Every year, a huge number of the population is affected by various neurological diseases like Alzheimer’s disease (1), Parkinson’s’ disease (PD), glioblastoma (2), different types of strokes (3), etc. The cases of such patients are continuously increasing worldwide, which could be attributed to the rapidly changing lifestyle, environmental factors, and genetic factors (4). Neurological disorders are disorders associated with the neurons and nervous system; one such disease affecting most of the population is PD (5, 6). PD is a progressive neurodegenerative disorder that is characterized by tremors, bradykinesia, rigidity, and postural instability and that mainly affects the motor system. PD significantly affects the quality of life (4, 7). PD mainly occurs due to the degeneration of dopamine-generating neurons in the substantia nigra. The substantial nigra a part of the brain which is vital for movement control. Due to the decline in dopamine levels in the brain, the communication between neurons gets disrupted, further leading to the hallmark motor symptoms of PD. Besides this, there are certain mon-motor symptoms, which include cognitive decline, mood disorders, and autonomic dysfunction, which also contribute to the disease burden. So, there is a requirement for precise diagnosis and treatment of PD (8, 9).
Currently, medical practitioners are focussing on symptom management rather than curing of the PD. Several conventional treatments, such as pharmacological, non-pharmacological, surgery, and emerging and experimental approaches, are available for treating PD (10). Among these, the pharmacological treatment-based approach is widely used to treat PD. The most extensively used pharmacological treatment is levodopa (11). Levodopa is a precursor to dopamine, which often combines with carbidopa to increase its effectiveness and reduce side effects. Besides this, dopamine agonists, monoamine oxidase B (MAO-B) inhibitors, and catechol-O-methyltransferase (COMT) inhibitors are some of the few options for the pharmacological treatment of PDs (12, 13). All three drugs aim to enhance or mimic dopamine activity in the brain. Moreover, surgical treatment involves deep brain stimulation (DBS) and lesioning surgery, out of which the former could be effective for patients of PD in the advanced stage who no longer respond adequately to the medicines. Even after treatment with such conventional approaches, the patients may experience fluctuating symptoms, side effects from the medicines, and disease progression (14, 15). So, there is a need for novel therapeutic strategies for PD that could overcome all the above-mentioned issues faced in conventional treatment. Several emerging novel techniques for treating PD include gene therapy (GT), nanomedicine, stem cells, and neuroprotective agents. Even though most of these techniques are in experimental stages only and need to go under clinical trials and evaluation, among all these emerging technologies, nanotechnology-based nanomedicines are gaining popularity among researchers for the theragnostic purposes of PD (16). Nanomedicine is the branch of nanosciences and nanotechnology that applies nanotechnology or nanoparticles (NPs) in medicine and offers promising new possibilities for diagnosing and treating PD (17). By developing the controlled size NPs, it is possible to deliver the drugs more effectively and precisely target affected regions in the brain. NPs are materials whose sizes fall from 1 to 100 nm (18, 19). Due to the small size and high surface area to volume ratio (SVR) of the NPs, they could easily cross the biological hurdles, like the blood-brain barrier (BBB) (20, 21). These BBBs traditionally restrict the efficacy of several treatments for neurological disorders. Furthermore, the NPs can be readily surface-modified with diverse organic compounds, ligands, and other agents to achieve target specificity, augmenting their efficacy. Additionally, certain NPs may function as nanocarriers, transporting drugs for PD on their surfaces, thereby facilitating the controlled and prolonged release of therapeutic agents. Furthermore, this focused drug administration should enhance therapeutic bioavailability and diminish side effects, thereby revolutionizing the management of PD (22, 23).
Recent theragnostic applications of nanomedicine for PD treatment include the formulation of various nanocarrier systems such as liposomes, nano gels, dendrimers, and solid lipid nanoparticles (SLNs) (24). All these nanocarrier systems are capable of encapsulating therapeutic agents for PD. The encapsulated therapeutic agents are shielded from enzymatic degradation, ensuring their delivery to the target site of action. Furthermore, nanomedicine presents opportunities for neuroprotective strategies by employing antioxidant and anti-inflammatory NPs to protect and repair damaged neurons. One more technique that demonstrates high possibilities for the treatment of PD is GT. GT involves the application of NPs as carriers for the delivery of desired genes that could correct or mitigate the underlying causes of PD (25). Besides this, integrating CRISPR/Cas9 technology with nanotechnology can address the root causes of neurodegeneration in PD. The integrated approaches significantly advance the quest for more effective and targeted therapies. The advancement of research in nanomedicine-based approaches promises to improve symptom management and patient outcomes and potentially slow the advancement of PD. The future of PD treatment lies in the successful translation of nanomedicine from the laboratory to clinical practice, opening the way for a new era in neurological care (25).
In the present review article, investigators have emphasized the basics of Parkinson’s disease, their pathophysiology, and current available treatment methods. Further emphasis was given to the drawbacks of the current drug for Parkinson’s disease. The authors have further focused on the role of nanotechnology, nanoparticles, and nanomedicine in treating Parkinson’s disease. The authors have highlighted the role of newly emerging techniques like gene therapy and CRISPR/Cas9 for the possible treatment of PD. Finally, emphasis was given to the possible integration of the nanoparticles with other emerging technologies for the symptomatic management of PD. Such investigation will pave the way for advanced technology and a new PD neurological care era.
2 Parkinson’s at a glance and their pathophysiologyPD is a chronic and progressive neurological disease that generally impacts the cortico-basal ganglia-thalamic circuitry in the brain (26). It is characterized by the degeneration of dopaminergic neurons in the substantia nigra, resulting in a deficiency of dopamine, a neurotransmitter vital for motor function (27). While PD is usually considered a disease that affects individuals over the age of 65, cases of early-onset Parkinson’s can also occur (28). Annually, it impacts approximately 3% of older people and ranks second behind Alzheimer’s disease (1). There is initial damage in β-oxidation in PD, reducing long-chain acylcarnitine levels (29). Consequently, there is a progressive accumulation of the presynaptic protein α-synuclein within intracellular fibres. The loss of dopamine neurons in the midbrain substantia nigra causes resting tremor, bradykinesia, and stiffness (30). The major problem is that no effective method exists to find the cause.
2.1 Pathophysiology of Parkinson’s disease 2.1.1 Mechanism of neurodegenerationPD is caused by the progressive degradation of dopaminergic nerve cells in the substantia nigra, a part of the brain that is essential for controlling movement (31). Dopamine levels rapidly decline due to neuronal death, disturbing the equilibrium between motor and non-motor functions. Furthermore, Lewy bodies and abnormal aggregates of the protein alpha-synuclein contribute to neuronal dysfunction and the clinical symptoms of the disease. A concise description of the pathogenesis of PD is provided. Initially, a significant advancement in comprehending PD involves elucidating the neurodegenerative process. The precise aetiology of this neuronal death is indeterminate; nonetheless, it is presumed to stem from the intricate interplay of genetic, environmental, and mitochondrial influences. Multiple critical pathways contribute to neurodegeneration in PD, oxidative stress (OS), mitochondrial failure, and neuroinflammation (32, 33).
OS is a prevalent phenomenon in the brain in PD, resulting from an imbalance between the production of deleterious chemicals known as reactive oxygen species (ROS) and the brain’s capacity to neutralize them (34). The increased OS in PD damages cellular components (lipids, proteins, and DNA), resulting in neuronal malfunction and death. This issue is further intensified by mitochondrial dysfunction, which impairs energy production and increases the creation of ROS. The characterization of neuroinflammation is carried out by the activation of microglia and the release of pro-inflammatory cytokines. The pro-inflammatory cytokines also play a crucial role in the neurodegenerative method, contributing to neuronal injury and cell death (35, 36).
2.1.2 Role of alpha-synucleinA small soluble protein (alpha-nuclein) is present in the brain in large amounts, mainly at the presynaptic terminals, and is expected to plays an important role in synaptic function and neurotransmitter release (37). Various pieces of literature have found that alpha-synuclein undergoes pathological aggregation in PD, forming insoluble fibrils. These insoluble fibrils accumulate as Lewy bodies and Lewy neurites within the nerve cells. The aggregation of such fibrils disrupts cellular function and contributes to neuronal death through several phenomena (38, 39).
Moreover, these proteins may misfold, further impair proteasomal and autophagic pathways. As a result, there will be an accumulation of damaged proteins and organelles. The synaptic function of the alpha-synuclein could also be disrupted by interfering with vesicle trafficking and the release of the neurotransmitter. Besides this, alpha-synuclein aggregates can spread from cell to cell, which may propagate pathology throughout the brain in a prion-like fashion. The presence of these aggregates is a hallmark of PD and is closely associated with the progression and symptomatology of the disease (38).
2.1.3 Dopaminergic pathwaysThe dopaminergic pathways, mainly the nigrostriatal pathway, are critically involved in the motor symptoms of PD. Dopaminergic pathways consist of dopaminergic neurons that project from the substantia nigra pars compacta to the striatum, a brain area associated with coordinating movement (40). If these neurons are lost, there will be a significant lowering in dopamine levels in the striatum. As a result, there will be a disruption in the balance of excitatory and inhibitory signals needed for smooth and controlled movements. Dopamine is very important for modulating the activity of the basal ganglia. The basal ganglia are a group of nuclei that play a valuable role in motor control. Depleting the dopamine level in PD will lead to overactivity of the indirect pathway and under activity of the direct pathway within the basal ganglia circuitry (41). This imbalance increases inhibitory output to the thalamus and motor cortex. As a result, there will be characteristic motor symptoms of PD, like bradykinesia, rigidity, and tremors. The degeneration of dopaminergic neurons affects motor symptoms and non-motor functions. This is because dopaminergic pathways regulate mood, cognition, and autonomic functions. This explains the wide range of non-motor symptoms noticed in PD patients, including autonomic dysfunction, depression, anxiety, and cognitive impairment (42, 43).
It could be concluded that the pathophysiology of PD involves the complex phenomenon of neurodegeneration, the pathological role of alpha-synuclein, and the disruption of dopaminergic pathways. Understanding these processes is vital for developing effective therapeutic strategies to slow or halt PD progression and improve patients’ quality of life. Figure 1 shows the pathophysiology of PD.
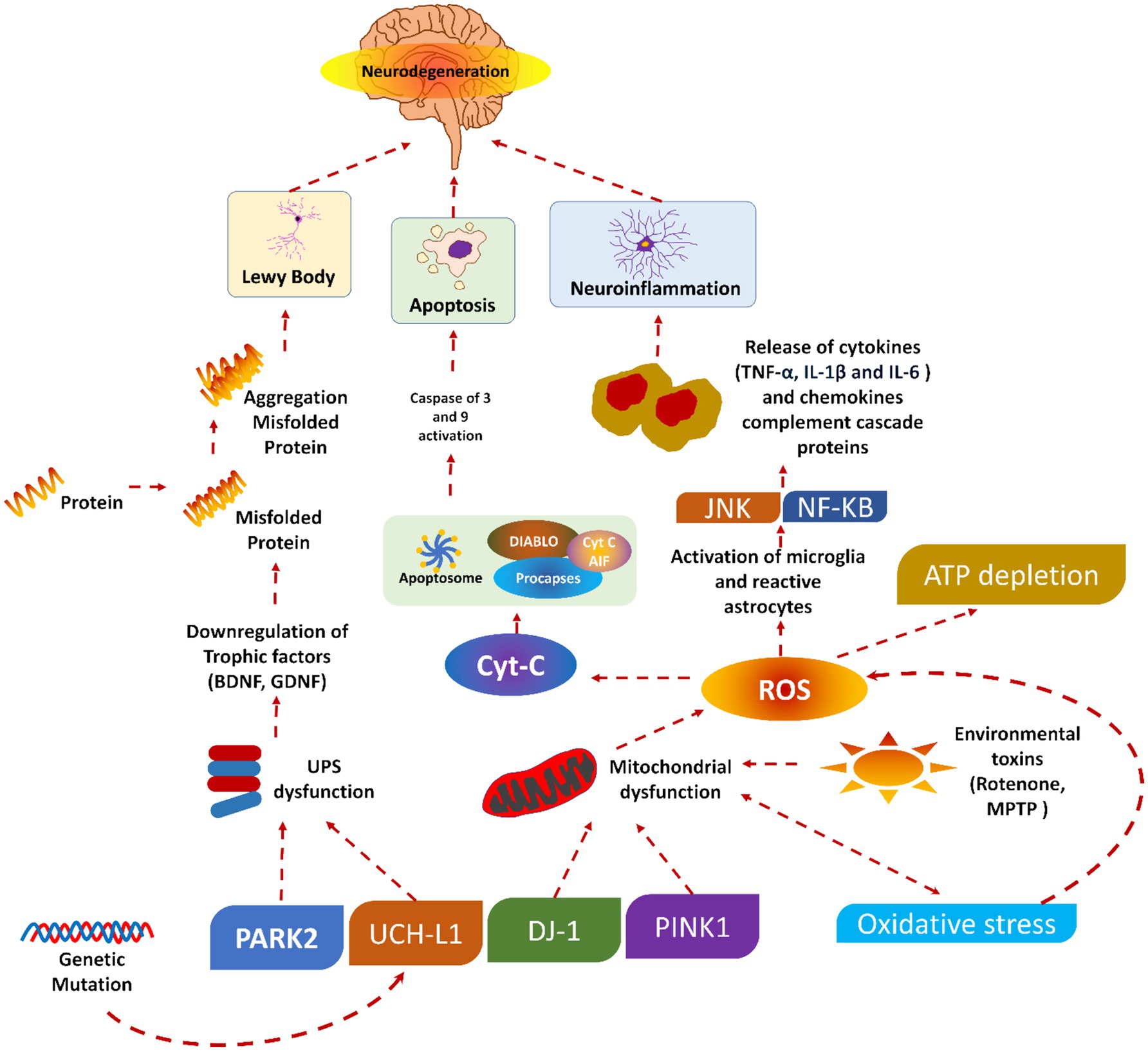
Figure 1. Pathophysiology of Parkinson’s diseases, reproduced from Doke et al. (189) with permission.
A summarized presentation of various genes involved in PD and their respective mechanisms of action is given in Table 1. Despite this, several pieces of information are still unknown, which warrants further investigation.
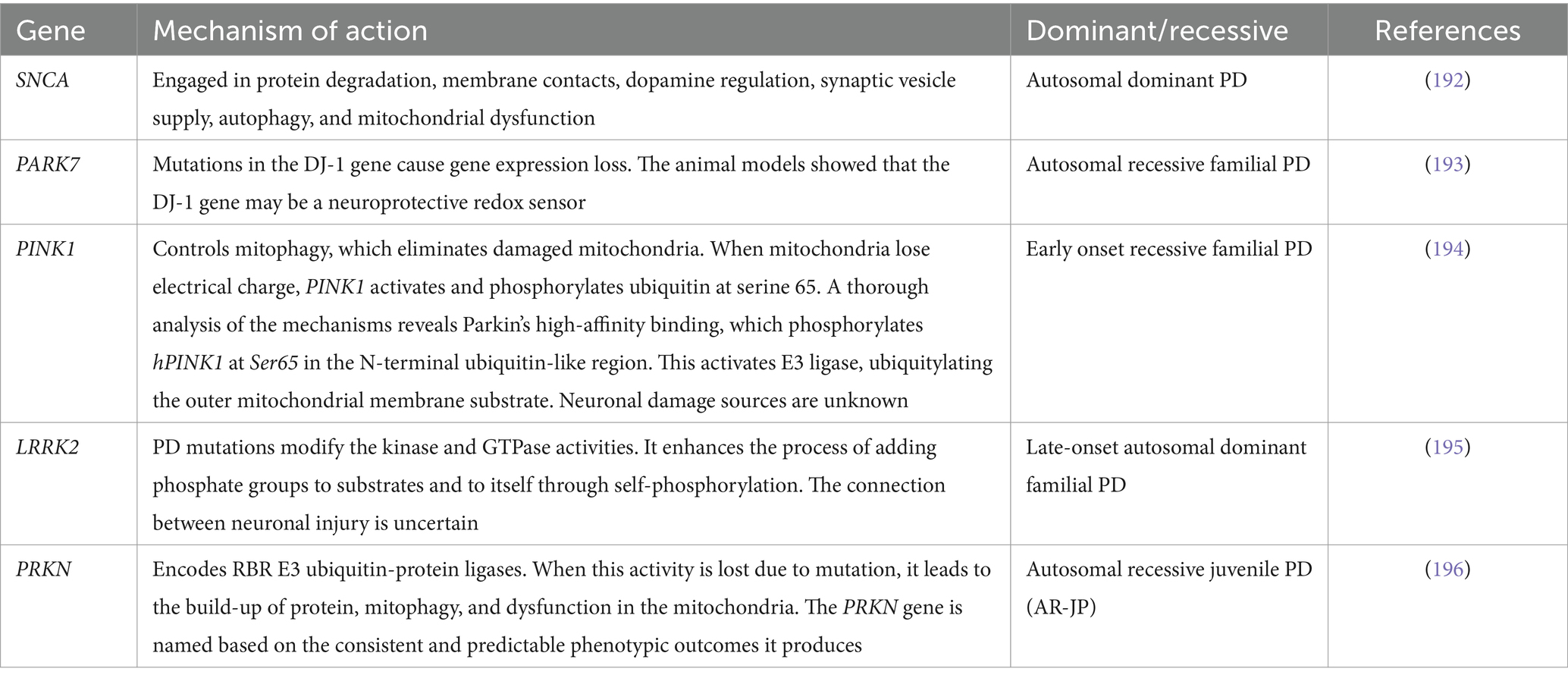
Table 1. Genes commonly implicated in the onset of Parkinson’s disease.
3 Current treatment approaches for the PDsTraditional approaches, such as pharmacological, non-pharmacological, and surgical, and some emerging treatment techniques, GT, stem cell therapy, neuroprotective agents, and nanomedicine, exist for treating PD (44). Figure 2 shows the broader categories of treatment options for PD, while Figures 3, 4 show various existing treatment strategies for PD.

Figure 2. Treatment options for PD adapted from Church (190).
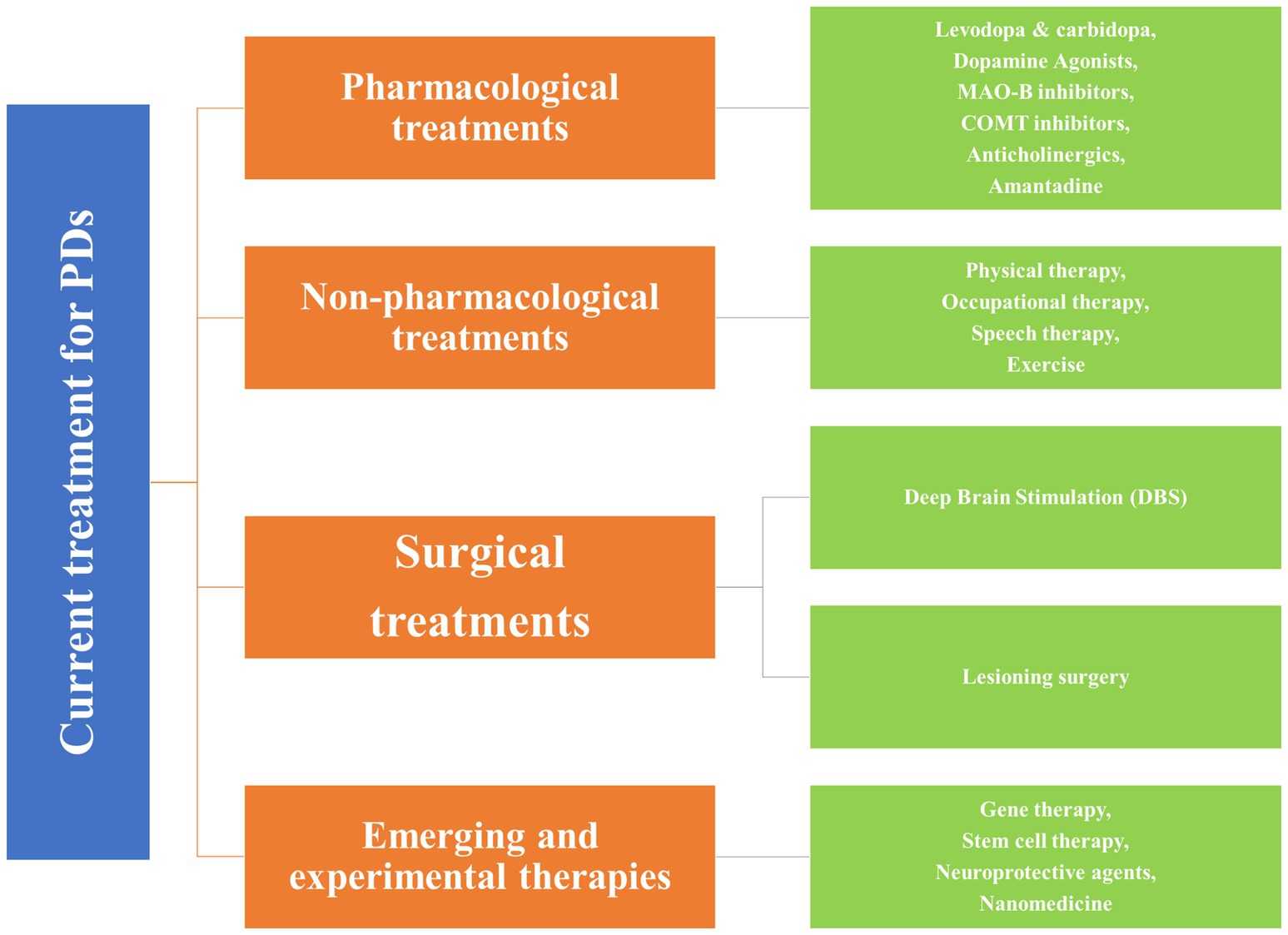
Figure 3. Current treatment approaches for the PDs.
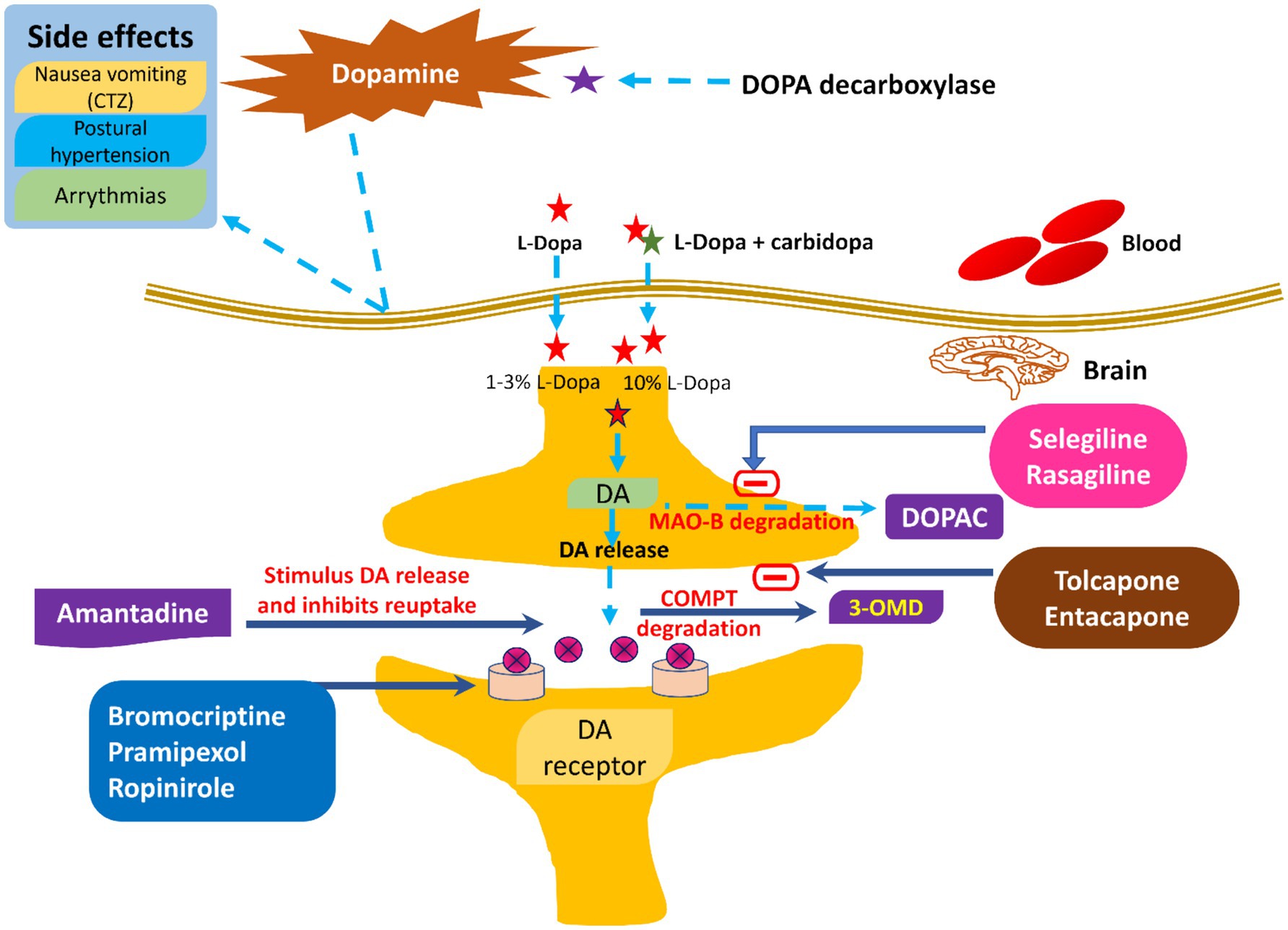
Figure 4. Current therapeutic management of PD along with their side effects.
3.1 Pharmacological treatments of PDsPharmacological treatments for PD encompass levodopa and carbidopa, dopamine agonists, MAO-B inhibitors, COMT inhibitors, anticholinergics, and amantadine (45). Levodopa is a highly effective and commonly utilized drug for PD, functioning as a precursor to dopamine that traverses the BBB and is subsequently converted to dopamine within the brain (46). Carbidopa is administered with levodopa to inhibit its early conversion to dopamine outside the central nervous system, thereby minimizing side effects like nausea and enhancing the quantity of levodopa penetrating the brain (47). The commonly utilized dopamine agonists are pramipexole, ropinirole, and rotigotine. These drugs replicate dopamine’s effects by directly activating dopamine receptors in the brain. These drugs may be utilized independently or in conjunction with levodopa to mitigate fluctuations in motor control. Selegiline and rasagiline are both inhibitors of MAO-B. MAO-B is an enzyme responsible for the degradation of dopamine in the brain, which subsequently leads to elevated dopamine levels and enhancement of motor symptoms (11, 48).
Both entacapone and tolcapone are COMT inhibitors that inhibit catechol-O-methyltransferase. COMT is an enzyme that breaks down levodopa (49). Besides this, COMT inhibitors are combined with levodopa to prolong its effects and reduce “off” periods. Both benztropine and trihexyphenidyl are anticholinergics that help control tremors and rigidity by decreasing acetylcholine activity (50). Acetylcholine is a neurotransmitter that can become overactive in PD due to the loss of dopamine (6, 51, 52). Amantadine is mainly an antiviral drug where the amantadine helps in reducing the symptoms of PD, particularly dyskinesia (involuntary movements) associated with prolonged use of levodopa. It could be concluded that there is little evidence to exhibit that dopamine prodrugs and levodopa would help alleviate dopamine neuron degeneration (28). It has been reported that such medical treatment causes several side effects like arrhythmia, gastrointestinal discomfort, extreme emotional changes, etc. (53). To date, not even a single drug is available, even after neuroprotective tests, which could delay or stop the progression of the disease. In addition to this, there are no effective biomarkers in PD, drugs having secondary hallucination and delusion effects, and the resistance of BBB. All these factors minimize the diagnosis and treatment of PD. Figure 5 shows various pharmacological treatment methods for PD (54).

Figure 5. Common pharmacological drugs for treating symptoms of PD.
Table 2 shows the classification of dopamine agonists, which are classified into two main classes: ergot-derived and non-ergot-derived. Among the ergot-derived dopamine agonists, bromocriptine, marketed as Parlodel, is administered orally. Cabergoline and lisuride are oral drugs in this category, though their market names are not specified. Pergolide, known as Permax, is another ergot-derived agent available in oral form (55, 56).
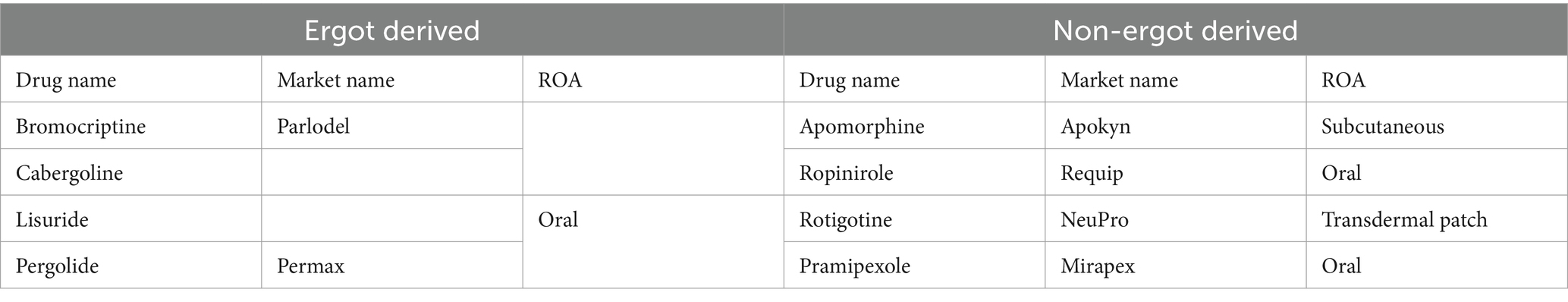
Table 2. Classification of dopamine agonists.
In contrast, the non-ergot-derived dopamine agonists include apomorphine, marketed as Apokyn, which is administered subcutaneously. Ropinirole, sold under the brand name requip, is an oral drug. Similarly, pramipexole, marketed as Mirapex, is also taken orally. Another notable non-ergot-derived agent is rotigotine, available as a transdermal patch under neupro (56, 57). This classification highlights the diversity of dopamine agonists in terms of their origin and routes of administration (ROA).
The pharmacological management of PD aims to enhance motor function by increasing dopamine levels. Additionally, there are limited treatments for non-motor symptoms, which can be significantly debilitating. Few treatments can impede or alter the progression of the disease. Levodopa and dopamine agonists are commonly utilized drugs for PD, although they may induce adverse consequences. Each patient’s treatment plan is personalized based on their symptoms, side effects, and requirements (43).
3.2 Non-pharmacological treatments of PDsThe non-pharmacological treatments of PDs include various approaches like physical, occupational, speech, and nutritional therapy and exercise (58, 59). Physical therapy focuses on improving mobility, balance, and strength through exercises, gait training, and assistive devices. Occupational therapy helps patients maintain independence by improving their ability to perform daily activities and recommending adaptive strategies and equipment (60). The difficulties associated with speech and swallowing could be addressed by speech therapy. Speech therapy provides exercises so that articulation, volume, and speech clarity, as well as techniques to manage dysphagia (difficulty swallowing), could be improved (61). In nutrition therapy, emphasis is given on the proper nutrition for managing symptoms and maintaining overall health. Advice from a well-trained dietitian could be very helpful for optimizing food choices. Moreover, a dietician could also help ensure adequate nutrient intake and manage gastrointestinal tract symptoms. Moreover, exercise could also help improve motor symptoms, balance, and overall well-being (62). The exercises may include regular physical activity like aerobic exercises, strength training, and flexibility exercises (63, 64).
Kalbe et al. (65) recently emphasized the significance of non-pharmacological interventions in managing PD. These therapies are considered supportive or “add-on” strategies primarily designed to mitigate motor symptoms. Physiotherapy, speech-language, and occupational therapy have increasingly emerged as essential to comprehensive PD management. Additionally, non-pharmacological interventions such as cognitive training, cognitive behavioural therapy, and art or light therapy, previously deemed “exotic,” are now beginning to be integrated into therapeutic guidelines. Additionally, several advancements have been noted in this field, including (i) the expansion of intervention types, (ii) the standardization of intervention protocols, (iii) the development of digital intervention forms, (iv) the scientific evaluation of intervention feasibility and effects, (v) the understanding of underlying mechanisms of therapy-induced plasticity methods, (vi) the integration of non-pharmacological interventions into patient care concepts, and (vii) transition from merely symptomatic to preventive treatments (66). Figure 6 summarizes the various non-pharmacological treatments of PDs.
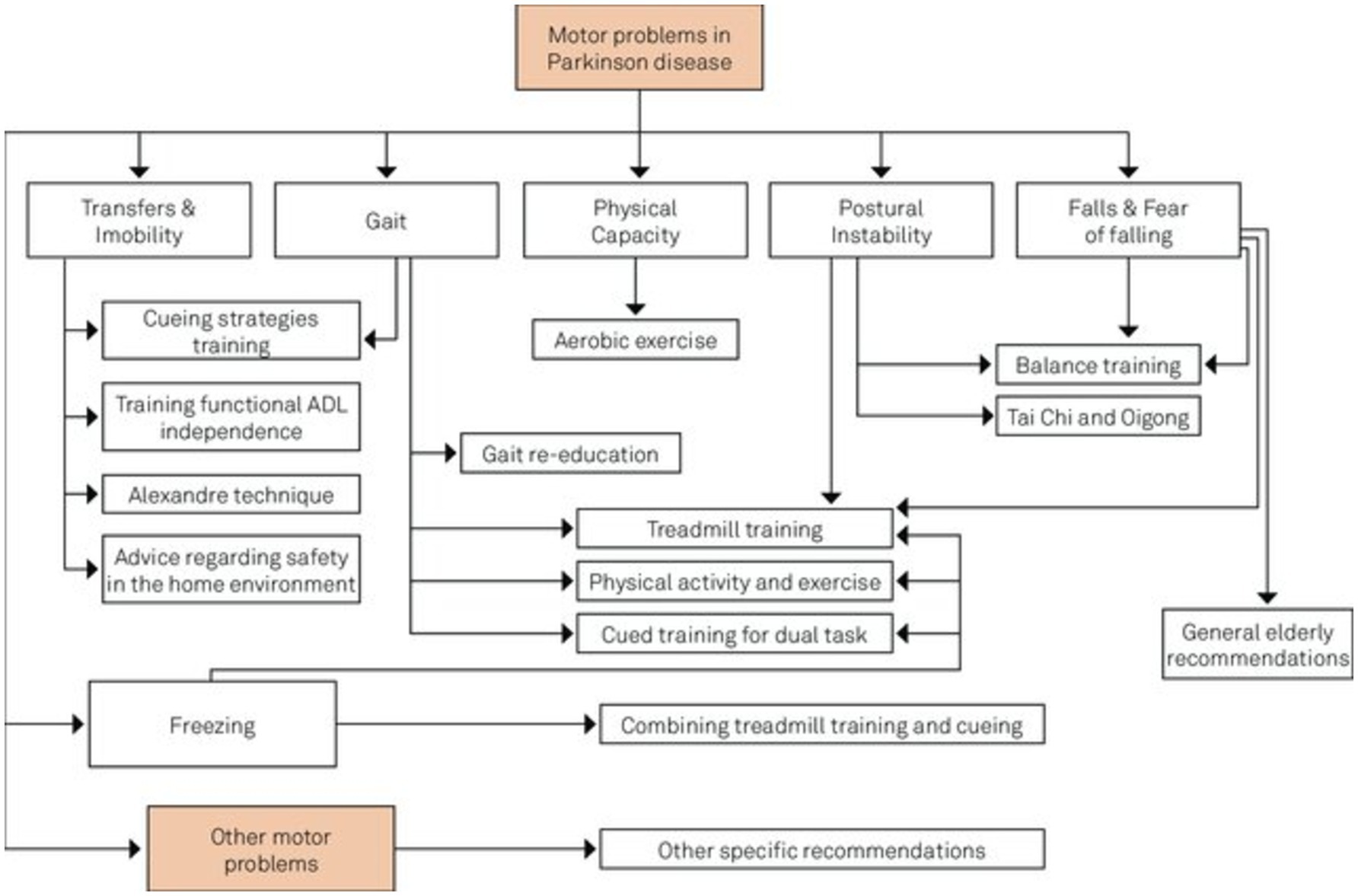
Figure 6. Intersection of non-pharmacological treatment interventions for PD adapted from Domingos et al. (191).
3.3 Surgical treatment of PDPD could also be treated by using surgical therapy, which mainly includes DBS and lesioning surgery (67). DBS is a surgical procedure involving the implantation of electrodes into the subthalamic nucleus or globus pallidus internus of the brain. Additionally, these electrodes are linked to a pulse generator implanted in the thoracic cavity. Further, these pulse generators send electrical impulses to the brain to help regulate abnormal impulses. In lesioning surgery techniques (pallidotomy and thalamotomy), a small lesion is created in the targeted areas of the brain to disrupt abnormal brain activity (68). DBS is preferred over lesioning surgery from both available surgical therapies (14).
Sharma et al. (69) reviewed DBS and lesioning techniques for PD, addressing indications, selection processes, and management strategies. The investigators proposed that a team make the decision regarding surgical treatment for PD, taking into account the patient’s clinical characteristics, treatment efficacy, ease of use, associated risks, and overall quality of life. Still, DBS is the most preferred surgical technique, but there is a growing interest in new techniques like MR-guided focused ultrasound.
Recently, Foltynie et al. (70) presented the latest evidence in support of the optimal treatment of PD. Further investigators described an expert approach to several aspects of treatment choice with insufficient evidence base. Here, the investigators suggested treatment for different phases of PD. For instance, firstly, there should be initial treatment of motor PD; secondly, adjunctive treatment of motor PD; thirdly, non-oral therapies for the complex phase of PD; and finally, treatment of non-motor symptoms. The first treatment for PD should involve a detailed discussion about the diagnosis, treatment options, and how to maintain the patient’s independence and quality of life based on their specific symptoms. Some patients may not get enough relief from symptoms with initial treatment, and if certain symptoms persist, it may be necessary to consider other types of PD. Continuous dopaminergic or electrical stimulation targets the limited therapeutic range between symptom relief and peak dose dyskinesia, which arises during the advanced stages of PD. Non-motor symptoms in PD can affect many areas like mood, sleep, and pain and may be more challenging than movement issues, often linked to low dopamine levels and needing careful management.
3.4 Emerging and experimental approaches for the treatment of PDsAbove all, some emerging and experimental approaches for treating PDs include GT, stem cell therapy, neuroprotective agents, and nanomedicine (71). Literateurs have proven that in GT, genetic material has been introduced into the brain to correct or modify the underlying genetic causes of PD. Several clinical trials in their early stages explore using viral vectors to deliver genes. These genes may either increase the production of dopamine or protect nerve cells. Stem cell therapy is new to this field, and extensive research is being done on using these stem cells to replace lost dopaminergic neurons in the brain. Still, such approaches are in their experimental stages (72). Neuroprotective agents are also in their infancy, and several investigations are being conducted to formulate drugs that could help protect the neurons from degeneration. These neuroprotective agents include antioxidants, anti-inflammatory drugs, and agents targeting mitochondrial function (73). Besides this, nanomedicine is another technique in its infancy, but it has a huge potential for treating PDs. By utilizing nanotechnology, enhanced drug delivery and targeting could be achieved (25). Besides this, nanotechnology may improve efficacy and reduce the side effects of current treatment methods. Several nanocarriers (liposomes, nanoparticles) have been studied for their potential to cross the BBB and deliver therapeutic agents directly to affected brain regions. In contrast, Yuan et al. (74) explore the neurotoxic effects of SiO2 NPs, particularly in promoting PD-like pathology both in vitro and in vivo, suggesting a potential link between SiO2 NPs exposure and the initiation and progression of PD. While these emerging approaches hold promise, it’s important to acknowledge that they also have their limitations. This awareness is crucial for understanding the current challenges in PD treatment. Some of the complications associated with the current treatment approaches are summarized below in Table 3.
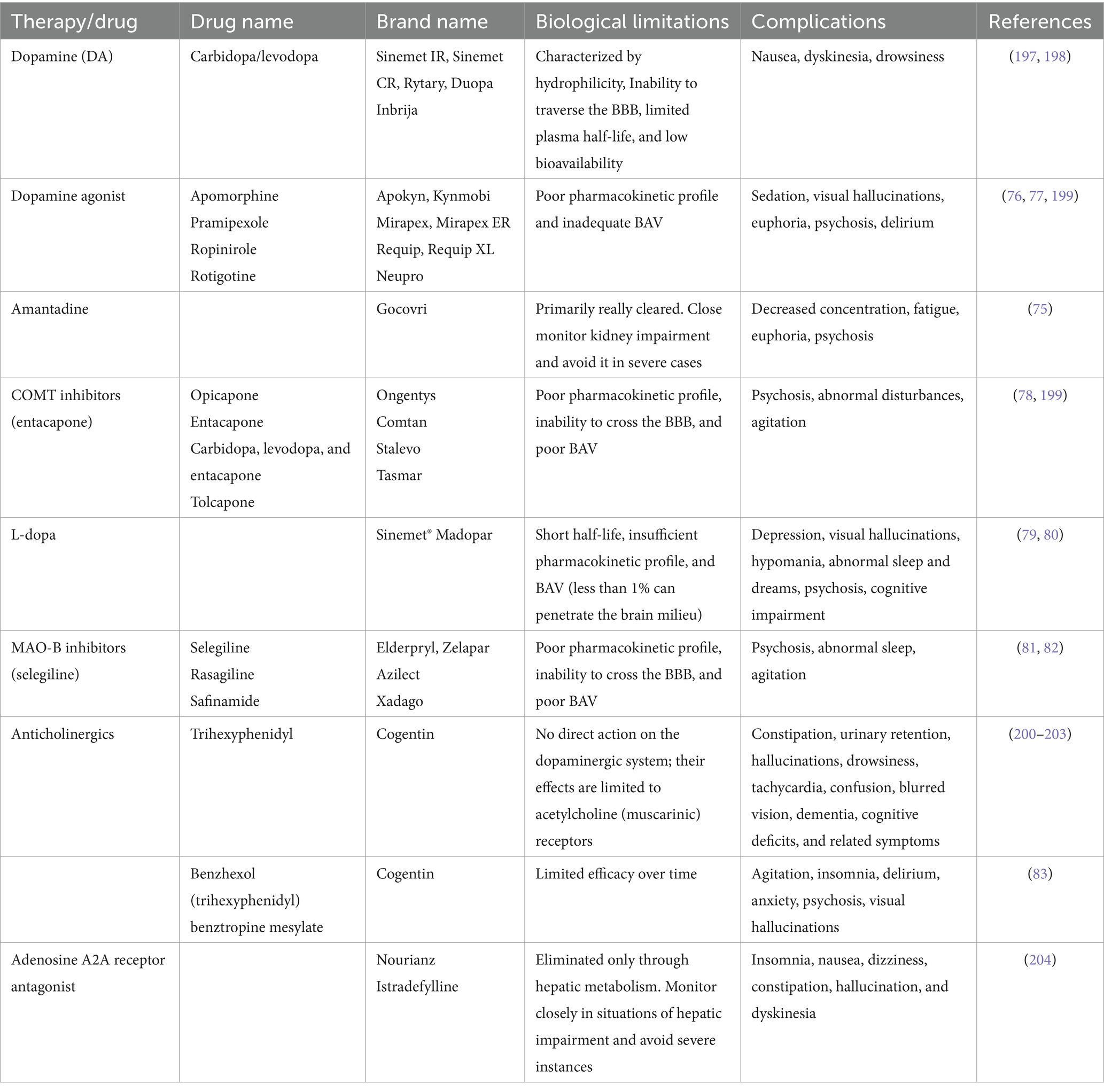
Table 3. Biological limitations and complications associated with the current treatment approaches for PD.
From the above table, it could be concluded that even though each drug is beneficial in the treatment of PDs, it also has several complications. For instance, amantadine may lead to decreased concentration and fatigue, along with the possibility of euphoria and psychosis (75). Dopamine agonists exhibit efficacy in managing PD symptoms but can be associated with sedation, visual hallucinations, euphoria, and psychosis, potentially leading to delirium (76, 77). COMT inhibitors like entacapone may induce psychosis, abnormal disturbances, and agitation (78). L-dopa, a cornerstone in PD treatment, may lead to depression, visual hallucinations, hypomania, abnormal sleep patterns, psychosis, and cognitive impairment (79, 80). Similarly, MAO-B inhibitors (selegiline) can lead to psychosis, abnormal sleep, and agitation (81, 82). Finally, benzhexol may induce agitation, insomnia, delirium, anxiety, and visual hallucinations (83). In clinical practice, weighing the therapeutic benefits against the potential adverse effects is crucial in individualizing treatment approaches for patients with PD.
4 Nanoparticles for the treatment of PDNPs offer a promising approach to treating PD due to their small size. Besides this, the NPs could be easily engineered for specific targeting and the potential to improve drug bioavailability and efficacy (84). The NPs could be of various shapes, sizes, and origins, i.e., metallic or organic, solid or liquid, and carbon-based. The widely used NPs for treating PDs are lipid-based NPs, polymeric NPs, metal NPs, and carbon-based NPs, described below in detail. NMs hold substantial promise for progressing diagnostics in PD through three vital strategies (85, 86). Firstly, NMs offer the potential for governing crucial biomarkers such as α-synuclein, a protein associated with PD pathology (87). By preparing nanoscale tools and sensors, the detection and quantification of α-synuclein levels can be improved, assisting early diagnosis and disease monitoring. Secondly, NMs enable the monitoring of active dopamine neurons, providing beneficial insights into the status of dopaminergic neurotransmission, which is central to PD. NMs are efficient carriers for delivering therapeutic agents to specific target regions in the brain. The current NMs demonstrate effectiveness by addressing dopamine deficiency, caspase-3 activation, α-synuclein accumulation, OS, mitochondrial dysfunction, inflammation, and growth factor supplementation (88). The findings indicate a broad spectrum of potential approaches for Parkinson’s disease therapy (89). Generally, NPs can be classified as inorganic, organic, or carbon-based NPs (Figure 7).
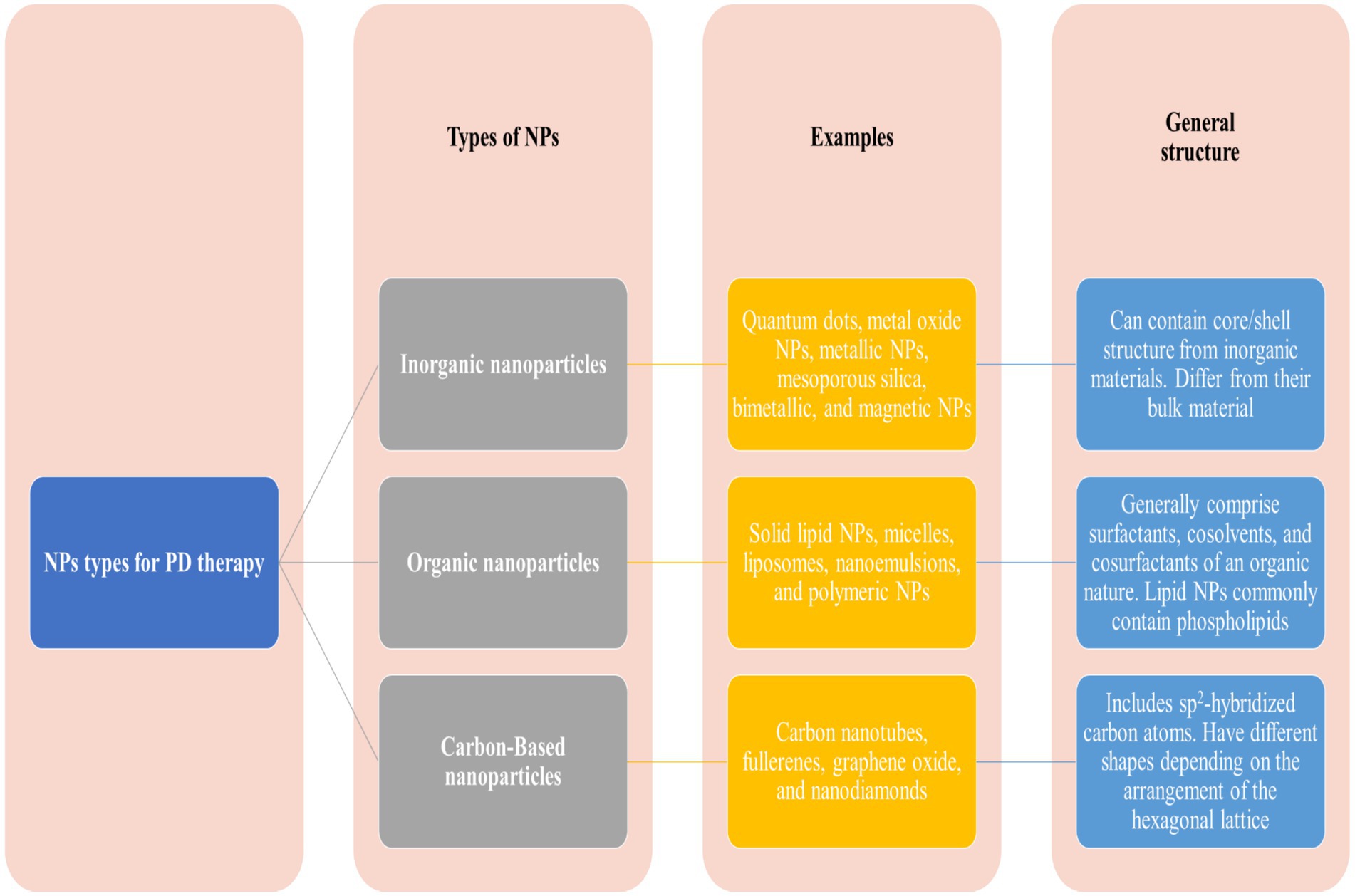
Figure 7. Major classes of nanoparticles used in nanomedicine.
4.1 Lipid-based nanoparticlesLipid-based NPs mainly comprise liposomes and SLNs, which are commonly investigated for treating PD. Such NPs encapsulate hydrophilic and hydrophobic drugs, protect them from degradation, and enhance their delivery across the BBB (90). Liposomes are spherical vesicles made up of lipid bilayers and are particularly advantageous due to their biocompatible nature and ability to fuse with cell membranes (Figure 8). The fusion of the liposomes with the cell membrane facilitates the release of the drug directly into the target cells. SLNs are made up of solid lipids, which offer a stable alternative to liposomes and can be applied for the effective delivery of lipophilic drugs effectively (91).
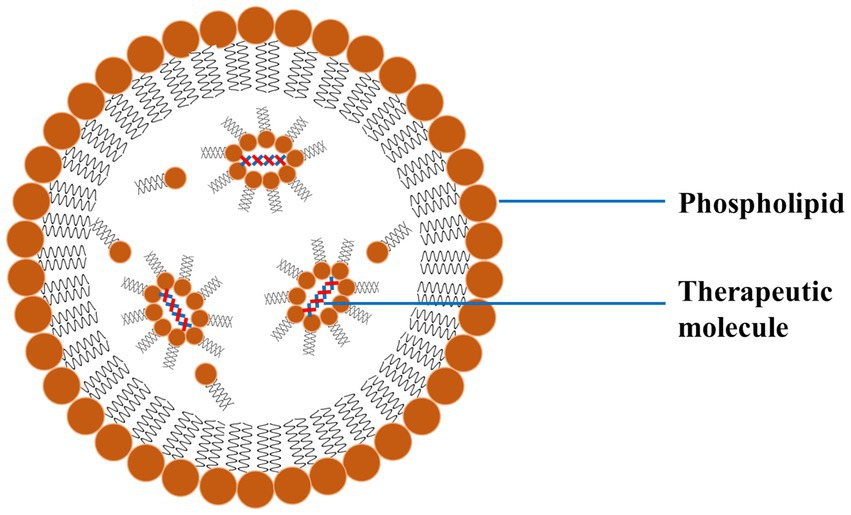
Figure 8. A schematic diagram of LNP exhibiting the outer phospholipid layer and the encapsulated therapeutics reproduced from Jagaran et al. (145) with permission.
4.2 Polymeric nanoparticlesPolymeric NPs are organic compounds composed of biocompatible and biodegradable polymers like polylactic acid (PLA) and polyglycolic acid (PGA) (92). These polymeric NPs form an important class of NPs extensively applied for treating PD (92). One major advantage of using these NPs in PD treatment is their ability to release drugs in a controlled and sustained manner. This reduces dosing frequency and makes it easier for patients to stick to their treatment plan. These polymeric NPs constitute an important class of NPs that are widely used in the treatment of PD (93). One major advantage of using these NPs in PD treatment is their controlled and sustained drug release, which reduces dosing frequency and improves patient compliance (94, 95). Polymeric NPs are designed so that they get dissolved by themselves within the body. Due to the degradation of such polymeric NPs, the encapsulated drug is released over a predetermined period. Besides this, such polymeric NPs could also be functionalized with targeting ligands to enhance the specificity of dopaminergic neurons (96).
Various studies have investigated the administration of exogenous dopamine (DA) and L-dopa into the CNS to supplement the reduced levels of endogenous DA and counteract the loss of dopaminergic neurons. Advanced polymeric nanoparticulate formulations can potentially improve PD symptoms by delivering DA directly to the brain (97).
Arisoy et al. (12) developed a NP system using PLGA and WGA to deliver L-dopa through the nose, which helps the drug reach the brain directly and avoids the BBB. This method showed better drug delivery to the brain with fewer side effects than traditional oral or nasal methods, as seen in tests on mice with PD. The NPs system had a high drug retention rate of 73% and released L-dopa over 9 h, indicating its effectiveness and safety in the nasal cavity. However, using this delivery method may be difficult for patients with nasal issues like sinusitis or rhinitis, which could affect how the system works.
Ragusa et al. (98) effectively synthesized chitosan NPs that contained DA and evaluated the neuroprotective properties of the system in vitro using human neuroblastoma SH-SY5Y cells. They conducted additional research on the diverse impacts of DA, uncoated chitosan NPs, and chitosan-dopamine NPs in generating ROS. Monge-Fuentes et al. (99) created a new NP system loaded with DA (7 μg DA per mg of NPs) made up of albumin and PLGA (353 to 497 nm in diameter; PDI: 0.4 to 0.6; zeta potential −27 to −37 mV) that helps improve motor skills in mice with Parkinson’s disease by delivering dopamine and crossing the BBB effectively.
Vong et al. (100) developed a nano-polymeric drug system (NanoDOPA) to enhance the brain delivery and therapeutic efficacy of L-dopa in a mouse model of PD. The drug gradually released L-dopa through chymotrypsin degradation and significantly enhanced the in vivo pharmacokinetic profile of L-dopa in comparison to free L-dopa. The administration of NanoDOPA in the MPTP-induced PD mouse model led to significant enhancements in motor symptoms and reduced dyskinesia commonly linked to L-dopa treatment (see Table 4).
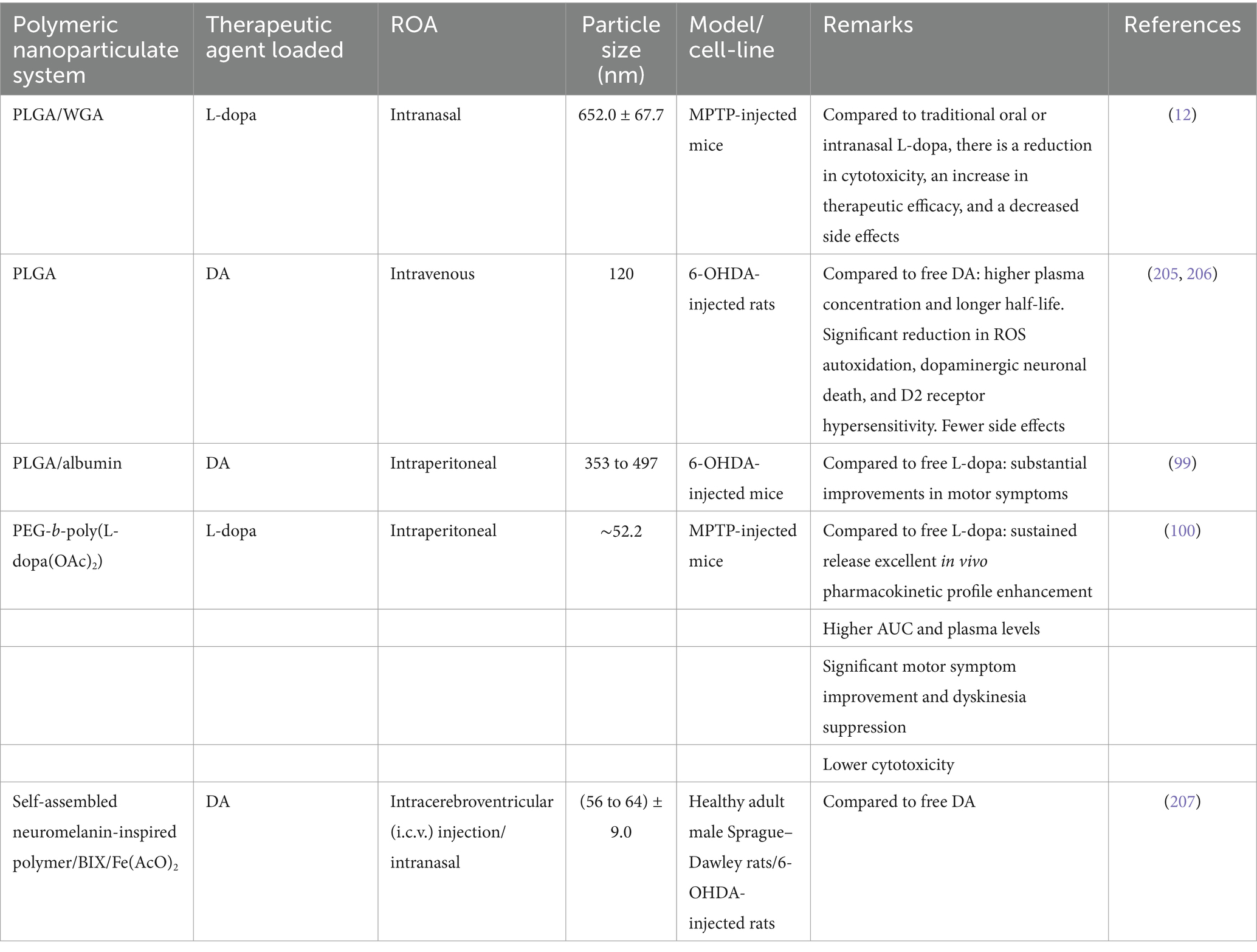
Table 4. The utilization of polymeric nanoparticulate systems to replace DA/L-dopa in PD.
4.3 Metallic nanoparticlesMetallic NPs (Ag, Au, Zn, Pt, Fe, etc.) also play a crucial role in treating PD due to their small size and unique optical and electronic properties (101, 102). Due to these features, such NPs could be exploited for therapeutic and diagnostic purposes in PD. The various metallic NPs used to treat PDs are gold (AuNPs) and silver nanoparticles (AgNPs) (103). For instance, AuNPs could be functionalized with drugs or targeting molecules and used for targeted delivery of drugs or as contrast agents for imaging. Besides this, such metallic NPs can be used to modulate the activity of enzymes engaged in OS. The OS is a key pathological PD property, offering neuroprotective effects (104, 105). Among metallic NPs, noble NPs like gold and silver have gained huge attention due to their high stability, easy synthesis routes, and easy surface functionalization.
AuNPs and AgNPs have demonstrated promise in inhibiting the aggregation of extracellular and intracellular protein aggregates in neurodegenerative disorders. Both the NPs can function as stabilizers by selectively attaching to certain areas of protein molecules, thereby preventing protein misfolding (106). This interaction hampers the proteins from assuming abnormal structures, creating harmful clusters. Furthermore, these noble NPs can act as “seeds” for protein aggregation. By emulating the protein’s original form, they entice the misfolded proteins and assist in their attachment to the NP surface. This process isolates the proteins from other molecules, decreasing their capacity to combine and create larger clusters. Moreover, both the NPs have exceptional catalytic capabilities. Both can facilitate the breakdown of misfolded proteins by catalytic mechanisms, such as the generation of ROS or the stimulation of cellular enzymes responsible for removing proteins. This augmented protein breakdown helps in preventing the buildup of harmful protein aggregates. The well-surface functionalized NPs can specifically engage with inflammatory cells in the brain, thus reducing the neuroinflammatory response. Due to the constant inflammation in neurodegenerative diseases, AuNPs and AgNPs have shown inherent antioxidative characteristics. Both of these NPs can eliminate ROS, which is known to play a significant role in causing neuronal damage through neuroinflammation (106). These NPs may improve brain inflammation caused by OS by decreasing levels of ROS. Moreover, it has been evident from the literature that both noble NPs might impede the synthesis and discharge of proinflammatory cytokines, including interleukin-1β (IL-1β), tumor necrosis factor-alpha (TNF-α), and IL-6. These cytokines play a vigorous role in neuroinflammation. NPs can potentially decrease the inflammatory cascade in the brain by inhibiting these cytokines. Furthermore, AuNPs and AgNPs regulate the nuclear factor-kappa B (NF-κB) pathway. NF-κB is a crucial transcription factor that activates many proinflammatory genes. Nps disrupt the NF-κB signalling pathway, inhibiting the synthesis of inflammatory mediators (104, 107).
AuNPs, as metallic NPs, are the most widely used in medicine due to their chemically inert nature, as in most cases, they do not undergo oxidation processes (108). This unique property makes them versatile and applicable in various medical contexts. Due to this reason, the surface of AuNPs can be easily functionalized with various types of chemical groups or dyes. Literature proves that AuNPs can act as a contrast and therapeutic agent in CNS disorders, further highlighting their potential in diverse medical applications (108, 109).
Hu et al. (110) developed composite materials of AuNPs (CTS@GNP-pDNA-NGF) by combining electrostatic adsorption and photochemical immobilization methods. These composites can potentially carry plasmid DNA (pDNA) and selectively target particular cell types. The Au NPs were introduced into cells through endocytosis to suppress the death of PC12 cells and dopaminergic neurons. Further, Au NPs composites are utilised in in vivo PD models, where they may effectively penetrate the BBB and accumulate in the brains of mice. It is concluded that the AuNP composites exhibit favourable therapeutic effects for in vitro and in vivo models of PDs.
AuNPs have also been employed in PD models. For instance, a study led by da Silva Córneo et al. (111) demonstrated that administering AuNPs to a mouse model of PD produced by reserpine significantly reduced OS markers and improved motor symptoms. The administration of AuNPs resulted in a partial improvement of neurotrophic factors. Specifically, the therapy at a concentration of 20 nm with a dosage of 2.5 mg/L successfully reversed the symptoms of the generated PD without causing any harmful effects.
A study by Xue et al. (112) exhibited that AuNPs synthesized from the root extract of Paeonia moutan effectively remove ROS and reduce the levels of inflammatory cytokines in microglial cells, as demonstrated in vitro. Moreover, the in vivo findings confirmed that therapy with AuNPs effectively avoided neuroinflammation in dopaminergic neurons and elevated dopamine levels. As a result, the mice with PD were protected from motor abnormalities. Both studies showed that therapy with AuNPs reversed the behavioural symptoms caused by PD, indicating the effective protective impact of AuNPs on dopaminergic neurons without any toxic effects. This treatment also prevented side symptoms associated with the condition.
The compatibility and interaction of AuNPs with pharmacological drugs render anti-inflammatory and other targeted drugs significant. These drugs are utilized in the treatment of neurodegenerative diseases, such as PD and AD. A study demonstrated that L-dopa, when associated with AuNPs, can mitigate the adverse effects of the drug while enhancing its therapeutic efficacy and prolonging its effect duration without compromising potency. The potential applications of Au NPs are effective and promising, broadening the scope of NP utilization in biomedical contexts as a novel treatment (113). Besides these two noble metal NPs, several other metal oxide NPs (iron oxide and silica) have also been used as therapeutic and contrast agents for PDs.
4.4 Carbon-based nanoparticlesBesides polymeric and metallic NPs, certain carbon-based NPs are widely used to treat PD. These carbon-based NPs include carbon nanotubes (CNTs), fullerenes, and graphene. The CNTs could be either single-walled (SWCNTs) or multi-walled (MWCNTs) (114). All these carbon-based NPs have high surface area, high mechanical strength, and the ability to penetrate cell membranes, which hold a promising potential for treating PD. Moreover, the CNTs could be further surface functionalized with various molecules to increase their solubility and biocompatibility. Surface-functionalized and biocompatible CNTs could be used to target the delivery of therapeutic agents (115). Both graphene and its derivatives have excellent conductivity and flexibility, which could be used to deliver drugs. Besides this, graphene and its derivatives act as scaffolds for neuronal growth, potentially facilitating the repair of damaged neural tissues (116).
One study used molecular dynamics simulation tools to investigate the impact of 4 forms of last-generation graphene-based nanostructures on preventing α-synuclein amyloid fibrillation. This study found that nitrogen-doped graphene (N-graphene) is the best in preventing the formation of harmful protein aggregates linked to PD, making it a promising option for treatment. This was so because N-graphene is the most effective at disrupting the structure of α-synuclein amyloids, showing strong interactions and causing significant instability compared to other monolayers (117, 118).
4.5 Metal-organic frameworksThe investigators have found that enzyme-integrated metal-organic frameworks (MOFs) had antioxidant activity and chiral-dependent BBB trans endocytosis. These frameworks may treat PD as anti-neuroinflammatory drugs (119). By adding extremely small platinum nanozymes (Ptzymes) to L-chiral and D-chiral imidazolate zeolite frameworks, chiral nanozymes are generated. In male PD mouse models, Ptzyme@D-ZIF accumulated more brain tissue than Ptzyme@L-ZIF. This was due to longer plasma presence and more BBB crossing routes, including clathrin- and caveolae-mediated endocytosis. These variables explain Ptzyme@D-ZIF’s increased therapeutic efficacy in reducing behavioural and pathological changes. Bioinformatics and biochemical studies show that Ptzyme@D-ZIF prevents neuroinflammation-induced apoptosis and ferroptosis (120) in damaged neurons. In enzyme-integrated chiral ZIF platforms, live organisms, metabolism, and therapeutic effects vary, suggesting the potential for PD medicines (121).
4.6 Mechanisms of action of NPsThe therapeutic effect of the NPs could be exerted on the PD through various processes. One basic feature is enhancing the drug delivery across the BBB, which ensures that a higher concentration of the therapeutic agent reaches the brain (122). This particular feature has an important role in PD as several drugs have limited ability to cross the BBB on their own (123). Besides this, NPs could also provide a controlled and sustained release of drugs, which maintains the therapeutic levels over extended periods and reduces side effects involved with peak-dose fluctuations. In addition to this, NPs can be designed to target specific cellular pathways associated with PD pathology. It has been found that customized NPs could deliver antioxidants or anti-inflammatory agents to lower the OS and neuroinflammation, which are the major contributors to neuronal degeneration. Besides this, NPs could also be used to deliver neurotrophic factors or GT agents. The neurotrophic factors or GT agents promote neuronal survival and repair, which offers potential disease-modifying effects (124). Table 5 exhibits a summarized form of the implications of nanomedicine on PD.
留言 (0)