Epithelioid hemangioendothelioma (EHE) is a rare malignant vascular tumor, that often occurs in soft tissues/bones or visceral sites, such as the liver and lung. Histologically, it is composed of epithelioid endothelial cells arranged in cords, nests, or single cells in the myxohyaline stroma. Tumor cells don’t have strong angiogenic properties commonly. In approximately 90% of EHEs, there is recurrent WWTR1::CAMTA1 gene fusion resulting from t(1;3)(p36;q25). YAP1::TFE3 gene fusion of EHE, a rare and morphologically distinct subtype, has been identified recently. This subtype is characterized by prominent vascular spaces, brightly eosinophilic cytoplasm, solid growth pattern, and a better prognosis (1, 2). Here, we describe an ultra-rare case of hepatic EHE with TFE3 rearrangement and review the subset of EHE occurring at various sites in the literature, with the aim of better understanding of EHE and its unique subtype of TFE3 rearrangement among clinicians, radiologists, and pathologists.
2 Case presentation2.1 Materials and methods2.1.1 Clinical materialsA 36-year-old female presented with chest tightness two years ago, whose chest and abdominal CT revealed liver multiple occupations incidentally considered to be benign in an outside hospital. The patient did not receive any treatment. Subsequently, the patient was referred to our institution for further evaluation and management. A recent CT performed in our hospital showed multiple circular low-density shadows with ring-shaped enhancement (“bull’s-eye sign”) in the liver, the largest one was 28mm in diameter (Figures 1A, B). MRI showed multiple round-like low signals on T1WI and high signals on T2WI in the liver, with well-defined and ring-shaped enhancement (Figures 1C, D). Multiple metastatic tumors in the liver were diagnosed based on CT and MRI images. The patient underwent a CT-guided core needle biopsy.
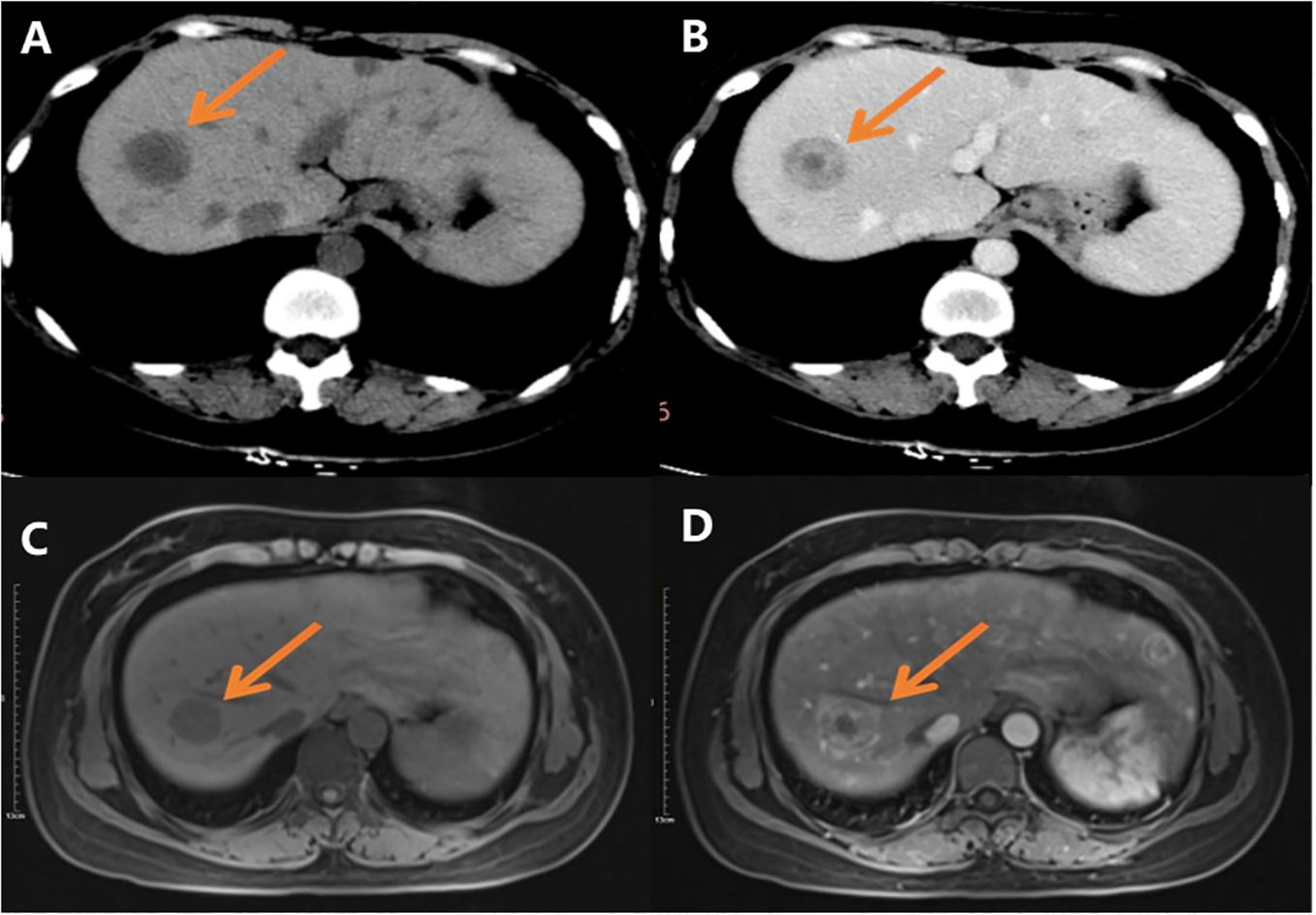
Figure 1. CT and MR images: (A) CT scan shows multiple round low-density shadows in the liver; (B) CT scan shows there is ring-shaped enhancement, and the “bull’s eye sign” can be seen after contrast administration; (C) Slightly low signal shadows in T1WI; (D) Slightly high signal shadows in T2WI.
2.1.2 Methods2.1.2.1 Hematoxylin&eosin and immunohistochemistryThe biopsy specimens were routinely sampled, fixed in 10% neutral formalin, embedded in paraffin, sectioned, subjected to H&E and immunohistochemical staining, and finally microscopically observed. Immunohistochemical staining was performed by the EnVision method using antibodies to CD34, CD31, ERG, INI-1, FLi-1, AE1/AE3, Hepatocyte, CK7, CD117, DOG-1, and Ki-67. Negative and positive controls were set up for all experiments.
2.1.2.2 FISHTFE3 gene rearrangement was detected by fluorescence in situ hybridization (FISH) using the LBP TFE3 gene breakage probe, and the specific operation method was carried out regarding the instruction manual of the kit. The reagents for this test were purchased from Guangzhou Anbipin Medical Laboratory Company.
3 Results3.1 Gross viewTwo punctured tissues, 1.4 and 1.8cm in length separately, grayish red.
3.2 Microscopic viewA Focal fibromyxoid matrix was observed in the liver tissue under low-power microscopy (Figure 2A). Epithelioid cells and plump spindle cells were seen in the myxohyaline stroma, hepatic sinusoids, and inner blood vessels. Lesional cells were arranged in scattered, nested, or cords. High-power microscopy showed tumor cells have moderate eosinophilic cytoplasm and round to plump spindle nuclei with inconspicuous nucleoli, histocyte-like, partially lipoblast-like cells with vacuolated cytoplasm (Figure 2B). Tumor cells infiltrated the hepatic sinusoids, with single vacuoles and erythrocytes in some cells (Figure 2C). A few tumor cells grew in distinct arborizing structure lined with tombstone or hobnailed tumor cells rather than typical well-formed vascular spaces. This morphology mimics the papillary intravascular lymphangioendothelioma (PILA) (Figure 2D). The tumor was accompanied by a small number of lymphocytic infiltrations, with no mitotic activity or necrosis in the core needle biopsy sample.
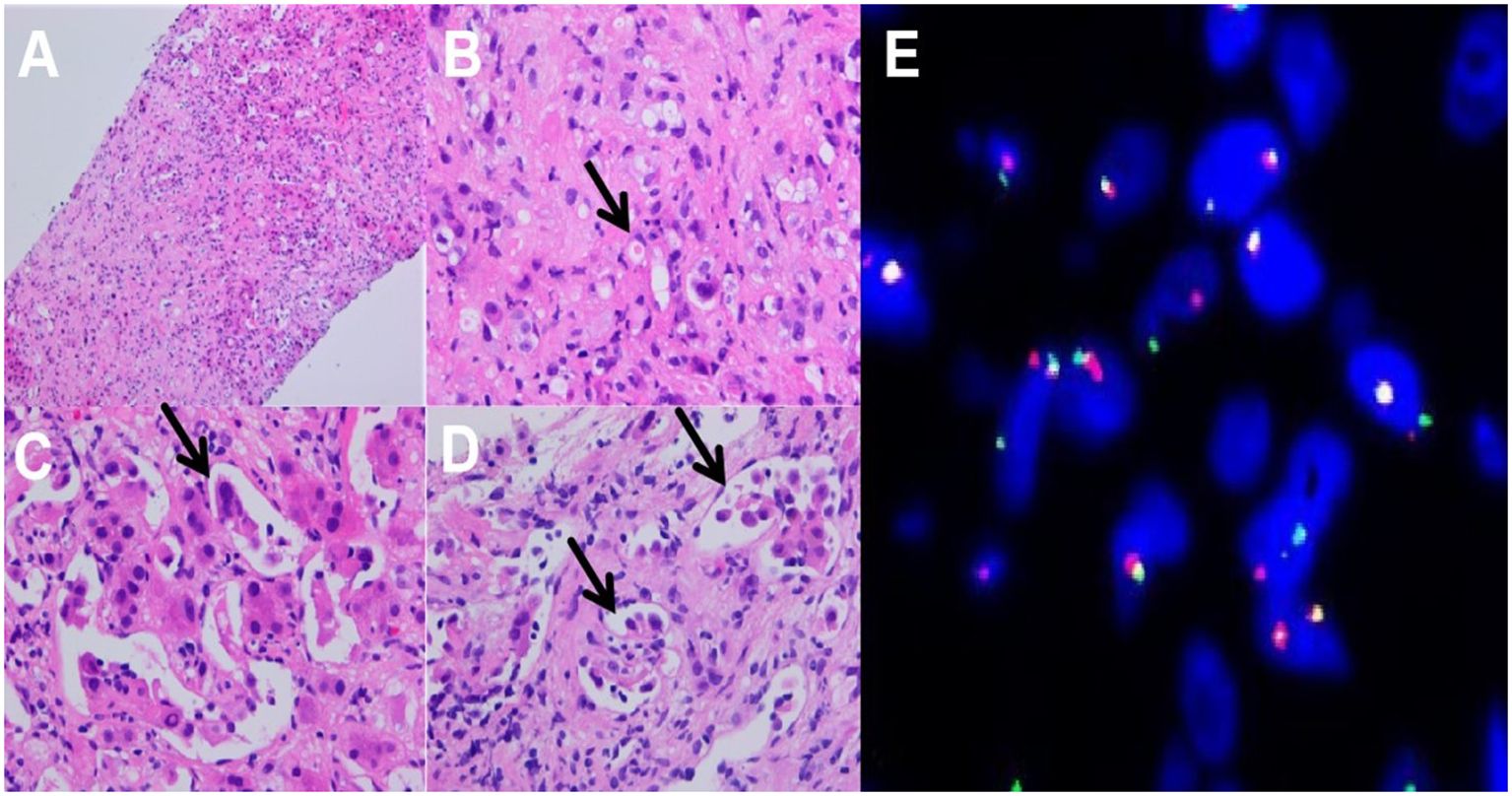
Figure 2. Histopathological features: (A) Focal fibrosis in liver tissue (HE-100X); (B) Tumor cells with cytoplasmic vacuoles or abundant eosinophilic cytoplasm, with small nucleoli, histiocyte-like, lipoblast-like, mildly heterogeneous, primitive vascular lumens, erythrocytes are seen in primitive lumens, single, striated, nested distributions, (HE-400X); (C) Tumor cells infiltrate to hepatic sinusoids (HE-400X); (D) high-power view showing arborizing structures lined with a tombstone or hobnailed tumor cells (HE-400X); (E) FISH testing:TFE3 gene breakage rearrangement.
3.3 ImmunophenotypeThe tumor cells exhibited an endothelial cell phenotype with diffuse strong positivity for CD34, CD31, ERG, and FLi-1 (Figures 3A–D), and diffuse and strong nuclear reactivity to TFE3 (Figure 3E). INI-1 was retained. There was no expression of AE1/AE3(-), Hepatocyte (-), CK7(-), CD117(-), and DOG-1(-). Ki-67 label index was 15% (Figure 3F).
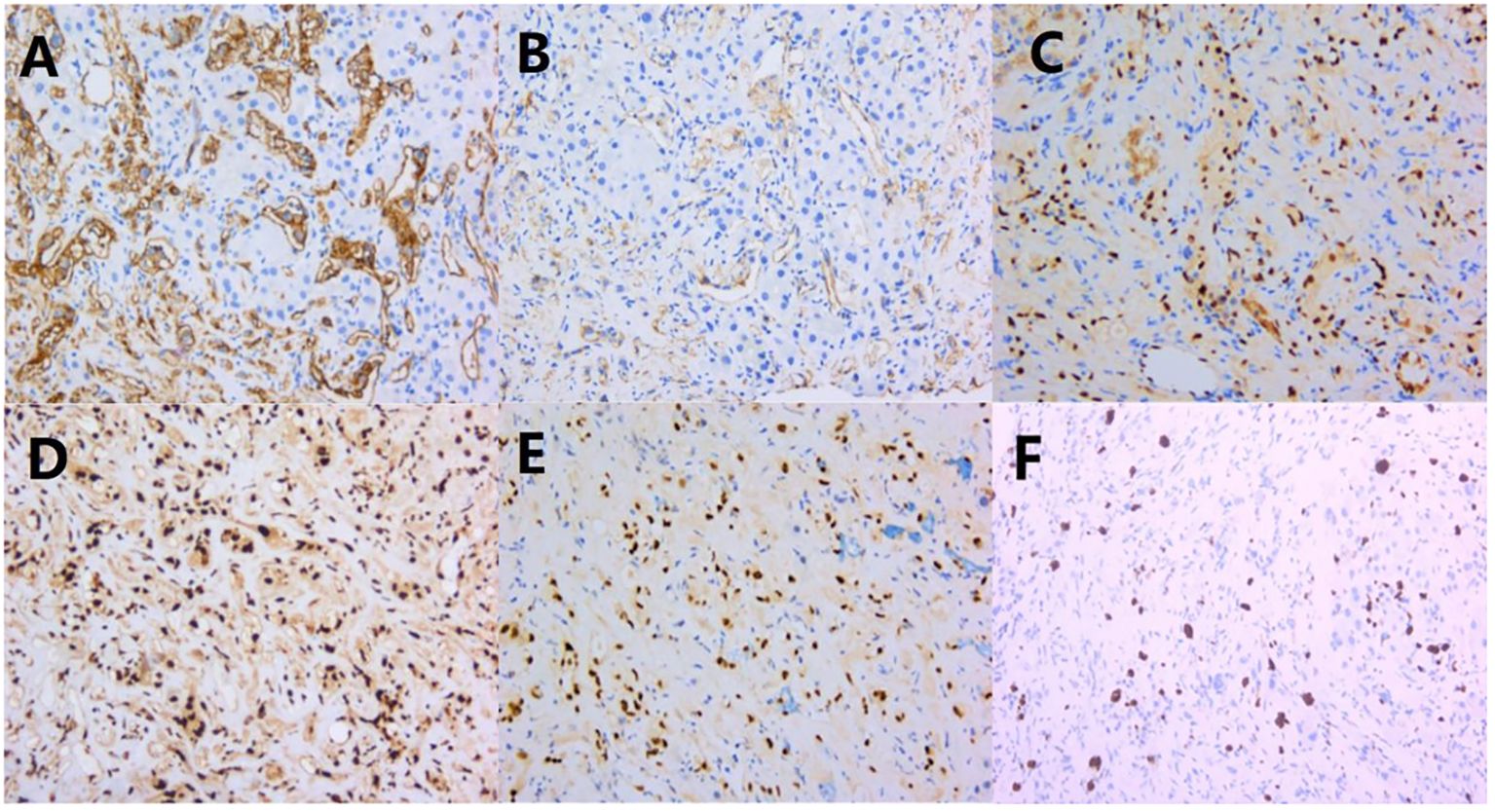
Figure 3. Immunohistochemical results: (A–D) CD34, CD31, ERG, and FLi-1 were diffusely and strongly positive; (E) TFE 3 was diffusely and strongly positive; (F) Ki-67 proliferation index was around 15%.
3.4 FISH testingThe results showed a broken rearrangement of the TFE3 gene (Figure 2E).
3.5 Pathological diagnosisHepatic Epithelioid hemangioendothelioma with TFE3 rearrangement.
4 Discussion4.1 Clinical featuresEHE of the liver is much rare, with an incidence of about 1/1 million (3, 4). The etiology and pathogenesis of hepatic EHE are still unclear. It may be related to alcohol consumption, oral contraceptives, Crohn’s disease, hepatitis, exposure to chemical substances such as polyvinyl chloride (5), monocyte chemotactic protein-1, and chronic Bartonella infection (6, 7).
Since TFE3 rearrangements in EHE were first reported in 2013 by Antonescu et al. (8), they were found in various sites, such as soft tissue, bone, lung, liver, and inguinal lymph nodes (9–11). We summarized 50 cases of EHE with TFE3 rearrangement in the literature including 3 hepatic ones. It occurred more frequently in young adults (mean age 39.5 years, range 14-65 years) with female predominance (Table 1). Its symptoms depend on the anatomical site of involvement and usually lack specificity. It may present as a painful mass in soft tissue. It is hard to recognize the primary malignant nature, frequently mistaken as benign or metastatic. For limited hepatic epithelioid hemangioendothelioma (HEHE) materials, patients do not have obvious symptoms, are often overlooked, and most of them are found incidentally during physical examination or routine US. A few patients may have abdominal pain, ascites, hepatomegaly, jaundice, emaciation, etc., with normal or slightly elevated blood AFP, CEA and tumor markers (CA199, CA125) (4, 21). Our patient was a young female who presented clinically with chest tightness, no abdominal distension or pain, and no significant change in weight. The lesions were located in the liver, presenting as multiple or metastatic, with normal blood AFP, CEA, CA199, and CA125. The clinical presentation is consistent with most of the literature.
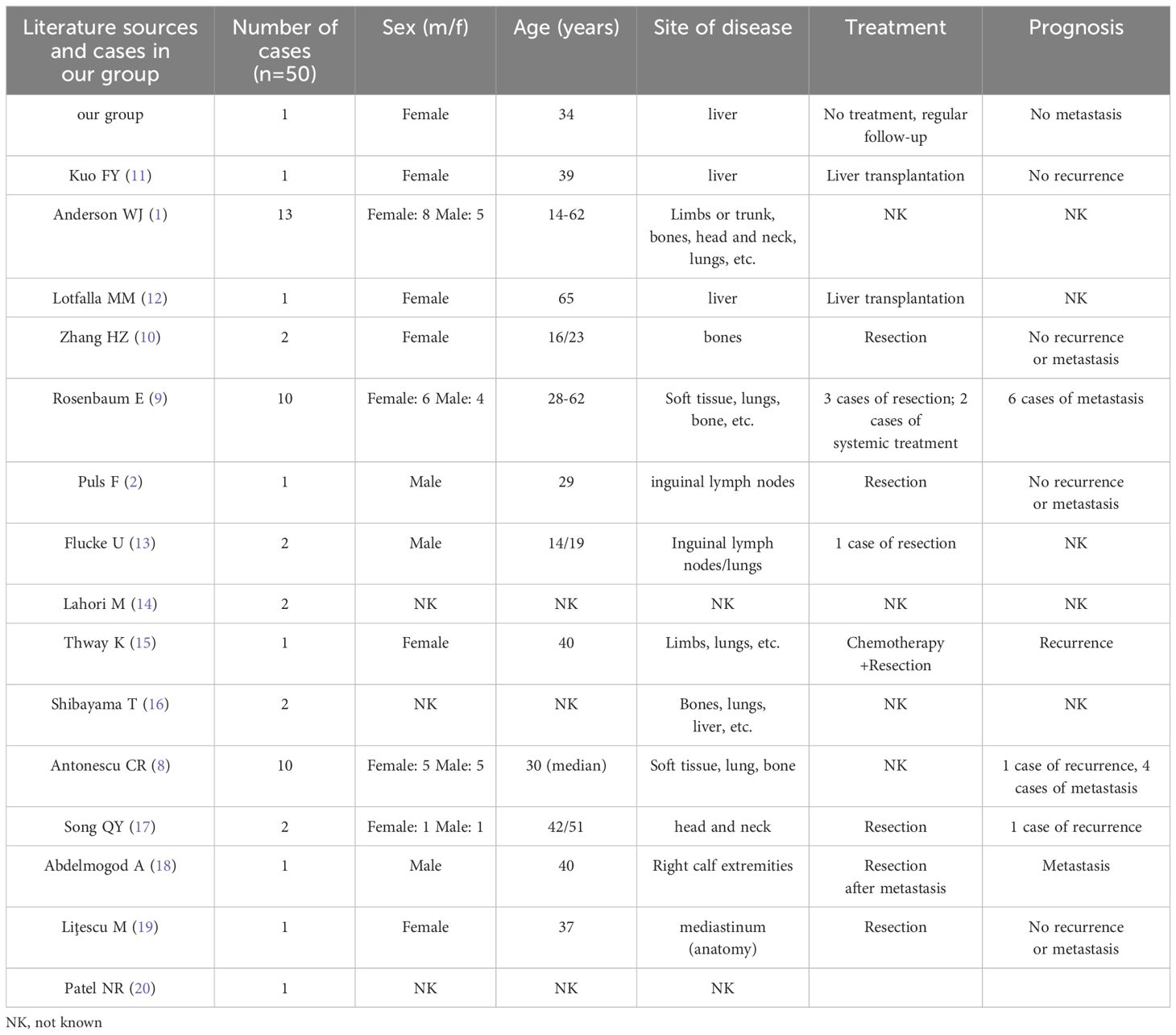
Table 1. Clinical features associated with EHE with TFE3 rearrangement.
4.2 Imaging findingsThe imaging manifestations of the HEHE are diverse, ranging from single, multiple nodular to diffuse fusion lesions. Following the disease progression, multiple nodules can fuse into patches at a late stage, which may be related to hepatic vascular invasion. The US shows most of them are hypoechoic nodules with clear borders, hyperechoic nodules are seldom and imply hemangiomatous nodules. CT scans often reveal heterogeneous density nodules with low density center, forming the typical “halo sign”. Ring enhancement is seen in the arterial phase without central enhancement. On MRI, T1WI shows slightly hypointensity, and the central signal is even lower when the tumor is necrotic. T2WI shows hyperintensity with a higher central signal, and the tumor tissues are significantly enhanced. The enhancement modes of HEHE: ring enhancement, inhomogeneous enhancement, and flocculent enhancement. Among them, ring enhancement is usually the most common. At present, it’s believed that HEHE has multiple helpful imaging signs for the diagnosis. For example, black or white target sign, “pericardial crumpling sign”, early “intratumoral vascular sign”, and late “Lollipop sign” (22). The “pericardial crumpling sign” is important but not specific. The “Lollipop sign” was a characteristic manifestation of HEHE (23). Although we did not find the “pericardial crumpling sign” and “Lollipop sign” in our case, CT showed a “bull’s-eye sign” and MRI showed ring enhancement, which was consistent with literature reports.
HEHE needs to be differentiated from liver metastases, hepatocellular carcinoma (HCC), cholangiocellular carcinoma, and hepatic hemangioma before surgery. The enhancement manifestations of liver metastases are diverse, with circular enhancement being more common and easily confused with HEHE. Therefore, it is particularly important to closely combine the history of primary malignant tumors. Patients with HCC usually have a history of hepatitis B and/or liver cirrhosis, and the enhancement of the tumor is characterized by “fast-in-fast-out” enhancement without ring-shaped enhancement. AFP is commonly elevated in HCC, however, it is normal or mildly elevated in HEHE. In addition, most benign and malignant tumors of the liver rarely show the “lollipop sign”. Hepatic hemangioma has typical progressive cardiac enhancement characteristics, with homogeneous enhancement in the delayed phase, and usually does not show the “pericardial crumpling sign”. Although cholangiocellular carcinoma may present with “peritoneal crumpling sign”, features such as thickening of the bile duct wall, dilation of the bile duct lumen, and elevation of CA199 can be distinguished from HEHE. As the CT and MRI images of HEHE have certain imaging features, they help in the diagnosis and differential diagnosis of the lesions. In addition, PET-CT which is used to detect living tumor tissue and assess disease activity, is an essential imaging modality for tumor detection. However, probably due to the rarity of the disease, no PET-CT features of TFE3-rearranged EHE have been reported to date.
4.3 Histopathologic featuresHistologically, EHE is a malignant tumor composed of vascular endothelial cells accompanied by a distinctive myxoid-hyaline transformation of the mesenchyme with WWTR1::CAMTA1 and YAP1::TFE3 fusion genes (8, 24, 25). Classic EHE often shows epithelioid or histiocyte-like cells arranged in a striated, nested, or single scattered pattern, with spindle, round, oval, or imprinted nuclei with fine chromatin, visible intracytoplasmic vacuoles containing erythrocytes. They generally do not form mature vascular lumens and are often characterized by WWTR1::CAMTA1 fusion (13). The t(11; X)(q13;p11) translocation that occurs in rare cases forms the YAP1::TFE3 fusion gene, and this subtype of EHE has several specific morphologic features, including the typical characteristics of solid or pseudo-glandular vesicle-like arrangement, well-formed blood vessels, and broad eosinophilic cytoplasm. The absence of well-formed vessels has also been reported in this rare type.
Nested, pseudo vesicular, partly solid growth pattern, occasional pseudo inclusion bodies, and interstitial inflammatory cell infiltration were observed in a single case (2, 8, 10). The core biopsy tissue in this case showed scattered, nest or cord arrangement of epithelioid to spindle cells, lipoblast-like or histiocyte-like cells, with eosinophilic cytoplasm with vacuolated cytoplasm. Our EHE with TFE3 rearrangement is unique in the presence of clear cells, lipoblast-like cells and focal PILA-like morphology, but without the characteristic well-defined vessels. The PILA-like morphology first reported by song et al. is ultra rare in TFE3-rearranged EHE (17). The PILA-like tumor cells express TFE3 to the same extent as other tumor cells. We also consider it as PILA-like morphology rather PILA.
Immunoreactivity for keratins (CK7 and CK18) is observed in the majority of EHEs (50% and 100%, respectively), while CK8 was seen in 10% cases and CK14 and CK19 in none. Epithelial membrane antigen (EMA) was occasionally detectable in EHE (26). TFE3 immunoreactivity was positive but lack of specific, which can also be positive in WWTR1::CAMTA1 EHE. TFE3 rearrangement requires FISH assay confirmation (13). Positive expression of endothelial cell markers such as CD31, CD34, ERG, and FLI-1 are commonly detected by immunohistochemistry. CD34 has high sensitivity but poor specificity. The positive rates of CD34 and CD31 are 94% and 86%, respectively (27). CD34 and CD31 should be used as the first choice for diagnosis. Ki-67 has a low proliferation index. FISH assay showed that the TFE3 gene was broken and rearranged. We diagnose this case as EHE with TFE3 rearrangement based on immunophenotype and the FISH result. Regarding prognosis, characteristics such as nuclear pleomorphism, mitotic activity, necrosis, and Ki-67 index greater than 10-15% were correlated with aggressive behavior for EHE. Rosenbaum E et al. proposed the two-grade histologic system of classical EHE based on nuclear pleomorphism, mitotic activity, and necrosis in WWTR1::CAMTA1 fused EHE (9). There is no distinctive histologic grading of EHE with TFE3 rearrangement. Our patient had mild nuclear pleomorphism, a lack of mitotic figure, and no necrosis. It may be grade 1, If we apply the classic EHE grading system.
4.4 Differential diagnosisTFE3-rearranged EHE often need to be differentiated from other similar tumors: (1) Epithelioid angiosarcoma (EA) is a malignant tumor of vascular endothelial origin. Immunohistochemistry is not helpful in differentiating between the two tumors. EA is common in older men instead of young women in EHE. Both of them can appear as epithelioid cells with solid growths. EA is much more malignant, with more striking atypical. MYC amplification occurs in a subset of EA. YAP1::TFE3 fusions is suggestive of EHE. (2) Epithelioid sarcoma (ES) is a malignant mesenchymal tumor with predominantly epithelioid, spindle-shaped, and rhabdomyosarcoma-like cells. The mitotic figure is often <5/10HPFs. ES show immunoreactivity for keratin and EMA, especially CK8 and CK19. Diffuse loss of SMARCB1(INI-1) expression is specificity. FLi-1, ERG, and CD34, nonspecific markers of vascular differentiation, could be expressed in more than half of ESs. In contrast, ES is negative for CD31 (28). SMARCB1 deletion detected by FISH or NGS is characteristic. (3) Pseudomyogenic hemangioendothelioma (PMHE) is similar to TFE3-rearranged EHE in morphology, with epithelioid to plump spindle-shaped cells, eosinophilic cytoplasm, and inflammatory cell infiltration in the stroma. Immunohistochemically they both express ERG and Fli-1, differing in that PMHE expresses the highly sensitive marker FOSB and does not express CD34. The FISH assay revealed a rearrangement of the FOSB gene, and its NGS showed a SERPINE1::FOSB gene fusion with some specificity (29). (4) Adenocarcinoma, especially intrahepatic cholangiocarcinoma (iCCA) in the liver, may appear as multifocal lesions on imaging. Diagnosis is quite challenging in small core biopsy, especially when the nuclei atypia is mild. Immunohistochemistry staining is essential to distinguish them. iCCA expresses biliary lineage-defining markers (CK7 and CK19). EHE can be easily misdiagnosed as iCCA especially in puncture samples. They both express CK7, while EHE shows negative for CK19. It is important to do a panel of CK7 and CK19 as well as vascular markers in atypical cases to determine the diagnosis of iCCA. Notably, it is important to rationally interpret TFE3 rearrangements in the context of morphology, as TFE3 rearrangements are also seen in other tumors such as alveolar soft tissue sarcomas, Xp11.2 translocation-associated renal cell carcinomas, and perivascular epithelioid cell tumors.
4.5 TreatmentHalf of the untreated EHE patients had stable disease when the lesions first appeared in the liver or lungs. For single lesions, especially in bone or liver, 75% of patients showed no evidence of recurrence after timely surgical resection. Systemic therapy is necessary for symptomatic multifocal, metastatic, observation-failed or postoperative recurrent patients. Antiangiogenesis agents, cytotoxic agents, and anti-PD-1 antibody are recommended (9). EHE patients with TFE3 rearrangements are generally younger and have a much better prognosis than the classical WWTR1::CAMTA1 type, with longer survival but a higher risk of metastasis (9, 19). TFE3 rearranged EHE is still an aggressive neoplasm with a 6% (3/50) local recurrence rate and a 22% (11/50) metastasis rate, even after the surgery (Table 1). Multifocal hepatic EHE with WWTR1::CAMTA1 is monoclonal representing metastatic implants of the same neoplastic clone rather than a synchronous occurrence of multiple neoplastic clones (30). However, whether multifocal TFE3 rearranged EHE of liver represents a pattern of metastasis or multiple separate primary tumors remains to be elucidated. Due to its rarity, more cases should be collected for further research in multicenter. There are only two cases of hepatic EHE with TFE3 rearrangement reported in the English literature. Both presented multiple liver masses and underwent liver transplants. Postoperative pathology confirmed EHE. One patient showed no signs of recurrence or metastasis during the 13 years of follow-up after liver transplants (11). Our patient denied any treatment and received regular CT examination. After 3.5 years of follow-up, the lesion stabilized (Table 2). Further research is warranted to determine if the TFE3 rearrangement predicts a less aggressive course than the WWTR1::CAMTA1 fusion.
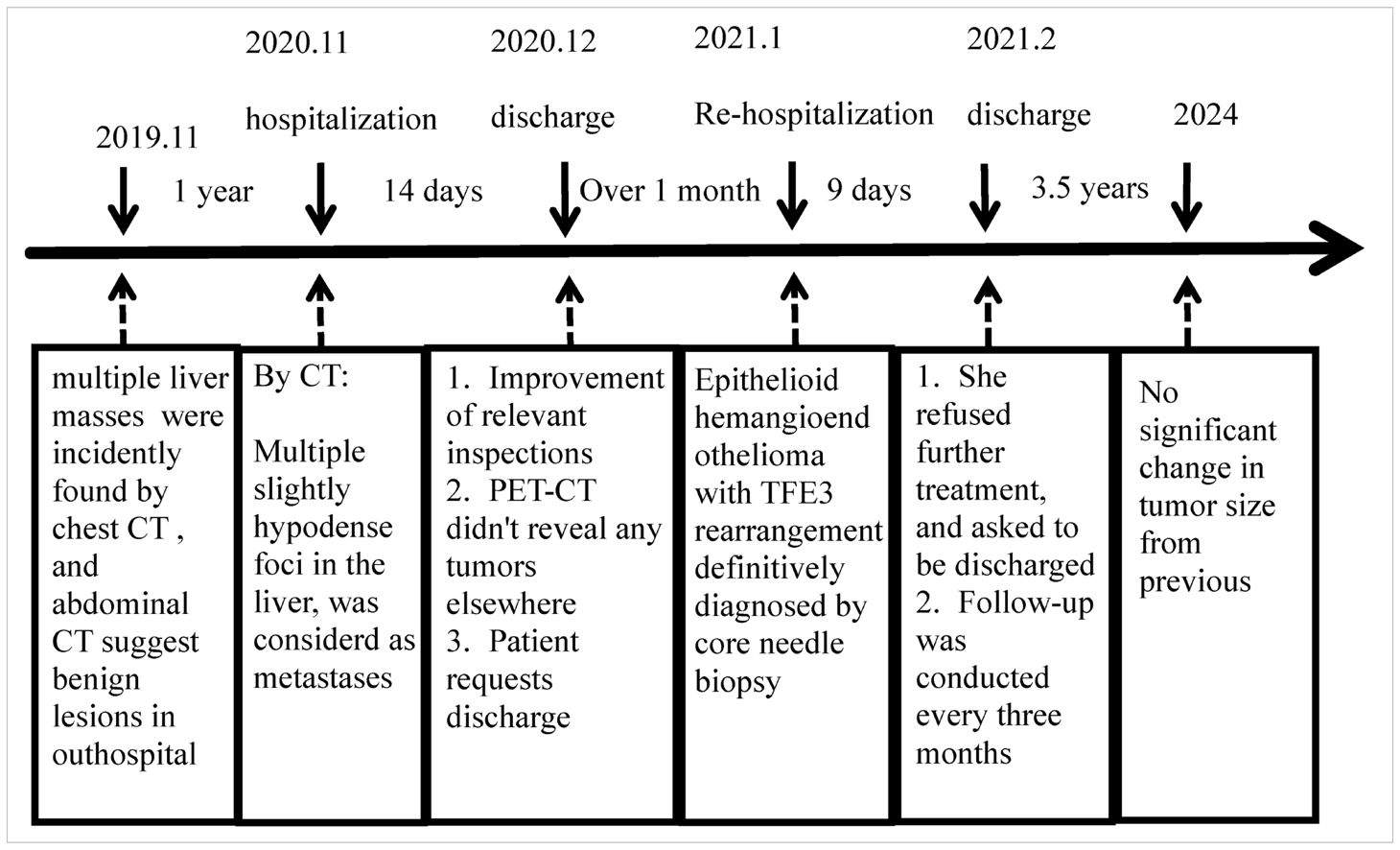
Table 2. Patient treatment process.
TFE3 rearrangements are characteristic of alveolar soft part sarcomas (ASPS), Xp11.2 translocation renal cell carcinomas (Xp11-RCC), besides EHE with TFE3 rearrangement. TFE3 immunohistochemistry lacks specificity and sensitivity in the diagnosis of TFE3 rearrangement. Final diagnosis needs fluorescent in-situ hybridization (FISH) and other molecular tests (31). Although the fusion partner of TFE3 is unknown in this case, previous studies of other tumors have shown that TFE3 always fused to the house-keeping gene. TFE3 contributes its DNA-binding domain, with imputed function in the fusion. House-keeping’s promoter leads TFE3 misexpression to promote tumorigenesis (32). However, TFE3 is not readily targetable. The mammalian target of rapamycin (mTOR) as TFE3 upstream regulator is targetable in some TFE3 rearranged tumor, like Xp11-RCC and ASPS. mTOR inhibitors (sirolimus) maybe an efficacious systemic treatment for EHE (33, 34). Correct diagnosis is essential for treatment. Unlike the less malignant EHE, hepatic angiosarcoma is a highly aggressive vascular tumor for which surgery is the most effective treatment. However, radical resection is difficult for multifocal lesions. Chemotherapy, tyrosine protein kinase inhibitors (TKI) therapy and immunotherapy may be effective.
There are some shortcomings in our study. First, although TFE3 rearrangement was confirmed by the FISH technique. Next-generation sequencing and RT-PCR were not performed to detect and validate TFE3’s partner gene. We can’t further study the oncogenic mechanism underlying the fusion of TFE3 and its partner and likely involved signal pathway. Second, we were unable to obtain samples from different liver nodules to verify whether multifocal lesions were monoclonal or polyclonal, and failed to figure out if it was metastasis or multiple primary tumors.
In conclusion, Hepatic multiple lesions with ring-enhancement should be considered as a possibility for EHE, besides metastases and primary liver cancer in imaging. Histologically,TFE3-rearranged EHE has distinct morphology features. PILA-like morphology is rarely described. Absence of well-formed vessels adds the difficult to diagnosis as well, especially in core biopsy. TFE3 immunostaining can be used as a screening tool, but is not specific for TFE3-rearranged EHE, and the final diagnosis is dependent on molecular testing. At present, both hepatic EHE with TFE3 rearrangement present multifocal lesions, and it is unclear whether they are metastatic or multiple primary tumors. More cases should be collected to verify this issue. Further study on the oncogenic mechanism underlying the fusion of TFE3 and its partner and the likely involved signal pathway is warranted to uncover its pathogenesis and target therapy. We recommend every 3 to 6 months follow-up for 2 to 3 years by CT and/or MRI with contrast according to National Comprehensive Cancer Network guidelines. Systemic treatment is recommended in the presence of multiple lesions or symptoms. Antiangiogenesis agents, cytotoxic agents, and anti-PD-1 antibody are recommended. mTOR inhibitors (sirolimus) maybe an efficacious systemic treatment for EHE as well. If acceptable, liver transplantation may be curative.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statementThe studies involving humans were approved by Research involving human subjects was approved by the Ethics Committee of the Second Affiliated Hospital of Dalian Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributionsKM: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. XY: Data curation, Writing – review & editing. SG: Data curation, Writing – review & editing. JT: Conceptualization, Project administration, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Anderson WJ, Fletcher CDM, Hornick JL. Loss of expression of YAP1 C-terminus as an ancillary marker for epithelioid hemangioendothelioma variant with YAP1-TFE3 fusion and other YAP1-related vascular neoplasms. Mod Pathol. (2021) 34:2036–42. doi: 10.1038/s41379-021-00854-2
PubMed Abstract | Crossref Full Text | Google Scholar
2. Puls F, Niblett A, Clarke J, Kindblom LG. McCulloch T.YAP1-TFE3 epithelioid hemangioendothelioma: a case without vasoformation and a new transcript variant. Virchows Arch. (2015) 466:473–8. doi: 10.1007/s00428-015-1730-y
PubMed Abstract | Crossref Full Text | Google Scholar
3. Ishak KG, Sesterhenn IA, Goodman ZD, Rabin L, Stromeyer FW. Epithelioid hemangioendothelioma of the liver: a clinicopathologic and follow-up study of 32 cases. Hum Pathol. (1984) 15:839–52. doi: 10.1016/s0046-8177(84)80145-8
PubMed Abstract | Crossref Full Text | Google Scholar
4. Mehrabi A, Kashfi A, Fonouni H, Schemmer P, Schmied BM, Hallscheidt P, et al. Primary Malignant hepatic epithelioid hemangioendothelioma: a comprehensive review of the literature with emphasis on the surgical therapy. Cancer. (2006) 107:2108–21. doi: 10.1002/cncr.22225
PubMed Abstract | Crossref Full Text | Google Scholar
6. Mascarelli PE, Iredell JR, Maggi RG, Weinberg G, Breitschwerdt EB. Bartonella species bacteremia in two patients with epithelioid hemangioendothelioma. J Clin Microbiol. (2011) 49:4006–12. doi: 10.1128/JCM.05527-11
PubMed Abstract | Crossref Full Text | Google Scholar
7. Tanas MR, Sboner A, Oliveira AM, Erickson-Johnson MR, Hespelt J, Hanwright PJ, et al. Identification of a disease-defining gene fusion in epithelioid hemangioendothelioma. Sci Transl Med. (2011) 3:98ra82. doi: 10.1126/scitranslmed.3002409
PubMed Abstract | Crossref Full Text | Google Scholar
8. Antonescu CR, Le Loarer F, Mosquera JM, Sboner A, Zhang L, Chen CL, et al. Novel YAP1-TFE3 fusion defines a distinct subset of epithelioid hemangioendothelioma. Genes Chromosomes Cancer. (2013) 52:775–84. doi: 10.1002/gcc.22073
PubMed Abstract | Crossref Full Text | Google Scholar
9. Rosenbaum E, Jadeja B, Xu B, Zhang L, Agaram NP, Travis W, et al. Prognostic stratification of clinical and molecular epithelioid hemangioendothelioma subsets. Mod Pathol. (2020) 33:591–602. doi: 10.1038/s41379-019-0368-8
PubMed Abstract | Crossref Full Text | Google Scholar
10. Zhang HZ, Dong L, Wang SY, Yang XQ. TFE3 rearranged epithelioid hemangioendothelioma of bone: a clinicopathological, immunohistochemical and molecular study of two cases. Ann Diagn Pathol. (2020) 46:151487. doi: 10.1016/j.anndiagpath.2020.151487
PubMed Abstract | Crossref Full Text | Google Scholar
11. Kuo FY, Huang HY, Chen CL, Eng HL, Huang CC. TFE3-rearranged hepatic epithelioid hemangioendothelioma-a case report with immunohistochemical and molecular study. Apmis. (2017) 125:849–53. doi: 10.1111/apm.12716
PubMed Abstract | Crossref Full Text | Google Scholar
12. Lotfalla MM, Folpe AL, Fritchie KJ, Greipp PT, Galliano GG, Halling KC, et al. Hepatic YAP1-TFE3 rearranged epithelioid hemangioendothelioma. Case Rep Gastrointest Med. (2019) 2019:7530845. doi: 10.1155/2019/7530845
PubMed Abstract | Crossref Full Text | Google Scholar
13. Flucke U, Vogels RJ, de Saint Aubain Somerhausen N, Creytens DH, Riedl RG, van Gorp JM, et al. Epithelioid Hemangioendothelioma: clinicopathologic, immunhistochemical, and molecular genetic analysis of 39 cases. Diagn Pathol. (2014) 9:131. doi: 10.1186/1746-1596-9-131
PubMed Abstract | Crossref Full Text | Google Scholar
14. Lahori M, Dehghani A, Wilson C, Law W, Agaram N, Murali R, et al. Cytopathologic features of epithelioid hemangioendothelioma including touch imprints for rapid on-site evaluation. Cytojournal. (2023) 20:29. doi: 10.25259/Cytojournal_57_2022
PubMed Abstract | Crossref Full Text | Google Scholar
15. Thway K, Mentzel T, Perrett CM. Calonje E.Multicentric visceral epithelioid hemangioendothelioma, with extremity dermal deposits, unusual late recurrence on the nasal bridge, and TFE3 gene rearrangement. Hum Pathol. (2018) 72:153–9. doi: 10.1016/j.humpath.2017.08.020
PubMed Abstract | Crossref Full Text | Google Scholar
16. Shibayama T, Makise N, Motoi T, Mori T, Hiraoka N, Yonemori K, et al. Clinicopathologic characterization of epithelioid hemangioendothelioma in a series of 62 cases: a proposal of risk stratification and identification of a synaptophysin-positive aggressive subset. Am J Surg Pathol. (2021) 45:616–26. doi: 10.1097/PAS.0000000000001660
PubMed Abstract | Crossref Full Text | Google Scholar
17. Song QY, Zhu XM, Song GX, Li X, Fan QH, Zhang ZH, et al. [Epithelioid hemangioendothelioma with TFE3 translocation in soft tissue: a clinicopathological study]. Zhonghua Bing Li Xue Za Zhi. (2021) 50:1151–6. doi: 10.3760/cma.j.cn112151-20210119-00054
PubMed Abstract | Crossref Full Text | Google Scholar
18. Abdelmogod A, Papadopoulos L, Riordan S, Wong M, Weltman M, Lim R, et al. A matched molecular and clinical analysis of the epithelioid haemangioendothelioma cohort in the stafford fox rare cancer program and contextual literature review. Cancers (Basel). (2023) 15:4378. doi: 10.3390/cancers15174378
PubMed Abstract | Crossref Full Text | Google Scholar
19. Liţescu M, Abduraim T, Paverman L, Vrabie CD, Dina I, Pleşea IE, et al. Epithelioid hemangioendothelioma - an unexpected diagnosis of a mediastinal tumor with extensive local thrombosis. Rom J Morphol Embryol. (2022) 63:197–202. doi: 10.47162/RJME.63.1.21
PubMed Abstract | Crossref Full Text | Google Scholar
20. Patel NR, Salim AA, Sayeed H, Sarabia SF, Hollingsworth F, Warren M, et al. Molecular characterization of epithelioid haemangioendotheliomas identifies novel WWTR1-CAMTA1 fusion variants. Histopathology. (2015) 67:699–708. doi: 10.1111/his.12697
PubMed Abstract | Crossref Full Text | Google Scholar
21. Zhou L, Cui MY, Xiong J, Dong Z, Luo Y, Xiao H, et al. Spectrum of appearances on CT and MRI of hepatic epithelioid hemangioendothelioma. BMC Gastroenterol. (2015) 15:69. doi: 10.1186/s12876-015-0299-x
PubMed Abstract | Crossref Full Text | Google Scholar
24. WHO Classification of Tumours Editorial Board. WHO classification of tumours. soft tissue and bone tumours. 5th ed. Lyon: IARC Press (2020). p. 172.
25. Errani C, Zhang L, Sung YS, Hajdu M, Singer S, Maki RG, et al. A novel WWTR1-CAMTA1 gene fusion is a consistent abnormality in epithelioid hemangioendothelioma of different anatomic sites. Genes Chromosomes Cancer. (2011) 50:644–53. doi: 10.1002/gcc.20886
PubMed Abstract | Crossref Full Text | Google Scholar
26. Miettinen M, Fetsch JF. Distribution of keratins in normal endothelial cells and a spectrum of vascular tumors: implications in tumor diagnosis. Hum Pathol. (2000) 31:1062–7. doi: 10.1053/hupa.2000.9843
PubMed Abstract | Crossref Full Text | Google Scholar
27. Kou K, Chen YG, Zhou JP, Sun XD, Sun DW, Li SX, et al. Hepatic epithelioid hemangioendothelioma: Update on diagnosis and therapy. World J Clin cases. (2020) 8:3978–87. doi: 10.12998/wjcc.v8.i18.3978
PubMed Abstract | Crossref Full Text | Google Scholar
28. Stockman DL, Hornick JL, Deavers MT, Lev DC, Lazar AJ, Wang WL. ERG and FLI1 protein expression in epithelioid sarcoma. Mod Pathol. (2014) 27:496–501. doi: 10.1038/modpathol.2013.161
PubMed Abstract | Crossref Full Text | Google Scholar
29. Kosemehmetoglu K, Rekhi B, Wakely PE Jr, Pant V, Dervisoglu S. Aydingoz U.Pseudomyogenic (epithelioid sarcoma-like) hemangioendothelioma of bone. Clinicopathologic features of 5 cases. Ann Diagn Pathol. (2019) 41:116–23. doi: 10.1016/j.anndiagpath.2019.06.003
PubMed Abstract | Crossref Full Text | Google Scholar
30. Errani C, Sung YS, Zhang L, Healey JH, Antonescu CR. Monoclonality of multifocal epithelioid hemangioendothelioma of the liver by analysis of WWTR1-CAMTA1 breakpoints. Cancer Genet. (2012) 205:12–7. doi: 10.1016/j.cancergen.2011.10.008
PubMed Abstract | Crossref Full Text | Google Scholar
31. Sharain RF, Gown AM, Greipp PT, Folpe AL. Immunohistochemistry for TFE3 lacks specificity and sensitivity in the diagnosis of TFE3-rearranged neoplasms: a comparative, 2-laboratory study. Hum Pathol. (2019) 87:65–74. doi: 10.1016/j.humpath.2019.02.008
PubMed Abstract | Crossref Full Text | Google Scholar
32. Kobos R, Nagai M, Tsuda M, Merl MY, Saito T, Laé M, et al. Combining integrated genomics and functional genomics to dissect the biology of a cancer-associated, aberrant transcription factor, the ASPSCR1-TFE3 fusion oncoprotein. J Pathol. (2013) 229:743–54. doi: 10.1002/path.4158
PubMed Abstract | Crossref Full Text | Google Scholar
33. Pozner A, Li L, Verma SP, Wang S, Barrott JJ, Nelson ML, et al. ASPSCR1-TFE3 reprograms transcription by organizing enhancer loops around hexameric VCP/p97. Nat Commun. (2024) 15:1165. doi: 10.1038/s41467-024-45280-5
PubMed Abstract | Crossref Full Text | Google Scholar
34. Robinson D, Leonard H, Baldi GG, Tap WD, Jones RL, Stacchiotti S, et al. The patient perspective on sirolimus for epithelioid hemangioendothelioma (EHE): results of a community survey highlighting the importance of equitable access to treatments. Front Oncol. (2024) 14:1367237. doi: 10.3389/fonc.2024.1367237
留言 (0)