Immune checkpoint inhibitors (ICIs) are monoclonal antibodies that, as their name suggests, inhibit checkpoints in the immune system so as to restore immune response against tumor cells. Over the last decade, their use has expanded in the field of oncology because they can present equal or better results alone or in combination with conventional therapies, with lower rates of serious adverse effects and death (1). This class of medications includes antibodies targeting programmed death receptor 1 (PD-1), such as Nivolumab, and those directed against cytotoxic T-lymphocyte antigen 4 (CTLA-4), exemplified by Ipilimumab, among others (1). Given that ICIs disrupt immune system checkpoints and stimulate immune activation, they can induce the development of immune-related adverse effects (IRAEs). These adverse effects can occur in various systems, with several clinical manifestations (1).
Thus, multiple IRAEs can be related to impaired consciousness, including delirium (1). Delirium is a syndrome that poses challenges for clinicians, due to its multiple causes, and the need for a multifactorial approach (2). Notably, thyroid disorders can manifest with altered consciousness, particularly in the elderly (2, 3). Therefore, in the context of oncology patients receiving immune checkpoint inhibitors (ICIs), the differential diagnosis for delirium must encompass a broader spectrum of potential causes, including usual neurological ones (such as stroke, central nervous system infections, etc), systemic diseases (infections, myocardial infarctions, respiratory diseases, etc, just to name a few), and those derived of the underlying oncological processes, as well as the adverse effects of oncological interventions, including IRAEs.
The following section describes a case of delirium due to peripheral hyperthyroidism secondary to thyroiditis, deemed to be an IRAE caused by ICIs used for palliative treatment in a patient with mesothelioma. This case delves into the diagnostic process for cognitive impairment in individuals undergoing immune checkpoint inhibitor (ICI) therapy, shedding light on how immune-related adverse events (IRAEs) influence treatment strategies and clinical outcomes for these patients.
2 Case descriptionA 64-year-old female patient with a history of predominantly epithelioid pleural mesothelioma, treated with pemetrexed and cisplatin chemotherapy for 6 cycles between 2016-2017. At the 6th year of follow-up, she evolved with locoregional progression. Subsequently, she commenced palliative immunotherapy with Nivolumab at a dose of 180 mg (equivalent to 3 mg/kg) and Ipilimumab at 60 mg (corresponding to 1 mg/kg). Additional pertinent elements of her medical history comprised a history of inactive rheumatoid arthritis, fibromyalgia, depression, and rheumatic mitral valve disease. Her current medication regimen included methadone, morphine, celecoxib, pregabalin, esomeprazole, bisoprolol, sertraline, and clonazepam.
She was admitted to the hospital due to a 2-month history of progressive episodes of disorientation, inattention, frequent forgetfulness, confusion, sporadic nocturnal visual hallucinations, and occasional difficulties in naming objects, notable for the absence of evident aphasia but exhibiting fluctuating patterns throughout the day. The onset of symptoms coincided with the initiation of immunotherapy with nivolumab and ipilimumab. Additionally, she reported asthenia, adynamia, mild gait instability, heat intolerance, nocturnal sweating, palpitations, tremors, low mood, as well as a deterioration in her quality of life and functionality. The patient denied any falls, fever, fluctuations in weight, or other significant symptoms.
At evaluation, the patient was partially disoriented, inattentive, with mild ataxia and dysarthria upon neurological examination. Her vital signs and the rest of the physical examination were without relevant findings. An extensive study of potential causes for delirium was conducted. A mild urinary tract infection was found and treated with amikacin. Hepatic encephalopathy, renal failure, intoxication, drug or medication withdrawal, and urinary or fecal retention were ruled out. Considering the history of mesothelioma, and the recent initiation of immunotherapy, a comprehensive neurological study was performed: Contrast-enhanced brain MRI revealed non-confluent supratentorial white matter microangiopathy, without evidence of vascular lesions or mesothelioma dissemination; electroencephalogram was normal; lumbar puncture showed no signs of encephalitis or leptomeningeal spread.
Further investigations revealed a suppressed thyroid-stimulating hormone (TSH), prompting a comprehensive evaluation of the thyroid axis. The results demonstrated elevated levels of peripheral thyroid hormones (total T4 and T3), absence of anti-thyroid-stimulating hormone receptor antibodies (TRAb) and anti-thyroid peroxidase (TPO), along with the presence of anti-thyroglobulin (Anti-Tg) antibodies. No additional endocrine irregularities were detected, and subsequent thyroid iodine uptake studies indicated low uptake. Ultimately, it was concluded that the etiology of the delirium was a thyrotoxicosis episode that developed in the context of autoimmune thyroiditis, secondary to ICI treatment.
Symptomatic treatment with beta-blockers was initiated, leading to a gradual amelioration of neurological manifestations. Upon discharge, the patient was scheduled for outpatient monitoring by an endocrinologist and advised to continue her oncological regimen without modifications. Figure 1 describes the clinical course of our patient.
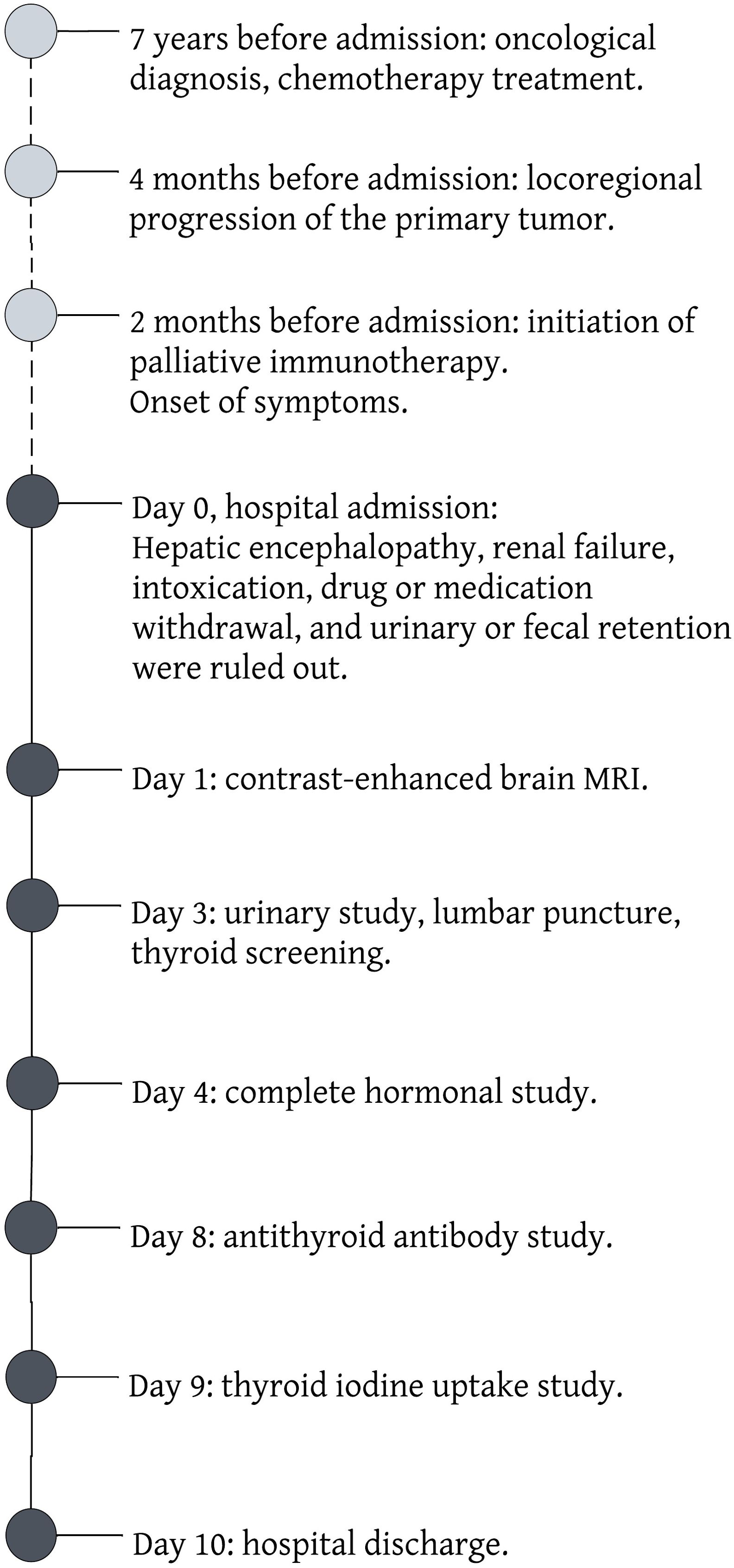
Figure 1. Timeline of the clinical case presented.
3 DiscussionQualitative impairment of consciousness (i.e., delirium) and neuropsychiatric symptoms can be manifestations of ICI adverse reactions (4–6). Additionally, patients can have multiple causes of impaired consciousness (7). Currently, there is a lack of consensus regarding a standardized approach to managing impaired consciousness in individuals undergoing therapy involving ICIs.
Delirium can be a difficult syndrome to diagnose in the presence of neuropsychiatric symptoms, such as emotional lability, delusions, and hallucinations, but it always presents with the particular characteristic of being fluctuating over time (3). Among the possible precipitating causes of delirium are infections, myocardial infarctions, inflammatory diseases (e.g., acute pancreatitis), neurological diseases (e.g. strokes), surgeries, organic dysfunctions (renal, hepatic, endocrine, hematological such as anemia, etc.), pain, sleep deprivation, dehydration, and electrolyte imbalances, medications and drugs or their withdrawal, urinary and/or fecal retention, and environmental changes (2). Neuropsychiatric symptoms and psychoses can be manifestations of medical conditions that should be actively ruled out, including thyroid diseases, vitamin B12 or thiamine deficiency (B1, Wernicke-Korsakoff Encephalopathy), Wilson’s Disease, neurosyphilis, HIV infection, brain tumors, some epilepsies, adverse effects of medications (such as systemic corticosteroids), and even rare diseases, such as lysosomal diseases (8, 9). In managing patients on immune checkpoint inhibitors (ICIs), clinicians must also explore potential complications directly linked to cancer, including neurological disease progression, intracranial metastases, leptomeningeal involvement, or the emergence of paraneoplastic neurological syndromes impacting the central nervous system (such as encephalitis, encephalomyelitis). Additionally, they should consider chemotherapy-related complications, like heightened infection risks or direct toxic effects. ICIs increase the likelihood of IRAEs, whether they be neurological (e.g., autoimmune encephalitis) or from other systems, where the symptoms relate to the central nervous system, such as endocrinopathies, renal failure, liver failure, etc. (4, 7, 10, 11). In Figures 2, 3 we propose a sequential approach to the oncologic patient that consults because of impaired consciousness, considering the list of causes previously described.
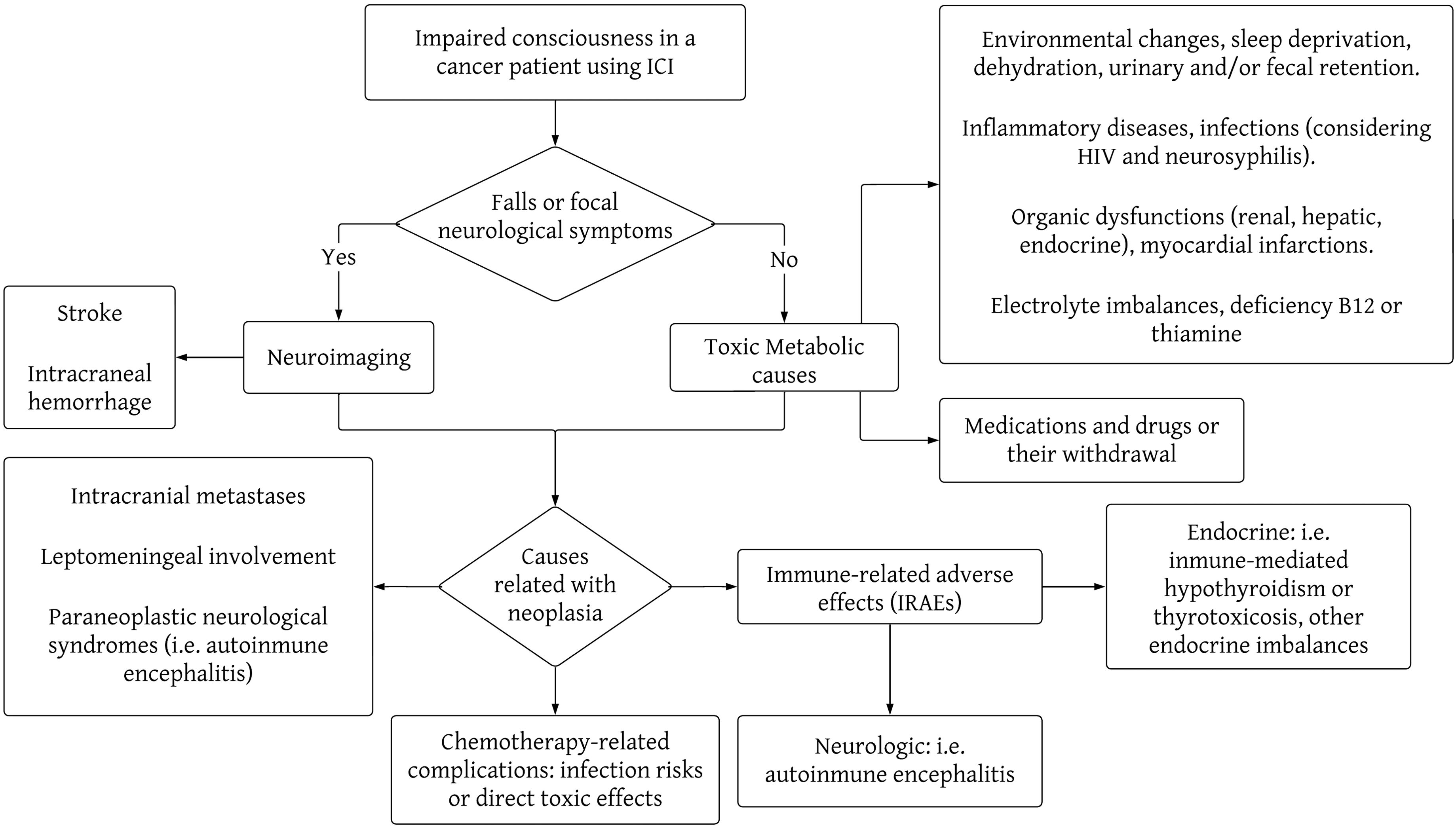
Figure 2. Flowchart of the most important causes of impaired consciousness in cancer patients undergoing therapy with immune checkpoint inhibitors. This diagram is not intended as a comprehensive list of all the causes of impaired consciousness, but a schematic approach to the diagnosis reasoning behind the study of these clinical causes.

Figure 3. Proposed multistep approach for the laboratory and imaging workup of cancer patients using immune checkpoint inhibitors that consult because of impaired consciousness. This diagram should not be taken as rigid, and the approach should be modified and adapted to the clinical case being faced, according to the pre-test probability of each entity.
In our patient, although the initial study indicated a possible low urinary tract infection, this did not justify the diversity of neuropsychiatric symptoms associated with palpitations or heat intolerance, nor a temporal correlation with the initiation of ICI therapy. This finding prompted us to explore other possible causes following the list of etiologies previously described, seeking an alternative diagnosis that could unify the presentation of impaired consciousness in a patient with advanced cancer under ICI treatment. Therefore, we ruled out neurological causes, such as autoimmune encephalitis, intracerebral lesions, and leptomeningeal involvement secondary to oncological pathology, and studied endocrinological causes, confirming thyrotoxicosis.
The involvement of the thyroid in ICI-related IRAEs is very common, observed as asymptomatic laboratory abnormalities, primary immune-mediated hypothyroidism (13-16% in users of combined immunotherapy), thyrotoxicosis due to autoimmune glandular destruction (up to 8 to 10% of patients using combined immunotherapy) or Basedow-Graves disease. In general, these develop during the first months of treatment (1, 12, 13). Thyrotoxicosis refers to the clinical scenario that arises from an excess of thyroid hormones, associated with a wide variety of symptoms (14, 15). Among the most commonly reported neurological symptoms are mood alterations, such as irritability, anxiety, and depression, while psychotic symptoms have been very rarely reported: there is a series of 18 cases presented by the group led by B. Brownlie in 2000, and some isolated cases reports (16–21). Of these, stand out a case of thyrotoxicosis with hyperactive delirium and another one with hypomania and paranoia; we also noticed that the majority of cases reported with psychotic symptoms are of Graves disease in patients with preexistent psychiatric diseases, leading us to the conclusion that psychoses associated to thyrotoxicosis not caused by Graves disease is rare. With the previous evidence the incidence of psychoses in thyrotoxicosis has been estimated as approximately 1% (16). In our patient, thyrotoxicosis was secondary to thyroiditis associated with ICI use, which is consistent with the lower iodine uptake and the profile of autoantibodies found, a situation that we find unique with the actual available evidence.
Endocrine IRAEs can occur in 12 to 34% of patients, potentially affecting any gland in the body (with the thyroid being frequently affected), but their lethality is relatively low. This contrasts with neurological IRAEs, such as autoimmune encephalitis, which have an incidence of less than 3%, but a lethality of 3 to 16% (1, 22, 23). The oncological prognosis, beyond direct mortality from the complication itself, is also diametrically different between these two groups: recent evidence shows that the occurrence of thyroid IRAEs is associated with better treatment response, and even longer survival, compared to patients who do not have them (24–26); on the other hand, the occurrence of neurological IRAEs requires ICI discontinuation, and the initiation of treatment involving potent immunosuppression with systemic corticosteroids and intravenous immunoglobulins, among other options, that can further worsen oncologic prognosis (1, 22, 27). In the case of thyroiditis, treatment consists of symptomatic management with beta-blockers, adding prednisolone only if there is associated neck pain, and temporarily suspending ICI use solely if the symptoms are poorly tolerated, without being an indication for discontinuing therapy (12, 27).
Unfortunately, follow-up was lost after discharge and we do not have information about further symptom resolution and TSH level monitoring after immune checkpoint inhibitor therapy was resumed. Although this poses a limitation for our case report, we were able to present our approach to oncologic patients being treated with ICIs that present with impaired consciousness, such as the one presented.
4 ConclusionThis case highlights the intricate diagnostic challenge of impaired consciousness in cancer patients who use ICIs, especially considering the wide spectrum of possible etiologies. In this context, the recognition of delirium symptoms in relation to thyrotoxicosis as an IRAE serves as a pertinent example, demonstrating the differential impact of endocrine IRAEs versus neurological ones on patient outcomes and oncological prognosis. An organized and multisystemic diagnostic approach is crucial to reach the correct diagnosis, and thus, implement appropriate management for both the acute pathology, and the underlying oncological disease.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statementFor the publication of this case report, informed consent was obtained from the patients legal tutor because of the patients impairment of consciousness, according to local and international ethical regulations.
Author contributionsDC: Writing – original draft, Writing – review & editing. CB: Writing – original draft, Writing – review & editing. TY: Writing – original draft, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Dirección de Investigación of Pontificia Universidad Católica de Chile, for translation and publishing costs.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. (2019) 16:563–80. doi: 10.1038/s41571-019-0218-0
PubMed Abstract | Crossref Full Text | Google Scholar
4. Zhou C, Peng S, Lin A, Jiang A, Peng Y, Gu T, et al. Psychiatric disorders associated with immune checkpoint inhibitors: a pharmacovigilance analysis of the FDA Adverse Event Reporting System (FAERS) database. EClinicalMedicine [Internet]. (2023) 59:101967. doi: 10.1016/j.eclinm.2023.101967
PubMed Abstract | Crossref Full Text | Google Scholar
5. Mavrotas M, Murray B. Mania as a possible complication of immunotherapy. Prog Neurol Psychiatry [Internet]. (2020) 24:9–13. doi: 10.1002/pnp.555
Crossref Full Text | Google Scholar
6. Jono M, Kinehara Y, Utsu Y, Tamura Y, Koseto M, Murakami T, et al. Neuropsychiatric immune-related adverse events induced by pembrolizumab in a patient with lung adenocarcinoma and systemic lupus erythematosus. Intern Med [Internet]. (2020) 59:569–72. doi: 10.2169/internalmedicine.3782-19
PubMed Abstract | Crossref Full Text | Google Scholar
7. Wei D, Zhou DJ, Datta P, Taraschenko O. Recognizing encephalopathy in immune checkpoint inhibitor therapy: a single-center experience. Cancer Med. (2021) 10:2978–86. doi: 10.1002/cam4.3818
PubMed Abstract | Crossref Full Text | Google Scholar
8. Freudenreich O, Schulz SC, Goff DC. Initial medical work-up of first-episode psychosis: a conceptual review. Early Interv Psychiatry. (2009) 3:10–8. doi: 10.1111/j.1751-7893.2008.00105.x
PubMed Abstract | Crossref Full Text | Google Scholar
11. Dubey D, David WS, Reynolds KL, Chute DF, Clement NF, Cohen JV, et al. Severe neurological toxicity of immune checkpoint inhibitors: growing spectrum. Ann Neurol [Internet]. (2020) 87:659–69. doi: 10.1002/ana.25708
PubMed Abstract | Crossref Full Text | Google Scholar
13. Nervo A, Ferrari M, Gruosso G, Migliore E, Basile S, D’Angelo V, et al. Immune-related thyroid dysfunctions during anti PD-1/PD-L1 inhibitors: new evidence from a single centre experience. Clin Exp Med. (2023) 23:4817–24. doi: 10.1007/s10238-023-01082-5
PubMed Abstract | Crossref Full Text | Google Scholar
16. Brownlie BE, Rae AM, Walshe JW, Wells JE. Psychoses associated with thyrotoxicosis - 'thyrotoxic psychosis.' A report of 18 cases, with statistical analysis of incidence. Eur J Endocrinol. (2000) 142:438–44. doi: 10.1530/eje.0.1420438
PubMed Abstract | Crossref Full Text | Google Scholar
17. Bennett B, Mansingh A, Fenton C, Katz J. Graves’ disease presenting with hypomania and paranoia to the acute psychiatry service. BMJ Case Rep CP. (2021) 14:e236089. doi: 10.1136/bcr-2020-236089
PubMed Abstract | Crossref Full Text | Google Scholar
18. Urias-Uribe L, Valdez-Solis E, González-Milán C, Ramírez-Rentería C, Ferreira-Hermosillo A. Psychosis crisis associated with thyrotoxicosis due to Graves' disease. Case Rep Psychiatry. (2017) 2017:6803682. doi: 10.1155/2017/6803682
PubMed Abstract | Crossref Full Text | Google Scholar
21. Emul M, Sakalli A, Erol TC, Ertan T. Thyrotoxic psychosis in an elderly woman and haloperidol use: a case report. Psychogeriatrics. (2013) 13:49–51. doi: 10.1111/j.1479-8301.2012.00404.x
PubMed Abstract | Crossref Full Text | Google Scholar
22. Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. (2018) 4:1721–8. doi: 10.1001/jamaoncol.2018.3923
PubMed Abstract | Crossref Full Text | Google Scholar
24. Lima Ferreira J, Costa C, Marques B, Castro S, Victor M, Oliveira J, et al. Improved survival in patients with thyroid function test abnormalities secondary to immune-checkpoint inhibitors. Cancer Immunol Immunother. (2021) 70:299–309. doi: 10.1007/s00262-020-02664-y
PubMed Abstract | Crossref Full Text | Google Scholar
25. Lee HJ, Manavalan A, Stefan-Lifshitz M, Schechter C, Maity A, Tomer Y. Permanent hypothyroidism following immune checkpoint inhibitors induced thyroiditis may be associated with improved survival: results of an exploratory study. Front Endocrinol. (2023) 14:1169173. doi: 10.3389/fendo.2023.1169173
PubMed Abstract | Crossref Full Text | Google Scholar
26. Iwamoto Y, Kimura T, Dan K, Ohnishi M, Takenouchi H, Iwamoto H, et al. Immune checkpoint inhibitor-induced hypothyroidism predicts treatment response in Japanese subjects. Front Endocrinol. (2023) 14:1221723. doi: 10.3389/fendo.2023.1221723
PubMed Abstract | Crossref Full Text | Google Scholar
27. Haanen J, Obeid M, Spain L, Carbonnel F, Wang Y, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. (2022) 33:1217–38. doi: 10.1016/j.annonc.2022.10.001
留言 (0)