Isocitrate dehydrogenase (IDH)-mutant astrocytoma central nervous system (CNS) World Health Organization (WHO) grade 3 is a subtype of adult type diffuse gliomas and is, in principle, an incurable disease. However, median estimated survival for affected individuals is about eight years with considerable inter-individual differences (1, 2). Treatment therefore needs to be delicately balanced, focusing on tumor control as well as an optimal quality of life. Standard therapy for patients with IDH-mutated diffuse astrocytoma CNS WHO grade 3 consists of maximally safe surgical resection followed by radiotherapy to a total dose of 59.4 Gray (Gy) and 12 adjuvant courses of temozolomide (3–5). All these anti-neoplastic therapies are prone to side effects, including radiotherapy, which is known to carry a risk of detrimental late effects (6–11). Proton therapy is an increasingly used radiotherapeutic modality with physical properties that facilitate improved preservation of healthy tissue compared to photon therapy (12–16). This is an appealing quality for patients with relatively favorable lifetime expectancies, such as diffuse gliomas CNS WHO grade 2 and 3. However, their diffuse infiltrative nature poses a potential hazard. The ongoing PRO-GLIO study investigates whether proton therapy is safe and beneficial for IDH-mutated diffuse gliomas grade 2 and 3 (17), thereby seeking to establish whether proton therapy should be implemented as standard of care for this patient group (18, 19).
With new treatment modalities, new clinical conundrums appear. Pseudoprogression is a phenomenon most often seen in high-grade gliomas following photon radiotherapy (20–25). It may, however, also appear after radiotherapy for diffuse grade 2 and 3 gliomas (22, 26–28). Pseudoprogression imaging characteristics, timing related to radiotherapy, susceptible locations, and incidence following proton radiotherapy might be different than after photon therapy, partly related to protons’ slightly higher relative biological effectiveness (RBE) (29–32). The most critical clinical task is distinguishing pseudoprogression from neoplastic progression, an endeavor that is often challenging and lacks universally accepted guidelines or criteria.
In the PRO-GLIO trial, a previously healthy young man diagnosed with an IDH-mutant astrocytoma CNS WHO grade 3 was randomized to protons given to the total dose of 59.4 Gy. Three months after the completion of radiotherapy, magnetic resonance imaging (MRI) showed what was highly suspicious of a distant recurrence. The patient was in excellent health with no new symptoms, and the new lesion was treated surgically as the appearance and location did not suggest pseudoprogression. This case highlights the need to include pseudoprogression as a differential diagnosis whenever new postradiation lesions appear.
2 Case presentation and diagnostics assessmentA young man in his twenties presented with epileptic seizures. He was otherwise healthy and had no prior medication. An electroencephalogram (EEG) conducted after a hyperventilation episode detected focal pathological activity in the left frontotemporal region, and a sleep-deprived EEG gave rise to suspicion of a structural abnormality in the same region. A nonenhancing lesion measuring 5 x 4 x 5 centimeters (cm), suspicious for a diffuse low-grade glioma, was identified in the left temporal lobe by MRI (Figures 1A, B). A few weeks later, the patient underwent surgery with awake craniotomy and the use of intraoperative MRI. Only a small residual neoplastic lesion in the left insular region identified on postoperative MRI remained. The patient’s preoperative Neurological Assessment in Neuro-Oncology (NANO; 33) score was 0, whereas a mild and transient postoperative expressive aphasia led to a NANO score of 0-1. He was in good general condition with Karnofsky Performance Status (KPS) score of 100 before and after surgery. Examination of the tissue specimen revealed an IDH-mutant astrocytoma CNS WHO grade 3 with an O-6 methylguanine-DNA methyltransferase (MGMT) promotor methylation level of 5.5%.
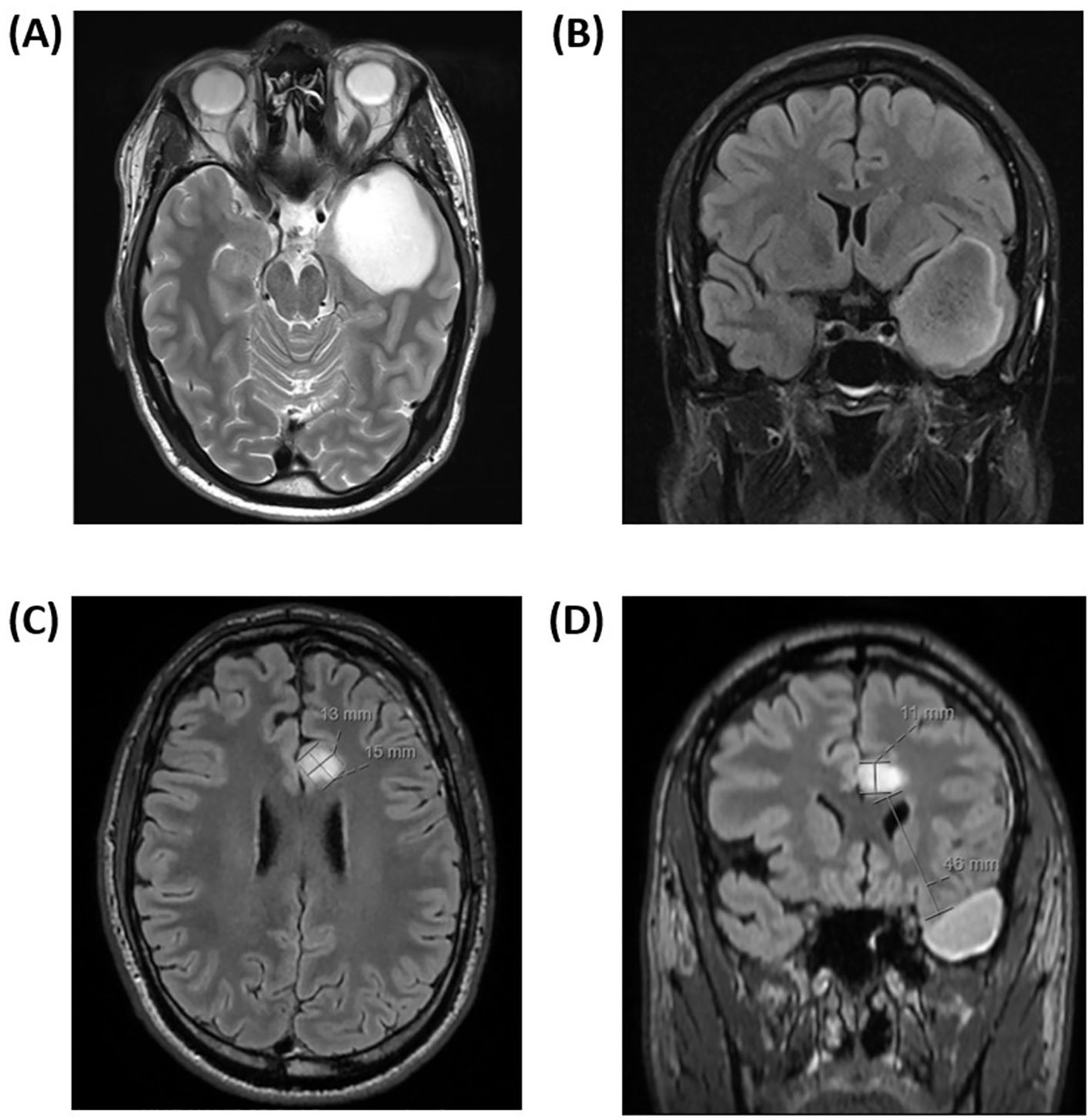
Figure 1. (A, B) Axial magnetic resonance contrast-enhanced T2/FLAIR image sequence prior to the first tumor resection showed a nonenhancing cystic lesion in the left temporal lobe, suspicious for diffuse low-grade glioma. The tumor measured 5 x 4 x 5 centimeters in its largest dimension. (C, D) Axial magnetic resonance contrast-enhanced T2/FLAIR image sequence three months after completion of proton beam therapy. A new 15 x 13 x 11 millimeters nonenhancing lesion, highly suspicious of distant neoplastic recurrence in the left subcortical, parasagittal left limbic area was found. The appearance of the lesion was relatively well-defined and expansive, and it was located 46 millimeters from the resection cavity.
Following resection, the patient was included in the PRO-GLIO trial and randomized to receive proton therapy, which commenced about two months following resection. Fractionation was 1.8 Gray (Gy) RBE x 33 to a total dose of 59.4 Gy RBE. Radiotherapy was delivered with two plans. The first plan was used for 23 of 33 fractions and a second plan contributed with the last 10 fractions. Replanning was done due to swelling of the skin in the patient’s left temporal region. In sum, radiation doses were within OAR tolerance doses according to the European Particle Therapy Network (EPTN) consensus (34) and standard clinical practice at the proton institution. Both plans used a 3-field technique with identical field angles; one field with a 320 gantry degree and a 90 couch degree, a second with a 70 gantry degree and a couch degree of 10, and a third with a gantry degree of 100 and a couch degree of 0. Monitor units (MU) per fraction for the first plan was 207.9, 256.2, and 249.8 for the three fields, and for the second plan 272.9, 293.4, and 289.0, respectively. Radiotherapy was well tolerated, with only mild fatigue at the end of treatment.
One month after completion of radiotherapy, standard adjuvant chemotherapy with temozolomide was initiated and well tolerated. The first postradiation MRI undertaken three months after completion of radiotherapy showed stable disease in the primary tumor area; however, surprisingly, with a new nonenhancing lesion in the left anterior cingulate gyrus measuring 15 x 13 x 11 millimeters (mm; Figures 1C, D). The lesion appeared well-defined and expansive, with a high T2/FLAIR (Fluid-Attenuated Inversion Recovery) signal. Most of the lesion exhibited high diffusion (1.5 x 10-3 mm2/s) with a peripheral rim of low diffusion (0.9 x 10-3 mm2/s). The T2/FLAIR mismatch present in the primary lesion was not observed in this case. Apart from this, the new lesion appeared highly suspicious for a distant recurrence of the IDH-mutant astrocytoma. Figure 2 illustrates the radiotherapy beam angles in relation to the original GTV and CTV, as well as the location of the new lesion which was radiologically deemed suspicious of a recurrence. The patient was in excellent general condition with KPS score 100 without new symptoms. At this time point, he had received only two courses of temozolomide, using ondansetron as an anti-emetic only on the five treatment days. Apart from this, the only medication he used was an anti-epileptic (levetiracetam 1000 milligrams two times daily). A neuropsychological assessment conducted as part of the PRO-GLIO trial did not uncover any new cognitive deficits.
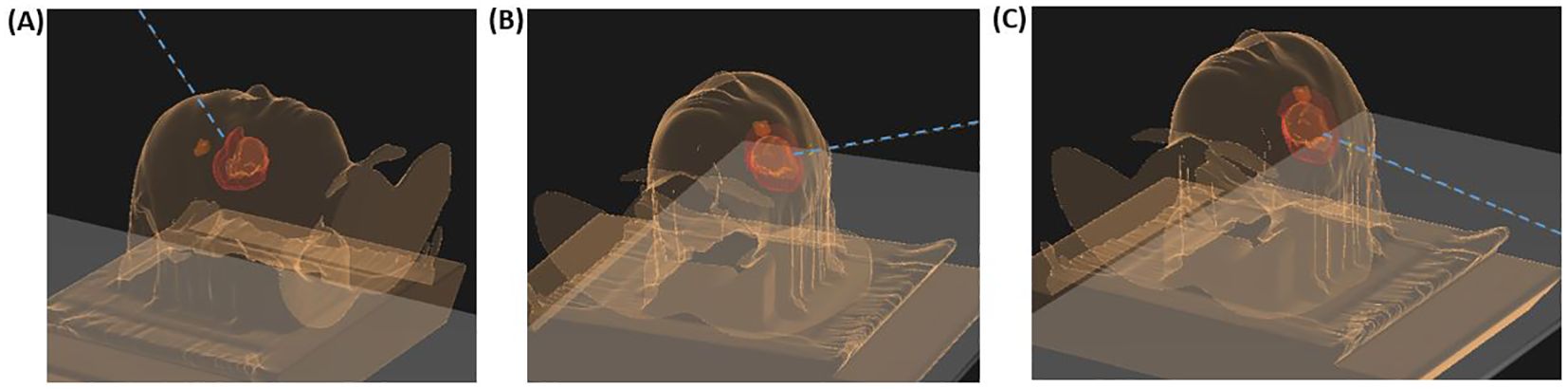
Figure 2. Beam angles for the patient’s proton plan in relation to the original gross target volume (GTV, orange), clinical target volume (CTV, red), and the location of the new lesion suspicious of a recurrence (brown). (A) displays the field with 320 gantry degree and a 90 couch degree, (B) the second field with a 70 gantry degree and a couch degree of 10, and (C) the third beam with a gantry degree of 100 and a couch degree of 0.
After a multidisciplinary discussion, it was decided to offer the patient a resection followed by new radiotherapy for the presumed distant neoplastic progression. A preoperative MRI was performed the day before the second resection and the lesion had increased in size compared to the MRI four weeks earlier; now measuring 21 x 14 x 8 mm and increasing the suspicion of tumor progression. The patient accepted, and a gross total resection (GTR) was achieved with a pre-and postoperative NANO score of 0. The second surgery was performed 7 months after his first resection. Surprisingly, histopathological and molecular biological examination of the tissue specimen did not reveal active neoplastic tissue. Macrophages and perivascular immune cell accumulations were seen - fitting well with inflammation, albeit not typical for postradiation changes. Retrospectively, D98% (radiotherapy dose received by 98%) of the new lesion was estimated to be 15 Gy RBE (Figure 3); however, parts of the new lesion had received up to 50 Gy RBE. A new multidisciplinary discussion decided against offering additional radiotherapy, opting instead to continue adjuvant temozolomide.
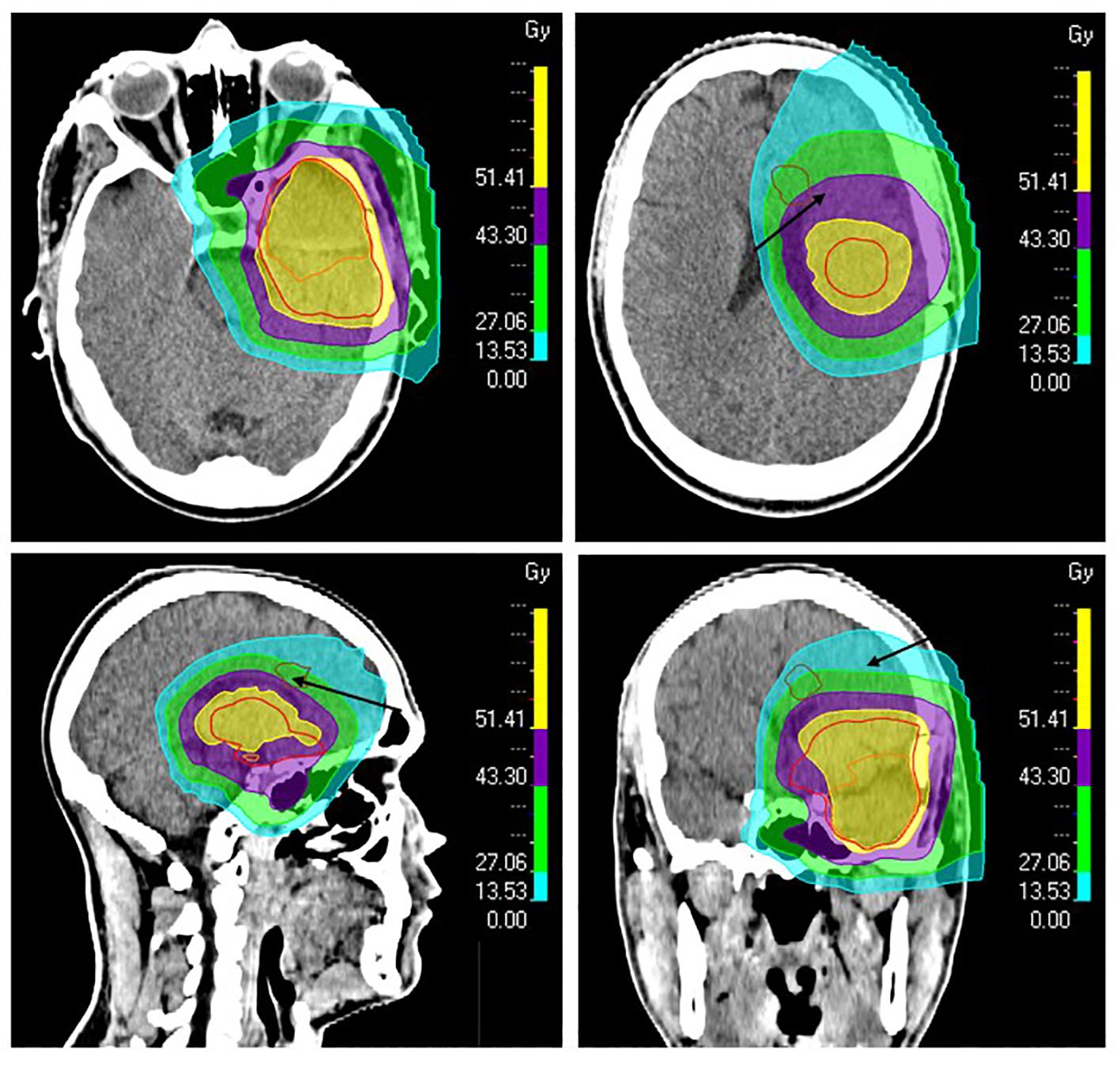
Figure 3. The patient’s proton plan showing isodose levels and radiotherapy target volumes. The resection cavity after primary surgery and the suspected residual tumor were delineated as gross target volume (GTV, orange). A margin of 15 mm was added to GTV to define the clinical target volume (CTV, red), which was also modified against natural anatomical barriers. The new lesion found by MRI three months after proton therapy was retrospectively delineated (brown, arrows) to calculate dose levels in the region of the lesion. The 51.4 Gray (Gy) (95%) isodose line is shown in yellow, 43.3 Gy (80%) in purple, 27.1 Gy (50%) in green, and 13.5 Gy (25%) in turquoise.
MRI two months after the second surgery showed no signs of neoplastic activity. The patient´s timeline is summarized in Figure 4. Fortunately, the patient has no sequelae following surgery. The last MRI taken one year following the second surgery shows no new lesions, and the patient is in excellent general condition.
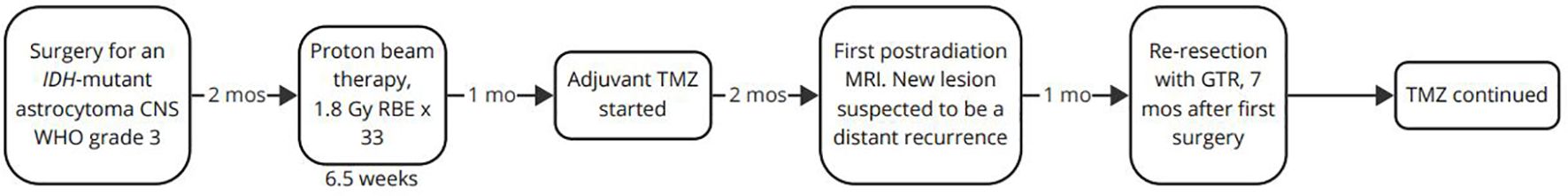
Figure 4. The patient’s timeline. CNS, central nervous system; GTR, gross total resection, Gy, Gray; IDH, isocitrate dehydrogenase; mo, month; mos, months; MRI, magnetic resonance imaging; RBE, relative biological effectiveness; TMZ, temozolomide; WHO, World Health Organization.
3 DiscussionPseudoprogression is a relatively common phenomenon following radiotherapy for brain neoplasms and is often hard to distinguish from actual neoplastic progression.
Three months following completion of proton therapy, our patient underwent resection for a new lesion that was highly suspicious for a distant neoplastic recurrence of an IDH-mutant astrocytoma CNS WHO grade 3. Surprisingly, examination of the tissue specimen showed no evidence of neoplasia but changes compatible with inflammation. Although not characteristic for postradiation changes, proton therapy is suspected to be the etiological basis for the lesion. Adjuvant chemotherapy with temozolomide was continued following surgery, as initially planned.
Usually, pseudoprogression for patients with lower-grade diffuse gliomas is defined as new or increased contrast-enhancement (27). Response Assessment in Neuro-Oncology (RANO) criteria propose that neoplastic progression is most likely when the majority of a new contrast-enhancing lesion is outside the radiation field (beyond the high-dose region/80% isodose line) (24, 35). However, it has also been argued that pseudoprogression can manifest as new or increased T2/FLAIR-signal hyperintensity (28, 36). As IDH-mutated lower grade gliomas most often are nonenhancing, increased T2/FLAIR-signal hyperintensity may mimic the primary disease more than new contrast-enhancing lesions. Pseudoprogression is often seen in the first 12 weeks following radiotherapy in glioblastomas which are per definition IDH wild-type, whereas onset may be later for IDH-mutant lower-grade gliomas, although timing is not uniform (28, 35).
Pseudoprogression is well-known following photon therapy, but is also known to occur following the less available proton therapy. Radiotherapy with protons is increasingly used for treatment of IDH-mutated diffuse gliomas grade 2 and 3, and understanding the appearances of pseudoprogression is therefore highly relevant. Ritterbusch et al. suggested criteria for characterizing pseudoprogression after proton beam therapy: location in the distal end of the proton beam, small lesions (<1 cm), often multifocal, and resolving without anti-neoplastic therapy (29). In a systematic review and meta-analysis by Lu et al., the incidence of pseudoprogression was 30% for adults with low-grade diffuse gliomas following proton beam therapy, compared to 18% of patients who received photon-based intensity-modulated radiotherapy (IMRT) (30). Bronk et al. failed to identify any difference between the two modalities (37).
In the study by Ritterbusch et al., mean time to development of pseudoprogression was 15 months, ranging from 7.0-27 months, and often appearing later than what is normally seen following photon therapy (29). The latter is in contradiction to the findings by Bronk et al. who found that pseudoprogression after proton beam therapy appeared earlier than with photon therapy for patients with oligodendrogliomas grade 2 and 3; the same was not observed in patients with diffuse astrocytomas (37). Others have found that pseudoprogression appeared at median 7.6 (proton) and 12 (photon) months following radiotherapy (27, 36).
Ritterbusch et al. found no association between pseudoprogression and sex, age, IDH-mutation, grade, MGMT promotor methylation, 1p/19q codeletion, or chemotherapy received (29). Somewhat contradictory, Dworkin et al. found an increased risk for pseudoprogression in patients with diffuse low-grade gliomas when temozolomide was given adjuvant following proton beam therapy (36). In a study by Harrabi et al., radiation-induced brain injuries for diffuse low-grade gliomas following proton beam therapy were almost exclusively seen in the distal part of the spread-out Bragg-peak. However, in this study, only contrast-enhanced lesions were considered (31). Besides at the distal part of the proton beam and within the high-dose region, new contrast-enhancing lesions are also often located in close proximity to the ventricular system (32).
In our patient, the new lesion was unifocal, measuring over 1 cm (15 x 13 x 11 mm), located 46 mm from the edge of the primary resection cavity (Figure 1), and most of it was located outside the high-dose region (Figure 3). The lesion was located close to the ventricular system, which is a predilection site for postirradiary changes, however, and more atypical - the lesion was nonenhancing with a high T2/FLAIR signal, and it appeared as early as three months after radiotherapy completion when the patient had only received two courses of temozolomide. The only medications used in addition to temozolomide were ondansetron and levetiracetam, none of which is thought to increase the risk of pseudoprogression. Most parameters suggested that the lesion was highly suspicious for a recurrence, which nonetheless turned out to be wrong.
Patients with IDH-mutant gliomas grade 2 and 3 are often young and have long expected survival. All therapeutic measures must be delicately balanced to avoid unnecessary side effects. Gaining better knowledge on pseudoprogression following proton beam therapy is essential to avoid superfluous and possibly harmful therapeutic measures. In the presented case, neoplastic progression was considered the most likely explanation for the new lesion, based mainly on timing, distance to the radiotherapy target volume and MRI appearance Therefore, resection was considered the most prudent approach. However, it could have been delayed or even avoided if an accurate radiological diagnosis could have been established. The case illustrates that pseudoprogression should always be considered a differential diagnosis when new lesions appear following radiotherapy of patients with IDH-mutant gliomas grade 2 and 3. More knowledge about radiotherapy-induced MRI changes in these patients is needed, and we hope that the PRO-GLIO trial will contribute to close this knowledge gap.
Data availability statementThe data analyzed in this study is subject to the following licenses/restrictions: Due to strict private policy data will not be made publicly available. Data is available on study center upon request. Requests to access these datasets should be directed to bGljYWhlQG91cy1oZi5ubw==.
Ethics statementPRO-GLIO has been approved by independent ethical committees in Norway (Regional Committee for Medical & Health Research Ethics, South East Norway, Section C: reference number: 265626) and Sweden (The Swedish Ethical Review Authority, Västra Götaland: reference number: Dnr 2021-04239 and Dnr 2022-01305-02) before trial start. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributionsLH: Investigation, Writing – original draft, Writing – review & editing. IB: Investigation, Writing – review & editing. HB: Writing – review & editing. CS: Investigation, Writing – review & editing. PR: Investigation, Writing – review & editing. PN: Investigation, Writing – review & editing. KW: Writing – review & editing. MB: Writing – review & editing. PB: Investigation, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This patient is included in the PRO-GLIO trial, which has received financial support from the South-Eastern Norway Regional Health Authority (Project number: 2021081), the Norwegian Cancer Society (Project number: 216158), Network in Radiation Oncology (NIRO), the Swedish Society of Medicine (SLS-890541), the Gothenburg Society of Medicine (GLS-887961), Jubileumsklinikens Cancerfond and Lions Cancer Research Fund of Western Sweden.
AcknowledgmentsWe are grateful to the patient for allowing us to publish this case report, as it brings valuable knowledge regarding distant pseudoprogression following proton beam therapy for an IDH-mutant astrocytoma CNS WHO grade 3. We would also like to acknowledge study nurse, Bente Amundsen, for following the patient in an exemplary manner.
Conflict of interestAuthor MB was employed by the company The Skandion Clinic.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Franceschi E, Tosoni A, Bartolini S, Minichillo S, Mura A, Asioli S, et al. Histopathological grading affects survival in patients with IDH-mutant grade II and grade III diffuse gliomas. Eur J Cancer. (2020) 137:10–7. doi: 10.1016/j.ejca.2020.06.018
PubMed Abstract | Crossref Full Text | Google Scholar
2. Olar A, Wani KM, Alfaro-Munoz KD, Heathcock LE, van Thuijl HF, Gilbert MR, et al. IDH mutation status and role of WHO grade and mitotic index in overall survival in grade II-III diffuse gliomas. Acta Neuropathologica. (2015) 129:585–96. doi: 10.1007/s00401-015-1398-z
PubMed Abstract | Crossref Full Text | Google Scholar
3. Weller M, van den Bent M, Preusser M, Le Rhun E, Tonn JC, Minniti G, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. (2021) 18:170–86. doi: 10.1038/s41571-020-00447-z
PubMed Abstract | Crossref Full Text | Google Scholar
4. van den Bent MJ, Tesileanu CMS, Wick W, Sanson M, Brandes AA, Clement PM, et al. Adjuvant and concurrent temozolomide for 1p/19q non-co-deleted anaplastic glioma (CATNON; EORTC study 26053-22054): second interim analysis of a randomised, open-label, phase 3 study. Lancet Oncol. (2021) 22:813–23. doi: 10.1016/S1470-2045(21)00090-5
PubMed Abstract | Crossref Full Text | Google Scholar
5. van den Bent MJ, Baumert B, Erridge SC, Vogelbaum MA, Nowak AK, Sanson M, et al. Interim results from the CATNON trial (EORTC study 26053-22054) of treatment with concurrent and adjuvant temozolomide for 1p/19q non-co-deleted anaplastic glioma: a phase 3, randomised, open-label intergroup study. Lancet. (2017) 390:1645–53. doi: 10.1016/S0140-6736(17)31442-3
PubMed Abstract | Crossref Full Text | Google Scholar
6. Scoccianti S, Detti B, Cipressi S, Iannalfi A, Franzese C, Biti G. Changes in neurocognitive functioning and quality of life in adult patients with brain tumors treated with radiotherapy. J Neuro-Oncology. (2012) 108:291–308. doi: 10.1007/s11060-012-0821-8
PubMed Abstract | Crossref Full Text | Google Scholar
7. Pearson CM, Ecklund-Johnson E, Gale SD. Neurosurgical Neuropsychology. The Practical Application of neuropsychology in the Neurosurgical Practice. Academic Press (2019).
8. Kiebert GM, Curran D, Aaronson NK, Bolla M, Menten J, Rutten EHJM, et al. Quality of life after radiation therapy of cerebral low-grade gliomas of the adult: results of a randomised Phase III trial on dose response (EORTC trial 22844). Eur J Cancer. (1998) 34:1902–9. doi: 10.1016/S0959-8049(98)00268-8
PubMed Abstract | Crossref Full Text | Google Scholar
9. van Coevorden-van Loon EMP, Coomans MB, Heijenbrok-Kal MH, Ribbers GM, van den Bent MJ. Fatigue in patients with low grade glioma: systematic evaluation of assessment and prevalence. J Neuro-Oncology. (2017) 133:237–46. doi: 10.1007/s11060-017-2454-4
PubMed Abstract | Crossref Full Text | Google Scholar
10. Di Perri D, Jmil S, Lawson TM, Van Calster L, Whenham N, Renard L. Health-related quality of life and cognitive failures in patients with lower-grade gliomas treated with radiotherapy. Cancer Radiothérapie. (2023) 27:219–24. doi: 10.1016/j.canrad.2022.10.004
PubMed Abstract | Crossref Full Text | Google Scholar
11. Frances SM, Velikova G, Klein M, Short SC, Murray L, Wright JM, et al. Long-term impact of adult WHO grade II or III gliomas on health-related quality of life: A systematic review. Neuro-Oncology Pract. (2021) 9:3–17. doi: 10.1093/nop/npab062
PubMed Abstract | Crossref Full Text | Google Scholar
12. van der Weide HL, Kramer MCA, Scandurra D, Eekers DBP, Klaver YLB, Wiggenraad RGJ, et al. Proton therapy for selected low grade glioma patients in the Netherlands. Radiotherapy Oncol. (2021) 154:283–90. doi: 10.1016/j.radonc.2020.11.004
PubMed Abstract | Crossref Full Text | Google Scholar
14. Jhaveri J, Cheng E, Tian S, Buchwald Z, Chowdhary M, Liu Y, et al. Proton vs. Photon radiation therapy for primary gliomas: an analysis of the national cancer data base. Front Oncol. (2018) 8. doi: 10.3389/fonc.2018.00440
PubMed Abstract | Crossref Full Text | Google Scholar
15. Shih HA, Sherman JC, Nachtigall LB, Colvin MK, Fullerton BC, Daartz J, et al. Proton therapy for low-grade gliomas: Results from a prospective trial. Cancer. (2015) 121:1712–9. doi: 10.1002/cncr.v121.10
PubMed Abstract | Crossref Full Text | Google Scholar
16. Tabrizi S, Yeap BY, Sherman JC, Nachtigall LB, Colvin MK, Dworkin M, et al. Long-term outcomes and late adverse effects of a prospective study on proton radiotherapy for patients with low-grade glioma. Radiotherapy Oncol. (2019) 137:95–101. doi: 10.1016/j.radonc.2019.04.027
PubMed Abstract | Crossref Full Text | Google Scholar
17. Heggebø LC, Borgen IMH, Rylander H, Kiserud C, NorDenmark TH, Hellebust TP, et al. Investigating survival, quality of life and cognition in PROton versus photon therapy for IDH-mutated diffuse grade 2 and 3 GLIOmas (PRO-GLIO): a randomised controlled trial in Norway and Sweden. BMJ Open. (2023) 13:e070071. doi: 10.1136/bmjopen-2022-070071
PubMed Abstract | Crossref Full Text | Google Scholar
18. Chambrelant I, Eber J, Antoni D, Burckel H, Noël G, Auvergne R. Proton therapy and gliomas: A systematic review. Radiation. (2021) 1:218–33. doi: 10.3390/radiation1030019
Crossref Full Text | Google Scholar
19. Thurin E, Nyström PW, Smits A, Werlenius K, Bäck A, Liljegren A, et al. Proton therapy for low-grade gliomas in adults: A systematic review. Clin Neurol Neurosurg. (2018) 174:233–8. doi: 10.1016/j.clineuro.2018.08.003
PubMed Abstract | Crossref Full Text | Google Scholar
21. Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in Malignant gliomas. Lancet Oncol. (2008) 9:453–61. doi: 10.1016/S1470-2045(08)70125-6
PubMed Abstract | Crossref Full Text | Google Scholar
22. Leao DJ, Craig PG, Godoy LF, Leite CC, Policeni B. Response assessment in neuro-oncology criteria for gliomas: practical approach using conventional and advanced techniques. Am J Neuroradiology. (2020) 41:10–20. doi: 10.3174/ajnr.A6358
PubMed Abstract | Crossref Full Text | Google Scholar
24. Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. (2010) 28:1963–72. doi: 10.1200/JCO.2009.26.3541
PubMed Abstract | Crossref Full Text | Google Scholar
25. Oberheim Bush NA, Cha S, Chang SM, Clarke JL. Chapter 55 - Pseudoprogression in Neuro-Oncology: Overview, Pathophysiology, and Interpretation. In: Newton HB, editor. Handbook of Neuro-Oncology Neuroimaging, 2nd ed. Academic Press, San Diego (2016). p. 681–95.
26. van den Bent MJ, Wefel JS, Schiff D, Taphoorn MJB, Jaeckle K, Junck L, et al. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. (2011) 12:583–93. doi: 10.1016/S1470-2045(11)70057-2
PubMed Abstract | Crossref Full Text | Google Scholar
27. van West SE, de Bruin HG, van de Langerijt B, Swaak-Kragten AT, van den Bent MJ, Taal W. Incidence of pseudoprogression in low-grade gliomas treated with radiotherapy. Neuro-Oncology. (2016) 19:719–25. doi: 10.1093/neuonc/now194
PubMed Abstract | Crossref Full Text | Google Scholar
29. Ritterbusch R, Halasz LM, Graber JJ. Distinct imaging patterns of pseudoprogression in glioma patients following proton versus photon radiation therapy. J Neuro-Oncology. (2021) 152:583–90. doi: 10.1007/s11060-021-03734-6
PubMed Abstract | Crossref Full Text | Google Scholar
30. Lu VM, Welby JP, Laack NN, Mahajan A, Daniels DJ. Pseudoprogression after radiation therapies for low grade glioma in children and adults: A systematic review and meta-analysis. Radiotherapy Oncol. (2020) 142:36–42. doi: 10.1016/j.radonc.2019.07.013
PubMed Abstract | Crossref Full Text | Google Scholar
31. Harrabi SB, von Nettelbladt B, Gudden C, Adeberg S, Seidensaal K, Bauer J, et al. Radiation induced contrast enhancement after proton beam therapy in patients with low grade glioma – How safe are protons? Radiotherapy Oncol. (2022) 167:211–8. doi: 10.1016/j.radonc.2021.12.035
PubMed Abstract | Crossref Full Text | Google Scholar
32. Bahn E, Bauer J, Harrabi S, Herfarth K, Debus J, Alber M. Late contrast enhancing brain lesions in proton-treated patients with low-grade glioma: clinical evidence for increased periventricular sensitivity and variable RBE. Int J Radiat Oncol Biol Phys. (2020) 107:571–8. doi: 10.1016/j.ijrobp.2020.03.013
PubMed Abstract | Crossref Full Text | Google Scholar
33. Nayak L, DeAngelis LM, Brandes AA, Peereboom DM, Galanis E, Lin NU, et al. The Neurologic Assessment in Neuro-Oncology (NANO) scale: a tool to assess neurologic function for integration into the Response Assessment in Neuro-Oncology (RANO) criteria. Neuro-Oncology. (2017) 19:625–35. doi: 10.1093/neuonc/nox029
PubMed Abstract | Crossref Full Text | Google Scholar
34. Lambrecht M, Eekers DBP, Alapetite C, Burnet NG, Calugaru V, Coremans IEM, et al. Radiation dose constraints for organs at risk in neuro-oncology; the European Particle Therapy Network consensus. Radiotherapy Oncol. (2018) 128:26–36. doi: 10.1016/j.radonc.2018.05.001
PubMed Abstract | Crossref Full Text | Google Scholar
35. Wen PY, van den Bent M, Youssef G, Cloughesy TF, Ellingson BM, Weller M, et al. RANO 2.0: update to the response assessment in neuro-oncology criteria for high- and low-grade gliomas in adults. J Clin Oncol. (2023) 41:5187–99. doi: 10.1200/JCO.23.01059
PubMed Abstract | Crossref Full Text | Google Scholar
36. Dworkin M, Mehan W, Niemierko A, Kamran SC, Lamba N, Dietrich J, et al. Increase of pseudoprogression and other treatment related effects in low-grade glioma patients treated with proton radiation and temozolomide. J Neurooncol. (2019) 142:69–77. doi: 10.1007/s11060-018-03063-1
PubMed Abstract | Crossref Full Text | Google Scholar
37. Bronk JK, Guha-Thakurta N, Allen PK, Mahajan A, Grosshans DR, McGovern SL. Analysis of pseudoprogression after proton or photon therapy of 99 patients with low grade and anaplastic glioma. Clin Trans Radiat Oncol. (2018) 9:30–4. doi: 10.1016/j.ctro.2018.01.002
留言 (0)