Jaundice is a common condition in newborn infants, affecting more than 80% of them (1). High bilirubin levels can lead to acute bilirubin encephalopathy and kernicterus, which are serious neurological complications. Therefore, careful monitoring of the cranial status in patients with neonatal hyperbilirubinemia is essential to prevent and manage these potential complications (2). CUS is an easily accessible and well-accepted first-line imaging modality that is widely used to screen preterm and high-risk infants for brain injury during the neonatal period (3). Various studies have sought to identify prognostic factors associated with CUAs. Preterm, intracranial infection, hypoxia and other factors have been shown to have independent predictive value (4). One potential predictive clinical variable is RDW-CV. RDW-CV is a vital blood parameter that serves as a key indicator of physiological health and plays a crucial role in assessing overall well-being and bodily function. Recently RDW-CV has been found to have prognostic value in subarachnoid hemorrhage and other intracranial diseases (5). In these studies, it was shown that increased value of RDW was an independent prognostic factor for poor outcome in these patients. We hypothesize that the incorporation of RDW will improve prediction of overall CUAs in neonatal hyperbilirubinemia. Although CUS screening is routinely performed for neonatal hyperbilirubinemia, the relationship between RDW-CV and CUAs in neonates remains unclear. Understanding the relationship between these factors is essential for improving medical interventions and treatment of neonates. Therefore, this study aimed to explore the potential correlation between neonatal RDW-CV and CUAs, with the goal of enhancing the care and management of neonatal health.
Materials and methods Study populationThis study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement. This retrospective cross-sectional study included 508 patients with neonatal hyperbilirubinemia requiring phototherapy, who were admitted to the NICU of Nanjing Lishui People's Hospital (NJLSPH) between July 1, 2021, and December 31, 2023, with a gestational age of ≥35 weeks. Neonatal records were obtained from the hospital and retrieved from the electronic medical record system. The exclusion criteria were missing RDW-CV, alanine aminotransferase (ALT), and total bilirubin (TBIL) values. Ultimately, 503 patients were analyzed (Figure 1). This study was approved by the Research Ethics Committee of NJLSPH (Approval No 2024KY0726-01). As this study had a retrospective design and only de-identified and anonymized participant information was used, the need for written informed consent was waived by NJLSPH. This study was registered at the Chinese Clinical Trial Registry Center (Registration Number: ChiCTR 2400087550).
Figure 1. Flowchart for the study population.
Laboratory, ultrasound data collection and measuresThe data of neonatal hyperbilirubinemia patients admitted to the NJLSPH between July 1, 2021, and December 31, 2023, were collected. The database contains information on demographics, laboratory values, and ultrasound findings. Neonatal demographics included sex, weeks of gestation, birth weight, weight at admission, age, blood type, parity, number of births, and mode of delivery. Laboratory values included hsCRP, RBC, HGB, HCT, MCV, MCH, RDW-CV, WBC, NEUT, LYMPH, MONO, PLT, PCT, MPV, TBIL, ALT, GGT, ALP, ALB, GLO, and A/G levels.
All data were tested in the biochemical laboratory of NJLSPH. Clinical chemistry and biochemical laboratory parameters (TBIL, ALT, GGT, ALP, ALB, GLO, and A/G) were measured using a biochemical analyzer (Beckman Coulter Trading Co. Ltd., China; Model: AU5800). Hematological variables (hsCRP, RBC, HGB, HCT, MCV, MCH, RDW-CV, and WBC) were measured using an instrument (BC-7500; Mindray Corporation, Shenzhen, China).
In accordance with the routine scheduling for CUS screenings, which are conducted on Tuesdays and Fridays, neonates admitted to the NICU underwent one or more ultrasound examinations during their hospital stay. Notably, the median age for these CUS examinations was on the second day of hospitalization. Ultrasonographic findings were categorized into two groups: normal and abnormal. CUAs included subependymal cysts, choroidal cysts, ventricular enlargement, periventricular-intraventricular hemorrhage (PVH-IVH), cerebellar hemorrhage, and cephalohematoma. PVH-IVH and cerebellar hemorrhage were classified as intracranial hemorrhage.
Statistical analysisAll statistical analyses were conducted using R Statistical Software (Version 4.2.2, http://www.R-project.org, The R Foundation) and the Free Statistics Analysis Platform (Version 1.9, Beijing, China, http://www.clinicalscientists.cn/freestatistics). Statistical significance was defined as a P-value <0.05. Histogram distribution and Kolmogorov-Smirnov tests were used to assess the normality of the variables. Normally distributed continuous variables are reported as mean ± standard deviation, while skewed continuous variables are presented as median with interquartile range (IQR). Categorical variables are presented as frequencies (%). Statistical comparisons of continuous variables between groups were conducted using the independent samples Student's t-test or Mann-Whitney U-test, depending on the normality of the distribution. Categorical data were analyzed using the chi-square test or Fisher's exact test, as appropriate. Logistic regression analysis was used to investigate the relationship between RDW-CV and CUAs. RDW-CV was analyzed as a continuous variable and in tertiles. Confounding variables were selected based on expert judgment, previous scientific literature, or established associations with the outcomes of interest, with a minimum impact of 10% on the effect estimate. A series of models were constructed to control for potential confounders. Model I was adjusted for age and sex. Model II included additional adjustments for birth weight, gestational age, and delivery mode. In model III (primary model), further adjustments were made for TBIL, WBC, HCT, PLT, and ALT levels. Trends were tested using multivariate regression models, in which the median value of each tertile of RDW-CV was included as a continuous variable. A restricted cubic spline model was employed to generate smooth curves in order to investigate potential dose-response relationships between RDW-CV and CUAs. In this model, RDW-CV was treated as a continuous variable with four knots (5th, 35th, 65th, and 95th percentiles), as recommended by Harrell.
Results Baseline characteristics of the study populationA total of 503 patients with a median age of 108.5 h (81.0, 155.7) were included in the analysis. The overall prevalence of CUAs was 26.04%. The baseline clinical characteristics of the patients included in the study are shown in Table 1.
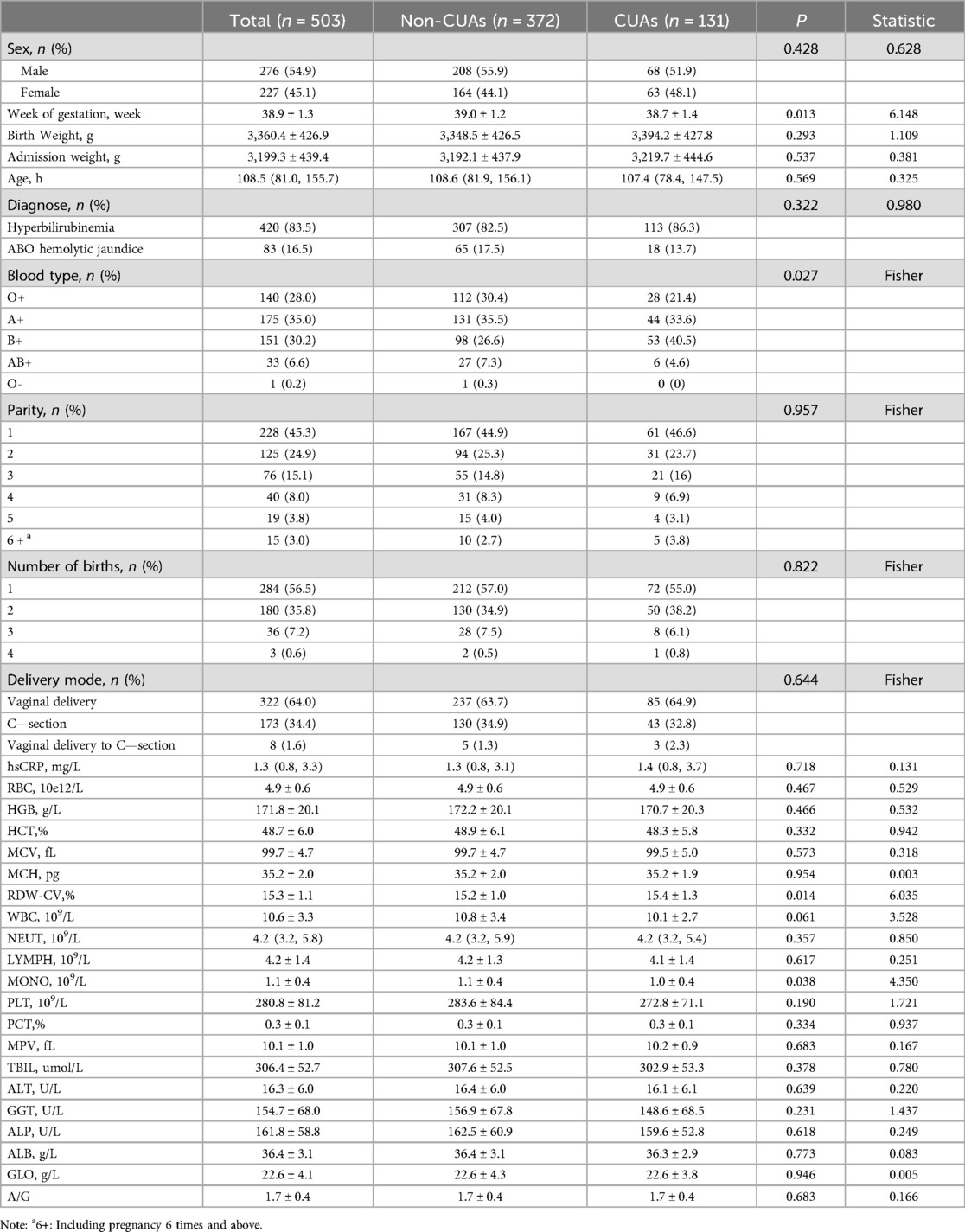
Table 1. Baseline characteristics of the study population.
Table 2 presents the results of the multivariate logistic regression analysis examining the association between RDW-CV and CUAs. A higher RDW-CV was significantly correlated with an increased risk of CUAs. In the crude model, each 1% increase in RDW-CV was associated with a 25% increase in the risk of CUAs. After adjusting for all potential confounders, the risk of CUAs was significantly associated with RDW-CV (OR, 1.23; 95%CI: 1.01–1.48).

Table 2. Logistic regression analysis of RDW-CV and the risk of CUAs in neonatal hyperbilirubinemia.
Evidence derived from the estimated dose-response curve demonstrated a significant linear relationship between RDW-CV and the risk of CUAs (Figure 2; P for nonlinearity = 0.891) based on restricted cubic spline analyses adjusted for multiple variables. With increasing RDW-CV levels, the risk of CUAs increased.
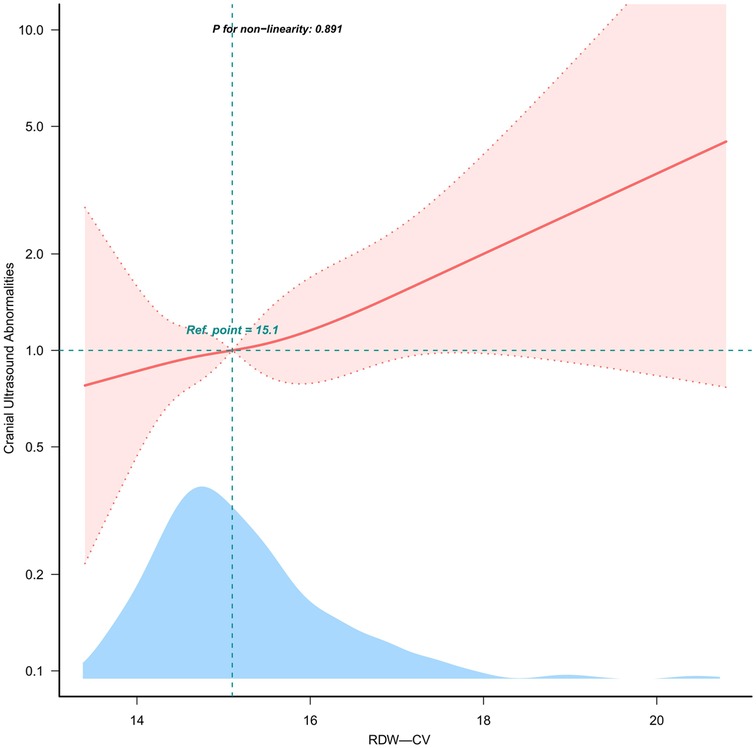
Figure 2. Linear dose-response relationship between RDW-CV and OR of CUAs. Note: Figure 2 presents restricted cubic spline plots illustrating the relationship between RDW-CV and CUAs outcomes after covariate adjustment. Background histograms (light blue) show the percentage of RDW-CV density distribution in the study population. The solid central lines represent the estimated adjusted odds ratios accompanied by shaded ribbons indicating 95% confidence intervals (CIs). The horizontal dashed lines represent the odds ratio of 1.0 (reference point). The reference point was the median RDW-CV (15.1%).
The E-value was calculated to evaluate the robustness of the findings against potentially unmeasured confounding factors. Our results were deemed statistically significant unless there was an unmeasured confounding variable, with the risk ratio for CUAs exceeding 1.452 (E-value). When RDW-CV was analyzed as a tertile variable, significant differences were observed between RDW-CV and the risk of CUAs after adjusting for all potential confounders (P for trend = 0.045). Compared with RDW-CV Q1 (13.4%≤RDW-CV<14.7%), the adjusted ORs for the risk of CUAs in Q2 (14.7%≤RDW-CV<15.5%) and Q3 (15.5%≤RDW-CV<20.8%) were 1.04 (95%CI: 0.60–1.79) and 1.66 (95%CI: 0.98–2.81), respectively.
DiscussionThis retrospective cross-sectional study, conducted in NJLSPH, included cases of neonatal hyperbilirubinemia at ≥35 weeks. This study found a significant association between RDW-CV and CUAs in neonatal hyperbilirubinemia (Table 2).
RDW is a hematologic parameter routinely measured in clinical blood tests, and it reflects the volume heterogeneity of peripheral red blood cells (RBCs) (6). Compared with observing RBCs on a blood smear with the naked eye, it can more objectively reflect the degree of the unequal size of red blood cells (7). Variations in RDW are attributed to multiple factors, including nutritional deficiency (vitamin B12), bone marrow suppression, hemolysis, and splenic sequestration (8). RDW is associated with various diseases (9, 10), such as cardiovascular disease, diabetes, and kidney disease. As a clinical biomarker, RDW plays an increasing role in predicting outcomes in hematologic malignancies, as well as lung, breast, and gastrointestinal cancer (11–13).
Limited research has been conducted on the relationship between RDW-CV and the nervous system, particularly in neonates. Chugh et al. examined the correlation between RDW-CV and functional outcomes in subarachnoid hemorrhage in a cohort of 40 patients. Their findings indicated that higher RDW-CV levels were linked to unfavorable outcomes, suggesting that RDW may serve as a valuable prognostic indicator in patients (5). Increased RDW is associated with hematoma expansion in patients with intracerebral hemorrhage (14, 15). Another study found that non-survivors of intracerebral hemorrhage had higher RDW levels during the first week than survivors (16). In this study, we investigated the correlation between RDW-CV and CUAs in patients with neonatal hyperbilirubinemia with a gestational age of ≥35 weeks. Our findings showed that the risk of CUAs tended to increase with increasing RDW-CV levels, consistent with the literature. We controlled for potential confounders in our multivariable analysis, as detailed in Sub-Table 1, to isolate RDW-CV's effect on CUAs risk. Sensitivity analysis confirmed the robustness of RDW-CV's association with CUAs, independent of common confounders. Other noteworthy findings emerged from this study. First, subventricular cysts accounted for the largest number of CUAs. Second, the incidence of left-hemisphere abnormalities was significantly higher than that of the right and bilateral hemispheres (Figure 3). The exact reason for this is not clear, and further investigation is warranted to validate our findings and elucidate the underlying mechanisms.
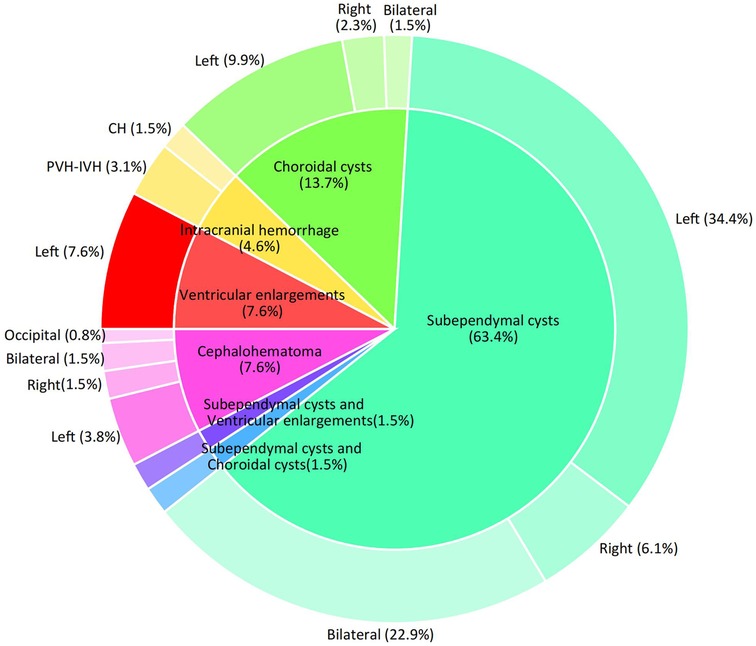
Figure 3. Disease distribution of CUS screening abnormalities. Note: Two cases of intraventricular hemorrhage were associated with subventricular cysts and one with a cephalohematoma.
However, Kelly et al. (17) reported that although RDW is a prognostic factor for survival in many inflammatory, prothrombotic, and neoplastic diseases, preoperative RDW was not associated with overall survival in patients with glioblastoma (GBM). These differences in findings may be related to differences in population heterogeneity, sample size, and diversity of unknown factors that could contribute to body development. The above studies suggest that although ultrasound screening is abnormal, further follow-up is needed to determine any complications.
Regarding potential mechanisms and clinical implications, emerging studies have suggested a link between RDW and inflammation. RDW has been shown to increase in inflammatory states (18, 19). Some studies have shown that specific inflammatory cytokines, including IL-6, ESR, and CRP, are positively correlated with RDW. A possible underlying mechanism is that pro-inflammatory cytokines suppress maturing RBCs, allowing newer and larger reticulocytes to enter the bloodstream, leading to increased RDW (20, 21).
Inflammation plays a vital role in neurological development. Many studies show the presence of an association between biomarkers of oxidative stress and brain damage in newborns (22, 23). Klein et al. (24) reported that systemic inflammation causes chronic activation of microglia and maldevelopment of the cerebellum in mice. Yanni et al. (25) noted that placental inflammation followed by postnatal systemic inflammation predisposes individuals to indicators of white matter damage on CUS, cerebral palsy, low developmental indices, and microcephaly. Therefore, inflammation may be a significant factor connecting RDW and traumatic brain injury. Further research is required to clarify the potential association between RDW and CUAs.
This study has several strengths. It investigated the correlation between RDW-CV and CUAs, an area that has not received substantial attention from clinicians. This analysis has the potential to enhance clinicians’ evaluation of neurological abnormalities in children with neonatal hyperbilirubinemia. Nonetheless, the present study has some limitations that warrant consideration. First, it is important to acknowledge that an observational study differs from a randomized controlled trial (RCT); thus, the findings obtained may diverge from the anticipated results of an RCT owing to inherent limitations in the study design. Therefore, our findings should be interpreted in the context of this distinction. Despite these limitations, the data obtained in our study offer valuable insights into the association between RDW-CV and CUAs, contributing additional evidence to this field of research.
ConclusionThis study revealed a proportional relationship between a higher RDW-CV and an increased risk of CUAs in neonatal hyperbilirubinemia. These results demonstrate the importance of RDW-CV in neonatal hyperbilirubinemia. This study showed that RDW-CV may serve as a risk factor and predictor of CUAs. Further research is required to confirm this hypothesis.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributionsHW: Writing – original draft, Writing – review & editing. XC: Data curation, Formal Analysis, Writing – original draft. JW: Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article from Jiangsu University Medical Education Collaborative Innovation Fund Program (JDYY2023102).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1488731/full#supplementary-material
AbbreviationsCUS, cranial ultrasonography; CUAs, cranial ultrasound abnormalities; NJLSPH, Nanjing Lishui People's Hospital; ChiCTR, Chinese Clinical Trial Registry Center; PVH-IVH, periventricular-intraventricular hemorrhage; CH, cerebellar hemorrhage; hsCRP, high-sensitivity C-reactive protein; RBC, red blood cell; HGB, hemoglobin; HCT, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; RDW-CV, red blood cell distribution width-coefficient of variation; WBC, white blood cell; NEUT, neutrophil; LYMPH, lymphocyte; MONO, monocyte; PLT, platelet; PCT, plateletcrit; MPV, mean platelet volume; TBIL, total bilirubin; ALT, alanine aminotransferase; GGT, gamma-glutamyl transferase; ALP, alkaline posphatase; ALB, albumin; GLO, globulin; A/G, albumin/globulin ratio; GBM, glioblastoma.
References1. Lieberman L, Callum J, Cohen R, Cserti-Gazdewich C, Ladhani N, Buckstein J, et al. Impact of red blood cell alloimmunization on fetal and neonatal outcomes: a single center cohort study. Transfusion. (2020) 60:2537–46. doi: 10.1111/trf.16061
PubMed Abstract | Crossref Full Text | Google Scholar
2. Kemper AR, Newman TB, Slaughter JL, Maisels MJ, Watchko JF, Downs SM, et al. Clinical practice guideline revision: management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. (2022) 150:E2022058859. doi: 10.1542/peds.2022-058859
PubMed Abstract | Crossref Full Text | Google Scholar
3. Mclean G, Malhotra A, Lombardo P, Schneider M. Cranial ultrasound screening protocols for very preterm infants. Ultrasound Med Biol. (2021) 47:1645–56. doi: 10.1016/j.ultrasmedbio.2021.03.006
PubMed Abstract | Crossref Full Text | Google Scholar
5. Chugh C, Nyirjesy SC, Nawalinski KP, Sandsmark DK, Frangos S, Maloney-Wilensky E, et al. Red blood cell distribution width is associated with poor clinical outcome after subarachnoid hemorrhage: a pilot study. Neurocrit Care. (2015) 23:217–24. doi: 10.1007/s12028-015-0117-x
PubMed Abstract | Crossref Full Text | Google Scholar
6. Wu K, Chen L, Huang H, Wu Z, Chen Q, Zhong W. Study on the application value of red blood cell distribution width and platelet distribution width in neonatal exchange transfusion with hyperbilirubinemia. J Matern Fetal Neonatal Med. (2022) 35:9811–5. doi: 10.1080/14767058.2022.2054321
PubMed Abstract | Crossref Full Text | Google Scholar
7. Lin H, Luo P, Liu C, Lin X, Que C, Zhong W. The application value of mean red blood cell volume and red blood cell volume distribution width combined with total Serum bilirubin in the early screening of neonatal hemolytic disease. BMC Pediatr. (2023) 23:19. doi: 10.1186/s12887-022-03812-2
PubMed Abstract | Crossref Full Text | Google Scholar
9. Zorlu A, Bektasoglu G, Guven FM, Dogan OT, Gucuk E, Ege MR, et al. Usefulness of admission red cell distribution width as A predictor of early mortality in patients with acute pulmonary embolism. Am J Cardiol. (2012) 109:128–34. doi: 10.1016/j.amjcard.2011.08.015
PubMed Abstract | Crossref Full Text | Google Scholar
10. Gang L, Lifang W. Association of the elevated red blood cell distribution width with the risk of developing diabetes mellitus. Intern Med. (2016) 55:1959–65. doi: 10.2169/internalmedicine.55.5956
PubMed Abstract | Crossref Full Text | Google Scholar
12. Albayrak S, Zengin K, Tanik S, Bakirtas H, Imamoglu A, Gurdal M. Red cell distribution width as A predictor of prostate cancer progression. Asian Pac J Cancer Prev. (2014) 15:7781–4. doi: 10.7314/APJCP.2014.15.18.7781
PubMed Abstract | Crossref Full Text | Google Scholar
13. Ay S, Eryilmaz MA, Aksoy N, Okus A, Unlu Y, Sevinc B. Is early detection of colon cancer possible with red blood cell distribution width. Asian Pac J Cancer Prev. (2015) 16:753–6. doi: 10.7314/APJCP.2015.16.2.753
PubMed Abstract | Crossref Full Text | Google Scholar
14. Altintas O, Duruyen H, Baran G, Baran O, Katar S, Antar V, et al. The relationship of hematoma growth to red blood cell distribution width in patients with hypertensive intracerebral hemorrhage. Turk Neurosurg. (2016) 27:368–73. doi: 10.5137/1019-5149.Jtn.16136-15.1
PubMed Abstract | Crossref Full Text | Google Scholar
15. Elkhatib T, Shehta N, Bessar AA. Hematoma expansion predictors: laboratory and radiological risk factors in patients with acute intracerebral hemorrhage: a prospective observational study. J Stroke Cerebrovasc Dis. (2019) 28:2177–86. doi: 10.1016/j.jstrokecerebrovasdis.2019.04.038
PubMed Abstract | Crossref Full Text | Google Scholar
16. Lorente L, Martín MM, González-Rivero AF, Pérez-Cejas A, Sabatel R, Ramos L, et al. Red blood cell distribution width and mortality of spontaneous intracerebral hemorrhage patients. Clin Neurol Neurosurg. (2020) 195:106066. doi: 10.1016/j.clineuro.2020.106066
PubMed Abstract | Crossref Full Text | Google Scholar
17. Kelly PD, Dambrino RJ, Guidry BS, Tang AR, Stewart TG, Mistry A, et al. Red blood cell distribution width in glioblastoma. Clin Neurol Neurosurg. (2022) 213:107096. doi: 10.1016/j.clineuro.2021.107096
PubMed Abstract | Crossref Full Text | Google Scholar
18. Förhécz Z, Gombos T, Borgulya G, Pozsonyi Z, Prohászka Z, Jánoskuti L. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am Heart J. (2009) 158:659–66. doi: 10.1016/j.ahj.2009.07.024
PubMed Abstract | Crossref Full Text | Google Scholar
19. Bazick HS, Chang D, Mahadevappa K, Gibbons FK, Christopher KB. Red cell distribution width and all-cause mortality in critically ill patients. Crit Care Med. (2011) 39:1913–21. doi: 10.1097/CCM.0b013e31821b85c6
PubMed Abstract | Crossref Full Text | Google Scholar
20. Pierce CN, Larson DF. Inflammatory cytokine inhibition of erythropoiesis in patients implanted with A mechanical circulatory assist device. Perfusion. (2005) 20:83–90. doi: 10.1191/0267659105pf793oa
PubMed Abstract | Crossref Full Text | Google Scholar
22. Perrone S, Grassi F, Caporilli C, Boscarino G, Carbone G, Petrolini C, et al. Brain damage in preterm and full-term neonates: serum biomarkers for the early diagnosis and intervention. Antioxidants (Basel). (2023) 12:309. doi: 10.3390/antiox12020309
PubMed Abstract | Crossref Full Text | Google Scholar
23. Wang B, Wang F, Wu D, Xu X, Yang L, Zhu J, et al. Relationship between tnf-Α and the risk of cerebral palsy: a systematic review and meta-analysis. Front Neurol. (2022) 13:929280. doi: 10.3389/fneur.2022.929280
PubMed Abstract | Crossref Full Text | Google Scholar
24. Klein L, Van Steenwinckel J, Fleiss B, Scheuer T, Bührer C, Faivre V, et al. A unique cerebellar pattern of microglia activation in a mouse model of encephalopathy of prematurity. Glia. (2022) 70:1699–719. doi: 10.1002/glia.24190
PubMed Abstract | Crossref Full Text | Google Scholar
25. Yanni D, Korzeniewski SJ, Allred EN, Fichorova RN, O’shea TM, Kuban K, et al. Both antenatal and postnatal inflammation contribute information about the risk of brain damage in extremely preterm newborns. Pediatr Res. (2017) 82:691–6. doi: 10.1038/pr.2017.128
留言 (0)