 Chengcheng Zhang1†
Chengcheng Zhang1† Linling Wang2†
Linling Wang2† Qianzhen Zhang2
Qianzhen Zhang2 Junjie Shen2
Junjie Shen2 Xia Huang2
Xia Huang2 Meiling Wang2
Meiling Wang2 Yi Huang2
Yi Huang2 Jun Chen2
Jun Chen2 Yanmin Xu2
Yanmin Xu2 Wenxu Zhao2
Wenxu Zhao2 Yanan Qi2
Yanan Qi2 Yunyan Li2
Yunyan Li2 Yanjiao Ou1
Yanjiao Ou1 Zhi Yang2*
Zhi Yang2* Cheng Qian2*1Department of Hepatobiliary Surgery, Southwest Hospital, Army Medical University, Chongqing, China2Chongqing Key Laboratory of Gene and Cell Therapy, Institute of Precision Medicine and Biotechnology, Chongqing Precision Biotech Co. Ltd., Chongqing, China
Cheng Qian2*1Department of Hepatobiliary Surgery, Southwest Hospital, Army Medical University, Chongqing, China2Chongqing Key Laboratory of Gene and Cell Therapy, Institute of Precision Medicine and Biotechnology, Chongqing Precision Biotech Co. Ltd., Chongqing, ChinaIn the published article, there was an error in Figure 7A as published. Among a large amount of pictures for selection, we found two graphs identical since they were not classified correctly. The corrected Figure 7A and its caption appear below.
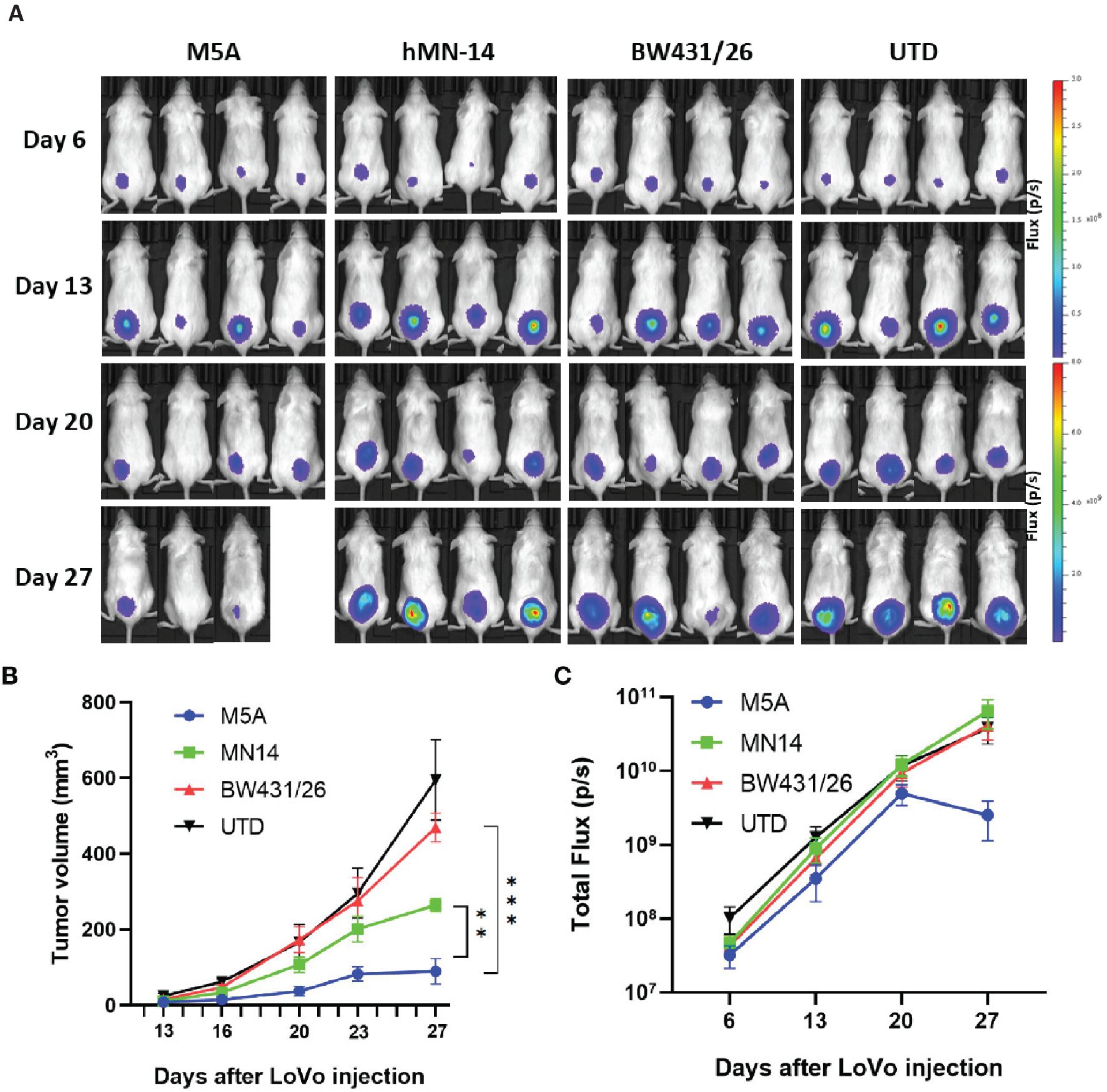
Figure 7. M5A CAR-T cells exhibited superior tumor suppression in the xenograft model in NOG mice. (A) Each mouse was implanted with 1× 106 LoVo cells (Luc+) on day 1 and injected i.v. with 1 × 107 CAR-T cells on day 7. Mice were imaged weekly. (B) Tumor growth was assessed by calculating the tumor volume. The values are presented as the means ± SEMs. The growth of tumors treated with M5A CAR-T cells was potently controlled compared with that of tumors in the other groups. (C) The total bioluminescence values were also recorded and compared. The values are presented as the means ± SEMs. Statistical analysis was performed by one-way ANOVA. * = p < 0.05; ** = p < 0.01; and ns, not significant.
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: chimeric antigen receptor T cells, carcinoembryonic antigen, single-chain fragment variable, affinity, cell therapy
Citation: Zhang C, Wang L, Zhang Q, Shen J, Huang X, Wang M, Huang Y, Chen J, Xu Y, Zhao W, Qi Y, Li Y, Ou Y, Yang Z and Qian C (2025) Corrigendum: Screening and characterization of the scFv for chimeric antigen receptor T cells targeting CEA-positive carcinoma. Front. Immunol. 16:1548247. doi: 10.3389/fimmu.2025.1548247
Received: 19 December 2024; Accepted: 10 January 2025;
Published: 31 January 2025.
Copyright © 2025 Zhang, Wang, Zhang, Shen, Huang, Wang, Huang, Chen, Xu, Zhao, Qi, Li, Ou, Yang and Qian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cheng Qian, Y3FpYW44NjM0QGdtYWlsLmNvbQ==; Zhi Yang, eXoyMDAzY2FuQDEyNi5jb20=
†These authors have contributed equally to this work
留言 (0)