At the end of 2019, China informed the World Health Organization (WHO) that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the virus that causes COVID-19 (1). This is a highly contagious infectious disease with contact-household transmission mechanisms, which damages the lung tissue induced by a new strain of the virus from the genus Coronavirus SARS CoV-2 (2). Highly contagious COVID-19 has affected the healthcare system and patients with certain preexisting medical conditions including cancer. Due to the fast-growing spread and high infection rates, lockdowns and restrictions were performed, which affected the detection and diagnosis of cancer. Therefore, cancer screening programs were suspended in many countries, and preventive care services including cancer screening programs showed a gradual decline worldwide (3–5).
Breast cancer is the most widespread cancer type worldwide. In 2020, 2.3 million new cases were diagnosed and 685,000 deaths were registered around the world (5). Early screening and detection can reduce mortality by up to 65% among breast cancer patients (5). Screening models vary in different countries. Beginning in 2008, breast cancer screening became mandatory in Kazakhstan for women at the ages of 50, 52, 54, 56, 58, and 60 until 2017. Since 2018, all women between the ages of 40 and 70 years with an interval of 2 years are subject to mandatory screening. For the screening test, the target population receives two projections of mammography of each breast. Kazakhstan implemented screening by international standards in 2011, including double reading, interpretation according to the Breast Imaging-Reporting and Data System (BI-RADS), and test result (mammogram) archiving (6).
The COVID-19 pandemic also led to a reduction in cervical cancer screening and population-based prevention activities including human papillomavirus (HPV) vaccinations. Studies have reported that HPV vaccinations declined by >70% in March 2020, and cervical cancer screening dropped by 94% due to the pandemic restrictions (7). In Kazakhstan, a major concern regarding smears according to the Bethesda System was introduced in 2011. The liquid-based cytology method was gradually introduced in 2012. Both methods are currently used for the screening test for the women age group 30–70 years with an interval of one time in 4 years. During the COVID-19 pandemic, our healthcare system also faced delays in care and cervical cancer screening tests, which led to disruptions in cancer prevention systems.
Colorectal cancer is considered the second cause of cancer deaths in the world with a mortality rate of 9.4% (8). During the COVID-19 pandemic, in 2020, more than 1.9 million new cases and 935,000 deaths occurred due to this type of cancer, which shows the significance of early screening in order to improve the mortality rate (8). Colorectal cancer screening was introduced in Kazakhstan in 2011 following the Health Development State Program of the Republic of Kazakhstan. The target population group included both sexes aged 50 to 60 years until 2017 and was expanded to 70 years in 2018, with the screening offered every 2 years. The detection of hidden blood in the stool has been used as a primary test type; however, beginning in 2013, an immunochemical (immunofluorescence) test has been used, and the in-depth diagnosis includes total colonoscopy (9). COVID-19 had a significant impact on the diagnosis of new cases of colorectal cancer and delayed onset of treatment and is significantly associated with increased mortality rates.
In 2020–2021, the COVID-19 pandemic changed approaches in the diagnostics and cancer care continuum. Fear and anxiety caused by the COVID-19 pandemic from patients and healthcare workers delayed screening for breast, cervical, and colorectal cancers, which led to diagnoses at a later stage with poorer prognoses (10). Screening rates for breast, cervical, and colon cancers were slightly below baselines, and cancer cases were lower than expected (11). Moreover, the perception of the existing barriers to screening procedures among the population worsened during COVID-19, and the disparity has grown (12, 13).
Although healthcare workers around the world adapted to meet the needs, data indicating screening availability in Kazakhstan during the quarantine are lacking (14). To our knowledge, this is the first study to describe the incidence and mortality rates of breast, cervical, and colorectal cancers during the pandemic period in Kazakhstan, even in Central Asia, using big population register data. Therefore, this study aimed to assess the epidemiological situation for cancer screening sites among the population of Kazakhstan before and during the COVID-19 pandemic. Notably, we aimed to analyze epidemiological changes in breast, cervical, and colorectal cancers in Kazakhstan, including early-onset cancer diagnosed between the ages of 20 and 49 years using data from the oncological service of the Republic of Kazakhstan for the 2017–2022 period.
2 Materials and methods2.1 Study design and populationThis is a retrospective study. The data were retrieved from statistical and analytical materials of the oncological service of the Republic of Kazakhstan for the 2017–2022 timeframe (15–20). The study population comprised patients aged 20 to 49 years [early-onset cancer (EOC)] and 50 years and older [late-onset cancer (LOC)] from the total number of patients diagnosed each year during the study period for breast, cervical, colon, and rectal cancers. There were four screening locations analyzed: breast and cervix for women and the two colorectal cancer (CRC) localizations, colon (the ascending colon, transverse colon, descending colon, and caecum) and rectum (the rectosigmoid junction, rectum, and anal canal), for women and men together and separately.
2.2 Statistical analysesFor the intent of this study, several indicators from the Kazakhstan Cancer Registry were calculated, and standardized indicators were analyzed according to the WHO World Standard (21). Categorical variables were described as frequencies and percentages, and continuous variables were expressed as averages. Selected indicators included standardized incidence per 100,000 population, standardized mortality per 100,000 population, mortality-to-incidence ratio in %, 1-year mortality as a percentage, mortality among the contingent of patients registered and under supervision at the end of the corresponding year, 5-year patient survival as a percentage, population and distribution by stages of newly diagnosed cancers, and early/advance stage ratio. These indicators were separately analyzed for every year in the period 2017–2022. The age-based detection study included all cancer patients registered in the oncological registry in Kazakhstan, according to data from official analytical reports of the oncological centers and “Form No. 7” and registration of patients diagnosed for the first time in their lives “Form No. 90” from 2017 to 2022. Annual percentage change (APC) was calculated by taking the difference in incidence between 1 year and the next by calculating the corresponding percentage. The average APC (AAPC) was obtained by adding all the APCs and dividing by the total number of years of the study period. APC indicates a difference in one-time intervals, whereas AAPC characterizes the global trend over the entire study period. Statistical analysis was performed using the Stata MP2 16.1 version. To determine the difference between one categorical variable and another continuous variable, analysis of variance (ANOVA) was used. p-Values are two-sided and reported as statistically significant at <0.05 for all analyses.
2.3 Ethics approvalThe Ethics Committee at JSC “Kazakh Research Institute of Oncology and Radiology” (protocol No. 4-2021) approved this project. The study was performed according to both international and local ethics guidelines and regulations.
3 Results3.1 Breast cancerFigure 1 depicts the epidemiological data of the breast cancer cohort. The incidence increased by 5.2% from 2017 to 2022. During the COVID-19 restrictions, the incidence dropped to 36.6 per 100,000 in 2020, which was 14.9% lower than in 2019 and 15.0% lower than in 2021. Mortality decreased by 25.7% from 2017 to 2022. During the COVID-19 restrictions, the mortality rate was 9.2 per 100,000 in 2020, which was 3.2% higher than that from 2019 to 2021. The ratio of mortality to incidence decreased by 8.3, which was 29.4% lower than that from 2017 to 2022. During the COVID-19 restrictions, this ratio was 25.1, which was 14.6% higher than in 2019 and 8.4% higher than in 2021. One-year mortality decreased by 0.1, which was 2.5% lower than that from 2017 to 2022. During the COVID-19 restrictions, 1-year mortality was 4.1%. Mortality follow-up decreased by 2.3%, which was 36.1% lower than that from 2017 to 2022. During the COVID-19 restrictions, mortality follow-up was 2.7%. The 5-year survival rate increased by 3.9% (growth rate was 7.3%), from 53.2% in 2017 to 57.1% in 2022.
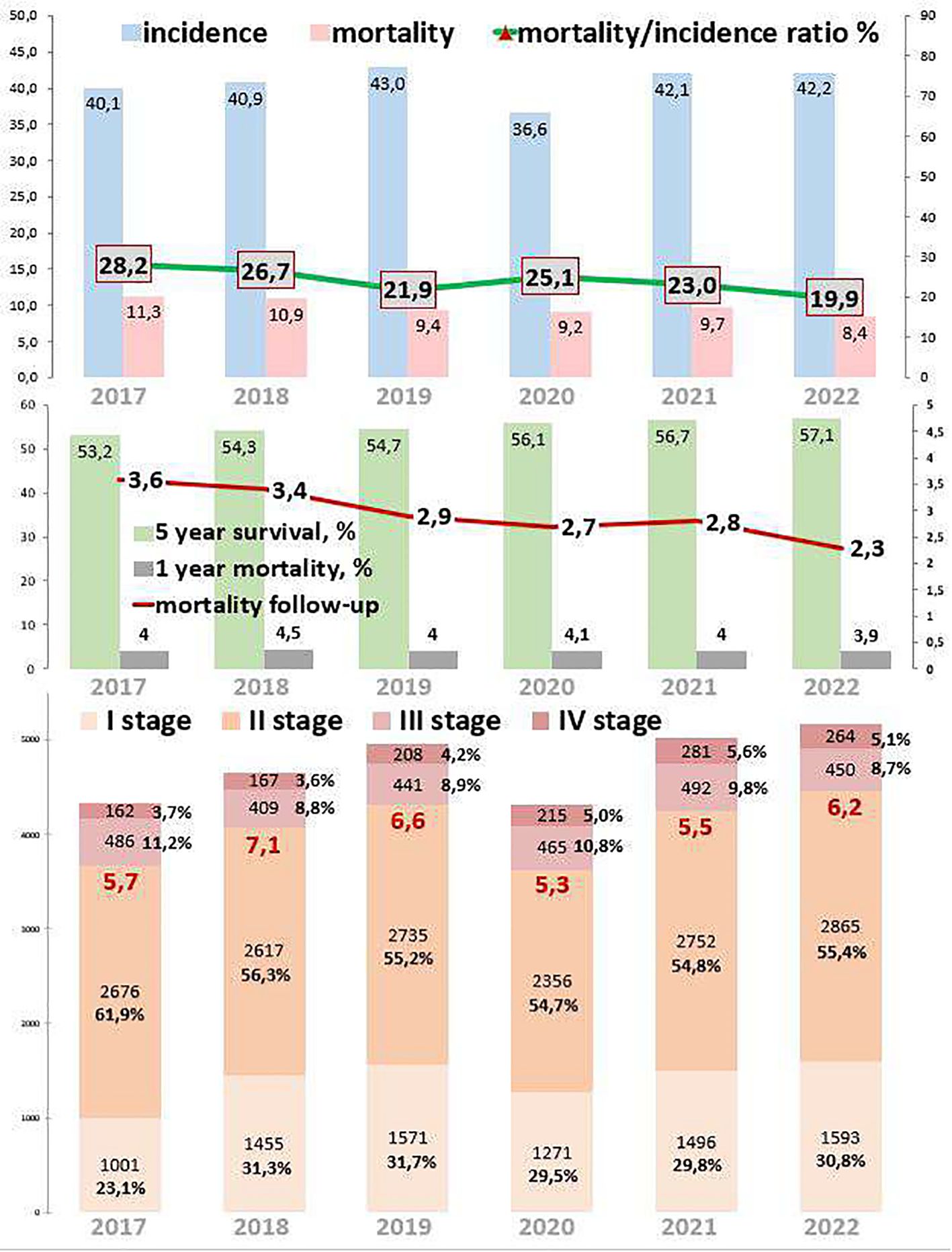
Figure 1. Key epidemiological indicators for breast cancer in Kazakhstan for 2017-2022.
Out of 121,769 breast cancer patients, 26,485 (21.7%) had EOC while 93,958 (77.2%) had LOC, and 1,326 (1.1%) patients were aged 0–19 years. Comparing 2022 to 2017, the new cases increased by 22.8% for EOC and 15.9% for LOC. The highest APC for EOC was 8.7% in 2018, and for LOC, it was 19.8% in 2021. The AAPC increased by 4.3% for EOC and 3.6% for LOC. During the COVID-19 restriction period, breast cancer detection rates decreased by 6.1% for EOC and 15.6% for LOC (Tables 1, 2).
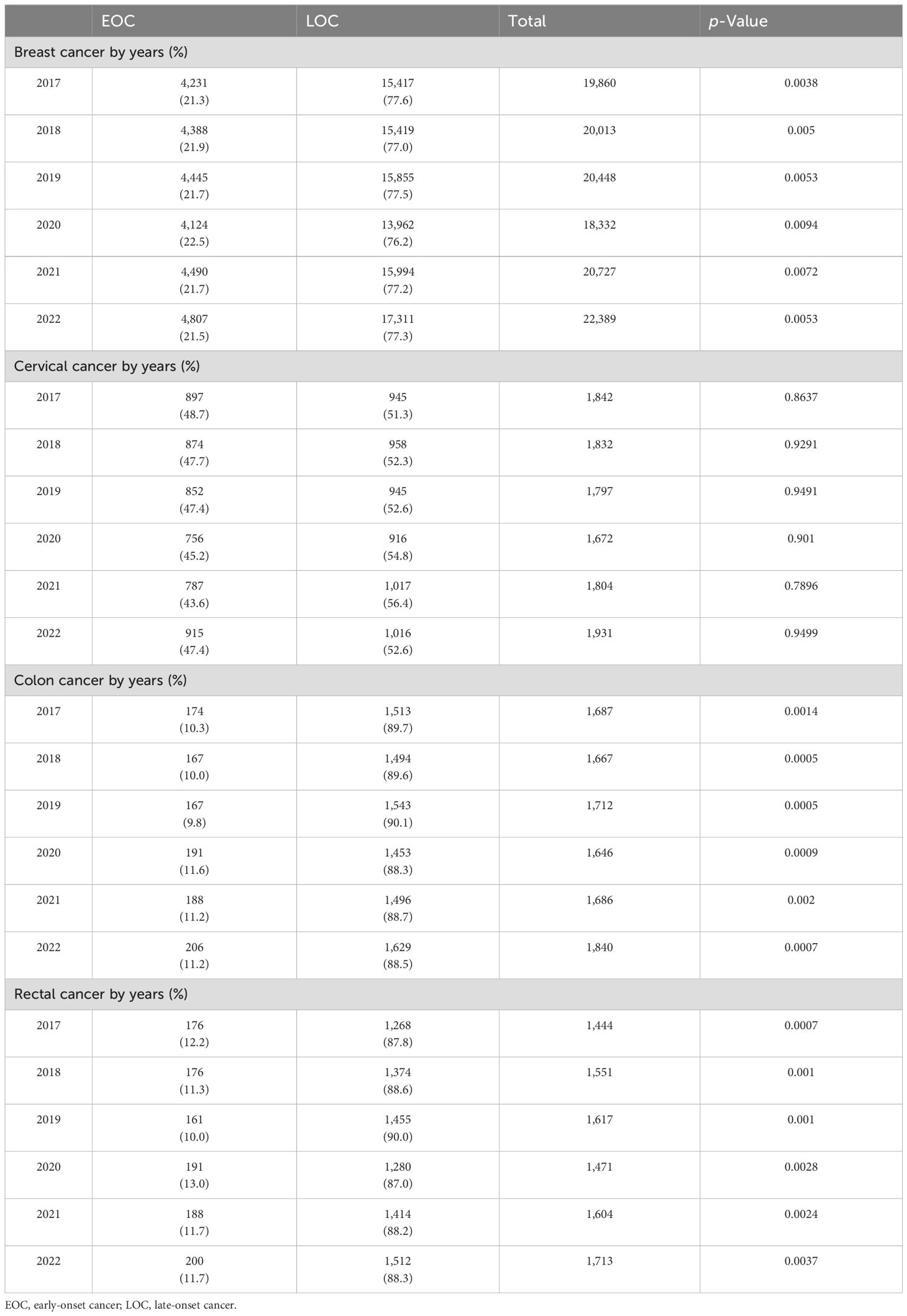
Table 1. Incidence of breast, cervical, colon, and rectal cancers by EOC (20–49 years) and LOC (≥50 years) in Kazakhstan between 2017 and 2022.
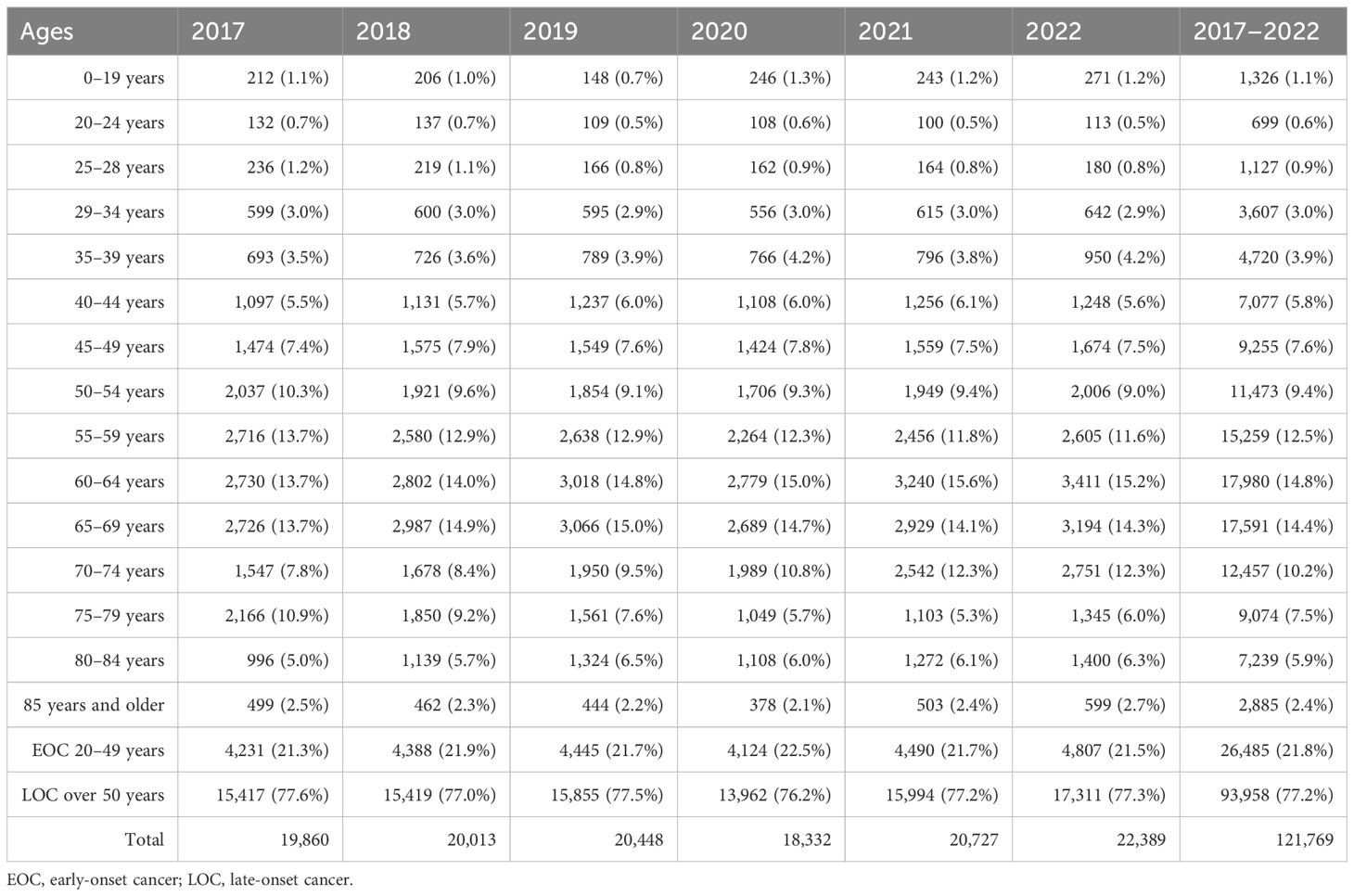
Table 2. Incidence by age for breast cancer in Kazakhstan between 2017 and 2022.
3.2 Cervical cancerFigure 2 describes cervical cancer epidemiological trends. The incidence decreased by 4.1% from 2017 to 2022. During the COVID-19 restrictions, the incidence dropped to 14.7 in 2020, which is 14.5% lower than in 2019 and 6.1% lower than in 2021. Mortality decreased by 10.7% from 2017 to 2022. During the COVID-19 restrictions, mortality was 5.1 in 2020, and it remained stable from 2019 to 2021. The ratio of mortality to incidence decreased by 2.3%, which was 20.6% lower than that from 2017 to 2022. During the COVID-19 restrictions, this ratio was 34.7, which was 10.9% higher than in 2019 and 7.5% higher than in 2021. One-year mortality decreased by 2.1%, which was 15.6% lower than that from 2017 to 2022. During the COVID-19 restrictions, 1-year mortality was 11.1%. Mortality follow-up decreased by 1.0%, which was 21.3% lower than that from 2017 to 2022. During the COVID-19 restrictions, mortality follow-up was 4.0%. The 5-year survival increased by 7.1% (growth rate by 17.0%), from 52.4% in 2017 to 61.3% in 2022.
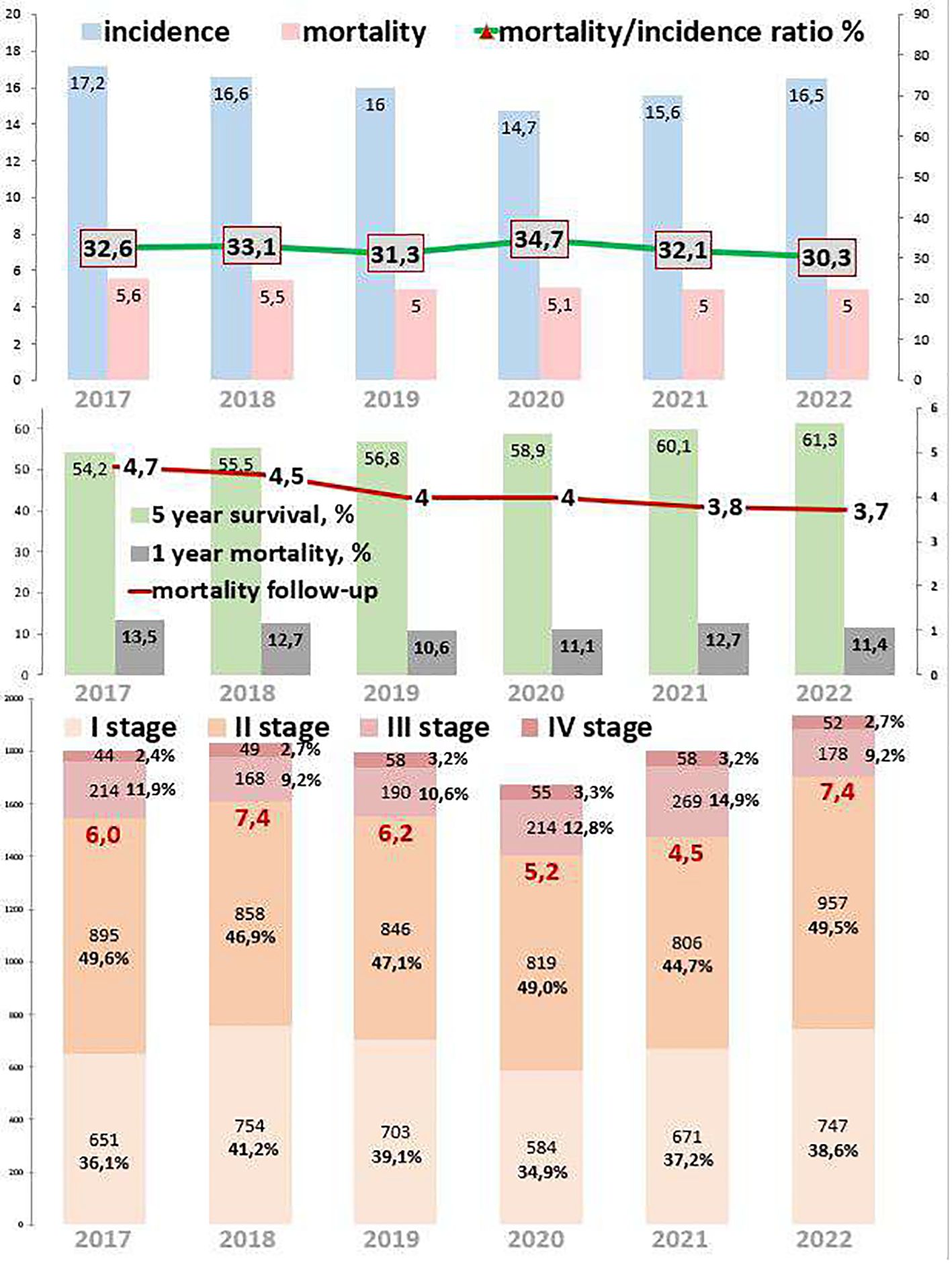
Figure 2. Key epidemiological indicators for cervical cancer in Kazakhstan from 2017 to 2022.
Out of 10,878 cervical cancer patients, 5,081 (46.7%) had EOC, while 5,797 (53.3%) had LOC. New cases of cervical cancer increased by 5.0% over the study period in both age groups. Comparing 2022 to 2017, the new cases increased by 2.3% for EOC and 7.5% for LOC. The highest APC for EOC was 16.6% in 2022, and for LOC, it was 11.0% in 2021. The AAPC increased by 0.9% for EOC and 1.6% for LOC. During the COVID-19 restriction period, cervical cancer detection rates decreased by 11.3% for EOC and 3.1% for LOC (Tables 1, 3). However, there was no statistically significant difference in the incidence of cervical cancer for the period of 2017 and 2022.

Table 3. Incidence by age for cervical cancer in Kazakhstan for 2017–2022.
3.3 Colon cancerColon cancer epidemiological trends are presented in Figure 3. The incidence decreased by 2.2% from 2017 to 2022. During the COVID-19 restrictions, the incidence was 7.8 cases per 100,000 in 2020, which was 4.9% lower than in 2019 and the same as in 2021. Mortality decreased by 23.7% from 2017 to 2022. During the COVID-19 restrictions, mortality was 3.6 cases per 100,000 in 2020, which was 2.9% higher than in 2019 and 13.9% higher than in 2021. The ratio of mortality to incidence decreased to 9.4, which was 22.0% lower than that from 2017 to 2022. During the COVID-19 restrictions, this ratio was 46.2, which was 8.2% higher than in 2019 and 14.1% higher than in 2021.
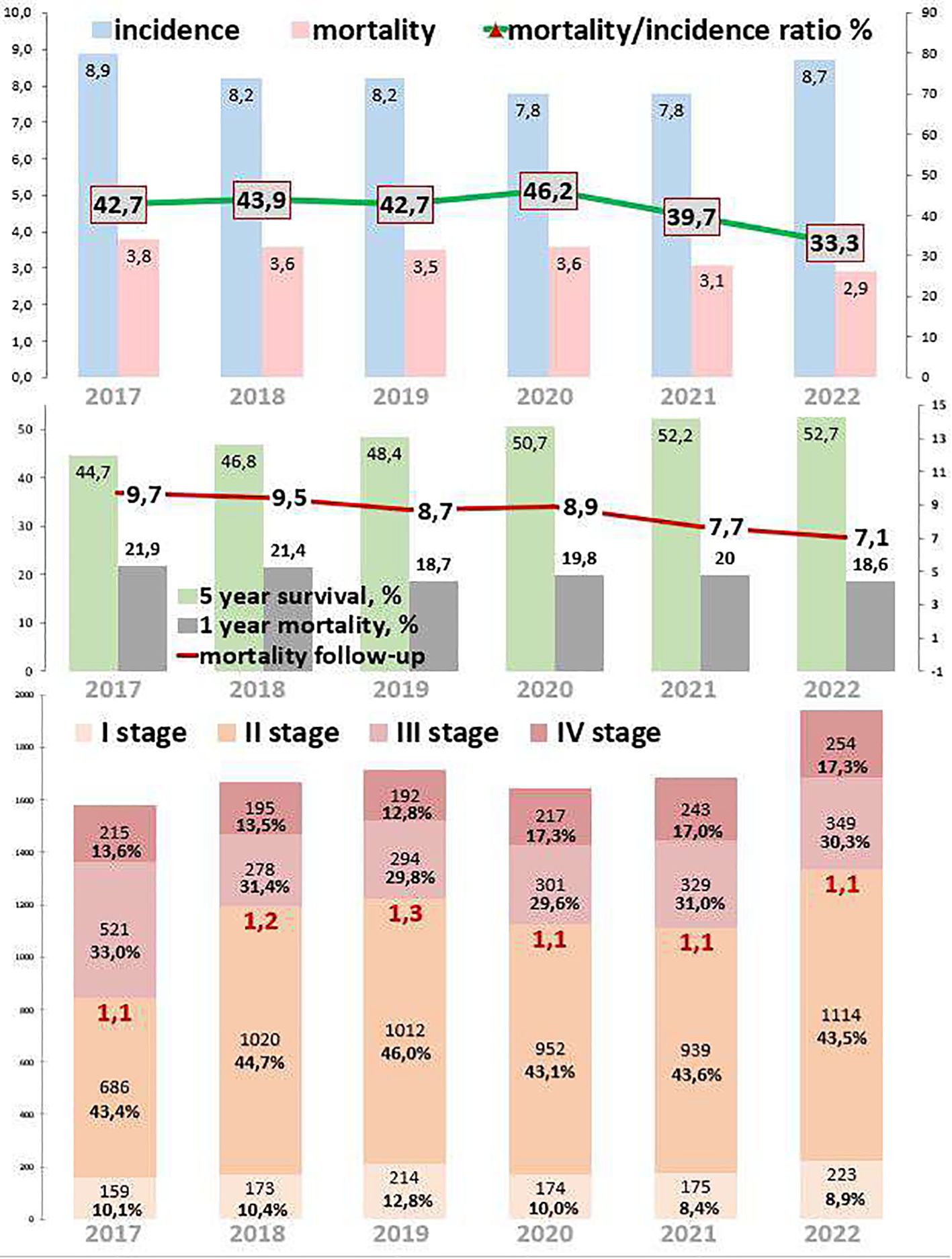
Figure 3. Key epidemiological indicators for colon cancer in Kazakhstan from 2017 to 2022.
Out of 10,238 colon cancer patients, 1,093 (10.7%) had EOC, while 9,128 (89.2%) had LOC, and 17 (0.2%) patients were aged 0–19 years (Tables 1, 4). Comparing 2022 to 2017, new cases increased by 18.4% for EOC and by 14.3% for LOC. The highest APC for EOC was 14.4% in 2020, and for LOC, it was 15.6% in 2022. The AAPC increased by 3.7% for EOC and by 2.9% for LOC. During the COVID-19 restriction period, colon cancer detection rates increased by 14.4% for EOC and decreased by 5.8% for LOC.
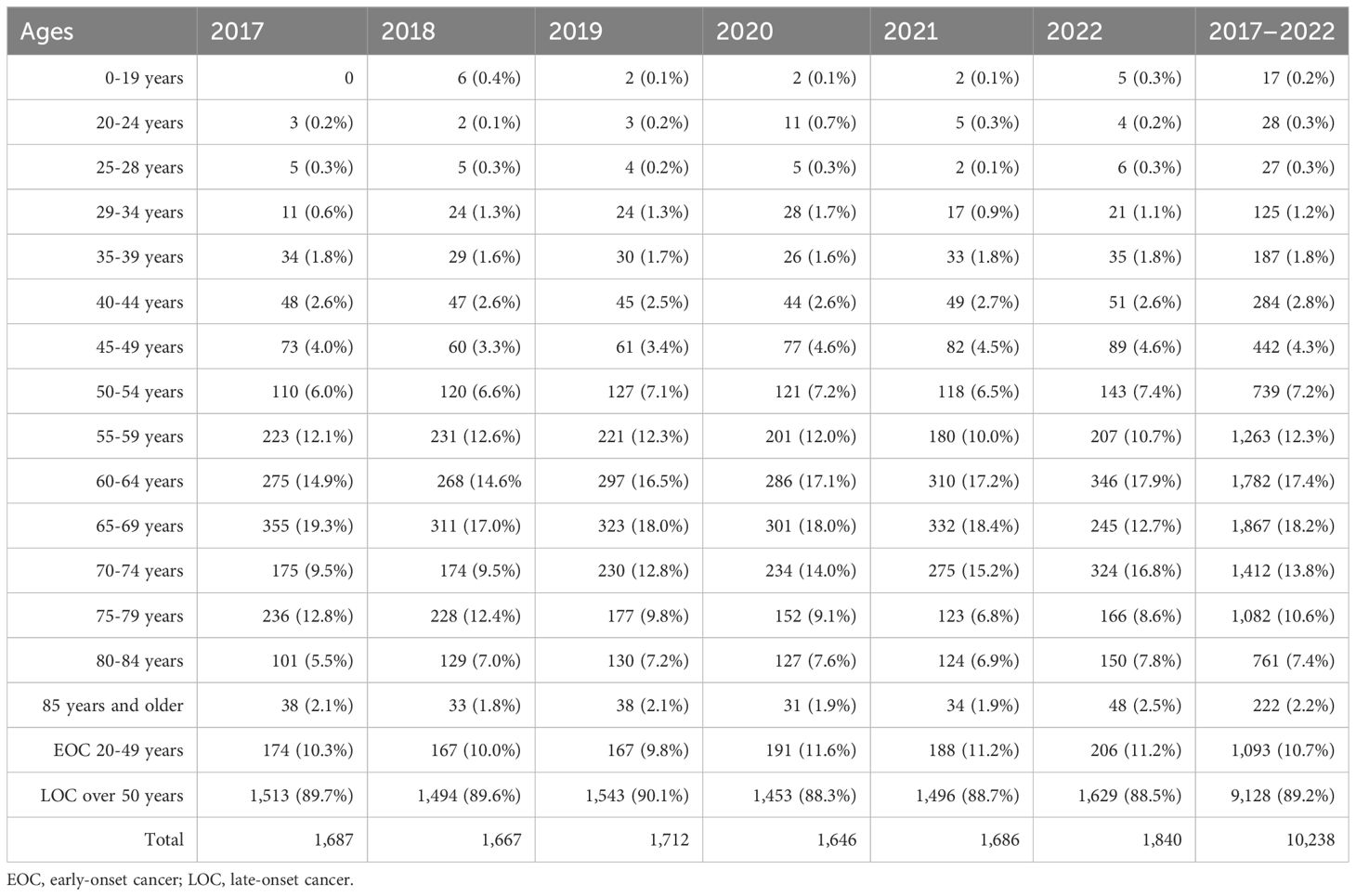
Table 4. Incidence by age for colon cancer of both sexes in Kazakhstan for 2017–2022.
3.4 Rectal cancerRectal cancer epidemiological trends are represented in Figure 4. The incidence of rectal cancer increased by 2.2% from 2017 to 2022. During the COVID-19 restrictions, the incidence dropped to 6.9 cases per 100,000 in 2020, which was 11.5% lower than that in 2019 and 7.2% lower than that in 2021. The mortality decreased by 22.5% from 2017 to 2022. However, during the COVID-19 restrictions, mortality increased to 3.5 cases in 2020, which was 9.4% higher than that in 2019 and 2.9% higher than that in 2021. The ratio of mortality to incidence decreased by 12.3, which was 23.4% lower than that from 2017 to 2022. During the COVID-19 restrictions, this ratio increased to 50.7, which was 23.7% higher than that in 2019 and 9.5% higher than that in 2021. One-year mortality decreased by 4.6%, which was 20.6% lower than that from 2017 to 2022. During the COVID-19 restrictions, 1-year mortality was 18.8%. Mortality follow-up decreased by 3.3%, which was 26.8% lower than that from 2017 to 2022. However, during the COVID-19 restrictions, mortality follow-up increased to 10.4%. The 5-year survival increased by 4.2% (growth rate by 9.9%), from 44.7% in 2017 to 52.7% in 2022.
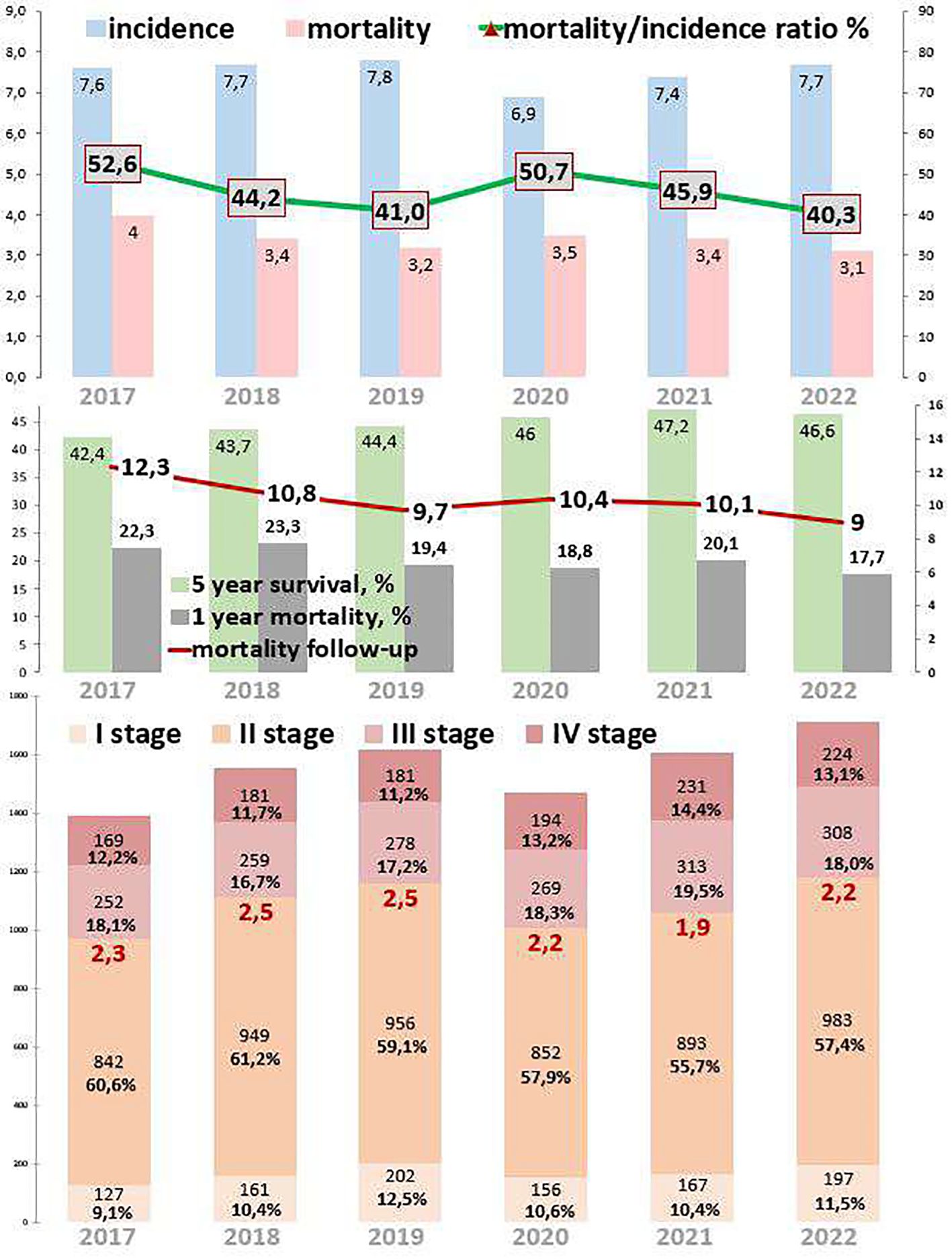
Figure 4. Key epidemiological indicators for rectum cancer in Kazakhstan from 2017 to 2022.
Out of 9,400 rectal cancer patients, 1,092 (11.6%) had EOC, while 8,303 (88.3%) had LOC, and five (0.1%) patients were aged 0–19 years (Tables 1, 5). Comparing 2022 to 2017, new cases increased by 13.6% for EOC and by 19.2% for LOC. The most significant APC for EOC was 18.6% in 2020, and for LOC, it was 10.5% in 2021. The AAPC increased by 3.0% for EOC and by 3.9% for LOC. During the COVID-19 restriction period, rectal cancer detection rates for EOC increased by 18.6%, while detection rates for LOC decreased by 12.0% (Table 6).
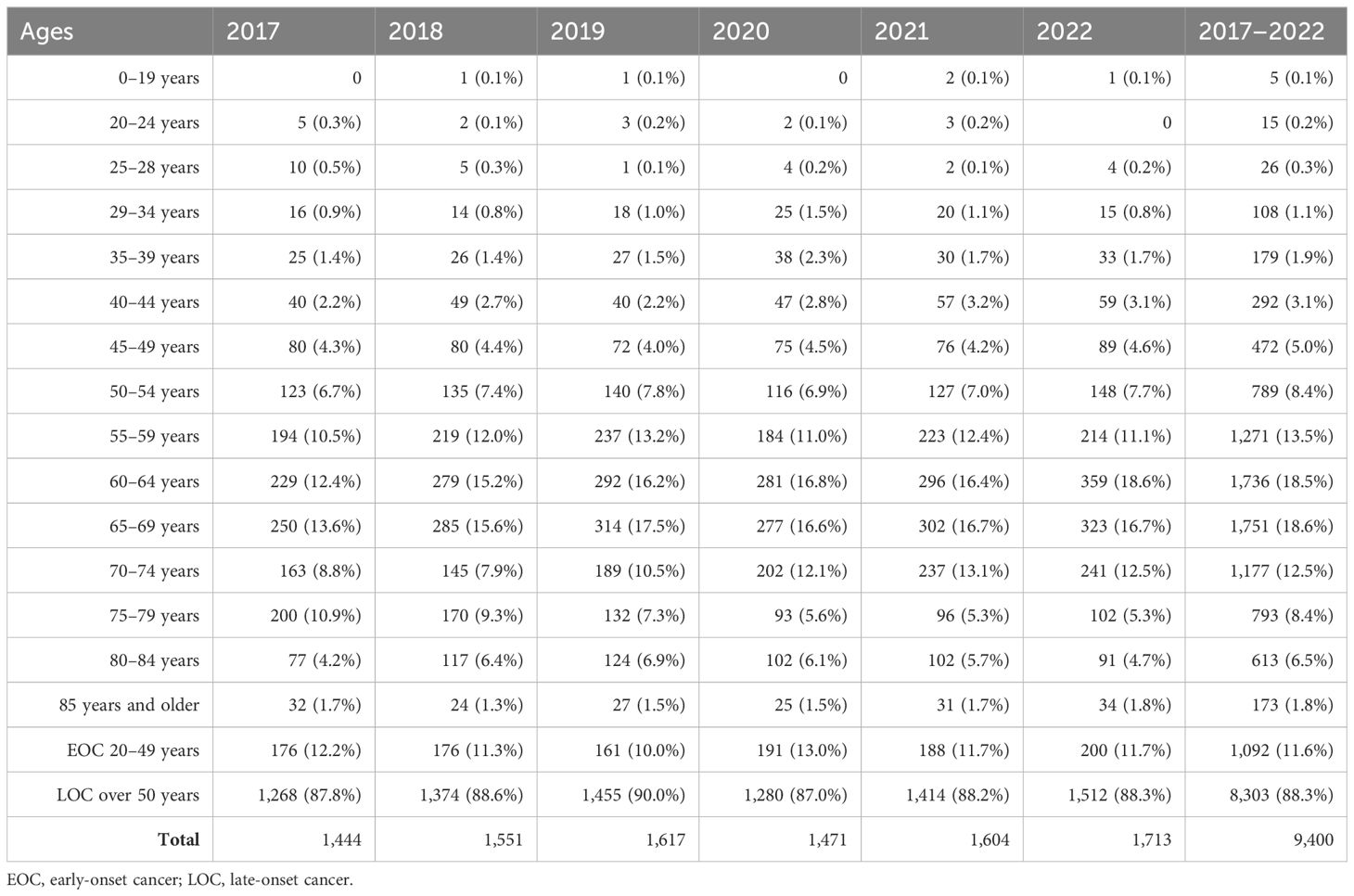
Table 5. Incidence by age for rectal cancer of both sexes in Kazakhstan for 2017–2022.
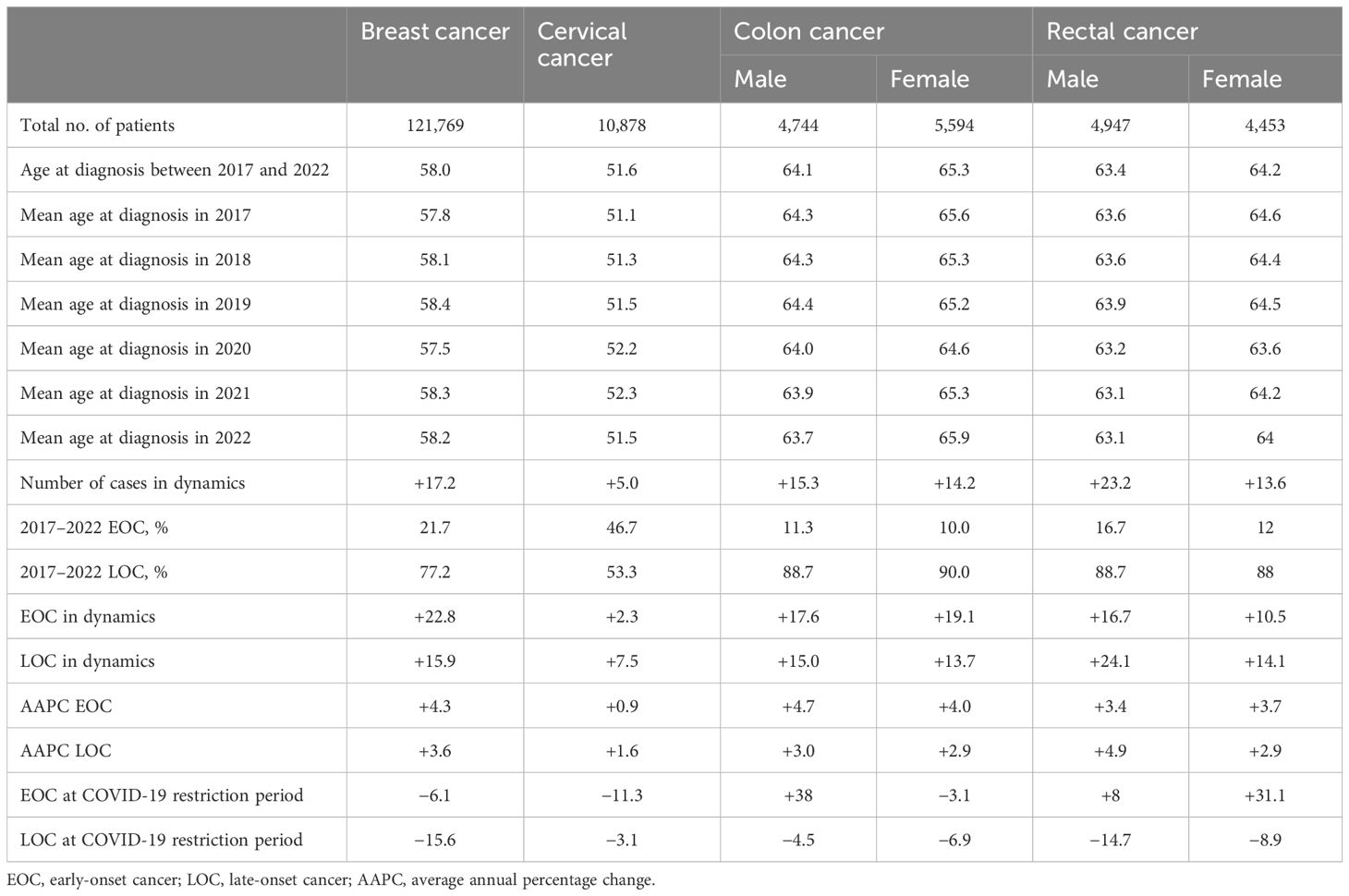
Table 6. Summary for incidence of early- and late-onset cancer for screening of different types of cancer by gender disparities.
Table 6 provides an insight summary into the trends and dynamics of cancer incidence, emphasizing the impact of COVID-19 restrictions on cancer detection rates by gender disparities.
4 DiscussionThis is the first study to describe the incidence and mortality rates of breast, cervical, and colorectal cancers during the pandemic period in Kazakhstan, even in Central Asia, using big population register data. This is a global public health problem that requires the utmost attention to reduce cancer incidence in young patients. This study investigated overall decreased incidence and mortality rates of breast and cervical cancers and increased detection rate of colorectal cancer during the COVID-19 restriction period. These findings, which may reflect similar situations in other countries also experiencing a decline in screening and diagnostic procedures, may present the first decline in survival rates observed during the pandemic period. Routine cancer screening is a primary preventative care measure that decreased dramatically at the start of the pandemic, with a 94% decrease in both breast cancer screening and cervical cancer screening and an 86% decrease in colon cancer screening. Rates of breast and colon cancer screening remain slightly below historical baselines, down at 2.7% and 3.4%, respectively. However, cervical cancer screening rates are still 10% below historical baselines (22, 30).
According to the results of our study, the AAPC for early-onset cancer increased between 2017 and 2022. Notably, breast cancer increased by 4.3%, cervical cancer increased by 0.9%, colon cancer increased by 4.7% among men and 4.0% among women, and rectal cancer increased by 3.4% among men and 3.7% among women. Despite the increase in EOC and LOC between 2017 and 2022, during the COVID-19 restrictions, for breast cancer, the detection rate of EOC decreased by 6.1% and that for LOC decreased by 15.6%; for cervical cancer, the detection rate of EOC decreased by 11.3%, and that of LOC decreased by 3.1%. The same trend can be observed in several studies (23–27). Restrictions imposed by different countries in order to minimize the risk of being infected had a significant effect on cancer care all over the world, especially on cancer screening procedures. For instance, a study from the Netherlands revealed that restrictions resulted in a 50% decrease in the detection rate for breast cancer cases among the 50–74-year age group. In Slovenia, cervical cancer screening programs led to a 92% decline in screening, a 70% decline in follow-up visits, and a 68% decrease in HPV testing (4). Delays in screening programs, diagnosis, and treatment during the pandemic may have led to an increase in breast cancer incidence. This may have resulted in a higher proportion of LOC and an increase in excess mortality from cancer. Moreover, due to these effects, the decrease in mortality from some cancers may have slowed down or even reversed. That is why it is essential to resume cancer screening, diagnosis, and treatment procedures in order to hold cancer incidence and mortality rates at a level as it was before the pandemic period.
However, for colon and rectal cancers, quite a different trend was observed. During the COVID-19 restrictions, for colon cancer, the detection rate of EOC increased by 14.4% (for men, it increased by 38.0%, and for women, it decreased by 3.1%) and that of LOC decreased by 5.8% (for men, it decreased by 4.5%, and for women, it decreased by 6.9%); for rectal cancer, the detection rate of EOC increased by 18.6% (for men, it increased by 8.0%, and for women, it increased by 31.1%) and that of LOC decreased by 12.0% (for men, it decreased by 14.7%, and for women, it decreased by 8.9%), which is contrary for breast and cervical cancer results. Several studies have revealed increased use of fecal immunochemical test (FIT) during the COVID-19 pandemic, which is associated with an increased rate of detection of early colorectal cancer (8, 9, 13, 28). Despite the recommendation to perform colorectal cancer screening in many countries, due to the COVID-19 pandemic restrictions, cancer screening programs were disrupted for an unknown period of time, which led to a decreased detection rate of LOC of colorectal cancer cases (28, 29). Therefore, in order to compensate for this decrease and to prevent further decline in cancer screening, further recommendations need to be addressed for the government and public health workers. First, consider the symptoms of cancers seriously and refer to diagnostic centers in a timely manner. Second, replace invasive methods with non-invasive methods in cancer screening programs. Third, increase the capacity of screening centers in order to compensate for the reduction of detection rate during the restriction period. Fourth, implement free or low-cost screening programs.
This is the first study to describe incidence and mortality rates of breast, cervical, and colorectal cancers during the pandemic restriction period in Kazakhstan, even in Central Asia, using big population register data. Moreover, the study assessed the change in the epidemiological situation and its results among the population of Kazakhstan before and during COVID-19. However, there were several limitations of our study. First, there is no clear boundary about the end and impact of restrictions during the COVID-19 pandemic. The greatest restrictions began on March 13, 2020, and continued throughout 2020 with a peak in the summer of 2020. In 2021, the COVID-19 pandemic in Kazakhstan reached a new level, and new strains of the virus appeared. However, in February 2021 a vaccination campaign began, and the restrictions were already lesser than those in 2020. Since September 2021, a gradual easing of COVID-19 pandemic restrictions occurred in Kazakhstan. According to epidemiological indicators, it is clear that the greatest failure in diagnostics occurred in 2020, but some restrictions extended to 2021, which was not taken into account in this study. Officially, the end of the COVID-19 pandemic was announced only in May 2023. The second limitation of our study is that there was no database by surnames. Data from official reports of oncology centers and the oncology institute were used. Some patients could have moved from one region to another during the year, and contamination and duplication of indicators could have occurred. Unfortunately, it is impossible to calculate and exclude these data, but such cases, if they existed, could not affect the overall picture since after transfer they were put on dispensary records with the note “translocation”. The third limitation of our study is a lack of randomization and comparison with other oncological localizations for which screening examination was not performed.
5 ConclusionThe results of the study showed that epidemiological indicators of population cancer screening improved from 2017 to 2022 but worsened during the maximum restrictions of the COVID-19 pandemic. Despite the increase in early-onset cancer, for breast cancer during the COVID-19 restriction period, the detection rate of EOC decreased by 6.1%; for cervical cancer, EOC decreased by 11.3%, while there was an increase in EOC for colon cancer in men by 38.0% and rectal cancer in men by 8.0% and women by 31.1%. Moreover, the results of this study highlight the importance of large-scale responses that could mitigate the detrimental association between the COVID-19 pandemic and worse cancer outcomes in patients with breast, cervical, colon, or rectal cancer.
Data availability statementThe datasets presented in this article are not readily available because data collected cannot be shared outside of the research institution. Requests to access the datasets should be directed to corresponding author. Requests to access the datasets should be directed to https://drive.google.com/file/d/1lXye8lkJRg7G8Tn96gjGoI3brv4buv6e/view, https://drive.google.com/drive/folders/1S6EnWQcSF_6qiurff1uGipcf2Mq98FnR.
Ethics statementThe Ethics Committee at JSC “Kazakh Research Institute of Oncology and Radiology” (protocol № 4-2021) approved this project. The study was performed according to both international local ethics guidelines and regulations. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributionsYI: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing. DK: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing. SN: Conceptualization, Data curation, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. SO: Supervision, Validation, Writing – original draft, Writing – review & editing. KM: Formal analysis, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. NO: Supervision, Validation, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was conducted within the framework of the grant of the Ministry of Science and Higher Education of the Republic of Kazakhstan with individual registration numbers AP13068657 “An innovative approach to the management of cancer patients in the COVID-19 pandemic” and BR24992933 “Development and implementation of diagnostic models, treatment and rehabilitation techniques for cancer patients”. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or final approval of the manuscript; and decision to submit the manuscript for publication.
AcknowledgmentsWe thank all the staff from the oncological service of the Republic of Kazakhstan, the Cancer Registry of Kazakh Institute of Oncology and Radiology, and all persons in oncological centers who were involved in the screening, diagnosis, and treatment of cancer.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. (2020) 395:507–13. doi: 10.1016/S0140-6736(20)30211-7
PubMed Abstract | Crossref Full Text | Google Scholar
2. Hui DS, Azhar E. I, Madani TA, Ntoumi F, Kock R, Dar O, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. (2020) 91:264–6. doi: 10.1016/j.ijid.2020.01.009
PubMed Abstract | Crossref Full Text | Google Scholar
3. Cairns A, Jones VM, Cronin K, Yocobozzi M, Howard C, Lesko N, et al. Impact of the COVID-19 pandemic on breast cancer screening and operative treatment. Am Surg. (2022) 88:1051–3. doi: 10.1177/00031348221087920
PubMed Abstract | Crossref Full Text | Google Scholar
4. Kiss Z, Kocsis J, Nikolényi A, Horváth Z, Knollmajer K, Benedek A, et al. Opposite trends in incidence of breast cancer in young and old female cohorts in Hungary and the impact of the Covid-19 pandemic: a nationwide study between 2011-2020. Front Oncol. (2023) 13:1182170. doi: 10.3389/fonc.2023.1182170
PubMed Abstract | Crossref Full Text | Google Scholar
5. Lee R, Xu W, Dozier M, McQuillan R, Theodoratou E, Figueroa J. A rapid review of COVID-19’s global impact on breast cancer screening participation rates and volumes from January to December 2020. eLife. (2023) 12:e85680. doi: 10.7554/eLife.85680
PubMed Abstract | Crossref Full Text | Google Scholar
6. Saktaganov M, Kaidarova DR, Zhylkaidarova AZ, Suleimenova D. Effect of digital technologies on mammographic screening in the Republic of Kazakhstan. J Clin Oncol. (2021) 39. doi: 10.1200/JCO.2021.39.15_suppl.e13601
Crossref Full Text | Google Scholar
7. Wentzensen N, Clarke MA, Perkins RB. Impact of COVID-19 on cervical cancer screening: Challenges and opportunities to improving resilience and reduce disparities. Prev Med. (2021) 151:106596. doi: 10.1016/j.ypmed.2021.106596
PubMed Abstract | Crossref Full Text | Google Scholar
8. Mazidimoradi A, Tiznobaik A, Salehiniya H. Impact of the COVID-19 pandemic on colorectal cancer screening: a systematic review. J Gastrointest Cancer. (2022) 53:730–44. doi: 10.1007/s12029-021-00679-x
PubMed Abstract | Crossref Full Text | Google Scholar
9. Zhylkaidarova AZ, Kaidarova DR, Batyrbekov K, Shatkovskaya O, Begimbetova D. Trends of colorectal cancer prevalence in Kazakhstan related to screening. Clin Endosc. (2020) 54:32–7. doi: 10.5946/ce.2019.198
PubMed Abstract | Crossref Full Text | Google Scholar
12. Tsapatsaris A, Babagbemi K, Reichman MB. Barriers to breast cancer screening are worsened amidst COVID-19 pandemic: A review. Clin Imaging. (2022) 82:224–7. doi: 10.1016/j.clinimag.2021.11.025
PubMed Abstract | Crossref Full Text | Google Scholar
13. Randle HJ, Gorin A, Manem N, Feustel PJ, Antonikowski A, Tadros M. Colonoscopy screening and surveillance disparities during the COVID-19 pandemic. Cancer Epidemiol. (2022) 80:102212. doi: 10.1016/j.canep.2022.102212
PubMed Abstract | Crossref Full Text | Google Scholar
15. Ahmad OB, Boschi-Pinto C, Lopez AD, Murray CJL, Lozano R, Inoue M. Age standardization of rates: a new WHO standard Vol. 31. Geneva: World Health Organization (2001) p. 1–14.
16. Kaidarova DR ed. Indicators of the oncology service of the Republic of Kazakhstan, 2018 (statistical and analytical materials). Almaty (2019). p. 209.
17. Kaidarova DR ed. Indicators of the oncology service of the Republic of Kazakhstan, 2019 (statistical and analytical materials). Almaty (2020). p. 225.
18. Kaidarova DR ed. Indicators of the oncology service of the Republic of Kazakhstan, 2020 (statistical and analytical materials). Almaty (2021). p. 370. doi: 10.52532/978-601-7548-11-7-2020-1-370
Crossref Full Text | Google Scholar
19. Kaidarova DR ed. Indicators of the oncology service of the Republic of Kazakhstan, 2021 (statistical and analytical materials). Almaty (2022). p. 384. doi: 10.52532/1-11-2021-1-384
Crossref Full Text | Google Scholar
20. Kaidarova DR ed. Indicators of the oncology service of the Republic of Kazakhstan, 2022 (statistical and analytical materials). Almaty (2023). p. 430. doi: 10.52532/1-09-2023-1-430
Crossref Full Text | Google Scholar
21. Vrinten C, Gallagher A, Waller J, Marlow LAV. Cancer stigma and cancer screening attendance: a population based survey in England. BMC Cancer. (2019) 19:566. doi: 10.1186/s12885-019-5787-x
PubMed Abstract | Crossref Full Text | Google Scholar
22. Hui DS, Azhar E. I, Madani TA, Ntoumi F, Kock R, Dar O, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. (2020) 91:264–6. doi: 10.1016/j.ijid.2020.01.009
PubMed Abstract | Crossref Full Text | Google Scholar
23. Li T, Nickel B, Ngo P, McFadden K, Brennan M, Marinovich ML, et al. A systematic review of the impact of the COVID-19 pandemic on breast cancer screening and diagnosis. Breast. (2023) 67:78–88. doi: 10.1016/j.breast.2023.01.001
PubMed Abstract | Crossref Full Text | Google Scholar
24. Lofters AK, Wu F, Frymire E, Kiran T, Vahabi M, Green ME, et al. Cancer screening disparities before and after the COVID-19 pandemic. JAMA Network Open. (2023) 6:e2343796–e. doi: 10.1001/jamanetworkopen.2023.43796
PubMed Abstract | Crossref Full Text | Google Scholar
25. Lohfeld L, Sharma M, Bennett D, Gavin A, Hawkins ST, Irwin G, et al. Impact of the COVID-19 pandemic on breast cancer patient pathways and outcomes in the United Kingdom and the Republic of Ireland – a scoping review. Br J Cancer. (2024) 131:778. doi: 10.1038/s41416-024-02791-8
PubMed Abstract | Crossref Full Text | Google Scholar
26. Mak VP, White K, Wilkens LR, Cheng I, Haiman CA, Le Marchand L. The impact of COVID-19 on cancer screening and treatment in older adults: The Multiethnic Cohort Study. Elife. (2023) 12. doi: 10.7554/eLife.86562.sa2
PubMed Abstract | Crossref Full Text | Google Scholar
27. Nonboe MH, Napolitano G, Schroll JB, Vejborg I, Waldstrøm M, Lynge E. Impact of COVID-19 pandemic on breast and cervical cancer screening in Denmark: A register-based study. eLife. (2023) 12:e81605. doi: 10.7554/eLife.81605.sa2
留言 (0)