Neuroendocrine carcinoma of the cervix (NECC) is a rare and highly aggressive subtype of cervical carcinomas, accounting for 0.9%-1.5% of cervical cancer (CC) cases (1). It is characterized by aggressive behavior and poor prognosis, particularly in the case of mixed types, and is strongly associated with HPV infection (2). Due to its rarity and the lack of large randomized controlled trials, the treatment of NECC is mainly based on experience of cervical adenocarcinoma and squamous cell carcinoma and small cell neuroendocrine carcinoma (SCNEC) of the lung (3). However, NECC patients have a higher risk of death than patients with other pathological types of cervical cancer like cervical squamous cell carcinoma and cervical adenocarcinoma (4). A meta-analysis of 3538 of NECC showed that the 5-year overall survival (OS) was 34% (5), compared with a 5-year OS of 62.34% for all CC (6). Consequently, individualized prognostic risk assessment of NECC patients is crucial, as it can help clinicians guide appropriate treatment interventions.
Patients with NECC tend to be diagnosed at a young age (4). Vale et al. found a higher incidence rate was seen in NECC patients 25 years or younger compared with older patients (PR:6.10, 95%CI: 2.03-18.35) (7). Another retrospective study from Japan showed that the median age at diagnosis of patients with NECC was earlier than that of squamous cervical cancer and adenocarcinoma of cervix (43 vs 55 vs 51 years) (8). Given the longer life expectancy and greater tolerance to cancer treatments in young patients (9), a prognostic nomogram for young NECC patients is valuable to better tailor therapies.
However, few studies have investigated the individualized prognostic risk factors of young NECC patients up to now. Therefore, this study aims to develop the first individualized prognostic model with an external validation to help clinicians improve the prognosis of young NECC patients.
Materials and methodsPatient selectionThis study utilized a retrospective research methodology. Young (≤45 years) patients diagnosed with NECC between 2000 and 2020 were retrieved from the Surveillance, Epidemiology and End Results (SEER)-18 database using SEER*Stat software (version 8.4.3). Inclusion criteria (1): International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) histology codes of 8013/3, 8041/3, 8042/3, 8045/3, 8240/3, 8241/3, 8244/3, 8245/3, and 8246/3 (2). Primary sites were C53.0, C53.1, C53.8, and C53.9 (3). Diagnosis confirmed by pathology (4). Patients aged 18-45 years at diagnosis (5). Complete clinical data and follow-up information. Exclusion criteria (1): Patients with other primary tumors (2). FIGO 2018 stage unknown (3). Survival months equal to zero or unknown. Based on the above inclusion and exclusion criteria, the external validation cohort included young NECC patients at Fujian Provincial Cancer Hospital between January 2012 and December 2023. Figure 1 shows the flow chart of the study. Experiments involving humans were carried out following the ethics policy approved by the Ethics Committee of Fujian Cancer Hospital (Approval No. K2024-121-01).
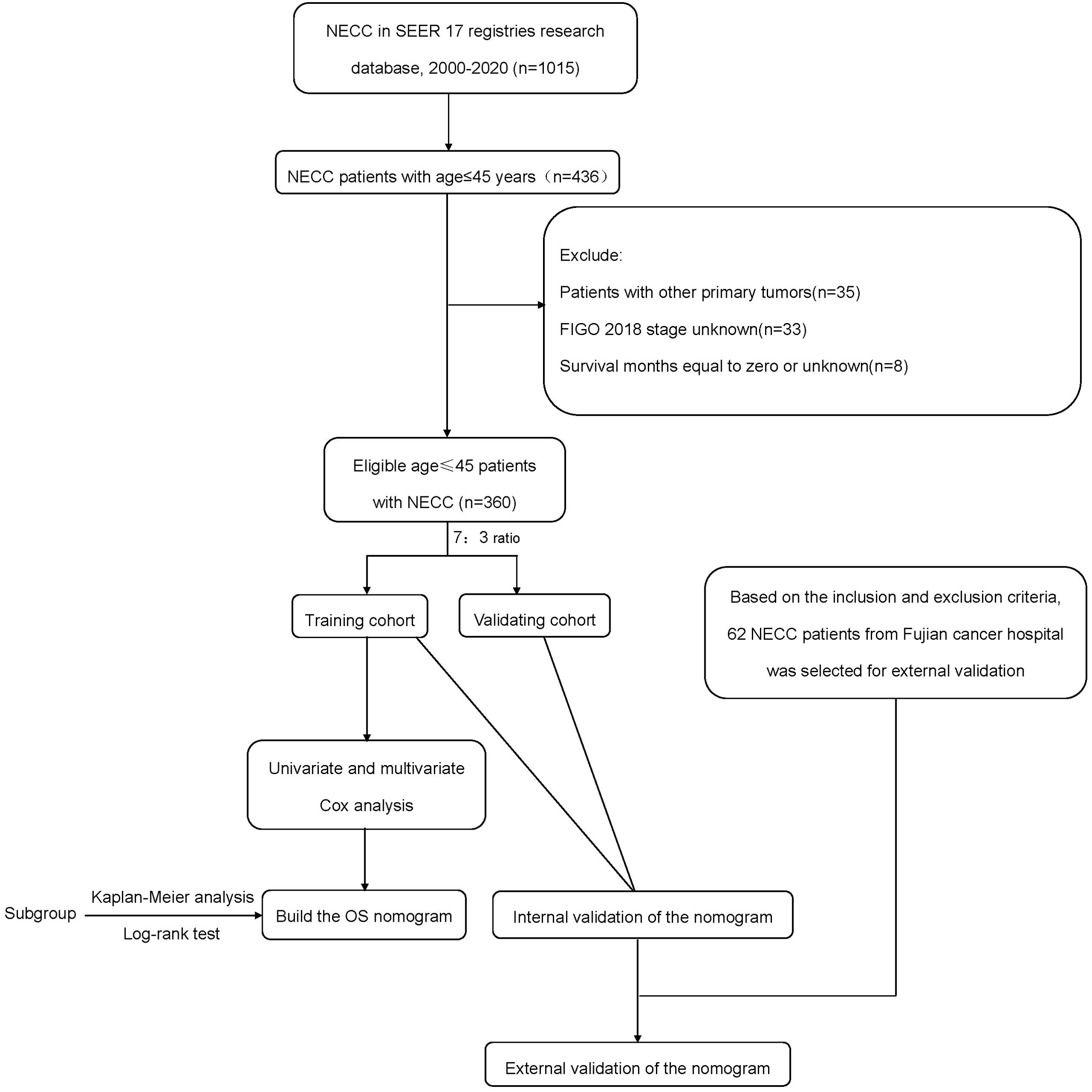
Figure 1. The flow chart of the study.
Data collectionClinicopathological information collected from patients included race, marital status, age at diagnosis, pathological type, grade, tumor size, FIGO 2018, surgery, radiotherapy, and chemotherapy. The primary endpoint of was OS, defined as the time from pathological diagnosis to death from any cause or the last follow-up. All follow-up information was acquired by telephone, inpatient records and outpatient records.
Statistics analysisAll analyses were analyzed by SPSS 26.0 and R software (version 4.3.0, http://www.r-project.org). Patients in the SEER database were randomized into a training cohort and an internal validation cohort according to a 7:3 ratio. The optimal cut-off value for age was determined based on the time-dependent receiver operating curve (timeROC). The χ2 test or Fisher exact test was used to analyze the differences in baseline characteristics between the groups. Univariate and multivariate Cox regression analysis were employed to estimate prognostic risk factors of young NECC. The predictive accuracy of the prognostic nomogram was determined by the area under the ROC curve (AUC) and consistency index (C-index). Calibration ability was assessed by the calibration plots. Decision curve analysis (DCA) was used to assess the clinical utility. According to the nomogram, the prognostic index (PI) of each patient was calculated. The patients were divided into low-risk and high-risk groups based on the PI score. Survival differences between the two subgroups were assessed by Kapla-Meier curves and Log-rank tests. Results were presented as hazard ratios (HRs) with 95% confidence intervals (CIs). All statistical tests were two-sided, and P values less than 0.05 were considered statistically significant.
ResultsClinical characteristics of patients360 young (≤45 years old) NECC patients in the SEER database were randomly divided into the training cohort (n=251) and the internal validation cohort (n=109). External validation was performed using 62 young NECC patients from Fujian Cancer Hospital. Table 1 summaries the clinicopathology characteristics of the training, internal validation and external validation cohorts. The median follow-up was 103 months in the training cohort, 100 months in the internal validation cohort and 67 months in the external validation cohort. The median age in each cohort was 37 (range: 21-45 years), 37 (range: 22-45 years) and 39 (range: 25-45 years), respectively.
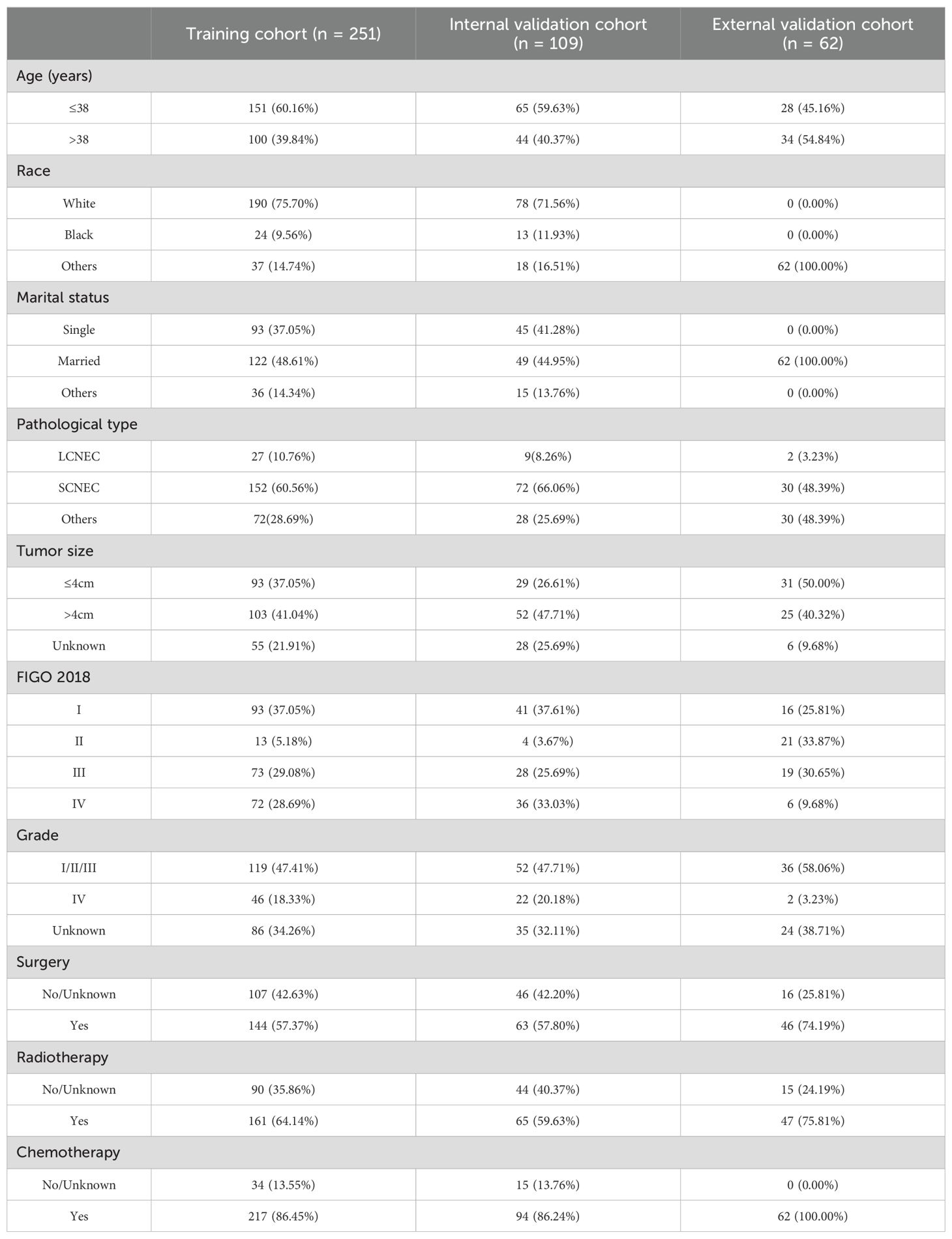
Table 1. Clinicopathology of young NECC patients.
The univariate and multivariate Cox analysisThe univariate analysis showed that age, pathological type, tumor size, FIGO 2018, and surgery were significantly associated with the prognosis of young NECC (P<0.05). However, only pathological type, FIGO 2018, and surgery were independent risk factors by multivariate analysis (P<0.05) (Table 2). For young NECC patients, SCNEC and surgery were independent prognostic protective factors, while FIGO stage was prognostic risk factors.
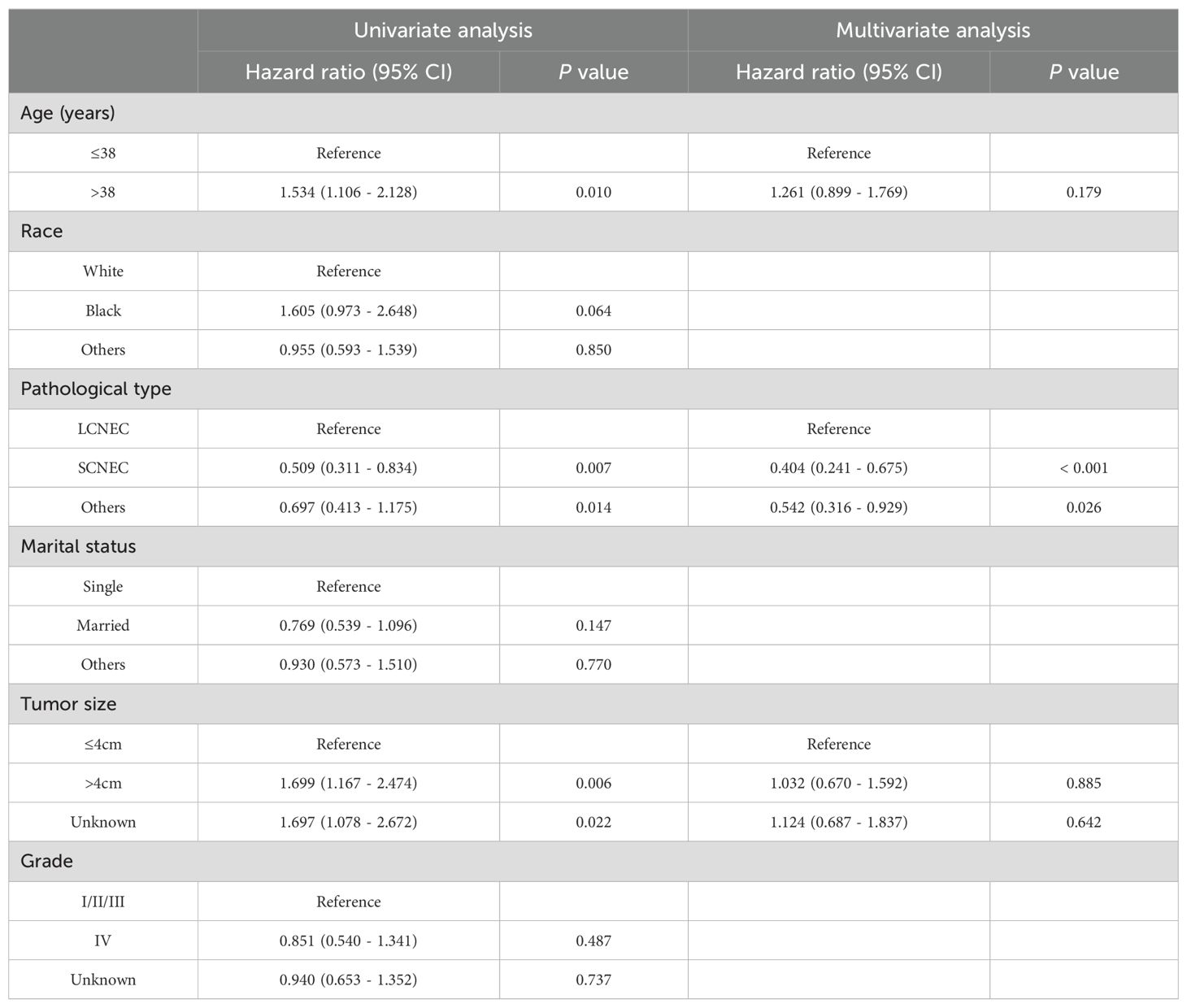
Table 2. Univariate and multivariate Cox regression analysis of OS in young NECC patients.
Development and validation of nomogramBased on the multivariate Cox analyses, FIGO 2018, pathological type, and surgery were included in the development of the prognostic nomogram (Figure 2). The nomogram has a relatively high predictive accuracy. The AUC of 1-, 3-, and 5-year OS of the training cohort were 0.843 (95%CI: 0.783-0.903), 0.793 (95%CI: 0.737-0.849), and 0.805 (95%CI: 0.747-0.862), respectively (Figures 3A–C). In the internal validation cohort, the AUC for predicting 1-, 3-, and 5-year OS were 0.838 (95%CI: 0.762-0.915), 0.803 (95%CI: 0.717-0.890), and 0.798 (95%CI:0.700-0.896) (Figures 3D–F). Similarly, the AUC for 1-, 3-, and 5-year OS of the external validation cohort were 0.817 (95%CI: 0.665- 0.970), 0.795 (95%CI: 0.668-0.922) and 0.872 (95%CI: 0.772-0.972) (Figures 3G–I). The C-index of nomogram was 0.745 (95%CI: 0.706-0.784), 0.744 (95%CI: 0.689-0.784), 0.755 (95%CI: 0.665-0.845) in the training, internal validation and external validation cohorts, respectively. The calibration curves showed good agreement between the predicted and the actual probability (Figure 4). In addition, the DCA plots demonstrated favorable positive net benefit of the nomogram (Figure 5).
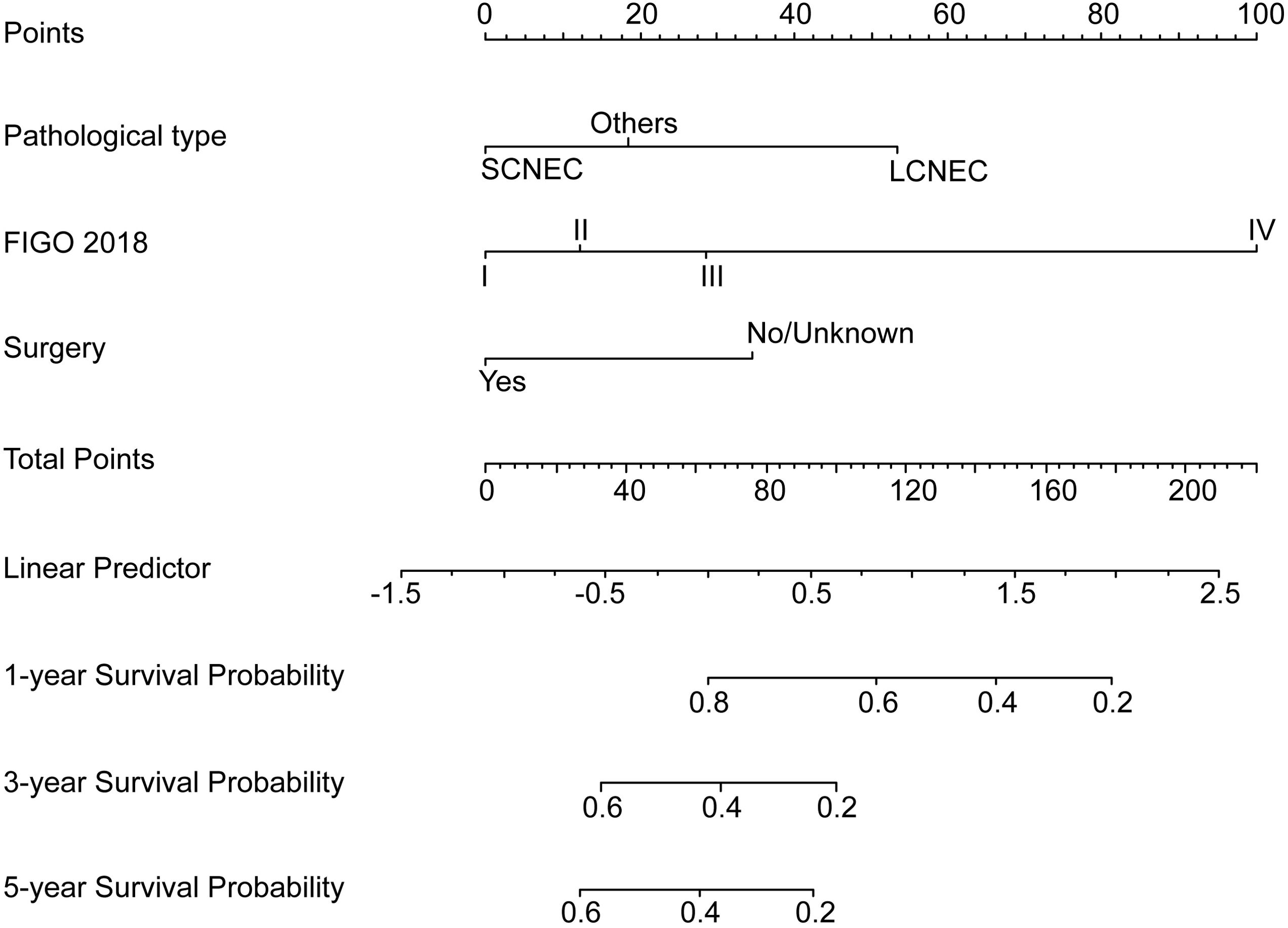
Figure 2. Prognostic nomogram for young NECC.
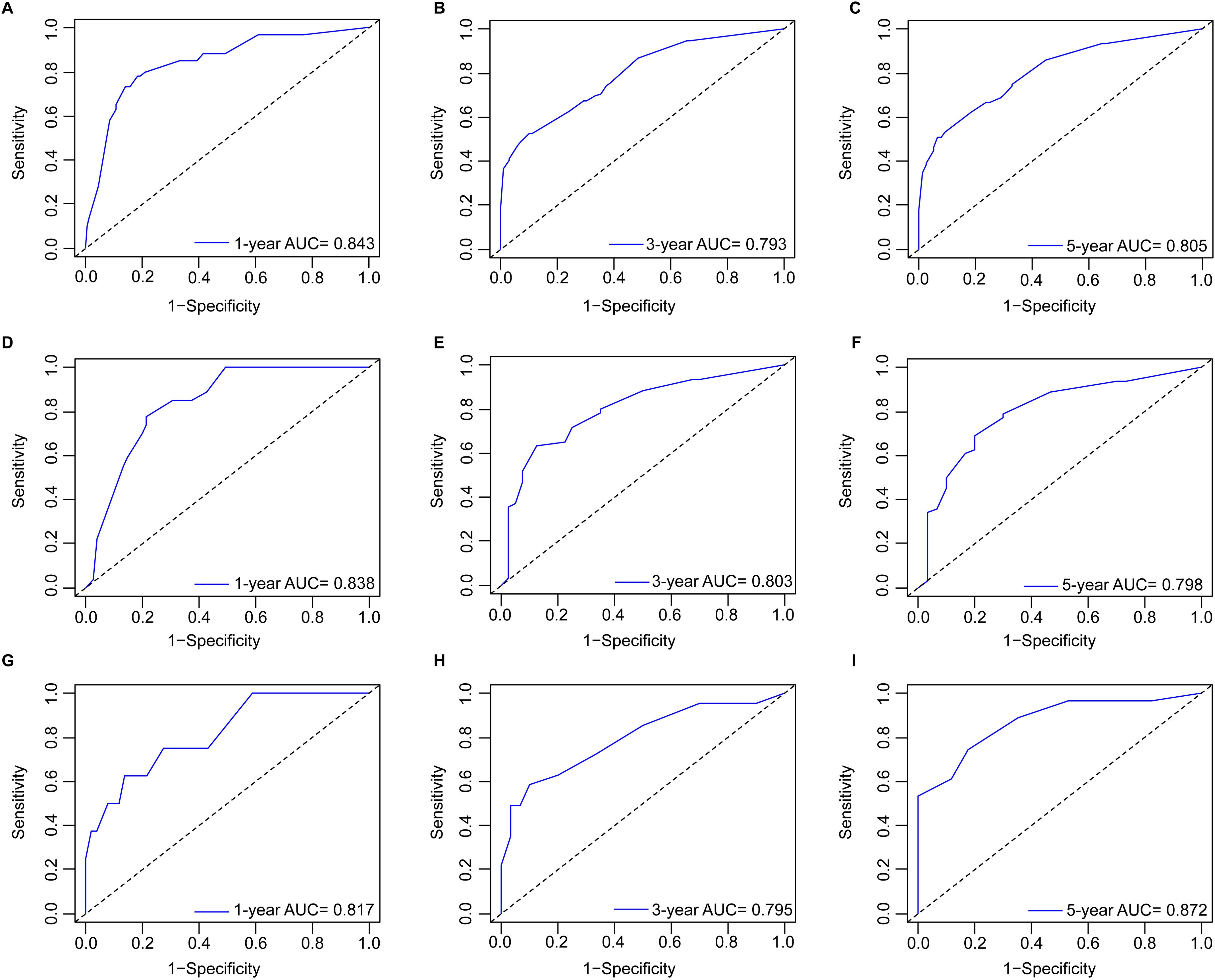
Figure 3. ROC curves by nomogram for 1-, 3-, and 5-year OS in young patients with NECC: (A–C) the training cohort; (D–F) the internal validation cohort; (G–I) the external validation cohort.
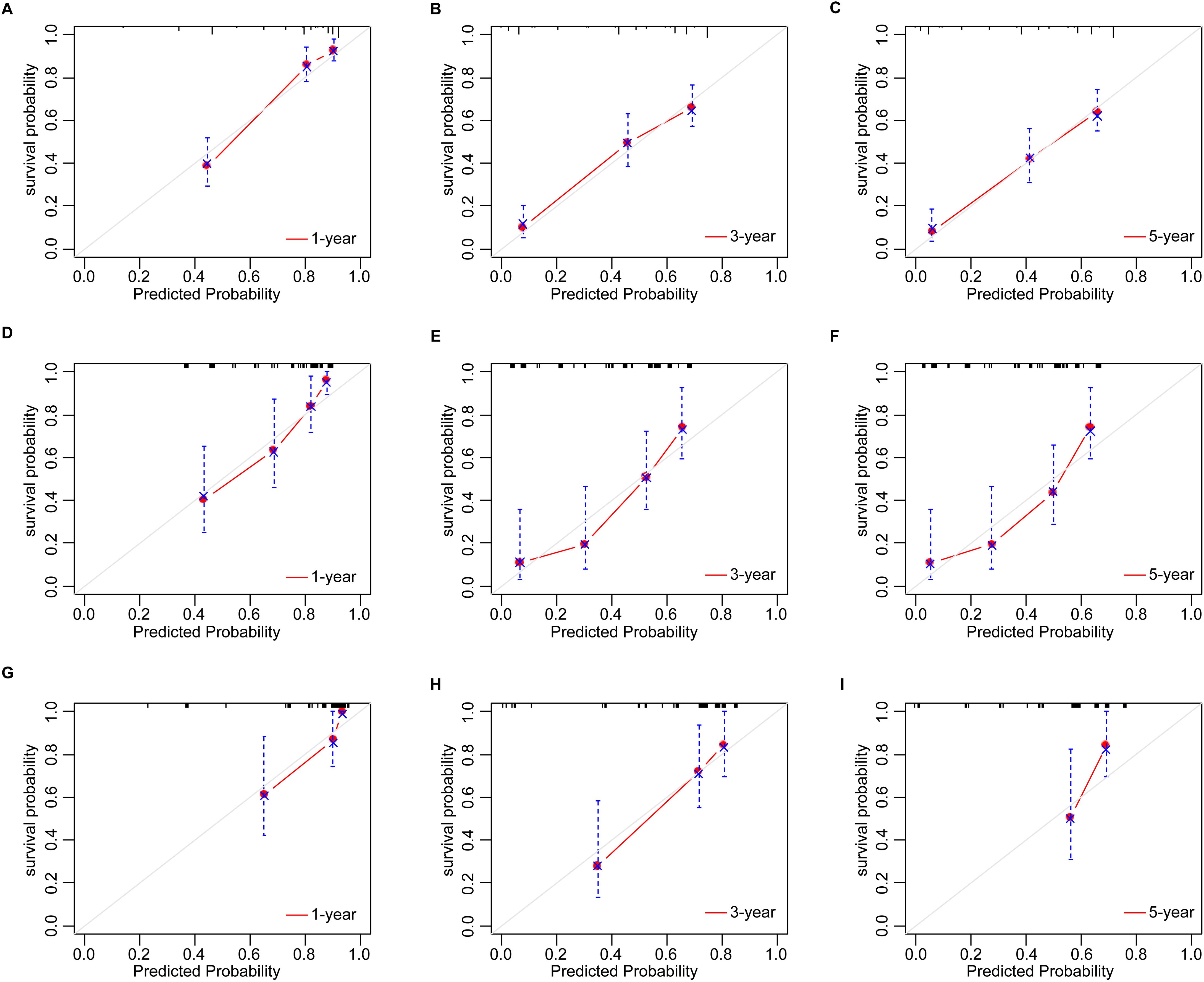
Figure 4. The calibration plots by nomogram for 1-, 3-, and 5-year OS in young patients with NECC: (A–C) the training cohort; (D–F) the internal validation cohort; (G–I) the external validation cohort.
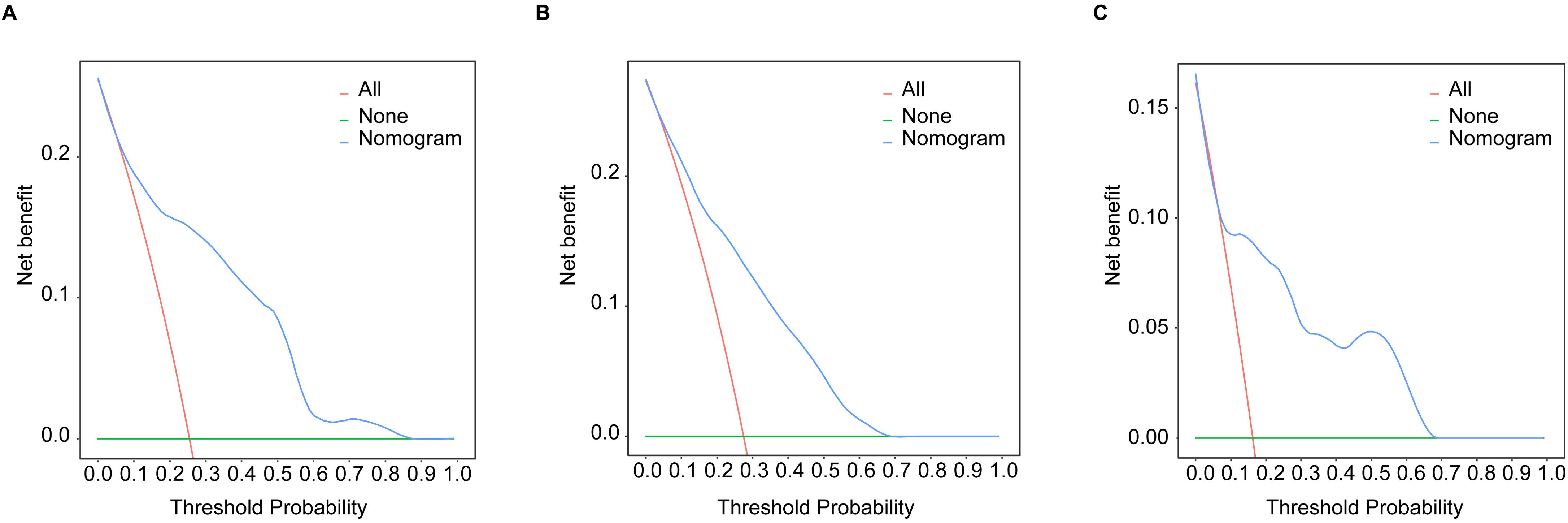
Figure 5. The DCA curves by nomogram in young patients with NECC: (A) the training cohort; (B) the internal validation cohort; (C) the external validation cohort.
Simplification of the scoring systemFor clinical use, a simplified scoring system was created based on the nomogram. The PI of each patient was the sum of those risk factors score, ranging from 0 to 187.9. According to the median PI (median PI=18.6), all young NECC patients were categorized into high-risk group and low-risk groups, respectively. (Figure 6). The result showed that the prognosis of high-risk group was significantly worse than that of low-risk group (P<0.05). The 5-year OS rates of the low-risk and high-risk groups were 59.2% and 19.2% in the training cohort. In the internal validation cohort, the 5-year OS rates were 57.8% and 15.0% in the low-risk and high-risk groups, respectively. For low-risk and high-risk groups in the external validation cohort, the 5-year OS rates were 63.0% and 0.0%.
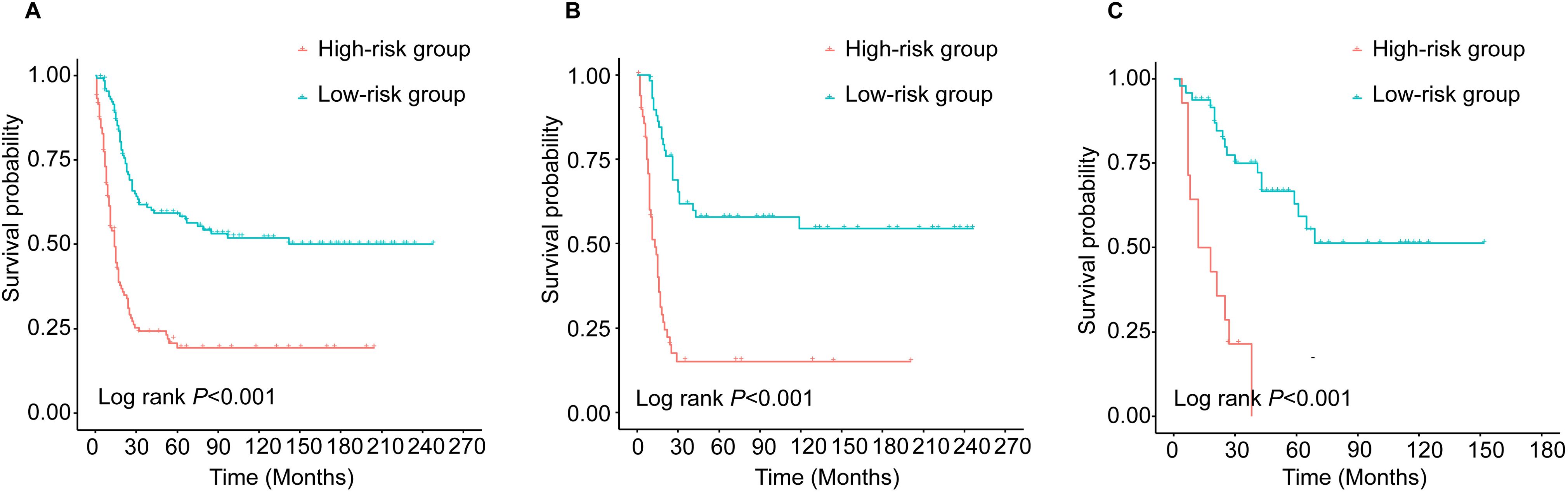
Figure 6. Kaplan-Meier survival curves of young patients with NECC in different risk groups: (A) training cohort; (B) internal validation and (C) external validation.
DiscussionThe results of this study showed that pathological type, FIGO stage, and surgery were independent prognostic factors for young NECC patients. Based on the above three factors, an individualized nomogram was developed to predict the prognosis of young NECC patients. The prognostic nomogram demonstrated the superior predictive ability in the internal and external validation. The calibration plots and the DCA curves further confirmed that the nomogram had a high clinical net benefit.
Our study showed that surgery was an independent prognostic risk factor in young NECC patients (HR=0.564, P=0.004), which is consistent with previous studies (10). Lin et al (11) found that early-stage cervical SCNEC who underwent radical surgery had a better overall survival than those who received radical radiotherapy (P = 0.03), and simultaneous integrated boost (SIB) was used in patients who could not be undergo surgery (12). Additionally, several studies have reported that surgery is beneficial for the prognosis of patients with advanced NECC. A multicenter retrospective study involving 678 patients with cervical SCNEC showed that surgery was not only associated with a better prognosis for patients in IB-IIA2 stages (HR 0.17, 95%CI: 0.05-0.50), but was also a protective factor for locally advanced NECC (HR 0.59, 95% CI: 0.37 - 0.95) (13). For NECC patients with oligometastases, in addition to stereotactic body radiation therapy (SBRT) therapy (14), surgery also helps to prolong survival outcomes by reducing tumor load and metastasis (15). It has been generally recommended that NECC patients receive radical hysterectomy with adjuvant chemoradiotherapy regardless of pathological risk factors (16). Therefore, the question has been raised whether radical hysterectomy can be replaced by less aggressive surgical approaches in the multimodal treatment of NECC patients to minimize postoperative morbidity. Zeng et al (17) concluded that total hysterectomy or radical hysterectomy was not associated with survival prognosis in the multimodal treatment of all-stage NECC. A study of 64 young NECC patients found that ovarian conservation had no effect on disease-free survival (DFS) and OS (18). What’s more, long-term survival has been reported in patients with stage IB1 NECC who underwent radical abdominal trachelectomy in combination with adjuvant chemotherapy (19, 20). Exploring the feasibility of less aggressive surgical approaches is significant and should be investigated in future studies to reduce postoperative morbidity or preserve the organ in young NECC patients.
Pathological type is an important independent risk factor for the prognosis of young NECC (21). According to the 5th edition of the World Health Organization (WHO) classification, neuroendocrine tumors of the female reproductive system are divided into well-differentiated neuroendocrine tumors (NETs) and poorly differentiated neuroendocrine carcinomas (NECs) (22). Among NECs, SCNEC is the most common, followed by LCNEC (22). Previous studies have shown that the 5-year OS of NECC is significantly worse than that of squamous cell carcinoma and adenocarcinoma (23). However, given the rarity of NECC, few studies have explored the prognostic differences between different pathologic types of NECs, and the results of the studies are still controversial. SCNEC is generally considered to be more aggressive than LCNEC and has a worse prognosis. A study of high-grade neuroendocrine carcinomas of the gastrointestinal carcinoma showed that the 5-year OS of LCNEC was significantly higher than that of SCNEC (32% vs 6%, P < 0.05) (24). However, our data showed that cervical LCNEC was associated with a worse patient prognosis than cervical SCNEC. A supportive study also found that median survival for endometrial LCNEC was much lower than for endometrial SCNEC (8 months vs 25 months) (25). Moreover, Shao et al (26) found that ovarian LCNEC had a worse 5-year OS compared to SCNEC (21.8% vs 28.0%). It suggests that in gynecological tumors, LCNEC may be associated with a worse prognosis for patients and the mechanism needs to be further investigated.
Our research showed that FIGO stage was the most important independent prognostic risk factor for young NECC. Advanced clinical stage is often considered an independent prognostic risk factor for CC (27), which is consistent with our study. It has been reported that median OS for patients with early stage (IA1-IB2) cervical SCNEC was 31.2 months, compared with 6.4 months for patients with advanced stage (IIB-IV) (28). Cohen et al. found that the 5-year disease-free survival (DSS) of patients with FIGO I-IIA, FIGO IIB-IVA, and FIGO IVB stage cervical SCNEC was 36.8%, 9.8%, and 0%, respectively (P < 0.001) (29). Additionally, a retrospective study based on the National Cancer Database included 1,896 NECC patients and found that 5-year OS decreased from 55% in patients with stage IB to 24% in patients with stage IIIB (4). The possible reasons may be related to the higher tumor burden in advanced NECC compared to early-stage patients. And patients with advanced NECC tend to receive palliative care regimens, which have longer treatment cycles and are more disruptive to normal bodily functions.
A new simplified scoring system was constructed based on these three independent prognostic factors, which could discriminate between high- and low-risk NECC patients. For high-risk young NECC patients, traditional treatments frequently fail to improve their prognosis, prompting consideration of innovative therapeutic options like targeted therapy and immunotherapy to improve clinical outcomes (30, 31). Paraghamian et al (32) documented a case of an individual with recurrent, metastatic, and programmed death-ligand 1 (PD-L1) negative metastatic cervical SCNEC who achieved a complete response to nivolumab. Another patient with chemotherapy refractory stage IV cervical LCNEC who was treated with a PD-L1 inhibitor in combination with stereotactic body radiotherapy (SBRT), exhibited nearly complete disease resolution (33). Carroll et al (3) found that most high-grade NECC tumor tested expressed poly ADP-ribose polymerase (PARP)-1, suggesting that PARP inhibitors might be an effective treatment. Overall, immunotherapy and targeted therapy are promising treatments for high-risk young NECC patients to improve their prognosis, and further prospective studies are needed to confirm the efficacy of these therapies.
This is the first large retrospective cohort study to investigate prognostic risk factors in young NECC patients. Using the innovative prognostic nomogram, gynecological oncologists can determine the survival of young NECC patients. However, our study has several limitations. First, as a retrospective study, selection bias was inevitable. Second, the SEER database did not contain information on chemotherapy regimens and cycles, radiotherapy dose, targeted therapy, and immunotherapy, which may also affect the prognosis of young NECC. Future, multicenter retrospective and prospective studies will be conducted to validate our findings.
ConclusionThis study clarified that pathological type, FIGO stage, and surgery were independent risk factors for the prognosis of young NECC patients. The novel prognostic nomogram and risk stratification is expected to become an individualized and accurate tool for gynecological oncologists to evaluate the survival of young NECC patients. For high-risk young NECC patients, novel and effective therapeutic options such as targeted therapy and immunotherapy are being considered to improve the clinical prognosis.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementExperiments involving humans were carried out following the ethics policy approved by the Ethics Committee of Fujian Cancer Hospital (Approval No. K2024-121-01). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article because This is a retrospective observational study. An exemption from informed consent was obtained from the ethics committee of Fujian Cancer Hospital.
Author contributionsNX: Data curation, Formal analysis, Writing – original draft. HY: Formal analysis, Methodology, Validation, Writing – review & editing. JL: Investigation, Visualization, Writing – review & editing. SD: Data curation, Writing – review & editing. LL: Software, Writing – review & editing. YS: Conceptualization, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Major Scientific Research Program for Young and Middle-aged Health Professionals of Fujian Province, China (Grant No. 2022ZQNZD008) and the High-level Talents Training Project of Fujian Cancer Hospital (2022YNG04).
AcknowledgmentsWe would like to thank all the researchers and patients, as well as recognize YS, whose contributions significantly enhanced the quality of this publication.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Gadducci A, Carinelli S, Aletti G. Neuroendrocrine tumors of the uterine cervix: A therapeutic challenge for gynecologic oncologists. Gynecol Oncol. (2017) 144:637–46. doi: 10.1016/j.ygyno.2016.12.003
PubMed Abstract | Crossref Full Text | Google Scholar
2. Zhang X, Li M, Zhang G, Shen D. Neuroendocrine carcinoma of the cervix: A comprehensive clinicopathologic study and literature review. Gynecology Obstetrics Clin Med. (2023) 3:163–9. doi: 10.1016/j.gocm.2023.07.003
Crossref Full Text | Google Scholar
3. Carroll MR, Ramalingam P, Salvo G, Fujimoto J, Solis Soto LM, Phoolcharoen N, et al. Evaluation of PARP and PDL-1 as potential therapeutic targets for women with high-grade neuroendocrine carcinomas of the cervix. Int J Gynecol Cancer. (2020) 30:1303–7. doi: 10.1136/ijgc-2020-001649
PubMed Abstract | Crossref Full Text | Google Scholar
4. Margolis B, Tergas AI, Chen L, Hou JY, Burke WM, Hu JC, et al. Natural history and outcome of neuroendocrine carcinoma of the cervix. Gynecol Oncol. (2016) 141:247–54. doi: 10.1016/j.ygyno.2016.02.008
PubMed Abstract | Crossref Full Text | Google Scholar
5. Tempfer CB, Tischoff I, Dogan A, Hilal Z, Schultheis B, Kern P, et al. Neuroendocrine carcinoma of the cervix: a systematic review of the literature. BMC Cancer. (2018) 18:530. doi: 10.1186/s12885-018-4447-x
PubMed Abstract | Crossref Full Text | Google Scholar
6. Vali M, Maleki Z, Nikbakht H-A, Hassanipour S, Kouhi A, Nazemi S, et al. Survival rate of cervical cancer in Asian countries: a systematic review and meta-analysis. BMC Womens Health. (2023) 23:671. doi: 10.1186/s12905-023-02829-8
PubMed Abstract | Crossref Full Text | Google Scholar
7. Vale DB, Cavalcante LA, Andrade LALDA, Teixeira JC, Menin TL do R, Zeferino LC. Stage and histology of cervical cancer in women under 25 years old. J Gynecol Oncol. (2019) 30:e55. doi: 10.3802/jgo.2019.30.e55
PubMed Abstract | Crossref Full Text | Google Scholar
8. Takayanagi D, Hirose S, Kuno I, Asami Y, Murakami N, Matsuda M, et al. Comparative analysis of genetic alterations, HPV-status, and PD-L1 expression in neuroendocrine carcinomas of the cervix. Cancers (Basel). (2021) 13:1215. doi: 10.3390/cancers13061215
PubMed Abstract | Crossref Full Text | Google Scholar
9. Pan S, Jiang W, Xie S, Zhu H, Zhu X. Clinicopathological features and survival of adolescent and young adults with cervical cancer. Cancer Control. (2021) 28:10732748211051558. doi: 10.1177/10732748211051558
PubMed Abstract | Crossref Full Text | Google Scholar
10. Liu J, Lyu Y, He Y, Ge J, Zou W, Liu S, et al. Competing risk nomogram and risk classification system for evaluating overall and cancer-specific survival in neuroendocrine carcinoma of the cervix: a population-based retrospective study. J Endocrinol Invest. (2024) 47:1545–57. doi: 10.1007/s40618-023-02261-7
PubMed Abstract | Crossref Full Text | Google Scholar
11. Lin L-M, Lin Q, Liu J, Chu K-X, Huang Y-X, Zhang Z-K, et al. Prognostic factors and treatment comparison in small cell neuroendocrine carcinoma of the uterine cervix based on population analyses. Cancer Med. (2020) 9:6524–32. doi: 10.1002/cam4.3326
PubMed Abstract | Crossref Full Text | Google Scholar
12. Mazzola R, Ricchetti F, Fiorentino A, Levra NG, Fersino S, Di Paola G, et al. Weekly cisplatin and volumetric-modulated arc therapy with simultaneous integrated boost for radical treatment of advanced cervical cancer in elderly patients: feasibility and clinical preliminary results. Technol Cancer Res Treat. (2017) 16:310–5. doi: 10.1177/1533034616655055
PubMed Abstract | Crossref Full Text | Google Scholar
13. Chu T, Meng Y, Wu P, Li Z, Wen H, Ren F, et al. The prognosis of patients with small cell carcinoma of the cervix: a retrospective study of the SEER database and a Chinese multicentre registry. Lancet Oncol. (2023) 24:701–8. doi: 10.1016/S1470-2045(23)00185-7
PubMed Abstract | Crossref Full Text | Google Scholar
14. Cuccia F, Pastorello E, Vitale C, Nicosia L, Mazzola R, Figlia V, et al. The use of SBRT in the management of oligometastatic gynecological cancer: report of promising results in terms of tolerability and clinical outcomes. J Cancer Res Clin Oncol. (2021) 147:3613–8. doi: 10.1007/s00432-021-03802-4
PubMed Abstract | Crossref Full Text | Google Scholar
15. Zhang Y, Huang Y, Luo S, Li L, Yang H, Wang Z, et al. Therapeutic strategy analysis of patients with advanced stage high-grade neuroendocrine cervical cancer: A real-world multicenter study. Int J Gynaecol Obstet. (2022) 158:722–9. doi: 10.1002/ijgo.14125
PubMed Abstract | Crossref Full Text | Google Scholar
16. Zhang Y, Li L, Wang Z, Huang Y, Luo S, Peng Y, et al. Preferred method of therapy for patients with early-stage high-grade neuroendocrine carcinoma of the cervix. Am J Cancer Res. (2021) 11:4595–606.
PubMed Abstract | Google Scholar
17. Zeng F, Guo P, Xia M, He M. Total hysterectomy versus radical hysterectomy in neuroendocrine cervical cancer: a SEER-database analysis. J Cancer Res Clin Oncol. (2024) 150:236. doi: 10.1007/s00432-024-05773-8
PubMed Abstract | Crossref Full Text | Google Scholar
18. Xiang X, Zhang Y, Hua K, Ding J. Impacts of ovarian preservation on the prognosis of neuroendocrine cervical carcinoma: a retrospective analysis based on machine learning. World J Surg Oncol. (2023) 21:146. doi: 10.1186/s12957-023-03014-9
PubMed Abstract | Crossref Full Text | Google Scholar
19. Wu P-Y, Cheng Y-M, New GH, Chou C-Y, Chiang C-T, Tsai H-W, et al. Case report: term birth after fertility-sparing treatments for stage IB1 small cell neuroendocrine carcinoma of the cervix. BMC Womens Health. (2017) 17:56. doi: 10.1186/s12905-017-0404-0
PubMed Abstract | Crossref Full Text | Google Scholar
20. Singh S, Redline R, Resnick KE. Fertility-sparing management of a stage IB1 small cell neuroendocrine cervical carcinoma with radical abdominal trachelectomy and adjuvant chemotherapy. Gynecol Oncol Rep. (2015) 13:5–7. doi: 10.1016/j.gore.2015.04.004
PubMed Abstract | Crossref Full Text | Google Scholar
21. Liu Y, Zhang N, Yang Q. Predicting the recurrence of usual-type cervical adenocarcinoma using a nomogram based on clinical and pathological factors: a retrospective observational study. Front Oncol. (2024) 14:1320265. doi: 10.3389/fonc.2024.1320265
PubMed Abstract | Crossref Full Text | Google Scholar
23. Liu R, He X, Li Z. Positive clinical outcomes following therapy with programmed cell death protein 1/programmed cell death ligand 1 inhibitors in neuroendocrine carcinoma of the cervix. Front Pharmacol. (2022) 13:1029598. doi: 10.3389/fphar.2022.1029598
PubMed Abstract | Crossref Full Text | Google Scholar
24. Korse CM, Taal BG, van Velthuysen M-LF, Visser O. Incidence and survival of neuroendocrine tumours in the Netherlands according to histological grade: experience of two decades of cancer registry. Eur J Cancer. (2013) 49:1975–83. doi: 10.1016/j.ejca.2012.12.022
PubMed Abstract | Crossref Full Text | Google Scholar
26. Shao C, Guo H, Chen L, Chen J, Wang L, Wang H. Prognostic factors and clinic-pathologic characteristics of ovarian tumor with different histologic subtypes-a SEER database population study of 41,376 cases. Transl Cancer Res. (2023) 12:1937–50. doi: 10.21037/tcr-23-58
PubMed Abstract | Crossref Full Text | Google Scholar
27. Xie N, Lin J, Liu L, Deng S, Yu H, Sun Y. Nomograms constructed for predicting diagnosis and prognosis in cervical cancer patients with second primary Malignancies: a SEER database analysis. J Cancer Res Clin Oncol. (2023) 149:13201–10. doi: 10.1007/s00432-023-05192-1
PubMed Abstract | Crossref Full Text | Google Scholar
28. Zivanovic O, Leitao MM, Park KJ, Zhao H, Diaz JP, Konner J, et al. Small cell neuroendocrine carcinoma of the cervix: Analysis of outcome, recurrence pattern and the impact of platinum-based combination chemotherapy. Gynecol Oncol. (2009) 112:590–3. doi: 10.1016/j.ygyno.2008.11.010
PubMed Abstract | Crossref Full Text | Google Scholar
29. Cohen JG, Kapp DS, Shin JY, Urban R, Sherman AE, Chen L, et al. Small cell carcinoma of the cervix: treatment and survival outcomes of 188 patients. Am J Obstet Gynecol. (2010) 203:347.e1–6. doi: 10.1016/j.ajog.2010.04.019
PubMed Abstract | Crossref Full Text | Google Scholar
30. Yang Y, Liu Q, Shi X, Zheng Q, Chen L, Sun Y. Advances in plant-derived natural products for antitumor immunotherapy. Arch Pharm Res. (2021) 44:987–1011. doi: 10.1007/s12272-021-01355-1
PubMed Abstract | Crossref Full Text | Google Scholar
31. Ji X, Sui L, Song K, Lv T, Zhao H, Yao Q. PD-L1, PARP1, and MMRs as potential therapeutic biomarkers for neuroendocrine cervical cancer. Cancer Med. (2021) 10:4743–51. doi: 10.1002/cam4.4034
PubMed Abstract | Crossref Full Text | Google Scholar
32. Paraghamian SE, Longoria TC, Eskander RN. Metastatic small cell neuroendocrine carcinoma of the cervix treated with the PD-1 inhibitor, nivolumab: a case report. Gynecol Oncol Res Pract. (2017) 4:3. doi: 10.1186/s40661-017-0038-9
PubMed Abstract | Crossref Full Text | Google Scholar
33. Sharabi A, Kim SS, Kato S, Sanders PD, Patel SP, Sanghvi P, et al. Exceptional response to nivolumab and stereotactic body radiation therapy (SBRT) in neuroendocrine cervical carcinoma with high tumor mutational burden: management considerations from the center for personalized cancer therapy at UC san diego moores cancer center. Oncologist. (2017) 22:631–7. doi: 10.1634/theoncologist.2016-0517
留言 (0)