The rise of multidrug-resistant (MDR) bacteria further highlights an urgent need for alternative, next-generation antibacterial treatments. While antibiotics have historically revolutionized healthcare, their widespread use has led to substantial challenges, including disrupting human microbiota and the rise of antibiotic-resistant bacterial strains.
Beyond their pathogenic roles, bacteria are integral to human health, especially in the gut, contributing to immune modulation and digestion (Bull and Plummer, 2014; Yin et al., 2019; Arciola et al., 2018). Consequently, the modern healthcare system cannot fully eliminate infection risk, particularly in post-surgical settings (Oliva et al., 2021; Hedrick et al., 2006; van Seventer and Hochberg, 2017) and procedures involving biomaterials or medical devices (Arciola et al., 2018; Oliva et al., 2021; Hedrick et al., 2006). Infections occur when infectious agents enter human body tissues, multiply, and trigger host immune responses (van Seventer and Hochberg, 2017). Although infections cannot be entirely prevented due to inevitable interactions with environmental microbes, targeted measures can mitigate bacterial invasion and inhibit replication at potential infection sites.
The human microbiome, especially the gut microbiota, comprises numerous symbiotic bacterial species (e.g., Lactobacillus, Bacillus, Clostridium, Enterococcus, and Ruminococcus) that collectively represent approximately 90% of the gut flora (Rinninella et al., 2019; Martín et al., 2013; Eloe-Fadrosh and Rasko, 2013). These beneficial microorganisms are crucial in digestion, nutrient absorption, and immune defense. Importantly, they maintain a delicate balance, contributing to immune regulation and protecting against pathogens without causing harm to the host (Rinninella et al., 2019; Bogitsh et al., 2019; Hemarajata and Versalovic, 2013; Isolauri et al., 2001; Wieërs et al., 2020; Hills et al., 2019; Ding et al., 2019). However, the broad-spectrum use of antibiotics has disrupted this balance, weakening the microbiota’s natural protective functions and impairing the immune response, leading to gut dysbiosis—an environment conducive to antibiotic-resistant strains (Patangia et al., 2022).
While antibiotics effectively eliminate harmful pathogens, their indiscriminate targeting also affects beneficial bacteria, reducing microbial diversity and increasing the likelihood of antibiotic resistance (Lathakumari et al., 2024). Inappropriate antibiotic use across sectors, including clinical and animal health, has further escalated the global antibiotic resistance crisis, contributing to the emergence of MDR pathogens that resist multiple antibiotic classes (Yang et al., 2024).
The decreasing efficacy of antibiotics and the limited availability of alternative treatments underscore the urgent need for new classes of antibacterial therapeutics. Ideal alternatives would incorporate mechanisms of action that lower the risk of resistance. In this context, naturally derived biopolymers (NDBs) have attracted significant attention due to their unique antibacterial properties. Although long used in biomedical applications, interest in biopolymers as antibacterial agents has surged, with publications on their use rising by approximately 400% since 2015 (Web of Science, n.d.).
Approximately two decades ago, antibacterial biopolymers, e.g., those with intrinsic antibacterial activity, were first proposed as alternatives to antibiotics for treating bacterial infections (Muñoz-Bonilla and Fernández-García, 2012). Today, biopolymer-based strategies show potential for localized, non-antibiotic antibacterial applications that support the immune system and minimize impact on the natural microbiota. Such approaches could represent a sustainable innovation within modern healthcare.
Notably, NDBs disrupt bacterial membranes instead of targeting specific metabolic pathways, a mechanism less prone to resistance development (Bustamante-Torres et al., 2022; Kamaruzzaman et al., 2019). Numerous studies have documented the use of NDBs in biomedical devices, including drug delivery systems, contact lenses, and injectable cement, where they exhibit potent antibacterial activity and biocompatibility (Pahlevanzadeh et al., 2022; Sam et al., 2023; Coma, 2013). This review provides a comprehensive examination of the potential of antibacterial NDBs, analyzing recent literature to compare their effectiveness and applications with those of conventional antibiotics. By exploring the mechanisms, advantages, and limitations of NDBs, this review assesses whether these biopolymers could serve as reliable, antibiotic-free therapeutics capable of complementing or partially replacing traditional antibiotics in treating bacterial infections—or whether their promise remains largely theoretical.
2 Antibiotics and antibiotic resistanceIn the pre-antibiotic era, more than half of deaths were attributable to infections (Aminov, 2010). Since the 20th century, antibiotics have revolutionized antibacterial therapeutics in the history of medicine, drastically changing modern medicine and extending the average human lifespan (Johnston and Badran, 2022; Cook and Wright, 2022; Sikdar et al., 2021). Several groups and generations of antibiotics have been discovered and developed with specific target mechanisms of action on bacterial cells (Kapoor et al., 2017; Coates et al., 2011) (Table 1). Conventionally, antibiotics are classified as cell wall inhibitors, protein synthesis inhibitors, nucleic acid synthesis inhibitors, antimetabolites, and cytoplasmic membrane inhibitors (Pancu et al., 2021; Ullah and Ali, 2017) (Figure 1).
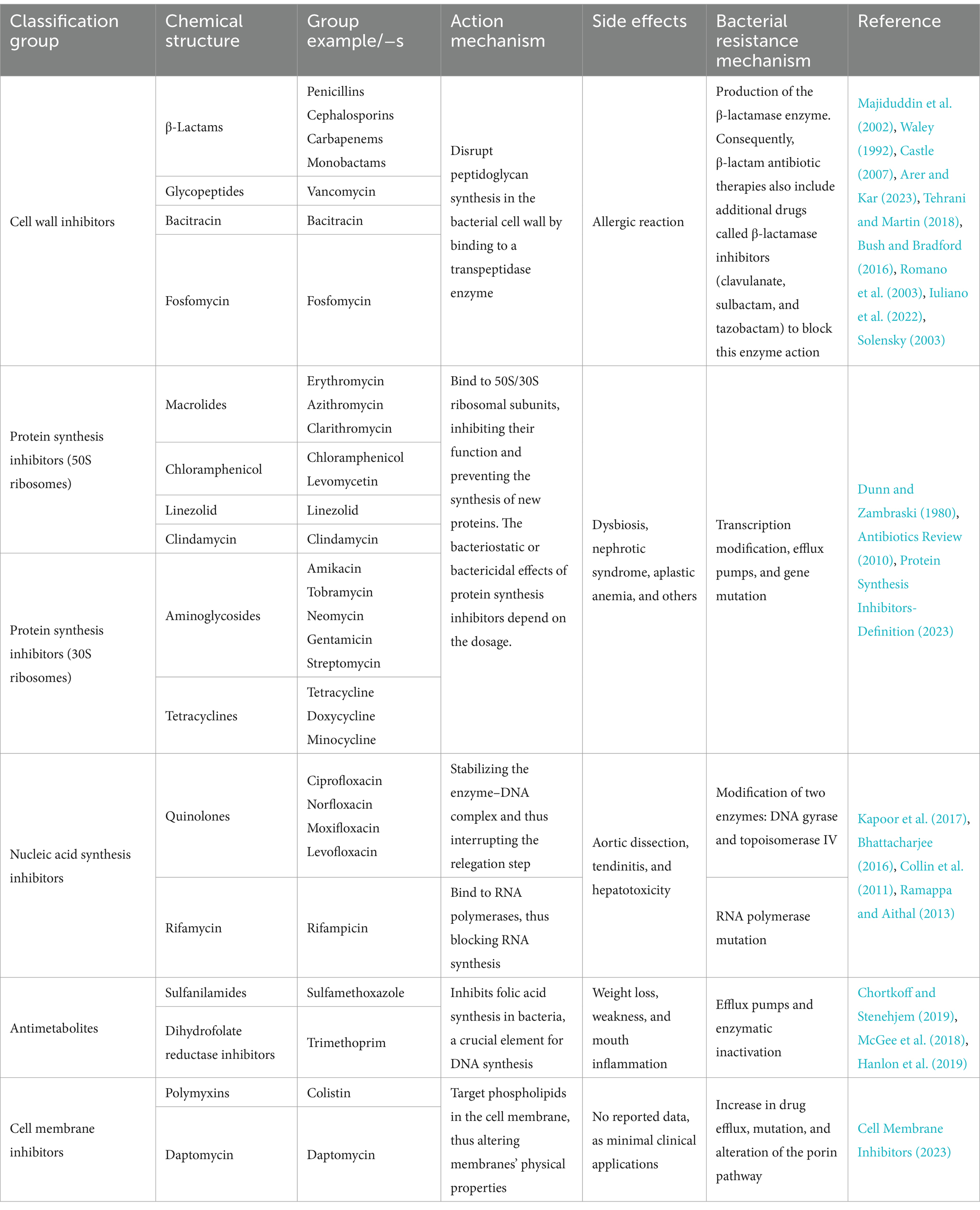
Table 1. Classification of antibiotics based on the mechanism of action and chemical structure with characterization: action mechanisms, reported side effects, and bacterial resistance mechanisms.
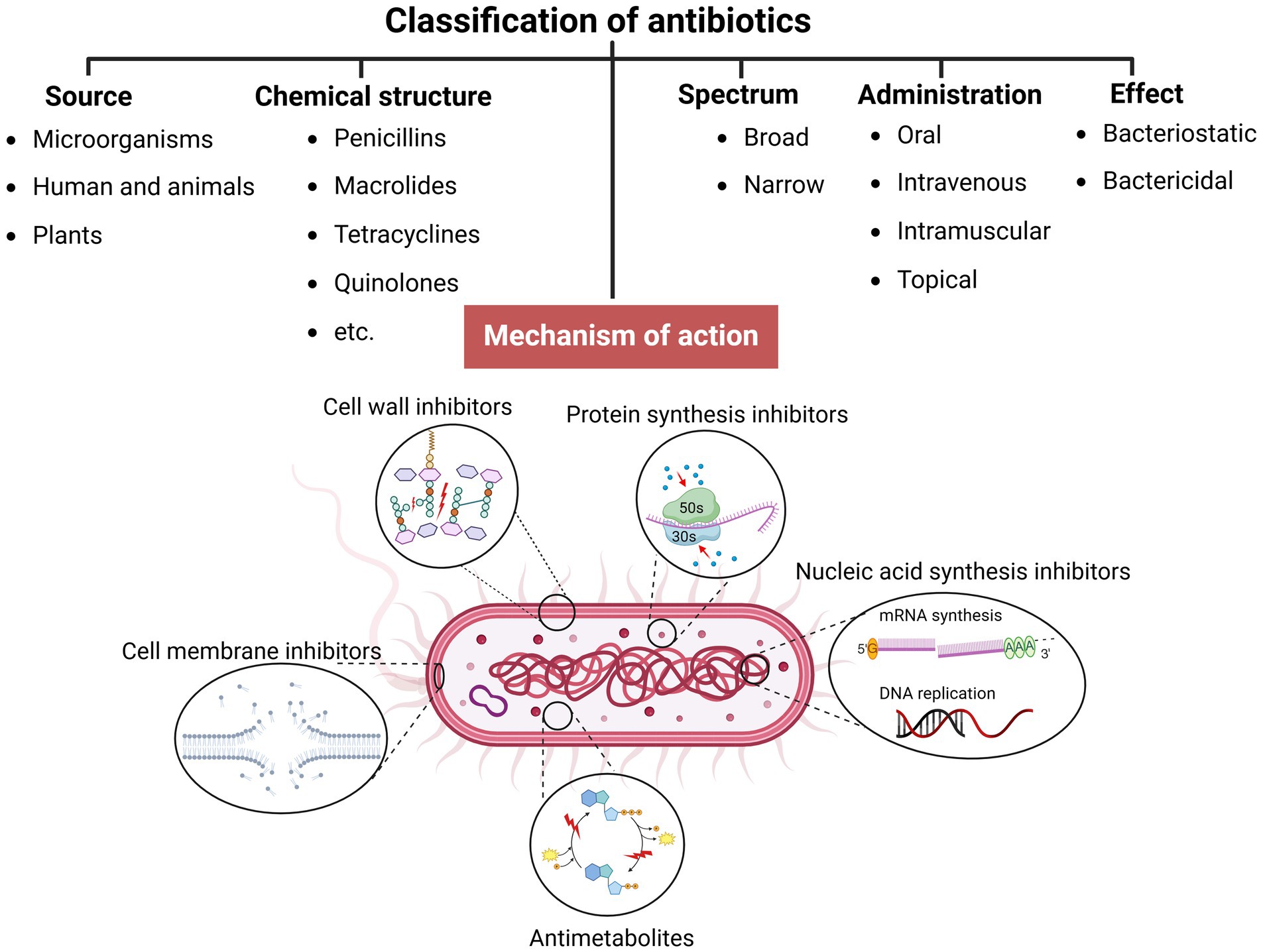
Figure 1. Classification of antibiotics (created by Biorender).
Other classification principles of antibiotics rely on their origin, spectrum of action, administration strategies, chemical structure, and mechanism of action (Figure 1). Antibiotics can be administered through various routes, including oral, intravenous, intramuscular, and topical applications (Buonavoglia et al., 2021; Enenkel and Stille, 1988). The topical application is particularly relevant for localized infections, such as skin wounds or mucosal infections, where direct delivery to the affected area can enhance efficacy and minimize systemic side effects. The effectiveness of these administration strategies depends on various factors, including bioavailability, drug formulation, gastrointestinal conditions, and systemic distribution, which, if not optimized, could compromise therapeutic outcomes and limit the antibiotic’s efficacy against targeted infections (McCarthy and Avent, 2020; Vinarov et al., 2021). Moreover, antibiotics exhibit specific behavioral characteristics, including whether they are bactericidal or bacteriostatic, as well as their spectrum, which can be broad or narrow. Antibiotics with a wide spectrum and bactericidal action may impact the microbiota within organisms’ niches (Dubourg et al., 2014; Blaser, 2011; Yang et al., 2021), resulting in dysbacteriosis conditions post-therapy and an increasing risk of secondary disease. In addition, the systemic use of antibiotics has been documented to affect various organ systems, leading to heightened organism toxicity (Berry et al., 1995; Grill and Maganti, 2011) (Table 1).
Currently, bacteriophage therapy is the only alternative as effective as antibiotics. Phage therapy relies on using naturally occurring bacteriophages (viruses) to infect and lyse bacteria at the site of infection (Lin et al., 2017). However, phage therapy must still be licensed in the majority of countries or used under exceptional situations (Yang et al., 2023). Thus, antibiotics remain the primary treatment option in clinics to combat bacterial infections. However, the development of antibiotics has begun an endless race against pathogenic microorganisms. As a side problem, the overuse and misuse of these lifesaving drugs have developed the top global public health crisis named antibiotic resistance occurring worldwide (Akram et al., 2023). Antibiotic resistance arose from the evolutionary development of primary (antibiotic target site is not presented in bacteria strain) and secondary (genome-related and plasmid-related) resistance mechanisms in bacteria (Urban-Chmiel et al., 2022; Nilsson, 2019; Zhang and Cheng, 2022) (Table 1). The dramatic report by the WHO has shown that by 2050, drug resistance could catch up to cancer and sufficiently damage the economy if actions are not taken (World Health Organization, 2019). The uncontrolled use of antibiotics in agriculture and inappropriate therapeutic practices has provided an evolutionary advantage to bacteria, such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus (VRE), and others that have become resistant to one or multiple types of antibiotics, contributing to the dramatic situation in healthcare (Figure 2).
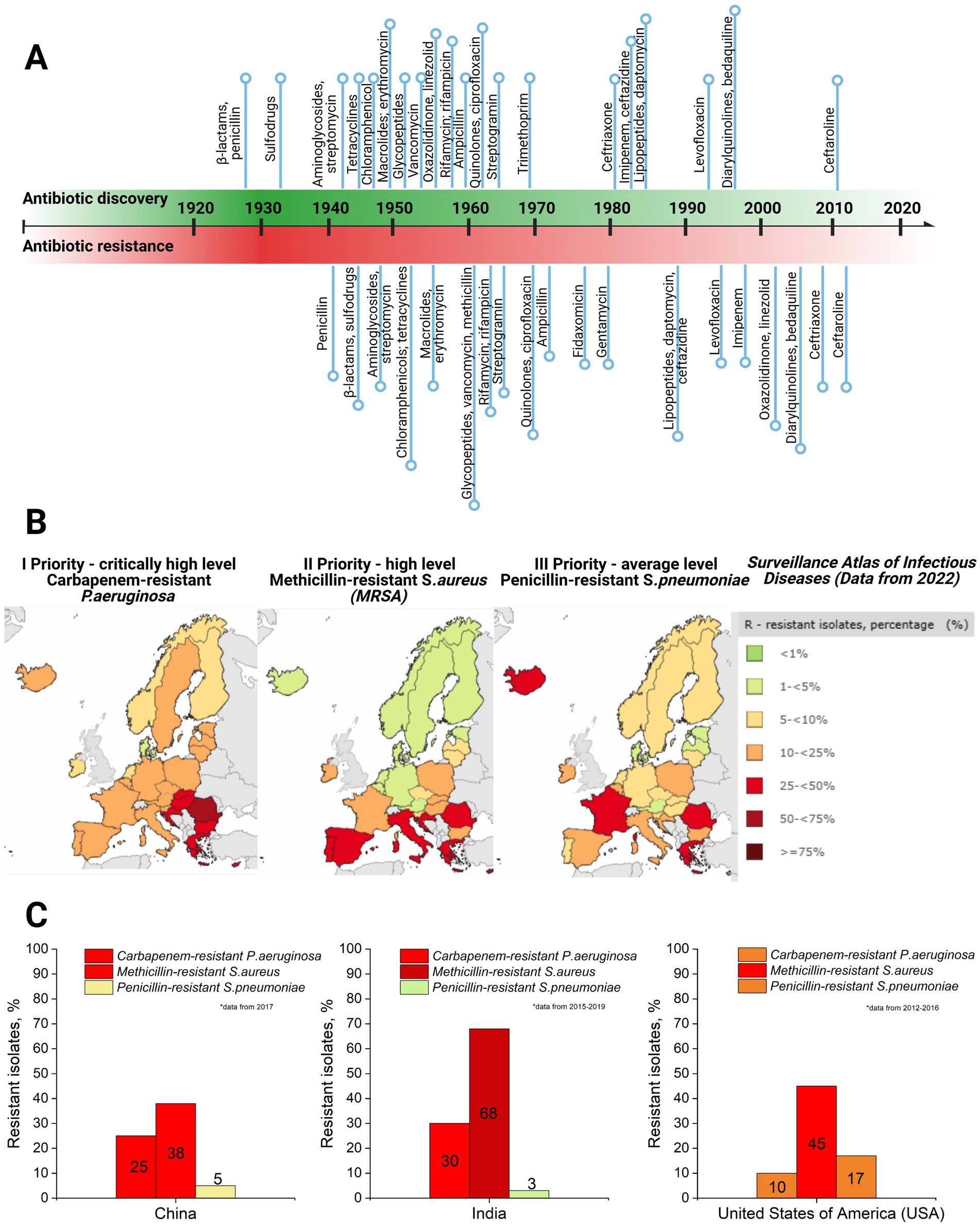
Figure 2. (A) Antibiotic discovery (title of antibiotics and relative point within timeline decade) and resistance reports timeline (resistance report against specific antibiotic with a title and relative point within timeline decade) (Dahal and Chaudhary, 2018). (B) Case map on three priority levels (I priority stands for critically high level; II priority – high level, and III – average level of resistance cases) resistant strains (carbapenem-resistant P. aeruginosa; methicillin-resistant S. aureus, and penicillin-resistant S. pneumoniae) in Europe, 2022, as well as a supporting table on resistant isolates’ case percentage and colorful indicator for priority, with green indicating low level and dark red indicating critically high level (Surveillance Atlas of Infectious Diseases, 2022). (C) Comparative graphs of the same three priority resistant strains with color indication in the top three most populated world countries: China, India, and the United States of America (USA) (ResistanceMap, n.d.) (created by Biorender).
The global threat of antimicrobial resistance (AMR) necessitates collaborative action to develop and implement effective strategies (Uchil et al., 2014). Several preventative measures have been established and continue to evolve, addressing AMR at international, national, community, hospital, and individual levels (Uchil et al., 2014). At the international level, efforts focus on enhancing collaboration among governments, non-governmental organizations, professional groups, and international agencies. Key initiatives include global networks for antimicrobial use and resistance surveillance, strategies to combat counterfeit antimicrobials, and programs to foster innovation in new drugs and vaccines. Strengthening global AMR control programs remains a priority. Nationally, dedicated committees and AMR policies have been introduced to monitor and manage AMR. These policies integrate geographical, social, and economic factors to provide tailored solutions. Educational initiatives, including training programs and certification courses, aim to equip healthcare professionals and the private sector with knowledge for the rational use of antibiotics. Regulatory controls to limit over-the-counter antibiotic sales further address misuse, a key driver of resistance. For example, a review revealed that non-prescription antibiotic use varies widely, from 3% in Northern Europe to 100% in some African regions (Uchil et al., 2014; Morgan et al., 2011). In addition, efforts are directed at improving standards in healthcare systems, microbiology laboratories, and pharmaceutical companies. Protecting existing antibiotic therapies remains critical, with ongoing research focused on developing new drugs to replace outdated ones and prolonging the effectiveness of current treatments. It has already been proven that synergy and drug combinations are a winning strategy in fighting multidrug-resistant bacteria and might help protect the existing drugs through antibiotic adjuvants. For instance, β-lactamase inhibitors have been used as adjuvants for penicillin group antibiotics as they block the resistance mechanism of bacteria against these antibiotics (see Table 1). Other examples include efflux pump inhibitors and outer membrane permeabilizers (Annunziato, 2019).
3 Naturally derived biopolymers with intrinsic antibacterial propertiesNaturally derived biopolymers (NDBs) are large macromolecules from living organisms such as plants and microorganisms. These polymers are formed through enzyme-catalyzed chain-growth polymerization processes of activated monomers (Sun et al., 2022). The molecular size of NDBs varies significantly based on their type and source, ranging from a few kilodaltons (kDa), as seen in polysaccharides such as chitosan (~10–50 kDa), to several megadaltons (MDa), such as cellulose and other structural polysaccharides (>1 MDa) (Moradali and Rehm, 2020; Yadav et al., 2015). This broad size range supports their diverse physicochemical properties and wide-ranging applications (Troy et al., 2021; Reddy et al., 2021).
The reason is that the source of these compounds is derived from living organisms through enzymatic polymerization, forming high molecular weight macromolecules. As a result, covalently bonded repetitive monomeric units form biodegradable compounds such as polysaccharides, polyamino acids, hydroxy fatty acids, polypeptides, and glycolipids (Moradali and Rehm, 2020; Yadav et al., 2015; Troy et al., 2021) (see Table 2). These compounds are classified as NDBs and have unique physical, chemical, and mechanical properties, which are exploited in biomedical applications. NDBs are commonly used in the development of drug delivery systems (Baranwal et al., 2022; Murali and Jayakumar, 2023; Atanase, 2021). In addition, NDBs such as collagen, gelatin, dextran, agarose/alginate, hyaluronic acid, cellulose, and fibrin are also being explored in various other biomedical applications, including open incision/wound suturing, fixing, adhesion, covering, occlusion, isolation, contact inhibition, cell proliferation, tissue guiding, and controlled drug administration (Baranwal et al., 2022). NDBs are of broad interest because of their potential to be used for developing environmentally friendly medical devices that perform high biocompatibility and serve as highly accurate biosensors, drug delivery systems, etc. (Manoukian et al., 2019). In addition to biocompatibility, biodegradation, bioadhesiveness, and biofunctionality of the NDBs, several drawbacks must be addressed, such as low stability, low melting point, high surface tension, structural complexity, and well-known immunological response from organisms (Ige et al., 2012; Jenkins et al., 1996; Reddy et al., 2021). Various NDBs such as chitosan, pectin, κ-carrageenan, alginate, ε-polylysine, and others have also been identified for their antibacterial activity (Muñoz-Bonilla et al., 2019; Li et al., 2021; Habeeb and Abdulkadhim, 2024; Hamidi et al., 2023) (see Table 2). Numerous studies have demonstrated the comparative effectiveness and potential advantages of NDBs over conventional antibiotics. For instance, Tin et al. (2009) reported that chitosan molecules with different molecular weights consistently exhibited a minimum inhibitory concentration (MIC) of 32 μg/mL against various strains of P. aeruginosa. In contrast, the MIC range for sulfamethoxazole was significantly broader, ranging from 64 to 2048 μg/mL (Tin et al., 2009). Similarly, Si et al. (2021) found that a chitosan derivative effectively inhibited Gram-negative and Gram-positive bacteria, with MIC values ranging from 8 to 32 μg/mL. Specifically, against A. baumannii, the chitosan derivative achieved an MIC of 32 μg/mL, compared to higher MIC values of 128 μg/mL for amikacin and tobramycin and 64 μg/mL for tazobactam. However, certain antibiotics outperformed NDBs in specific cases; for example, novobiocin demonstrated an MIC of 8 μg/mL, and carbenicillin and tobramycin were more effective against MRSA (Si et al., 2021). Another noteworthy example is ε-polylysine, which exhibited MIC values of 500, 800, 800, and 1,000 μg/mL against P. aeruginosa, K. pneumoniae, MSSA, and MRSA, respectively. Traditional antibiotics such as ampicillin, gentamicin, and tetracycline showed MIC values ranging from 35 to 250 μg/mL against the same bacterial strains (Sundaran et al., 2022). Importantly, combining NDBs with antibiotics has synergistic effects, significantly enhancing antibacterial activity and reducing the required antibiotic dosage (Si et al., 2021; Taheri-Araghi, 2024; Fayed et al., 2023; Baltimore et al., 1987; Khan et al., 2012; Kaur et al., 2022).
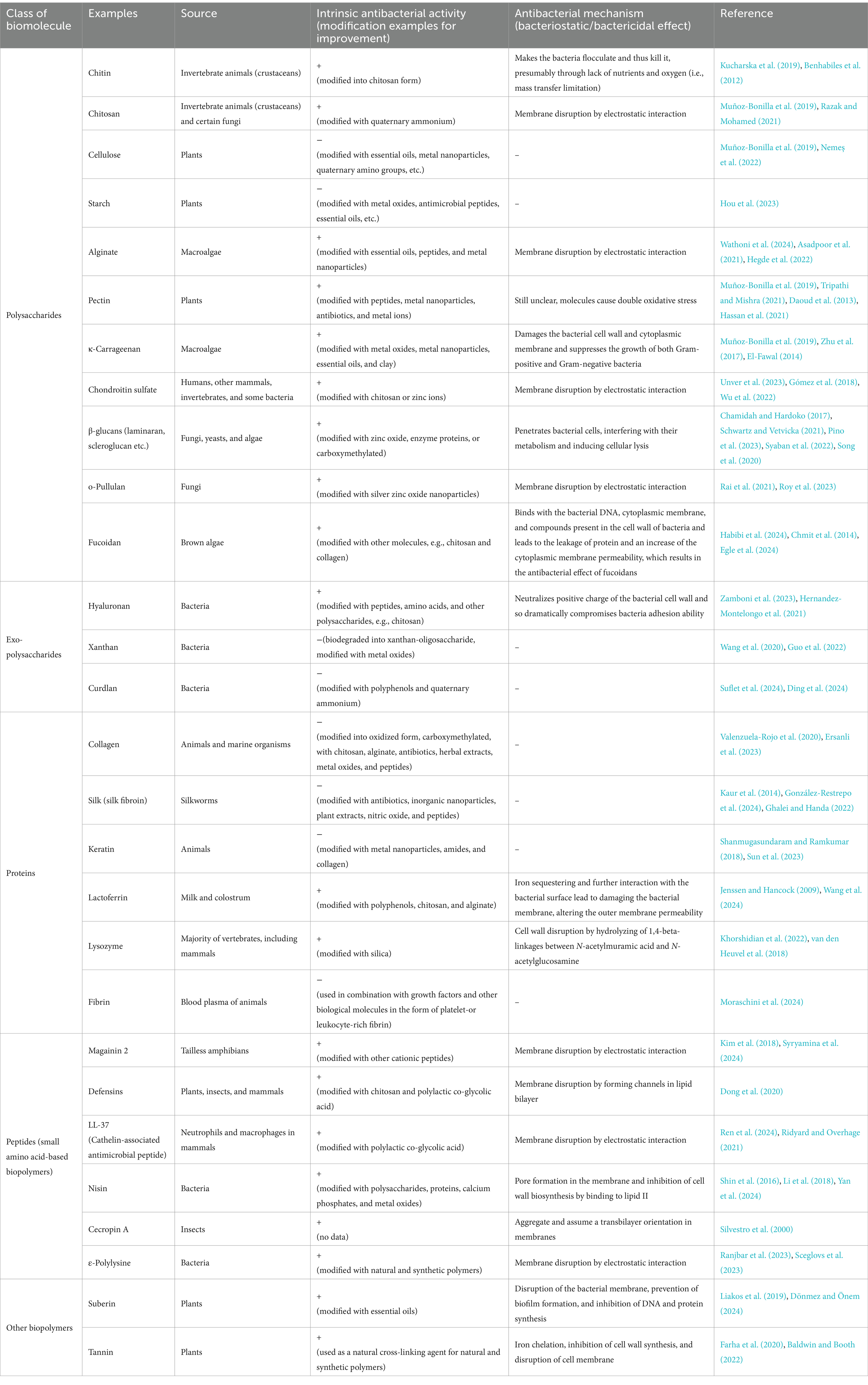
Table 2. Examples of naturally derived biopolymers (NDBs) and their antibacterial performance.
Considering the advantageous functionalities such as biodegradability, low immunogenicity, and non-toxicity of naturally derived biopolymer-based drug delivery systems, the antibacterial feature opens new horizons for developing local targeted antibacterial therapeutics based on antibacterial biopolymers. Countless reviews and studies have demonstrated the ability of NDBs to inhibit a broad spectrum of Gram-positive and Gram-negative bacteria, including bacterial strains currently being classified as “under urgent attention” due to their resistance to various antibiotics (Bustamante-Torres et al., 2022; Poznanski et al., 2023; Rofeal et al., 2022), as well as fungi (Poznanski et al., 2023; Ntow-Boahene et al., 2021) and viruses (Akbari et al., 2022; Bianculli et al., 2020). Several studies have highlighted that various naturally derived biopolymers (NDBs) exhibit notable antibiofilm activity (see Table 2). These antibiofilm mechanisms primarily involve disrupting biofilm exopolysaccharides (EPS), a critical component for biofilm stability. Such disruptions can lead to the detachment of bacterial cells or inhibit bacterial adhesion during the early stages of biofilm formation (Mishra et al., 2020; Melander et al., 2020). In addition, certain NDBs, such as lactoferrin-derived peptides, neutrophil peptides, and antimicrobial peptides (e.g., protegrin-1), have demonstrated antibacterial activity against intracellular pathogens, including Mycobacterium tuberculosis. These antibacterial effects are attributed to the disruption of the mycobacterial cell wall and enhanced membrane permeabilization (Khara et al., 2020; Jacobo-Delgado et al., 2023; Intorasoot et al., 2022). Despite these promising findings, significant challenges remain in translating NDBs into clinical applications. Key limitations include variability in their physicochemical and mechanical properties, which can impact reproducibility and reliability in therapeutic settings (Moradali and Rehm, 2020; Yadav et al., 2015). The limited physicochemical stability and difficulty tuning their biodegradation profiles further complicate their development as viable therapeutic solutions (Muñoz-Bonilla and Fernández-García, 2012; Pahlevanzadeh et al., 2022). In addition, the transition from laboratory-scale research to clinical application faces substantial barriers, including extensive preclinical testing to establish safety and efficacy, the complexities of large-scale manufacturing to ensure consistent quality, and the rigorous regulatory approval processes that demand considerable time and resources (Oliva et al., 2021; Murali and Jayakumar, 2023). Addressing these challenges will require interdisciplinary approaches and sustained efforts to optimize the properties of NDBs and streamline their development pipeline for clinical use (Arciola et al., 2018; Kong et al., 2023).
In further sections, the antibacterial potential of NDBs will be discussed to understand biopolymer interaction with bacterial cells, inhibition/bactericidal mechanism, and application specifics, to compare all these aspects with currently used conventional antibiotics, and to address the question posed in the title of this review.
4 Mode of delivery of NDBs for antibacterial treatmentBased on R&D reports, the most common local modes of delivery of antibacterial naturally derived biopolymers (aNDBs) to treat desired sites for various biomedical applications are summarized in Figure 3. Local application options and antibacterial activity are the main advantages reported in numerous studies for such biopolymers (Wu et al., 2022; Jarosz et al., 2023; Kong et al., 2023). Local delivery is preferable as it achieves the target site at the same concentration as it was prepared directly without passing through all the body barriers via the bloodstream and without losing biopolymer molecules. Such local delivery types include creams, ointments, and gels that are applied on the skin, burn or opened wounds, and surgery sites to prevent and treat infection (Zhao et al., 2023); intraoperative coatings and fillers (Ilić-Stojanović et al., 2023) are used for deeper surgical sites or dental sockets post-tooth extraction to avoid or combat already infected site; implant and catheters coating that allows preventing implant-and catheter-associated infections (Veiga and Schneider, 2013); and controlled delivery systems that achieve and bind to targeted site via different stimuli or due to specific conditions (temperature and pH), followed by antibacterial activity while providing controlled cell/ion/growth factor release from the matrix (Jacob et al., 2018) (Figure 3). This local delivery feature gives an advantage compared to conventional administration of antibiotics orally (pills and suspensions) or intravenously (in particular cases). While oral administration is convenient and suitable for at-home antibiotic therapy, it is associated with a decline in the concentration of the active compound upon reaching the infection site (Homayun et al., 2019; Kim and De Jesus, 2023). In addition, this strategy negatively impacts natural body microbiota, directly affecting the patient’s immune system response to continuous or new bacteria invasion (Konstantinidis et al., 2020). Nevertheless, it is worth acknowledging the efficacy of oral drug administration in systemic or severe infections at multiple sites (Cunha, 2006). However, various studies have reported that combined device development where aNDBs served as a drug delivery system with encapsulated antibiotics might have a promising synergistic effect to achieve the exact infection site (Liu et al., 2022; Khan et al., 2021; Meng et al., 2014; Hwang et al., 2023; Schrade et al., 2022).
5 Mechanism of antibacterial actionRegarding aNDBs, it is crucial to understand that these biopolymer molecules, unlike the previously described antibiotics, do not target specific synthesis pathways or molecules. First, it is worth mentioning that bacterial cell wall outer structures serve as adhesion and pathogenicity factors; for example, lipopolysaccharides and phospholipids of Gram-negative bacteria and teichoic and lipoteichoic acids of Gram-positive bacteria are negatively charged. Second, aNDBs consist of positively charged molecules (chondroitin sulfate, o-pullulan, alginate, ε-polylysine, chitosan, magainin-2, etc.), containing cationic groups such as quaternary ammonium, quaternary phosphonium, guanidinium, or tertiary sulfonium (Santos et al., 2016), which have a positive charge. As a result, the interaction between biopolymers and bacteria begins with mutual attachment caused by electrostatic forces (Haktaniyan and Bradley, 2022). As a result, if aNDB molecules and bacteria cells are close enough, oppositely charged molecules attract each other, leading to physical binding (Figure 4). Another essential fact is that not all cationic molecules are lethal to bacteria. The electrostatic interaction represents just the first step toward the bactericidal effect of aNDBs. Second, a specific concentration of the cationicity of aNDBs must be achieved to reach a multivalence effect (Smola-Dmochowska et al., 2023) that results in the simultaneous binding of aNDB molecules to the bacterial cell structures and moving to the next step.

Figure 4. Biopolymer (aNDBs)—bacterial cell interaction (left) and bactericidal mechanisms (right) (created by Biorender).
In the next step, aNDB mechanisms of action on bacterial cell walls are divided into pore-forming and micelle-forming mechanisms (Qiu et al., 2020; Zhou et al., 2023). The pore-forming mechanism can be further categorized into two models: barrel-stave pore and toroidal pore (Kumar et al., 2018; Pastore et al., 2020; Mihajlovic and Lazaridis, 2010). Within the barrel-stave model, the aNDB molecules are initially oriented parallel to the membrane but eventually inserted perpendicularly in the lipid bilayer (Hegde et al., 2022) (Figure 4). This promotes lateral peptide–peptide interactions such as membrane protein ion channels. Hydrophobic regions interact with membrane lipids, and hydrophilic residues form the lumen of the channels (Brogden, 2005). On the other hand, in the toroidal pore model, peptides are also inserted perpendicularly in the lipid bilayer, but specific peptide–peptide interactions are not present (Wimley, 2010). Instead, the peptides induce a local curvature of the lipid bilayer, with the pores partly formed by peptides and partly by the phospholipid head group. The dynamic and transient lipid–peptide supramolecule is the “toroidal pore.” The key distinguishing feature of this model, compared to the barrel-stave pore, is the net arrangement of the bilayer. In the barrel-stave pore, the hydrophobic and hydrophilic arrangement of the lipids is maintained, whereas, in toroidal pores, the hydrophobic and hydrophilic arrangement of the bilayer is disrupted. This provides alternate surfaces for interacting with the lipid tail and head group. As the pores are transient upon disintegration, some peptides translocate to the inner cytoplasmic leaflet, entering the cytoplasm and potentially targeting intracellular components (Kumar et al., 2018). Other features of the toroidal pores include ion selectivity and discrete size (Yeaman and Yount, 2003). Due to pore formation, joint cell wall integrity and permeability are disrupted, resulting in bacterial cell lysis.
The micelle-forming mechanism is usually called the “Carpet-like” model (Wimley, 2010; Huan et al., 2020; Shai, 2002). In this case, the aNDBs adsorb parallel to the lipid bilayer and reach a threshold concentration to cover the surface of the membrane, thereby forming a “carpet” (Figure 4). This leads to unfavorable interactions on the membrane surface. Consequently, membrane integrity is lost, producing a detergent-like effect, which eventually disintegrates the membrane by forming micelles, followed by bacterial cell death (Qiu et al., 2020; Zhou et al., 2023; Kumar et al., 2018).
6 Resistance development possibilityAnother crucial consideration lies in the potential for bacteria to develop resistance to antibiotics. As previously highlighted, different bacterial strains develop resistance to commonly used antibiotics. Resistance mechanisms are unique and depend on the antibiotic group and mechanism of action specifics. Still, overall mechanisms involve specific enzyme production, loss of targeted molecules, efflux pumps, mutation of the target site, increased cell permeability, etc. (Reygaert, 2018; Munita and Arias, 2016; Peterson and Kaur, 2018; Abushaheen et al., 2020). It has been conventionally assumed that this propensity for resistance is exclusive to antibiotics, and theoretically, bacteria cannot develop resistance to aNDBs. On the one hand, electrostatic attraction between aNDBs and bacterial outer structures seems inevitable. In addition, the aNDB mechanism of action is not explicitly targeted. Even after entering the inner environment, aNDBs could enter many metabolic pathways. Based on that, it is more likely that bacteria encounter challenges in impeding electrostatic interaction and developing resistance, given the biological expense associated with such a complex process.
Although thought to be improbable, alteration of bacterial membranes has been shown as a mechanism of resistance (Epand et al., 1858; Nawrocki et al., 2014). Such alterations include incorporating components with reduced anionic charge, which leads to the inability of peptides to aggregate on bacterial membranes and prevents them from entering the cell (Baltzer and Brown, 2011). For instance, studies have shown that Staphylococcus aureus modifies the anionic phospholipids in the cytoplasmic membrane with L-lysine, resulting in a reduction of the net negative charge of the bacterial membrane and leading to the repulsion and subsequent resistance to aNDBs (Peschel et al., 2001). Similarly, modification of Gram-negative bacteria’s lipopolysaccharides (LPS) is another bacterial mechanism contributing to resistance (Gunn, 2001; Guo et al., 1998). These modifications include incorporating fatty acids, thereby reducing the permeability of the outer membrane and increasing membrane structural stability (Peschel, 2002). Furthermore, bacteria can change the permeability of the cell wall, as is widely reported in the case of tetracyclines (Chmit et al., 2014); in addition, such non-specific structures as efflux pumps are also responsible for pumping out unfavorable molecules, and they are evidenced to work correctly against macrolides (Zhong and Shortridge, 2000).
7 Conclusion and future perspectiveThe highlighted findings in our review confirm that naturally occurring biopolymers with intrinsic antibacterial performance can be considered high-performance, sustainable, next-generation materials for biomedical field applications. Various studies have shown that hydrogels, biosensors, drug delivery systems, and implant coatings based on natural antibacterial biopolymers have promising physicochemical features. They possess excellent biocompatibility and are naturally derived, thus making them environmentally friendly. However, the main focus of this review was to elucidate the potential of naturally derived antibacterial biopolymers toward a specific aim—antibacterial therapy against bacterial infection in the human body—and second, to understand whether aNDBs are a future or a failure in replacing antibiotics. Multiple studies have reported these biopolymers in vitro; their antibacterial potential revealed action mechanisms. In addition, aNDBs have demonstrated substantial inhibitory effects against antibiotic-resistant bacteria strains. Based on the results, aNDBs could emerge as a novel weapon against bacterial infections to replace currently used antibiotics and antibiotic use approaches. Unique antibacterial action and the possibility of loading directly to the infection site (locally) open new horizons for this type of material.
However, learning from the past must be taken properly; many years ago, antibiotics were in the same situation. What is known for sure is that antibacterial biopolymers exhibit remarkable potential for combating bacteria and possess unique qualities. This material class is confined to research studies and is exclusively utilized for scientific purposes under controlled conditions. At the same time, antibiotics have already deserved the trust of medical doctors and have been proven effective antibacterial therapy in clinical care. Bacteria possess the biological mechanisms necessary for potential evolution, raising questions about the likelihood of encountering analogous issues. The problem associated with antibiotic resistance has emerged due to prolonged global exposure to antibiotics in the medical sector, inappropriate drug misuse or overuse, and the usage of antibiotics in agriculture. It is still being determined if antibacterial biopolymers will be opened to the world as much as antibiotics and undergo the same conditions. Will we face the same problem as now? Bacteria possess the biological mechanisms necessary for potential evolution, raising questions about the likelihood of encountering similar issues. In summary, antibacterial biopolymers are promising materials with many advantages, including their antibacterial potential. However, in light of various considerations and experiences, numerous questions must be answered, particularly considering the development of bacterial resistance. It is not merely a matter of substituting one for the other but a nuanced exploration of the complexities involved.
Author contributionsAS: Conceptualization, Resources, Writing – original draft, Writing – review & editing. IS: Writing – review & editing. MC: Writing – review & editing. JK: Supervision, Writing – review & editing. KS-A: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The authors acknowledge financial support for granting Open Access from the European Union’s Horizon 2020 research and innovation program under grant agreement No. 857287 (BBCE—Baltic Biomaterials Centre of Excellence) and from the C316 Implementation of Consolidation and Governance Changes at Riga Technical University, LiepU, RAT, LMA, and LMC to Advance Excellence in Higher Education, Science, and Innovation program under the grant agreement C4835.Dok.1025 No. 5.2.1.1.i.0/2/24/I/CFLA/003.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ReferencesAbushaheen, M. A., Muzaheed, A. J., Fatani, M., Alosaimi, W., Mansy, M., George, S., et al. (2020). Antimicrobial resistance, mechanisms and its clinical significance. Dis. Mon. 66:100971. doi: 10.1016/J.DISAMONTH.2020.100971
PubMed Abstract | Crossref Full Text | Google Scholar
Akbari, A., Bigham, A., Rahimkhoei, V., Sharifi, S., and Jabbari, E. (2022). Antiviral polymers: a review. Polym. 2022:1634. doi: 10.3390/POLYM14091634
Crossref Full Text | Google Scholar
Akram, F., Imtiaz, M., and ul Haq, I. (2023). Emergent crisis of antibiotic resistance: a silent pandemic threat to 21st century. Microb. Pathog. 174:105923. doi: 10.1016/J.MICPATH.2022.105923
PubMed Abstract | Crossref Full Text | Google Scholar
Annunziato, G. (2019). Strategies to overcome antimicrobial resistance (AMR) making use of non-essential target inhibitors: a review. Int. J. Mol. Sci. 20:5844. doi: 10.3390/IJMS20235844
Crossref Full Text | Google Scholar
Appanna, V. D. (2018). Human microbes-the Power within, vol. 2018. 1st Edn: Singapore: Springer Singapore, Springer Nature Singapore Pte Ltd.
Arciola, C. R., Campoccia, D., and Montanaro, L. (2018). Implant infections: adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 397–409. doi: 10.1038/s41579-018-0019-y
Crossref Full Text | Google Scholar
Asadpoor, M., Ithakisiou, G. N., van Putten, J. P. M., Pieters, R. J., Folkerts, G., and Braber, S. (2021). Antimicrobial activities of alginate and chitosan oligosaccharides against Staphylococcus aureus and group B Streptococcus. Front. Microbiol. 12:700605. doi: 10.3389/FMICB.2021.700605
PubMed Abstract | Crossref Full Text | Google Scholar
Atanase, L. I. (2021). Micellar drug delivery systems based on natural biopolymers. Polym. 2021:477. doi: 10.3390/POLYM13030477
Crossref Full Text | Google Scholar
Baltimore, R. S., Cross, A. S., and Dobek, A. S. (1987). The inhibitory effect of sodium alginate on antibiotic activity against mucoid and non-mucoid strains of Pseudomonas aeruginosa. J. Antimicrob. Chemother. 20, 815–823. doi: 10.1093/JAC/20.6.815
PubMed Abstract | Crossref Full Text | Google Scholar
Baltzer, S. A., and Brown, M. H. (2011). Antimicrobial peptides: promising alternatives to conventional antibiotics. J. Mol. Microbiol. Biotechnol. 20, 228–235. doi: 10.1159/000331009
PubMed Abstract | Crossref Full Text | Google Scholar
Baranwal, J., Barse, B., Fais, A., Delogu, G. L., and Kumar, A. (2022). Biopolymer: a sustainable material for food and medical applications. Polym. 14:983. doi: 10.3390/POLYM14050983
Crossref Full Text | Google Scholar
Benhabiles, M. S., Salah, R., Lounici, H., Drouiche, N., Goosen, M. F. A., and Mameri, N. (2012). Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocoll. 29, 48–56. doi: 10.1016/J.FOODHYD.2012.02.013
Crossref Full Text | Google Scholar
Berry, M., Gurung, A., and Easty, D. L. (1995). Toxicity of antibiotics and antifungals on cultured human corneal cells: effect of mixing, exposure and concentration. Eye 9, 110–115. doi: 10.1038/eye.1995.17
Crossref Full Text | Google Scholar
Bhattacharjee, M. K. (2016). Antibiotics that inhibit nucleic acid synthesis. Chem. Antibiot. Relat. Drugs, 109–128. doi: 10.1007/978-3-319-40746-3_5
Crossref Full Text | Google Scholar
Bianculli, R. H., Mase, J. D., and Schulz, M. D. (2020). Antiviral polymers: past approaches and future possibilities. Macromolecules 53, 9158–9186. doi: 10.1021/ACS.MACROMOL.0C01273
Crossref Full Text | Google Scholar
Biswas, M. C., Jony, B., Nandy, P. K., Chowdhury, R. A., Halder, S., Kumar, D., et al. (2022). Recent advancement of biopolymers and their potential biomedical applications. J. Polym. Environ. 30, 51–74. doi: 10.1007/s10924-021-02199-y
Crossref Full Text | Google Scholar
Brogden, K. A. (2005). Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 3, 238–250. doi: 10.1038/nrmicro1098
Crossref Full Text | Google Scholar
Bull, M. J., and Plummer, N. T. (2014). Part 1: the human gut microbiome in health and disease. Integr. Med. 13, 17–22.
Buonavoglia, A., Leone, P., Solimando, A. G., Fasano, R., Malerba, E., Prete, M., et al. (2021). Antibiotics or no antibiotics, That is the question: An update on efficient and effective use of antibiotics in dental practice, vol. 10. Basel, Switzerland: Antibiot.
Bustamante-Torres, M., Arcentales-Vera, B., Estrella-Nuñez, J., Yánez-Vega, H., and Bucio, E. (2022). Antimicrobial activity of composites-based on biopolymers. Macromolecules 2022, 258–283. doi: 10.3390/MACROMOL2030018
Crossref Full Text | Google Scholar
Castle, S. S. (2007). Beta-lactamase inhibitors. In: xPharm: The Comprehensive Pharmacology Reference, eds. S. J. Enna and B. David. (Netherlands: Bylund, Elsevier publisher) pp. 1–3.
PubMed Abstract | Google Scholar
Chamidah, A., and Hardoko, A. A. P. (2017). Antibacterial activities of β-glucan (laminaran) against gram-negative and gram-positive bacteria. AIP Conf. Proc. 1844:20011. doi: 10.1063/1.4983422/748336
Crossref Full Text | Google Scholar
Chmit, M., Kanaan, H., Habib, J., Abbass, M., Mcheik, A., and Chokr, A. (2014). Antibacterial and antibiofilm activities of polysaccharides, essential oil, and fatty oil extracted from Laurus nobilis growing in Lebanon, Asian Pac. J. Trop. Med. 7S1, S546–S552. doi: 10.1016/S1995-7645(14)60288-1
留言 (0)