Primary tic disorders (1), including Tourette syndrome (TS), chronic motor tic disorder, chronic vocal tic disorder, and transient tic disorder, are estimated to affect 0.77%, 1.65%, 0.69%, and 2.99% of the global population, respectively (2). Tics are the defining feature of these disorders, but the vast majority of individuals in these populations also experience premonitory urges. Premonitory urges are uncomfortable bodily sensations preceding tics, often building in the moments before a tic and resolving once the tic is performed. In Cohen and Leckman’s seminal 1992 study of sensory phenomena in TS, nearly 80% of participants endorsed premonitory urges, and nearly 60% reported their premonitory urges were more distressing than tics themselves (3). Subsequent research in other tic disorder samples has replicated these original findings, demonstrating that premonitory urges are common, particularly in adolescence and adulthood, and bothersome.
Since the initial publications describing premonitory urges more than thirty years ago, numerous studies have sought to characterize them, clarify their relationship with tics, identify their clinical and neurobiological correlates, and, to a lesser extent, treat them. As a result of these research endeavors, knowledge of premonitory urges has significantly advanced over the past three decades. However, given the complexity of the phenomenon, as well as the diverse methods employed and populations recruited to study it, much ambiguity remains concerning premonitory urges. Critically, the developmental and pathophysiologic interrelationship between premonitory urges and tics is unclear, and an effective treatment for premonitory urges has yet to be established. A comprehensive review of published research is needed to consolidate the breadth of current knowledge about premonitory urges and, equally important, to identify limitations and gaps in this knowledge. Reviews of the premonitory urge literature have previously been conducted but have focused on specific aspects of the phenomenon (4, 5) (e.g., neurobiological correlates) or adopted a non-systematic approach (6, 7). To address these limitations, we conducted a scoping review of premonitory urge in primary tic disorders.
2 MethodsThis scoping review was conducted in accordance with the Joanna Briggs Institute (JBI) methodology for scoping reviews (8). The study protocol was registered on Open Science Framework (OSF) (https://osf.io/b6tnf/files/osfstorage/65b18bb94aa63c067bdf2550). To be eligible for inclusion, studies had to be original research, published in English, and contain information about premonitory urge in primary tic disorders. There was no restriction based on study date. Studies involving participants with functional tic-like behaviors (9) were not included unless participants with primary tic disorders were also enrolled; for such studies, only data on individuals with primary tic disorders were reviewed and extracted. This scoping review considered experimental and quasi-experimental studies (i.e., randomized controlled trials, non-randomized controlled trials, before and after studies, and interrupted time-series studies), analytical observational studies (i.e., prospective and retrospective cohort studies, case-control studies, and analytical cross-sectional studies), descriptive observational studies (i.e., case series, individual case reports, and descriptive cross-sectional studies), and qualitative studies. Conference abstracts, grey literature, unpublished works, literature reviews of any type, and opinion papers were excluded.
PubMed (National Library of Medicine), Embase (Elsevier), PsycINFO (ProQuest), Web of Science (Clarivate), and Scopus (Elsevier) were searched for relevant articles. The journals Parkinsonism and Related Disorders, CNS Spectrums, Cognitive Neuropsychiatry, Behavioral Neurology, and Cognitive and Behavioral Neurology were also independently searched as they are not fully indexed in the included databases. A biomedical librarian (K.D.G.) developed the search strategy consisting of a combination of relevant keywords and database-specific subject terms. The search was conducted on January 9, 2023, and a revised search was conducted on January 24, 2024 after the authors identified studies not captured by the original search. The finalized search strategies are available in the Supplementary Material. Following the search, all identified citations were imported into Covidence (10), a systematic review management software. Covidence was also used to deduplicate results from the revised searched. Titles and abstracts of all imported publications were independently evaluated for inclusion or exclusion by two reviewers (D.A.I. and J.B.W.). Subsequently, the same two reviewers (D.A.I. and J.B.W.) independently assessed the full text of the included publications using the predefined inclusion criteria. Inter-reviewer disagreements at each stage of the selection process were resolved by the independent decision of a third reviewer (K.H.W.). Results of the search and study inclusion process are presented in a Preferred Reporting Items for Systematic Reviews and Meta-analyses extension for scoping review (PRISMA-ScR) flow diagram (Figure 1) (11).
Figure 1. Premonitory urge in tic disorders.
Data were extracted by two independent reviewers (D.A.I. and J.B.W.) using a data extraction tool (presented in the Supplementary Material) developed by the reviewers. The following data, if relevant and available, were extracted from eligible articles: 1) clinical characteristics of the study sample; 2) method of premonitory urge assessment; 3) qualitative and/or quantitative characteristics of premonitory urge; 4) associations between premonitory urge characteristics and other clinical characteristics; 5) premonitory urge response to treatment and/or tic suppression; and 6) neural correlates of premonitory urge and method for investigating such neural correlates. Within each of the above categories, additional specific data elements were extracted (as shown in the Supplementary Material). Following data extraction for all studies, the two reviewers consolidated their findings and arrived at a consensus as to the relevant data to be included from each article. All relevant findings were grouped into one of the above six categories. The overarching term “tic disorder” is used in this review to refer to all primary tic disorders including TS, chronic (persistent) motor tic disorder, chronic (persistent) vocal tic disorder, and transient tic disorder, but not functional tic disorder. The term “CTD” is used to refer collectively to TS, chronic motor tic disorder, and chronic vocal tic disorder.
3 Results3.1 Methods of premonitory urge ascertainmentOf the 155 studies meeting inclusion criteria, 25 examined methods to measure premonitory urge (Table 1 contains descriptions of assessment methods). The majority of these studies employed questionnaire-based methods, but a small minority employed urge monitors.
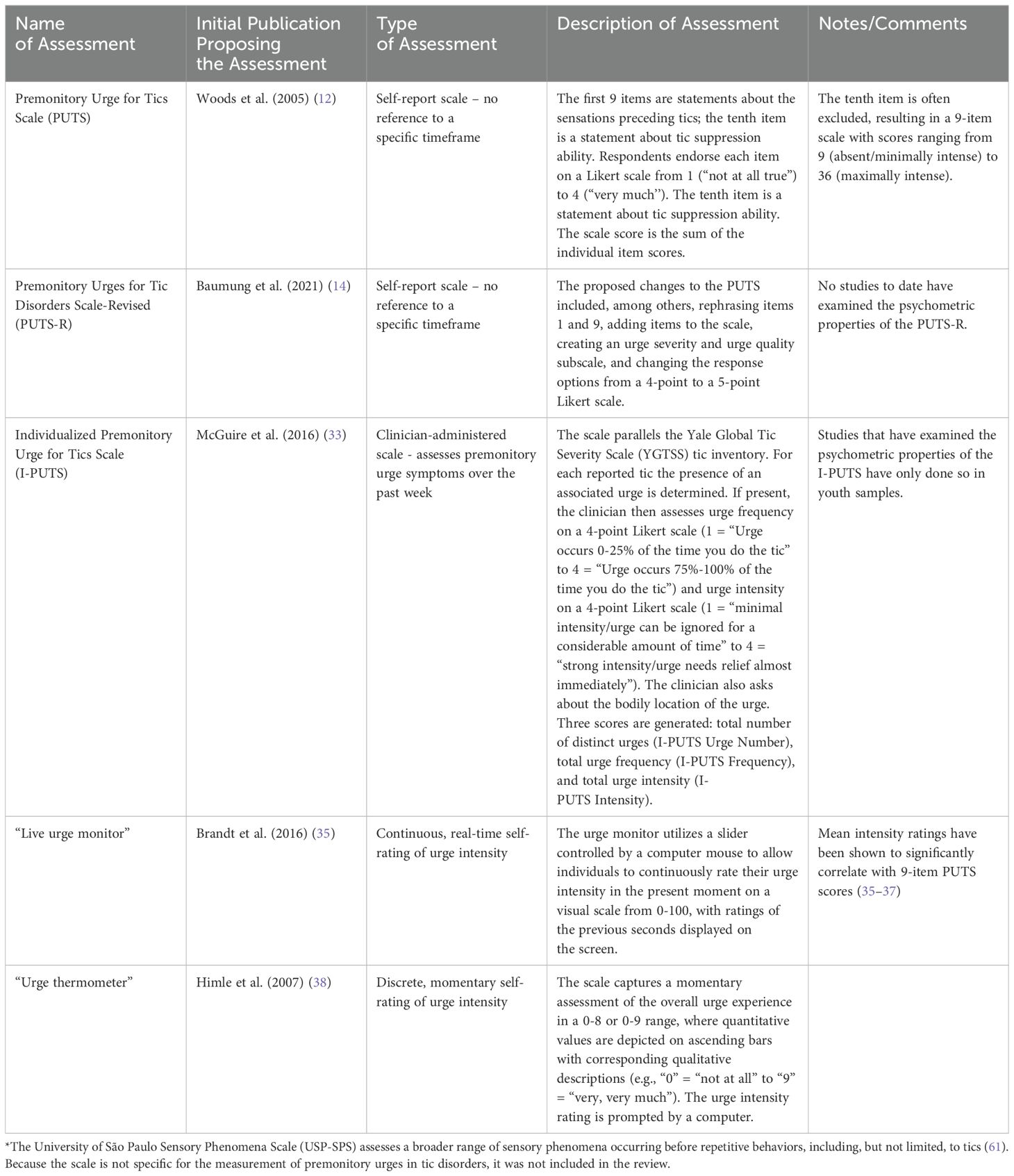
Table 1. Methods of premonitory urge measurement.
3.1.1 Premonitory urge for tics scaleThe most commonly used questionnaire was the Premonitory Urge for Tics Scale (PUTS), a 10-item, self-report scale developed by Woods et al. (12). The questionnaire contains 9 statements about premonitory urges and 1 statement about tic suppressibility. Respondents indicate their degree of agreement with each statement on a 4-point Likert scale ranging from 1 (“Not at all”) to 4 (“Very much”). Regarding content validity [i.e., the extent to which the phenomenon of interest is thoroughly represented by the questionnaire items (13)], a description of the two-stage method for PUTS item selection is provided in Woods et al’s original publication. In the initial validation study, PUTS item 10 (“I am able to stop my tics, even if only for a short period of time”) correlated poorly with the rest of the scale (12), a finding subsequently replicated in multiple other studies (14–19). Consequently, the tenth item is often excluded from the PUTS total score. Supplementary Table S1 contains a full summary of publications examining psychometric properties of the PUTS.
The original PUTS, developed in English, has been translated into multiple languages: German, Greek, Hebrew, Italian, Japanese, Korean, Mandarin/Cantonese, and Spanish. The translation process is described in some (20–26) but not all (14, 15, 17, 27) studies. No studies involving non-English versions of the PUTS reported re-assessing content validity of the translated scale, though this scoping review did not encompass non-English publications.
Twenty studies (12, 14–32) examined internal consistency of the PUTS, with Cronbach’s alpha values for the 9-item PUTS ranging from 0.75 (23, 27, 31) to 0.85 (24) in sample populations ranging in size from 22 (15) to 656 (17). Seven of these studies assessed internal consistency across children of different age groups within their sample, typically stratifying participants into those 10-years-old and younger and those older than 10 years (12, 17, 18, 20–23). Four of these studies found lower Cronbach’s alpha values (ranging from 0.57 to 0.70) in the younger age group (12, 18, 20, 21), but the other three studies did not observe this pattern (17, 22, 23). Three studies observed similar Cronbach’s alpha values between youth (< 18- years-old) and adults (14, 15, 24). Overall, the 9-item PUTS has shown acceptable internal consistency, with conflicting findings on the scale’s internal consistency in tic disorder populations younger than 10 years of age.
PUTS scores have been found to be temporally stable over a range of less than two weeks to four months (12, 19, 22, 23, 26), with between-timepoint correlations ranging from 0.49 (22) to 0.89 (23). In the largest of these studies, Li et al. observed a between-timepoint correlation of 0.89 at one month in 147 children with tic disorder (23), and Reese et al. observed a between-timepoint correlation of 0.79 at two weeks in 122 adolescents and adults with CTD (19). A single study examined differences in temporal stability across age groups, administering the Spanish version of the 9-item PUTS at baseline and 4-month follow-up (22). The correlation between scores was significant and positive for the subgroup older than 10 years of age and non-significant for the subgroup 10-years-old and younger (sample sizes of these subgroups were not reported). Notably, to assess temporal reliability of the PUTS, the above studies either employed Pearson’s correlations or did not report the specific correlation method used (12, 19, 23); no study reported intraclass correlations.
A number of studies conducted factor analyses to assess the dimensions of premonitory urge captured by the PUTS, with mixed results (14, 15, 17, 18, 22–24, 26). Study sample sizes varied widely, ranging from 38 (26) to 656 (17) participants, with many studies involving fewer than 100 participants (15, 18, 22, 24, 26). Multiple studies found that items 4 and 5 loaded onto a factor interpreted as “OCD-related items” or “just right phenomena” (17, 22, 24, 26). Other commonly proposed factors included “quality” (14, 15, 18, 22, 24, 26) and “intensity” (14, 15, 23, 24), although the items composing these factors were inconsistent across studies.
A single study, in a sample of 102 adults with TS, examined floor and ceiling effects of the PUTS (16). Both floor (3.9%) and ceiling (2.0%) effects were found to be small.
3.1.2 Individualized premonitory urge for tics scaleWhereas the PUTS is a self-report questionnaire, the Individualized Premonitory Urge for Tics Scale (I-PUTS) is a clinician-administered scale. The clinician assesses the presence, bodily location, frequency, and intensity of urges for individual tics experienced over the past week, employing a checklist analogous to the tic checklist in the Yale Global Tic Severity Scale (YGTSS). Each urge is rated on a 4-point Likert scale for both frequency and intensity. The scale generates three scores: urge number, urge frequency, and urge intensity (33). The authors note that the I-PUTS, in contrast to the PUTS, permits assessment of individual premonitory urges, quantifies premonitory urge in three separate dimensions, and specifies the timeframe in question.
In the initial validation study, McGuire et al. administered the I-PUTS to 75 children with tic disorders and found excellent inter-rater reliability (33). The I-PUTS scores did not significantly correlate with scores from scales assessing severity of common comorbid psychiatric symptoms. In contrast, the PUTS score, in the same study, did significantly correlate with some psychiatric symptom severity scores, suggesting that the I-PUTS possesses better divergent validity than the PUTS. The three I-PUTS scores showed small correlations (I-PUTS number: r = 0.28, p < 0.02, I-PUTS frequency: r = 0.23, p < 0.05, I-PUTS intensity: r = 0.19, p = 0.10) with the 9-item PUTS score. The study did not examine the test-retest reliability of the I-PUTS. Che et al. (34) translated the I-PUTS into Chinese, describing their methods for doing so, and then administered the resultant Chinese I-PUTS to 123 children with tic disorder. They found good internal consistency, both for children 8-10-years-old and children 11-14-years-old; good test-retest reliability at one month; and good inter-rater reliability for all three I-PUTS scores (34). The three Chinese I-PUTS scores exhibited significant, medium-sized positive correlations with the Chinese PUTS score (I-PUTS number: r = 0.433, p < 0.01, I-PUTS frequency: r = 0.486, p < 0.01, I-PUTS intensity: r = 0.489, p < 0.01).
3.1.3 Urge monitorsIn contrast to the above studies employing questionnaires or structured interviews, several studies have used a “live urge monitor” (15, 35–37) or an urge thermometer (38–42) to precisely gauge premonitory urge intensity over short time periods. In a factor analysis including real-time urge intensity (average live urge monitor score over five minutes) and the 10 PUTS items, real-time urge intensity loaded on the second of the factors, which the authors interpreted as “intensity” of premonitory urges (15).
3.2 Characteristics of premonitory urge3.2.1 Prevalence of premonitory urgesPrevalence estimates of premonitory urges in individuals with tic disorders range from 37% (43) to 93% (3, 19, 34, 43–51). Prevalence rates vary by age of the sample population. In a sample of 656 children with CTDs, prevalence of premonitory urges, as assessed by the PUTS, was 81% for children 7-years-old and younger, 95.5% for children 8-10-years-old, and 97.5% for children 10-years-old and older (17). The following two studies, which asked individuals categorical questions about the presence of premonitory urges (“yes/no” or “yes/no/don’t know” response options) rather than administering a scale, observed lower prevalence estimates. In Sambrani et al’s study of 1,032 individuals with tic disorders, premonitory urges were reported by 46.7% of those less than 10-years-old, 61.3% of those 10-12-years-old, and 79.7% of those 12-years-old and older (50). In Banaschewski et al’s study of 251 children and adolescents with TS, premonitory urges were reported by 24% of 8-10-year-olds, 34% of 11-14-year-olds, and 57% of 15-19-year-olds (43).
3.2.2 The nature and subjective quality of premonitory urgesIn a sample of 122 adolescents and adults with CTDs, the most frequently endorsed premonitory urge qualities from the PUTS were “an energy in my body that needs to get out” (>80%) and “an inner feeling of being wound up or tense” (>80%) (19). In another study of 656 youth with CTDs, “an energy in my body that needs to get out” was again the most frequently endorsed urge quality from the PUTS (17). Schunke et al. (52) administered a questionnaire to 14 adults with TS, allowing participants to select the most accurate descriptors for their premonitory urges from a list of 14 different qualities. The three most selected qualities were inner urge (100%), increased tension (71%), and urge to move (64%) (52). In a sample of 291 adults with CTDs, the following premonitory urge qualities were endorsed, in order of decreasing frequency (based on the publication’s Supplementary Table S1): “feelings of tension” (61%), “pressure” (45%), “feeling discomfort” (43%), “a feeling of energy that needs to be released” (40%), “a not-just-right feeling” (33%), “the feeling that something was building up” (31%), “an itch” (29%), and “incompleteness” (21%) (53). Participants also rated urge intensity on a 1-to-11-point scale in this study; urge intensity did not significantly differ between the above urge qualities. In contrast to the more quantitative approaches discussed above, two case reports and series provide narratives of individuals with tics describing their experience of premonitory urges (3, 54).
The quality of premonitory urges appears to differ in tic disorder subgroups with obsessive-compulsive disorder (OCD) and/or attention-deficit/hyperactivity disorder (ADHD) compared to those without these comorbidities. In particular, multiple studies have observed increased prevalence (47, 53) and/or intensity (19) of the “not-just-right” quality of premonitory urges in individuals with tic disorder and comorbid OCD or obsessive-compulsive symptoms. In a study of 74 individuals with CTDs, Edwards et al. found that the subgroup of participants with comorbid ADHD and/or OCD reported greater “tense” or “wound up” feelings (PUTS item 3) than those without comorbid ADHD or OCD, though PUTS total scores did not significantly differ between groups (32).
Based on only two small studies, premonitory urges are distressing for some individuals with tics. In Cohen and Leckman’s study, 57% of 28 participants reported their urges to be “more bothersome than the tics themselves” (3). In more recent interviews of 42 youth with TS, some found their urges to be “uncomfortable or painful” and perceived reducing their urges as “an important outcome of treatment” (55).
3.2.3 Number and frequency of premonitory urgesAmong individuals who experience premonitory urges, significant variability exists as to the number and frequency of the urges. In one study with 135 adults and children with tic disorders, participants endorsed an average of 8.7 distinct urges (48), while in a different study of 75 children with tic disorders, participants endorsed an average of 2.9 distinct urges (33). Among a sample of 50 children and adults with TS, 92% reported premonitory urges, but only 35% endorsed premonitory urges with all of their tics (47). In this same study, only 3% of participants reported urges with fewer than 25% of their tics. In a sample of 75 children with tic disorders, McGuire et al. found that for a given tic with an associated premonitory urge, participants “experienced urges over 50% of the time they had the tic” (33).
3.2.4 Premonitory urge locationIn their seminal study, Cohen and Leckman found that, of 28 children and adults with TS, 45% felt premonitory urges “in their mind,” while the other 55% felt the urge “in another body area” (3). In a subsequent study of children and adults with tic disorders, Leckman et al. found that, of 123 participants with premonitory urges, 89% reported their urges were “either partly or wholly a physical experience” as opposed to a mental one (48). O’Connor et al. posed a similar question to 60 adults with CTDs and found that, of the participants that experienced premonitory urges (>80%), only two reported the sensation was mental as opposed to physical (49). In their aforementioned study, Leckman et al. found that 40% of participants felt the urge exclusively in muscle, 24% felt it in both muscle and joints, and 8% felt it exclusively in joints (48). Similarly, Kwak et al. found that urges were more frequently perceived to occur in muscles than in joints or skin (47). Among 291 adults with CTDs, premonitory urges for motor tics were most frequently felt “exactly at the location or in direct proximity to the corresponding tic” (44). However, 52.6% of participants were unable to report a location for at least one premonitory urge (“diffuse, non-circumscribed premonitory urge”), and 3.9% of participants endorsed an urge located “outside the body” for at least one of their tics. In this study, a diffuse premonitory urge location was more common for complex tics than simple tics and more common for vocal tics than motor tics (44). In a large study of 700 children and adults with tic disorders who experienced premonitory urges, 64% reported their urges were localized, 16% reported their urges were diffuse, and 13.7% were unable to identify the precise nature of their urges (50). These findings generally align with those of a smaller study of 19 children and adults with TS: 53% reported their urges as focal sensations, 11% as generalized sensations, and 37% as both generalized and focal sensations (56).
The face and shoulders have tended to predominate as the most common bodily locations for premonitory urges (33, 34, 44, 47, 48, 52). Two studies utilized the I-PUTS to determine the relative intensity of premonitory urges across bodily locations. In these studies, urges in the “whole body/other region” (33) and the “neck/throat region” (34), respectively, were rated as most intense.
3.2.5 Development of premonitory urges over timeAs aforementioned, prevalence rates of premonitory urge increase over the course of development (17, 43, 50). The trajectory of premonitory urges, however, is unclear due to a paucity of longitudinal investigations. In their cross-sectional study of 135 children and adults with tic disorders, Leckman et al. found that individuals who experienced premonitory urges (n = 123) recalled first becoming aware of their urges at 10.0-years-old (SD = 6.2 years) (48). Awareness of premonitory urges emerged an average of 3.1 years (SD = 5.7 years) after tic-onset. Gulisano et al. administered the PUTS to 95 children with TS at baseline (mean age = 7.3 years, SD = 1.5 years) and at long-term follow-up (mean age = 13.1 years, SD = 3.7 years), observing that mean PUTS score was significantly higher at follow-up (24.1) than at baseline (13.5) (21).
Multiple cross-sectional studies have compared measures of premonitory urge severity between different age groups. Kyriazi et al. found that children with tic disorder who were 12-15-years-old (n = 13) had significantly higher PUTS scores than those who were 6-11-years-old (n = 39) (25). Yet, other studies have failed to identify significant differences in PUTS scores between older and younger youth (12, 18, 20, 23) or between adults and youth (24, 26). Langelage et al. (37) collected data using a live urge monitor over five minutes in 25 youth (8-18-years-old) with TS and compared their results to those from Schubert et al. (36), a similar study in 21 adults with TS (mean age = 30.5 years, SD = 10.6 years). They found that average urge monitor intensity ratings during the task did not significantly differ between youth and adult samples.
Other cross-sectional studies have examined the correlation between measures of premonitory urge severity and age. In some studies of children and adolescents, age positively correlated with premonitory urge severity, as indexed by the PUTS (29, 33), but other studies failed to identify a significant correlation using the PUTS (34, 57, 58) or the I-PUTS (33, 34). Most studies involving adults have not observed a significant correlation between age and PUTS score (16, 19, 59, 60), though one small study of 18 adults and children with TS did observe a significant positive association (61). In contrast, Brandt et al., assessing urge intensity on an 11-point Likert scale in 291 adults with CTDs, found that younger age was associated with more intense urges; however, older age was associated with a greater likelihood of experiencing urges (dichotomous variable), after accounting for gender and psychiatric comorbidity (53).
3.2.6 Premonitory urge relationship to ticsLikelihood of premonitory urges appears to vary by tic location, complexity, and severity. Tics of the head, neck, and shoulders were most commonly associated with a premonitory urge in a sample of 135 children and adults with tic disorders (48). In another study of 291 adults with CTD, premonitory urges were reported for 80% of complex motor tics compared to only 67% of simple motor tics (44). Among 240 individuals with CTDs, tics with an associated premonitory urge were on average rated as more severe compared to tics without an associated urge (62).
Evidence indicates that tics generally alleviate premonitory urges. Reese et al. found that 85% of 122 older adolescents and adults with CTD endorsed PUTS item 9 (“After I do a tic, [the sensation] goes away at least for a little while”) (19), and Essing et al. (44) found that 97% of 291 adults with CTD reported relief from their urges following at least one of their tics. In two studies involving both children and adults with TS (with sample sizes of 28 and 50), over three-fourths of participants (75% and 83%, respectively) reported relief from premonitory urge following a tic (3, 47). Moreover, 71% (3) and 67% (47) of participants believed their tics were voluntary responses to their premonitory urges. In the latter of these two studies, 88% of participants endorsed an increase in urge intensity if prevented from executing their motor tics (47).
A few studies have utilized a live urge monitor to examine the relationship between urges and tics in real time. Brandt et al. assessed real-time urge intensity in 16 adults with TS using a live urge monitor while two independent raters recorded tic occurrence (35). In both free ticcing and tic suppression conditions, urge intensity tended to increase prior to a tic and decrease after a tic. In a separate study of 22 adults with TS, number of tics during a five-minute live urge monitor task positively correlated with average real-time urge intensity (15). Two similar studies (36, 37) found that greater real-time urge intensity was associated with increased likelihood of real-time tic occurrence and tic intensity. In a study of 11 adolescents and adults with CTDs, Wellen et al. used a live urge monitor and video-based tic rating to assess urges and tics (63). They observed that real-time urge intensity was positively associated with tic occurrence in the subsequent second for nine (82%) of the participants. Additionally, a relationship between tic occurrence in the previous second and urge intensity in the subsequent second was significant for eight (73%) of the participants, but for seven of these eight participants the relationship was such that tic occurrence predicted an increase in urge intensity in the subsequent second. Six of these seven participants showed an eventual decrease of urge intensity following tics but at varying timepoints (four to nine seconds post-tic).
3.2.7 Associations between premonitory urge severity and tic severityResults are mixed concerning the cross-sectional association between premonitory urge severity and tic severity. Correlations of PUTS score with Yale Global Tic Severity Scale-Total Tic Score (YGTSS-TTS) and YGTSS Impairment Score range widely, from 0.08 (20) to 0.63 (64) and from 0.02 (58) to 0.60 (65), respectively. In studies with sample sizes of more than 100 participants, YGTSS-TTS consistently correlated with PUTS score, with correlation values of 0.13-0.27 in youth samples (23, 29, 46) and 0.20-0.32 in adult samples (19, 66). Supplementary Table S2 contains detailed results from studies examining the relationship between PUTS score and measures of tic severity. The relationship between I-PUTS scores and tic severity has been less extensively studied, but the existing data demonstrate a positive relationship between I-PUTS and YGTSS scores [(33, 34, 67, 68); Supplementary Table S3].
In the sole study to examine the relationship between premonitory urge severity and long-term tic severity, 80 CTD participants aged 16-30 years completed the PUTS and YGTSS at baseline and at a long-term follow-up (mean = 11.2 years later) (69). Baseline PUTS score did not significantly predict follow-up YGTSS-TTS or Impairment Score after controlling for selected covariates, nor did baseline PUTS score predict change in tic impairment over time. However, baseline PUTS score did significantly predict change in YGTSS-TTS between baseline and follow-up. That is, those with more severe premonitory urges at baseline experienced less reduction in tic severity over time.
3.2.8 Associations between premonitory urge severity and tic suppression abilitySeveral studies have evaluated the relationship between self-reported tic suppression ability and premonitory urge severity. Cohen and Leckman (3) found that 55% of their 28 participants felt their urges enabled them to better suppress their tics, but in Leckman et al.’s subsequent study (n = 132, age range 8-71-years-old, with 123 participants endorsing premonitory urge), only 20% of participants reported their urges enhanced their tic suppression ability (48). Among 1,032 children and adults with any tic disorder, presence of premonitory urge was positively associated with reported ability to suppress tics (50). Conversely, in a sample of 62 children and adults with TS, Matsuda et al. found no significant correlation between PUTS score and the tic suppression ability sub-score of the Tic Suppression Scale (24). However, in the subgroup of participants without comorbid OCD or ADHD (n = 37), these two scores showed a significant negative correlation, such that those with greater premonitory urge severity reported less ability to suppress tics. Within the 8-10-year-old subgroup (n = 81) of a sample of 254 youth with TS, those who endorsed premonitory urges were 2.75 times more likely to report tic suppressibility compared to 8-10-year-olds who did not endorse urges (43). In the other age groups, those who endorsed premonitory urges were not significantly more likely to report the ability to suppress tics.
Three studies have evaluated the relationship between objectively measured tic suppression ability and premonitory urge severity. In two studies of adults with TS, PUTS score did not significantly correlate with tic inhibition potency, as assessed by percent difference in the Modified Rush Video-Based Tic Rating Scale (MRVS) score during free-to-tic and tic inhibition conditions (70, 71). In their study of children with CTDs, Capriotti et al. included a condition in which participants were instructed to suppress tics but had the option to initiate a 10-second break during which they were free to tic (40). After removing one outlier, PUTS score positively correlated with average number of breaks taken.
3.2.9 Psychosocial impact of premonitory urgeTwo cross-sectional studies have examined the association between premonitory urge severity and the impact or consequences of ticcing. Zinner et al. administered a neuropsychiatric battery, including the PUTS, and a questionnaire assessing peer victimization and bullying behavior to 211 parent-child dyads, in which all children had a CTD (28). Based on questionnaire results, children were classified as “victims,” “bullies,” “bully-victims,” or “non-victims.” Severity of premonitory urge, as well as of tics, explosive outbursts, and internalizing symptoms, was greater in “victims” compared to “non-victims.” The authors suggested that peer victimization perceived to be tic-related may increase the aversive experience of tics, which in turn may intensify premonitory urges. Consistent with the above study results, Capriotti et al. observed in their questionnaire-based study of 118 youth with TS that tic-related impact score predicted PUTS score after adjusting for tic severity and comorbid psychiatric diagnoses (72).
3.3 Associations between premonitory urges and other clinical characteristics3.3.1 InteroceptionThree studies have examined the potential association between premonitory urge severity and interoceptive sensibility, defined as “self-reported sensitivity to bodily sensations” (73), with differing results. In a sample of 18 adolescents and adults with TS, PUTS score and Private Body Consciousness scale score did not correlate (74). In contrast, in 21 adults with TS, score on the “awareness” section of the Body Perception Questionnaire significantly positively correlated with PUTS score (73), and in 48 adults with CTDs, a Multidimensional Assessment of Interoceptive Awareness, Version 2 (MAIA-2) composite score was significantly associated with PUTS score after adjusting for tic and OCD symptom severity (75), indicating that participants with greater interoceptive sensibility reported more severe premonitory urges.
Several studies have examined the potential association between premonitory urge severity and interoceptive accuracy and/or interoceptive awareness. Interoceptive accuracy is a construct that reflects “objective interoceptive performance” (73) on tasks such as the heartbeat counting task, whereas interoceptive awareness is a construct defined as “insight into interoceptive ability, reflecting correspondence between subjective and objective measures” (73). In two studies assessing interoceptive accuracy in youth with CTDs (respective tic disorder sample sizes of 28 and 29), accuracy on a heartbeat perception task did not correlate with PUTS score (27, 30). One of these studies also assessed interoceptive accuracy (IAcc) by examining the correlation between self-rated muscle tension in the corrugator and masseter muscles and electromyography (EMG) activity, finding that PUTS score significantly positively correlated with Masseter IAcc but not Corrugator IAcc (27). In a study of 21 adults with TS, PUTS score did not correlate with participants’ accuracy in a heartbeat perception task or a heartbeat discrimination task nor did PUTS score correlate with metacognitive insight into accuracy on those two tasks (interoceptive awareness) (73). In contrast, in 19 adults with TS, better performance on a heartbeat counting task was significantly associated with higher PUTS score after adjusting for tic and OCD symptom severity (76).
A single study has examined the relationship between premonitory urge and embodiment. Rae et al. recruited 23 adults with TS to complete the rubber hand illusion task (77). PUTS score was not significantly associated with “body ownership plasticity” as indexed by the proprioceptive drift between synchronous and asynchronous conditions. However, PUTS score was correlated with embodiment prediction error, a metric generated from the difference between proprioceptive drift (objective measure of embodiment) and self-ratings of rubber hand embodiment during synchronous stimulation (subjective measure of embodiment). In this study, PUTS score was also associated with change in the “experience of rubber hand ownership” between synchronous and asynchronous conditions.
3.3.2 ExteroceptionSeveral studies have examined associations between premonitory urges and self-reported perception of the external environment, again with mixed results. In a study of 18 children and adults with TS, PUTS score did not significantly correlate with scores from the Sensory Gating Inventory or the Structured Interview for Assessing Perceptual Anomalies (61). In contrast, in a sample of 34 adults with CTDs, PUTS score significantly correlated with both Sensory Gating Inventory and Sensory Perception Quotient scores, indicating that participants with greater premonitory urge severity reported more sensory hypersensitivity, defined as “heightened awareness of and reactivity to external stimuli” (31). In a sample of 140 children and adults with TS, presence of premonitory urges was not significantly associated with presence of stimulus sensitization, defined as “heightened sensitivity to tactile, auditory, and visual stimuli that [result] in uncomfortable sensation, tension, or non-tic movement” (78). Whereas the above studies relied on self-reported sensory perception, Schunke et al. used the “Quantitative Sensory Testing” (QST) battery to objectively assess 13 sensory parameters, including thermal, mechanical, and pain thresholds, in 14 adults with TS (52). PUTS score did not significantly correlate with any of the sensory parameters (52).
3.3.3 Cognitive traits and functionsSeveral studies have examined the associations between premonitory urges and various aspects of cognition and metacognition. In a study of 122 adolescents and adults with CTD, PUTS score showed a small but significant positive correlation with intelligence quotient (IQ) as measured by the Wechsler Test of Adult Reading (19). Two other studies, with samples of 42 (12) and 29 (57) children and adolescents, found no such association.
Sections 3.2.8 and 3.4.1 review investigations of premonitory urge and tic suppression ability, but two studies have examined the relationship of premonitory urges with facets of inhibition outside the context of tic suppression. In a sample of 18 adolescents and adults with TS administered the standard and emotional Stroop tasks, PUTS score negatively correlated with standard Stroop time difference but not with standard Stroop error difference or emotional Stroop time difference or error difference (74). The authors suggested that the negative correlation between standard Stroop time difference and PUTS score may indicate reduced inhibitory interference in those with more severe premonitory urges. In a study of 40 adults with TS, average number of escape blinks during a blink suppression task, which included emotional and neutral facial expression conditions, was not associated with PUTS score (79).
Ganos et al. assessed the experience of intention during voluntary action in 27 adolescents with TS (80). Shorter time interval between the experience of intention and action performance was associated with higher PUTS score. The authors suggested that severe premonitory urges may interfere with self-perceived volition, as urges create perceptual noise from which signals of volitional action must be discriminated.
Arbuzova et al. conducted an assessment of metacognitive ability in 21 adults with TS, using a forced discrimination task of tactile stimuli (first-order) and confidence ratings regarding the accuracy of participants’ judgements in this task (second-order) to calculate the M-ratio, reflecting “the sensitivity of subjective second-order reports relative to objective first-order task performance” (81). They found no significant correlation between PUTS score and M-ratio. In a sample of 23 adults with TS, PUTS score significantly correlated with score from the Thinking About Tics questionnaire (“tic-related cognitions”) but not from the Meta-Cognitions Questionnaire (41).
3.3.4 Comorbid psychiatric symptoms/diagnoses, quality of life, and global functioningMany studies, involving children, adolescents, and/or adults with tic disorders, have observed significant associations between severity of premonitory urges and OCD symptoms [(12, 15, 17, 19–21, 23, 24, 26, 29, 32, 50, 53, 59, 61, 65, 66, 75, 76, 82, 83); Supplementary Table S5]. Results are more ambiguous from studies that have examined associations between severity of premonitory urges and severity of ADHD symptoms, depression, and/or anxiety [(12, 15, 17, 19–21, 26, 29, 33, 37, 53, 59, 75, 82–85); Supplementary Tables S6, S7]. A smaller number of studies have examined the association between premonitory urges and various behavioral, conduct, and emotional problems, including distress tolerance and externalizing behavior [(12, 17, 21, 33, 51, 58, 60, 86); Supplementary Table S8]. Of the studies that have assessed the relationship between premonitory urges and quality of life or global functioning, most indicate that more severe premonitory urges are associated with greater disability, poorer global functioning, and poorer health-related quality of life [(37, 53, 58, 59, 65, 66, 71, 72, 75, 82, 83, 87, 88); Supplementary Table S9].
3.3.5 Other characteristicsOf the eight studies that have examined differences in PUTS or I-PUTS scores between males and females, no between-sex differences were evident (16, 19, 29, 32, 34, 59–61). However, in a sample of 1,032 children and adults with any tic disorder (mean age = 20.9 years, SD = 12.9 years; male-female proportion of sample not reported), males were more likely to report presence of premonitory urges than females (males: 72.3%, females: 65.1%) (50). Conversely, in a sample of 291 adults with CTDs (mean age not reported but 33.3% of sample older than 35 years of age; 24% female), females were more likely to report premonitory urges than males, after adjusting for age and comorbid diagnoses (53). In their study of 74 youth and young adults with CTD, Edwards et al. found that the relationship between PUTS score and self-reported diagnoses of ADHD and OCD did not differ by sex (32).
In a study of 60 individuals (mean age = 32.2 years, SD = 14.1 years) with TS, Eddy and Cavanna administered the PUTS and assessed coprolalia and mental coprolalia (urge to swear as a tic) using a non-obscene socially inappropriate symptoms (NOSIS) questionnaire. PUTS score did not significantly correlate with the presence of coprolalia or mental coprolalia (89). In a separate study of 60 individuals (mean age 33 years, SD 14 years) with TS, Eddy and Cavanna found that those who reported NOSIS (n = 40) had significantly higher PUTS scores than those who did not (n = 20) (90).
A study of 1,032 children and adults with any tic disorder observed a significant association between the presence of premonitory urges and “not just right experiences” (50). In 111 adults with TS, scores from the Non-Just-Right-Experiences Questionnaire (NJRE-QR) and the Feelings of Incompleteness Questionnaire significantly correlated with PUTS score (66). Notably, PUTS item 4 (“Right before I do a tic I feel like something is not ‘just right’ “) score only moderately correlated with NJRE-QR score, suggesting that PUTS item 4 and the NJRE-QR may not index the exact same construct (66).
3.4 Premonitory urge response to intervention3.4.1 Response to tic suppressionMixed results have emerged from studies examining the effect of tic suppression on premonitory urge. Several have identified increased urge intensity during tic suppression. In a study of 13 children with CTDs, participants engaged in a tic suppression condition during which they had the option to initiate a 10-second break to tic freely (40). Participants rated their urge intensity on an “urge thermometer” at the initiation and termination of each break. Across participants, urge intensity significantly decreased between break initiation and termination. In this same study, the researchers also observed that average urge ratings were significantly higher in a reinforced tic suppression condition compared to the free-to-tic baseline condition. In a study of 45 children and adolescents with TS, participants completed a free-to-tic condition, a tic suppression condition, and an urge-acceptance condition, in which they were instructed to monitor “urges while practicing willingness to experience the urge and giving up any fight” (91). During each of these two-minute conditions, participants rated the intensity of each urge that occurred on a 1-9-point scale, allowing for assessment of both urge intensity and frequency. Urge intensity, but not frequency, was significantly higher during the tic suppression condition compared to the free-to-tic condition. Interestingly, both urge intensity and frequency were significantly lower in the urge acceptance condition compared to the free-to-tic condition. Brabson et al. conducted a study in which 12 children with CTD completed alternating baseline and differential reinforcement of zero rate behavior (DRO; i.e., incentivized tic suppression) conditions (42). Two participants showed an “M” pattern, with urge intensity lower in baseline conditions and higher in DRO conditions; three participants showed a “W” pattern, with urge intensity higher in baseline conditions and lower in DRO conditions; and the remaining participants showed other, less clear patterns of urge intensity across conditions. In a study of 16 adults with TS, participants rated their premonitory urge intensity on a live urge monitor during tic suppression and free-to-tic conditions (35). The timing of peak urge intensity differed between conditions: in the free-to-tic condition urge intensity peaked just after tic occurrence, while in the tic suppression condition urge intensity peaked just before tic occurrence. Notably, urge intensity tended to increase over the course of the suppression condition.
In contrast to the above, several studies have observed no change in premonitory urge intensity during tic suppression. In a study of 12 children and adolescents with CTD, participants completed three 10-minute free-to-tic conditions and two 40-minute DRO (incentivized tic suppression) conditions (39); urge intensity was rated every 10 seconds on an “urge thermometer.” Aggregate urge intensity did not significantly differ between baseline and DRO conditions, but tic frequency was significantly lower in DRO conditions. In a study of 11 individuals 17-60-years-old with CTD, participants engaged in multiple behavioral conditions, including free-to-tic (10 minutes each, 3 separate occurrences, though only first occurrence was used in analyses), tic suppression (30 minutes, 1 occurrence), and habit reversal tic suppression (tic suppression with a competing response; 30 minutes, 1 occurrence), while rating their urge intensity with a live urge monitor (63). Most participants showed similar urge levels across conditions, even though tic frequency was lower in the tic suppression and habit reversal tic suppression conditions compared to the free-to-tic condition. In a study of 22 adults with TS, participants completed the PUTS at baseline, suppressed their tics for 10 minutes, and then completed the PUTS again just before the end of the 10-minute task (70). The PUTS was also administered at 10 minutes, 20 minutes and 30 minutes post-suppression task. Mean PUTS score did not significantly differ between any of the timepoints. In study of 23 adults with TS, Hermann et al. assessed urge intensity in five conditions during which participants were instructed to focus their attention on different objects or phenomena: a picture of an empty room (baseline), a live video of themselves, a video of themselves ticcing, various objects within a room (distraction condition), and uncomfortable aspects of their tics (41). Participants completed each condition twice: once in a free-to-tic state and once in a tic suppression state. Attention condition, but not tic state, was significantly associated with urge intensity ratings. Relative to baseline, urge intensity was higher during the live feedback condition and the thoughts-about-uncomfortable-aspects-of-tics condition.
3.4.2 Response to behavioral interventionsNine studies have assessed whether various behavioral interventions, such as Comprehensive Behavioral Intervention for Tics (CBIT), exposure and response prevention (ERP), and habit reversal training (HRT), reduce premonitory urge severity (56, 92–99). Supplementary Table S10 provides detailed results of these studies. Most studies employed the PUTS and observed no effect of behavioral interventions on PUTS scores. In a randomized controlled trial comparing 10 sessions of CBIT versus psychoeducation and supportive therapy (PST) in 122 adults with CTD, PUTS scores did not significantly differ between treatment groups, though PUTS scores did significantly decrease over time across both groups (93). Interestingly, in the companion pediatric study of CBIT versus PST (n = 126), premonitory urge severity did not significantly change over time for either treatment group (93). Notably, one study, investigating the effectiveness of ERP in 18 children and adults with TS, indexed premonitory urge severity with subjective units of distress rather than the PUTS: subjective distress from premonitory urges decreased both within and across 10 ERP sessions (56).
3.4.3 Response to pharmacotherapyNineteen studies have assessed responsiveness of premonitory urges to medication (47, 84, 100–116). Supplementary Table S11 contains detailed results of these studies. In a randomized, double-blind, placebo-controlled trial of topiramate for children and adults with TS (20 individuals completed the study), premonitory urge severity (as measured by clinical global impression), as well as tic severity, improved in the topiramate arm (101). In contrast, premonitory urge severity did not improve during open-label treatment with aripiprazole (84) or ecopipam (100). Three studies examined the effect of cannabis on premonitory urges and observed some benefit (111–113), though each study had notable limitations: one study solely interviewed individuals about their experience using marijuana, another study enrolled a small sample (n = 18) of adults with TS for open-label cannabis treatment using varying doses and administration methods, and the only randomized controlled trial (cross-over design) enrolled a small sample (n = 12) of adults with TS for a single dose. Botulinum toxin injections improved premonitory urge severity in a number of case series (47, 103, 104, 106, 107) and in one randomized controlled trial (n=18) (105), though it is notable that none of these studies assessed premonitory urge with a validated, symptom-specific scale. In a combined pharmacotherapy-behavioral intervention, McGuire et al. randomized 20 youth with TS or chronic motor tic disorder to one session of HRT with either D-cycloserine 50 mg or placebo (taken one hour pre-HRT session). The D-cycloserine group exhibited significantly more improvement in I-PUTS frequency score, but not I-PUTS intensity score, than the placebo group (102).
3.4.4 Response to neural stimulationMost studies assessing response of premonitory urges to deep brain stimulation (DBS) have involved small case series and open-label studies, with the majority demonstrating some degree of improvement. Among 18 TS participants (age range: 17-47-years-old) with bilateral DBS in the centromedian–parafascicular (CM–Pfc) and ventralis oralis (Voa) nuclei of the thalamus, nine underwent blinded rating in “on” and “off” DBS conditions nine months post-implantation (117). In the “off” condition, a subset of participants (precise number not reported in the publication) experienced return of their premonitory urges. In two adults with refractory TS who underwent bilateral DBS in the CM-Pfc-ventral oral nuclei of the thalamus, PUTS scores improved after implantation, with sustained improvement two years post-implantation (118). In a case report of a 17-year-old female with TS who received bilateral globus pallidus internus (GPi) DBS, PUTS score reduced from 36 to 15 two months after surgery, with further reduction to 9 two years post-surgery (119). In contrast to the above studies, a nine-month, randomized, double-blind crossover trial comparing DBS of the centromedian-ventro-oral internus of the thalamus (CM-Voi), the posteroventral lateral globus pallidus internus (pvl GPi), and sham stimulation in nine adults with TS found no significant differences in PUTS or premonitory urge visual analogue scale scores across stimulation targets (120).
In the single pilot study to examine the impact of transcranial direct current stimulation (tDCS) on premonitory urge, Eapen et al. administered tDCS to the anterior supplementary motor area (SMA) in two adult males with TS over the course of six weeks (121). At three months post-treatment, PUTS scores had improved for both participants.
Median nerve stimulation (MNS) is a non-invasive intervention currently under study to reduce tic severity. Among 16 adolescents and adults with TS six, one-minute rounds of 10 Hz-MNS decreased live urge monitor ratings by 33% (not significant) during stimulation periods (122). In the same study, results of a stepwise multiple regression model found that higher PUTS score corresponded to greater reduction in tic intensity during MNS. The study also found that, during MNS, reduction in live urge monitor ratings was associated with reduction in tic frequency. Subsequently, Morera Maiquez et al. conducted a double blind, sham-controlled trial of home-administered MNS, obtaining complete data from 117 participants (123). Participants were randomized into one of three groups: active stimulation, sham stimulation, or treatment as usual. The treatment consisted of four weeks of a daily, 14-minute stimulation protocol. Reductions in premonitory urge severity, as assessed by the Premonitory Urges for Tic Disorders Scale-Revised (PUTS-R), did not significantly differ between groups.
Fu et al. conducted a sham-controlled trial of repetitive transcranial magnetic stimulation (rTMS) (0.5 Hz, intensity 90% of resting motor threshold) to the bilateral parietal cortices in 30 adolescents and adults with TS (124). Participants received three rounds of 400 pulses per side (10 minutes between rounds) for 10 consecutive days. Symptom rating scales were administered at baseline, at the end of treatment, one week after treatment, and one month after treatment. PUTS scores decreased significantly more in the rTMS group, with persistent improvements one-month post-treatment. A similar pattern emerged for YGTSS global scores (i.e., sum of Total Tic Score and Impairment Score), YGTSS motor tic scores, YGTSS vocal tic scores, and MRVS.
3.5 Neural correlates of premonitory urges
留言 (0)