Lung cancer is the leading cause of cancer-related deaths worldwide, with approximately 85% of cases attributed to non-small cell lung cancer (NSCLC) (1). Epidermal growth factor receptor (EGFR) belongs to a group of transmembrane receptors known as the HER/erbB family of receptor tyrosine kinases (RTK), which have the capability to homodimerize and/or heterodimerize with ligands, consequently activating the tyrosine kinase (TK) through autophosphorylation. This activation triggers downstream signaling pathways that ultimately promote tumor cell proliferation, angiogenesis, and migration (2, 3). Research suggested that more than 60% of NSCLC cases display elevated expression of EGFR, positioning EGFR as a promising target for NSCLC treatment (2). In 2004, research revealed a strong association between the efficacy of epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) and EGFR gene mutations (4). Approximately 20% of NSCLC cases harbor EGFR mutations, with exon 19 deletions or exon 21 L858R mutations accounting for over 90% of these mutations (5). First-generation EGFR-TKIs such as gefitinib and erlotinib, as well as second-generation EGFR-TKIs like afatinib and dacomitinib, have shown significant clinical efficacy. However, the majority of patients develop resistance, with the most common mechanism being the T790M mutation (6). Osimertinib is a third-generation EGFR-TKI, approved by the U.S. Food and Drug Administration (FDA) in 2015 and launched in China in 2017 (7). Osimertinib has the capability to form irreversible covalent bonds with the cysteine residue at position 797 (C797) within the ATP-binding site of specific EGFR mutants, including T790M, L858R, and exon 19 deletions, effectively overcoming acquired resistance to first-generation and second-generation EGFR-TKIs (8). Skin toxicity is frequently observed since osimertinib also targets EGFR located in the skin epithelium (9). Retrospective data from the FDA Adverse Event Reporting System (FAERS) (10) indicate that the preferred terms for skin and subcutaneous tissue disorders caused by osimertinib mainly include nail disorder, onychoclasis, skin disorder, dermatitis acneiform, erythema multiforme, onychalgia, ingrowing nail, nail discoloration, and nail bed disorder. Here, we report a case of osimertinib-induced cutaneous vasculitis and review the literature on cutaneous vasculitis induced by osimertinib and other EGFR-TKI drugs, aiming to provide a reference for the safe use of osimertinib. Informed consent was obtained from the patient in advance.
Case presentationA 49-year-old Chinese female was diagnosed with lung adenocarcinoma in June 2021. She has no notable medical history, family history, or history of psychosocial disorders, and there is no relevant genetic information available. She subsequently underwent curative surgery followed by 2 cycles adjuvant chemotherapy, which she did not complete due to intolerance of chemotherapy-related adverse effects. In May 2024, the patient developed pleural effusion, and adenocarcinoma cells were identified in the pleural fluid, with immunohistochemistry suggesting pulmonary origin. Both the surgical specimen and pleural fluid underwent next-generation sequencing, which revealed EGFR exon 21 L858R point mutation. On June 4, 2024, the patient began monotherapy with osimertinib at 80 mg once daily, and during this period, she did not take any other medications.
Six days after starting osimertinib treatment, the patient developed multiple patchy red rashes, initially concentrated on the anterior chest and then gradually extending to the abdomen and lower extremities. The skin was slightly warm to touch with mild itching, which she could tolerate, and she continued the osimertinib treatment. By the eleventh day of osimertinib treatment, the rash on her chest and abdomen had subsided, but the palpable purpura on her lower extremities worsened. The non-blanching palpable purpura, slightly presenting as papules, covered more than 10% of the body surface area, without significant itching or pain, and no pustules (Figure 1). Additionally, the patient did not experience fever, joint pain or swelling, abdominal cramps, hematuria, or other symptoms indicative of connective tissue disease.
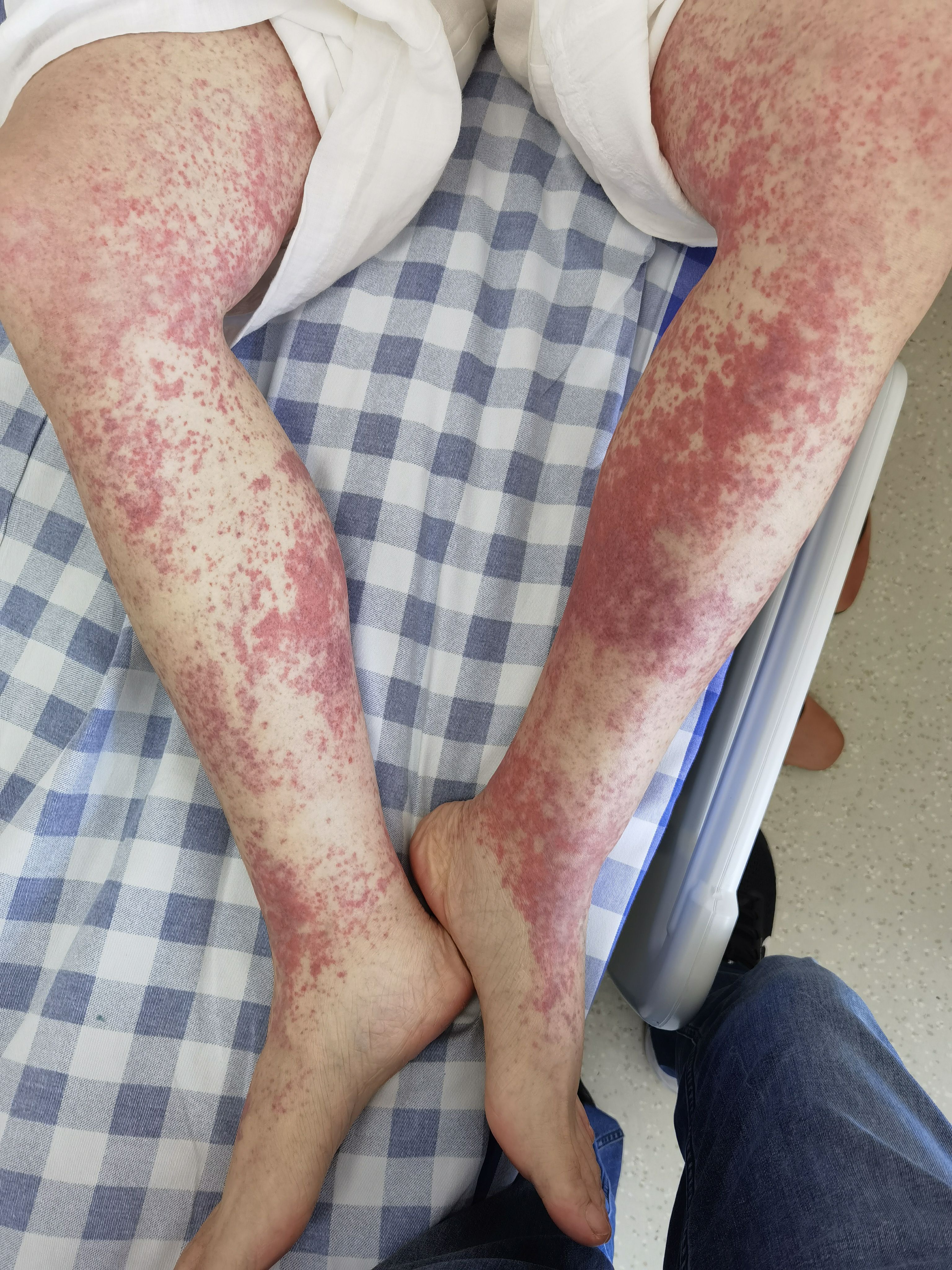
Figure 1. Palpable purpura, non-blanching over bilateral lower extremities.
Laboratory tests showed that platelet count, serum immunoglobulin A, coagulation function, renal function indices (serum creatinine, urea, electrolytes), liver function indices (serum transaminases, bilirubin), and urinary red blood cells, anti-streptolysin O test, anti-cyclic citrullinated peptide antibodies were all within the normal range. Urine protein, proteinase-3 anti-neutrophil cytoplasmic antibody (ANCA), and myeloperoxidase-ANCA were all negative (Table 1). Unfortunately, the patient refused a skin biopsy.
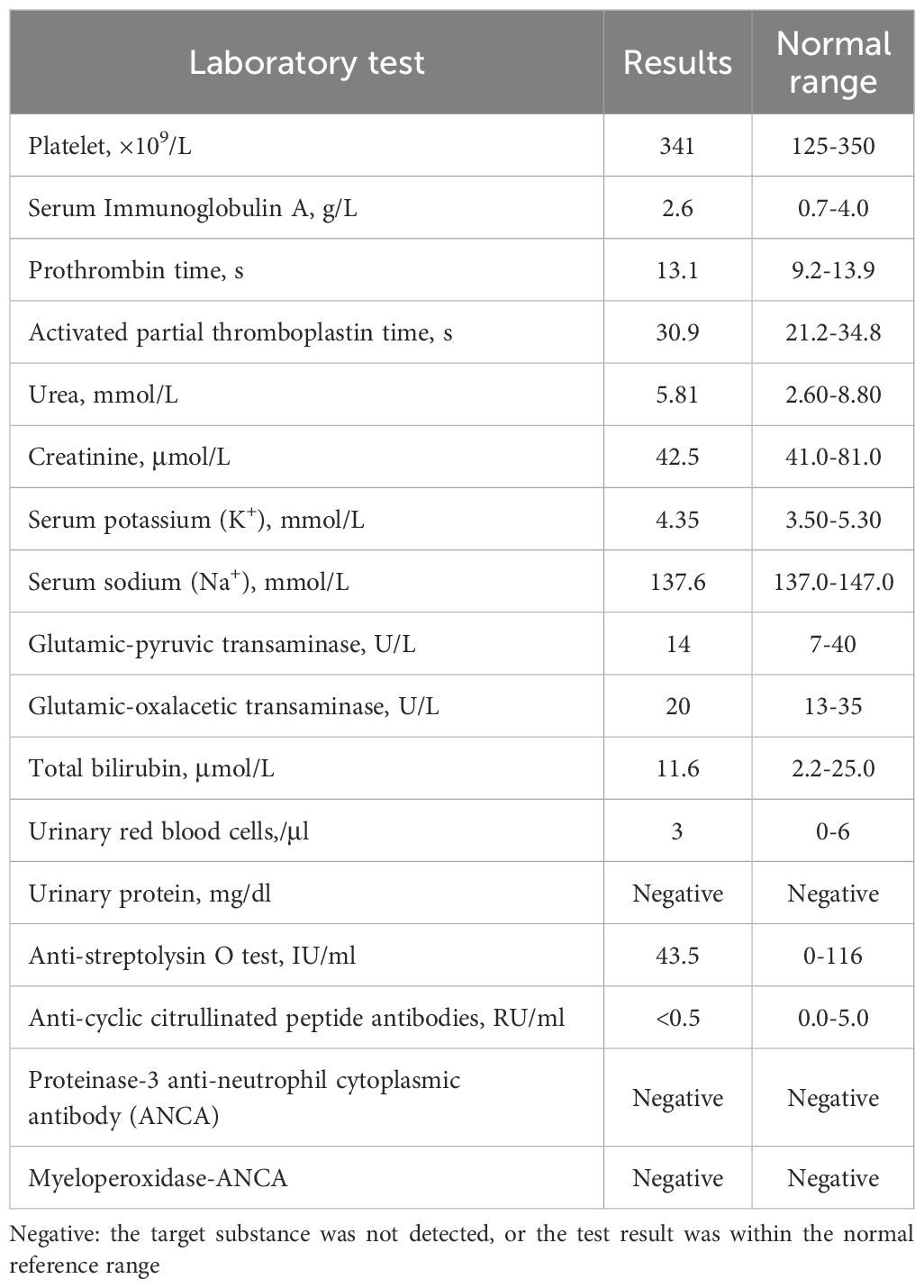
Table 1. The patient’s laboratory test results.
Based on the clinical presentation of purpura, a multidisciplinary team consisting of dermatology, hematology, oncology, and pulmonology collectively diagnosed the condition as cutaneous vasculitis. Subsequently, the patient discontinued osimertinib and was treated with intravenous methylprednisolone at 40 mg/day, combined with oral cetirizine at 10 mg/day. Eight days after stopping osimertinib, the patient’s multiple skin purpura completely subsided, thus it was considered to be osimertinib-induced cutaneous vasculitis. However, considering the patient’s EGFR mutation and the significant adverse effects of post-operative adjuvant chemotherapy, with the patient’s consent, we opted to rechallenge with another EGFR-TKI.
We administered a third-generation EGFR-TKI, almonertinib, developed in China, at a dose of 110 mg/day. Six days after starting almonertinib, the patient developed a mild rash on the inner side of her forearm, which blanched upon pressure. After a few days of observation, the rash did not worsen and later resolved on its own. She continues to take almonertinib at 110 mg/day. The timeline is shown in Figure 2.

Figure 2. timeline of disease, intervention, and outcome.
DiscussionCutaneous vasculitis is an inflammatory disease affecting the dermal blood vessel walls. According to the 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides, it includes cutaneous components of systemic vasculitides, skin-limited variants of systemic vasculitides, and single-organ cutaneous vasculitis (11). It is estimated that approximately 15%-20% of cutaneous vasculitis is drug-induced, occurs most often within 7-21 days after initiation of the suspected drug (12).
The skin is most susceptible to small vessel vasculitis, with the typical manifestation being palpable purpura, and it can also present as petechiae, urticarial papules, vesicles, and pustules (11, 13). The subcategories of cutaneous small vessel vasculitis include Henoch-Schönlein Purpura, Acute hemorrhagic edema of infancy, urticarial vasculitis, erythema elevatum diutinum, and cryoglobulinemic vasculitis (14). Leukocytoclastic vasculitis is a common pathophysiological change caused by different etiologies, with skin changes often localized to the lower extremities (12, 15). To our knowledge, there have been four reported cases of osimertinib-induced cutaneous vasculitis (Table 2).
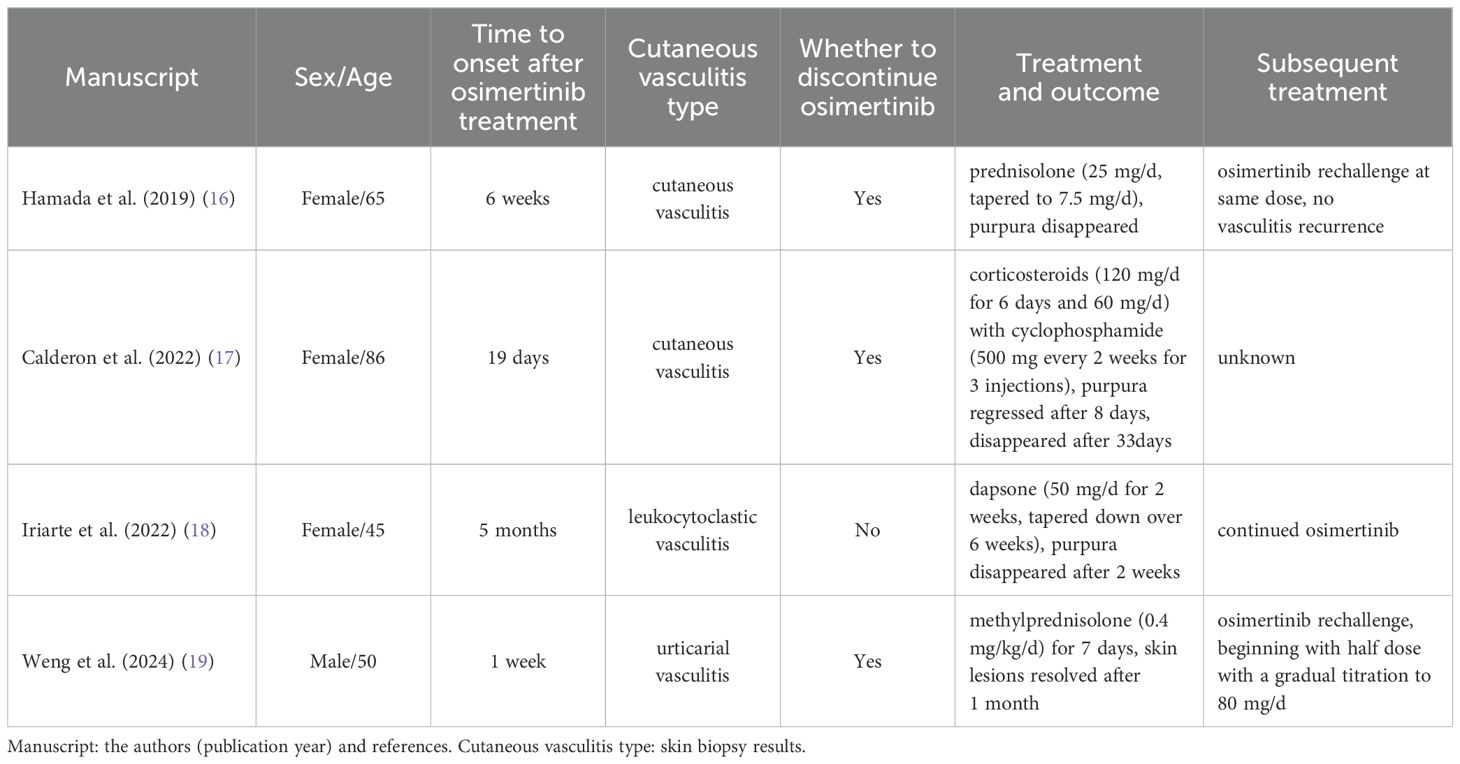
Table 2. Reported cases of osimertinib-induced cutaneous vasculitis.
We searched the PubMed, MEDLINE, Elsevier ScienceDirect, and Web of Science databases to investigate and review existing literature reports and review articles on cutaneous vasculitis induced by EGFR-TKIs. Over the past two decades, besides osimertinib, the primary EGFR-TKI drugs causing cutaneous vasculitis have been the first generation EGFR-TKIs, gefitinib and erlotinib, with a total of 14 cases reported (Table 3).
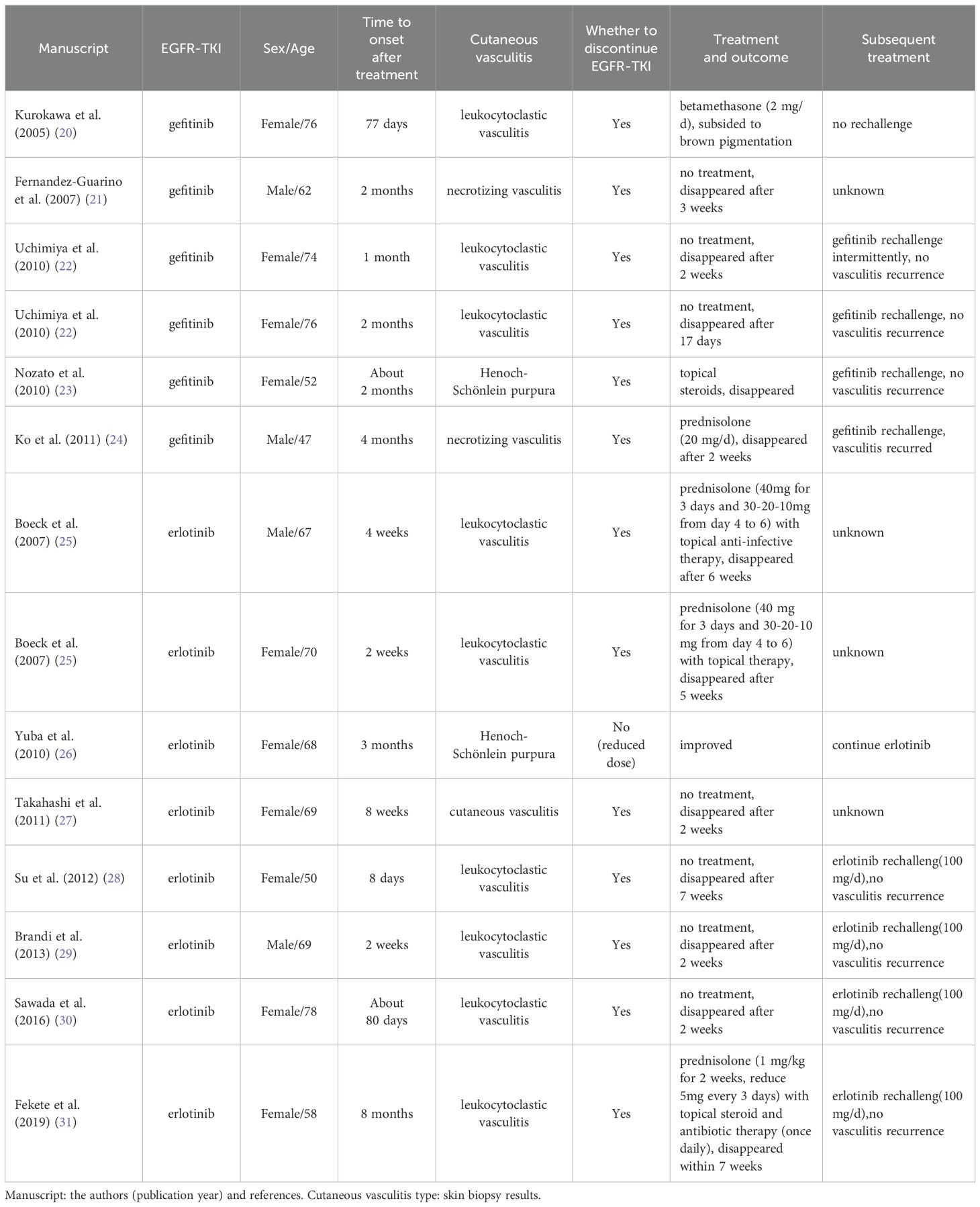
Table 3. Reported cases of other EGFR-TKIs-induced cutaneous vasculitis.
Currently, there are 18 reported cases of EGFR-TKI (Osimertinib, Gefitinib, Erlotinib) induced cutaneous vasculitis. Among these, there are 14 cases of lung cancer, 2 cases of advanced hepatocellular carcinoma, 1 case of metastatic pancreatic cancer, and 1 case of adenoid cystic carcinoma of the maxilla with multiple nodules in both lungs after surgery and radiotherapy. There are 13 female patients (72.2%) and 5 male patients (27.8%), with a median age of 65 ± 12 years, ranging from 45 to 86 years. The average time from the start of EGFR-TKI treatment to the onset of cutaneous vasculitis is 8.7 ± 7.8 weeks, with a range of 1 week to 8 months, indicating that clinical manifestations and signs may have a delayed onset. Almost all patients had clinical symptoms confined to the skin with no other systemic manifestations.
Drug-induced skin-limited vasculitis is typically characterized as cutaneous small vessel vasculitis, with a temporal association between the onset of symptoms and drug administration, and reversibility of symptoms upon suspected drug discontinuation (11). The pathogenesis of drug-induced cutaneous small vessel vasculitis is complex and generally considered to be a Type III hypersensitivity reaction. The drug acting as a hapten, binding to large molecules in the body to form immune complexes that deposit in small vessels, triggering complement cascade reactions, leading to the release of C3a and C5a, activation of inflammatory responses, neutrophil chemotaxis, and endothelial activation, ultimately leading to vascular damage (32–34). More than 50 drugs have been reported in the literature as potential inducers of cutaneous small vessel vasculitis, including antibiotics, NSAIDs, TNF-inhibitors, warfarin, immune checkpoint inhibitors, and others (14, 35). The mechanism by which EGFR-TKIs induced cutaneous vasculitis is not yet clear but is believed to be similar to other forms of cutaneous small vessel vasculitis, potentially mediated by immune complexes (21). In addition to being found in tumor cells, EGFR is also expressed in the endothelial cells of skin vessels. This has led some researchers to speculate that the skin toxicity observed with EGFR-TKIs may not be immune reaction-related, but rather associated with the inhibition of EGFR itself. Inhibition of the EGFR signaling pathway can potentially trigger endothelial inflammation, disrupt vascular tone, and increase vascular permeability. These effects may ultimately manifest as skin purpura and induce vasculitis (18, 21, 36). Some researchers have suggested that it may exhibit a dose-dependent pattern (31). Research on skin purpura has found that the inhibition of endothelial cell proliferation by EGFR-TKI is time-dependent. To compensate for the EGFR pathway in endothelial cells, IQGAP1, which is involved in various signaling pathways including cell-cell adhesion, may increase. Overexpression of IQGAP1 may disrupt adhesion junctions, leading to red blood cell extravasation (37).
In general, the most crucial step in managing drug-induced vasculitis is discontinuing the suspected drug, which typically leads to spontaneous resolution within days to weeks (33). Since cutaneous small vessel vasculitis is typically confined to the skin, supportive care is the mainstay of treatment. This includes rest, leg elevation, compression stockings, local application of corticosteroids, and NSAIDs (13). For severe symptoms, systemic corticosteroids may be administered, with a recommended dosage of 0.5-1 mg/kg/day of prednisone or an equivalent corticosteroid (13, 14). Other first-line treatment options include colchicine, dapsone, and azathioprine (13, 38, 39). Some researchers suggested that dapsone is an effective targeted therapy for cutaneous leukocytoclastic vasculitis that does not compromise the immune system and allows the continuation of EGFR-TKI therapy (18). Additionally, if urticarial symptoms are present, antihistamines can be used to alleviate itching.
In cases where patients have experienced drug hypersensitivity reactions, subsequent treatment options typically involve either using a non-cross-reacting alternative or undergoing desensitization therapy with the allergenic drug. Desensitization therapy involves gradually increasing the dosage of the drug, which can help patients develop temporary tolerance to the medication. This approach allows them to safely undergo drug treatment without triggering the hypersensitivity reaction (32). Among the 18 cases of EGFR-TKI-induced cutaneous vasculitis listed in the previous tables, 16 cases (88.9%) discontinued the EGFR-TKI. One case did not discontinue osimertinib and achieved complete remission with dapsone treatment without recurrence. Another case improved and did not recur after a dose reduction of erlotinib. Among the 16 cases discontinued TKI, after treatment, 3 cases were rechallenged with the original dose, and 6 cases rechallenged with a reduced dose or intermittent use of the original drug, all without recurrence of cutaneous vasculitis. However, there was 1 case experienced a recurrence of cutaneous vasculitis after rechallenge. One case did not rechallenge the original or similar drugs, and there was no mention of subsequent antitumor therapy in 5 cases.
Almonertinib is a structural modification of osimertinib, with the difference being that the indole 1-position substituent in almonertinib is a cyclopropyl group, while in osimertinib it is a methyl group (40). There has been a report in the literature of a patient who experienced severe rash with osimertinib was successfully switched to almonertinib (41). In our case, due to patient compliance, osimertinib was not rechallenged. After switching to the same class of EGFR-TKI, namely almonertinib, the patient experienced a mild, self-limiting rash and did not exhibit any further clinical signs of cutaneous vasculitis. This allowed for the continued use of almonertinib without any adverse reactions. Based on these observations, we lean towards considering cutaneous vasculitis caused by EGFR-TKIs is a drug-induced hypersensitivity reaction. The fact that altering the medication has allowed for the safe continuation of treatment further supports this conclusion.
A previous meta-analyses on gefitinib and erlotinib indicated that EGFR-TKI-associated rash may serve as a clinical marker for predicting the effective treatment response to EGFR-TKIs in NSCLC patients, including objective response rate (ORR) and disease control rate (DCR). Additionally, patients who develop a rash tend to have longer progression-free survival (PFS) and overall survival (OS) (42). The rash induced by EGFR-TKIs could be an external manifestation of their therapeutic efficacy. Similar results were also found in the research of the second-generation EGFR-TKIs afatinib and dacomitinib (43, 44). Nevertheless, it remains unclear whether skin adverse events induced by third-generation EGFR-TKIs, such as osimertinib, can serve as predictive indicators of their antitumor efficacy.
Our study has certain limitations that need to be acknowledged. First, we were unable to conduct a pathological examination to confirm the presence of cutaneous vasculitis due to the patient’s refusal for a skin biopsy. Instead, the case was managed by a multidisciplinary team based on clinical presentation and other available diagnostic information. It is important to note that skin lesions play a crucial role in the diagnosis of vasculitis, and the absence of a biopsy may impact the accuracy and certainty of the diagnosis (12). The presence of palpable purpura on the patient’s lower extremities is in line with the characteristic features of cutaneous small vessel vasculitis. These features include the extravasation of red blood cells, resulting in non-blanching purpura, the influence of gravity on the deposition of immune complexes that primarily affect the lower legs, and the indication of inflammatory cell infiltration (12, 13). In a study involving 32 patients with NSCLC who experienced purpuric drug eruptions due to EGFR-TKIs (gefitinib, erlotinib, and afatinib), the predominant characteristics of these eruptions were observed to be purpuric papules, pustules, or confluent plaques on the lower limbs. Pathological examination confirmed leukocytoclastic vasculitis in only 3 patients, while 63% (20/32) of the patients tested positive for Staphylococcus aureus in skin tests. All patients received systemic antimicrobial treatment (36). Furthermore, a case report documented a patient who developed a purpuric drug eruption attributed to afatinib. The patient exhibited purpura and pustules on the legs, accompanied by an ulcer and a crust. Pseudomonas aeruginosa and coagulase-negative Staphylococcus were detected in the pustules, while the skin pathology did not indicate vasculitis. The patient received systemic anti-infective treatment, which resulted in positive outcomes (45). In our case, the patient did not exhibit pustules, ulcers, or any comparable symptoms, nor did she receive any antimicrobial drugs during her treatment. Significantly, the purpura completely resolved. As a result, the possibility of a purpuric drug eruption is diminished, while the likelihood of cutaneous vasculitis as the diagnosis becomes more prominent. Moving forward, it is imperative to delve deeper into the mechanisms underlying cutaneous vasculitis, identify specific biomarkers to facilitate diagnosis, alleviate the discomfort associated with skin biopsies for patients, and enhance patient compliance.
ConclusionOur case can provide some safety alert information for clinical practice. Cutaneous vasculitis is a rare skin adverse reaction to EGFR-TKIs, and it is crucial to remain vigilant, identify it early, educate the patient, and manage it effectively. Additionally, skin adverse reactions may be related to the efficacy of EGFR-TKIs, but their predictive role warrants further investigation.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementEthical review and approval was not required for the study of human participants in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributionsYZ: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. ML: Conceptualization, Methodology, Visualization, Writing – original draft, Writing – review & editing. MW: Data curation, Writing – review & editing. YC: Conceptualization, Writing – review & editing. LZ: Formal analysis, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
AcknowledgmentsWe appreciate our patient for participating in this study.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References3. He J, Huang Z, Han L, Gong Y, Xie C. Mechanisms and management of 3rd−generation EGFR−TKI resistance in advanced non−small cell lung cancer (Review). Int J Oncol. (2021) 59:90. doi: 10.3892/ijo.2021.5270
PubMed Abstract | Crossref Full Text | Google Scholar
4. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. (2004) 350:2129–39. doi: 10.1056/NEJMoa040938
PubMed Abstract | Crossref Full Text | Google Scholar
5. Roskoski R. Small molecule inhibitors targeting the EGFR/ErbB family of protein-tyrosine kinases in human cancers. Pharmacol Res. (2019) 139:395–411. doi: 10.1016/j.phrs.2018.11.014
PubMed Abstract | Crossref Full Text | Google Scholar
6. Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer. (2019) 121:725–37. doi: 10.1038/s41416-019-0573-8
PubMed Abstract | Crossref Full Text | Google Scholar
10. Yin Y, Shu Y, Zhu J, Li F, Li J. A real-world pharmacovigilance study of FDA Adverse Event Reporting System (FAERS) events for osimertinib. Sci Rep. (2022) 12:19555. doi: 10.1038/s41598-022-23834-1
PubMed Abstract | Crossref Full Text | Google Scholar
11. Sunderkotter CH, Zelger B, Chen KR, Requena L, Piette W, Carlson JA, et al. Nomenclature of cutaneous vasculitis: dermatologic addendum to the 2012 revised international Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheumatol. (2018) 70:171–84. doi: 10.1002/art.40375
PubMed Abstract | Crossref Full Text | Google Scholar
12. Frumholtz L, Laurent-Roussel S, Lipsker D, Terrier B. Cutaneous vasculitis: review on diagnosis and clinicopathologic correlations. Clin Rev Allergy Immunol. (2021) 61:181–93. doi: 10.1007/s12016-020-08788-4
PubMed Abstract | Crossref Full Text | Google Scholar
16. Hamada K, Oishi K, Okita T, Chikumoto A, Ohteru Y, Murakawa K, et al. Cutaneous vasculitis induced by osimertinib. J Thorac Oncol. (2019) 14:2188–9. doi: 10.1016/j.jtho.2019.08.2507
PubMed Abstract | Crossref Full Text | Google Scholar
17. Calderon B, Vazquez L. Severe immune-related cutaneous vasculitis induced by osimertinib. Arch Clin Med Case Rep. (2022) 06:524–27. doi: 10.26502/acmcr.96550508
Crossref Full Text | Google Scholar
18. Iriarte C, Young JH, Rabin MS, LeBoeuf NR. Osimertinib-induced cutaneous vasculitis responsive to low-dose dapsone without interruption of anticancer therapy: A case report and review of the literature. JTO Clin Res Rep. (2022) 3:100415. doi: 10.1016/j.jtocrr.2022.100415
PubMed Abstract | Crossref Full Text | Google Scholar
19. Weng PC, Huang YL, Cheng CY. Osimertinib-induced urticarial vasculitis in a patient with lung cancer: A rare cutaneous toxicity. Eur J Cancer. (2024) 196:113432. doi: 10.1016/j.ejca.2023.113432
PubMed Abstract | Crossref Full Text | Google Scholar
21. Fernandez-Guarino M, Ryan AM, Perez-Garcia B, Gonzalez-Lopez C, Olasolo PJ. Necrotizing vasculitis due to gefitinib (Iressa). Int J Dermatol. (2007) 46:890–1. doi: 10.1111/j.1365-4632.2007.03275.x
PubMed Abstract | Crossref Full Text | Google Scholar
22. Uchimiya H, Higashi Y, Kawai K, Kanekura T. Purpuric drug eruption with leukocytoclastic vasculitis due to gefitinib. J Dermatol. (2010) 37:562–4. doi: 10.1111/j.1346-8138.2010.00896.x
PubMed Abstract | Crossref Full Text | Google Scholar
23. Nozato K, Morishima Y, Furuta J, Fujita J, Miyazaki K, Ogawa R, et al. A case of Henoch-Schönlein purpura which was difficult to distinguish from a skin rash associated with gefitinib. Nihon Kokyuki Gakkai Zasshi. (2010) 48:529–34.
PubMed Abstract | Google Scholar
24. Ko JH, Shih YC, Hui RC, Yang CH. Necrotizing vasculitis triggered by gefitinib: an unusual clinical presentation. J Clin Oncol. (2011) 29:e169–170. doi: 10.1200/JCO.2010.31.8352
PubMed Abstract | Crossref Full Text | Google Scholar
25. Boeck S, Wollenberg A, Heinemann V. Leukocytoclastic vasculitis during treatment with the oral EGFR tyrosine kinase inhibitor erlotinib. Ann Oncol. (2007) 18:1582–3. doi: 10.1093/annonc/mdm420
PubMed Abstract | Crossref Full Text | Google Scholar
26. Yuba T, Nagata K, Shiotsu S, Okano A, Hatsuse M, Murakami S, et al. Henoch-schönlein purpura induced by erlotinib (Tarceva): a case report. Nihon Kokyuki Gakkai Zasshi. (2010) 48:81–5.
PubMed Abstract | Google Scholar
27. Takahashi Y, Ebi N, Yamaguchi O, Fukusho R, Sugimoto Y, Tsuruno K. A case of cutaneous vasculitis caused by erlotinib treatment and a review of literature. Nihon Kokyuki Gakkai Zasshi. (2011) 49:663–6.
PubMed Abstract | Google Scholar
28. Su BA, Shen WL, Chang ST, Feng LY, Wu CJ, Feng YH. Successful rechallenge with reduced dose of erlotinib in a patient with lung adenocarcinoma who developed erlotinib-associated leukocytoclastic vasculitis: A case report. Oncol Lett. (2012) 3:1280–2. doi: 10.3892/ol.2012.647
PubMed Abstract | Crossref Full Text | Google Scholar
29. Brandi G, Venturi M, Dika E, Maibach H, Patrizi A, Biasco G. Cutaneous leukocytoclastic vasculitis due to erlotinib: just an adverse event or also a putative marker of drug efficacy? Cutan Ocul Toxicol. (2013) 32:336–8. doi: 10.3109/15569527.2013.780179
PubMed Abstract | Crossref Full Text | Google Scholar
31. Fekete GL, Fekete L. Cutaneous leukocytoclastic vasculitis associated with erlotinib treatment: A case report and review of the literature. Exp Ther Med. (2019) 17:1128–31. doi: 10.3892/etm.2018.6988
PubMed Abstract | Crossref Full Text | Google Scholar
33. Guzman AK, Balagula Y. Drug-induced cutaneous vasculitis and anticoagulant-related cutaneous adverse reactions: insights in pathogenesis, clinical presentation, and treatment. Clin Dermatol. (2020) 38:613–28. doi: 10.1016/j.clindermatol.2020.06.015
PubMed Abstract | Crossref Full Text | Google Scholar
34. Usman N, Annamaraju P. Type III hypersensitivity reaction. In: StatPearls. StatPearls Publishing, Treasure Island (FL (2023).
PubMed Abstract | Google Scholar
36. Cho YT, Chen KL, Sheen YS, Yang CW, Liau JY, Cheng YP, et al. Purpuric drug eruptions caused by epidermal growth factor receptor inhibitors for non-small cell lung cancer: A clinicopathologic study of 32 cases. JAMA Dermatol. (2017) 153:906–10. doi: 10.1001/jamadermatol.2017.0903
PubMed Abstract | Crossref Full Text | Google Scholar
37. Sheen YS, Lin MH, Tzeng WC, Chu CY. Purpuric drug eruptions induced by EGFR tyrosine kinase inhibitors are associated with IQGAP1-mediated increase in vascular permeability. J Pathol. (2020) 250:452–63. doi: 10.1002/path.5393
PubMed Abstract | Crossref Full Text | Google Scholar
38. Callen JP, Spencer LV, Burruss JB, Holtman J. Azathioprine. An effective, corticosteroid-sparing therapy for patients with recalcitrant cutaneous lupus erythematosus or with recalcitrant cutaneous leukocytoclastic vasculitis. Arch Dermatol. (1991) 127:515–22. doi: 10.1001/archderm.127.4.515
PubMed Abstract | Crossref Full Text | Google Scholar
39. Sais G, Vidaller. A, Jucglà A, Gallardo F, Peyrí J. Colchicine in the treatment of cutaneous leukocytoclastic vasculitis. Results of a prospective, randomized controlled trial. Arch Dermatol. (1995) 131:1399–402. doi: 10.1001/archderm.1995.01690240061009
PubMed Abstract | Crossref Full Text | Google Scholar
40. Nagasaka M, Zhu VW, Lim SM, Greco M, Wu F, Ou SI. Beyond osimertinib: the development of third-generation EGFR tyrosine kinase inhibitors for advanced EGFR+ NSCLC. J Thorac Oncol. (2021) 16:740–63. doi: 10.1016/j.jtho.2020.11.028
PubMed Abstract | Crossref Full Text | Google Scholar
41. Zhang Q, Xie P, Hou X, Zhao C, Duan L, Qiao H. Benefit from Almonertinib after Osimertinib treat EGFR 19 exon deletion NSCLC induced Severe rash: a case report. J Chemother. (2024) 36:334–42. doi: 10.1080/1120009X.2023.2276574
PubMed Abstract | Crossref Full Text | Google Scholar
42. Liu HB, Wu Y, Lv TF, Yao YW, Xiao YY, Yuan DM, et al. Skin rash could predict the response to EGFR tyrosine kinase inhibitor and the prognosis for patients with non-small cell lung cancer: a systematic review and meta-analysis. PloS One. (2013) 8:e55128. doi: 10.1371/journal.pone.0055128
PubMed Abstract | Crossref Full Text | Google Scholar
43. Kudo K, Hotta K, Bessho A, Nogami N, Kozuki T, Kuyama S, et al. Development of a skin rash within the first week and the therapeutic effect in afatinib monotherapy for EGFR-mutant non-small cell lung cancer (NSCLC): Okayama Lung Cancer Study Group experience. Cancer Chemother Pharmacol. (2016) 77:1005–9. doi: 10.1007/s00280-015-2910-9
PubMed Abstract | Crossref Full Text | Google Scholar
44. Pu X, Li J, Zhang B, Zhang J, Mok TS, Nakagawa K, et al. (2022). Efficacy in patients with EGFR-positive non-small-cell lung cancer treated with dacomitinib who had skin adverse events: post hoc analyses from ARCHER 1050. Future Oncol. (2024) 20:2971–82. doi: 10.1080/14796694.2024.2404762
PubMed Abstract | Crossref Full Text | Google Scholar
45. Oku A, Nakai K, Ikenaga T, Sato K, Mitsuoka S, Takahashi S, et al. Case of afatinib-induced severe purpuric drug eruption with gastrointestinal bleeding. J Dermatol. (2021) 48:e534–5. doi: 10.1111/1346-8138.16088
留言 (0)