Coccidioides is a type of dimorphic fungus that includes C. immitis and C. posadasii. Epidemiological data have shown that Coccidioides is mainly prevalent in central and southern California, the low-lying desert regions of Arizona, southeastern New Mexico, western Texas, the southwestern United States, Mexico, Central America, and South America, with strong geographical distribution patterns (1). Inhalation of arthroconidia into the lungs is the primary exposure factor. Coccidioidomycosis commonly manifests as an asymptomatic infection or a mild respiratory infection in humans and other vertebrate hosts. Occasionally, approximately 4.7% of coccidioidomycosis cases are reported to spread from the lungs to other organs, such as the skin, bone, spine, and meninges. This condition is known as disseminated coccidioidomycosis (2), which usually develops rapidly. Failure to diagnose and treat this condition in time can result in poor prognosis and even death (2, 3). Therefore, early and accurate diagnosis is crucial for the treatment of disseminated coccidioidomycosis.
Coccidioidomycosis is rarely reported in China. From 1958 to 2021, only 47 cases were reported, and the majority of these cases have a history of travel to endemic areas (4, 5). As it is rare in China, the majority of clinical laboratories do not carry out Coccidioides-specific routine detections. In addition, its clinical signs and imaging presentations are non-specific, making it easy to misdiagnosis as a tumor or another disease (1, 6, 7). Therefore, diagnosing coccidioidomycosis is difficult in non-endemic areas (8).
This article reports on a patient with unexplained recurrent fever and headache who was transferred to our hospital after receiving ineffective treatment at a local hospital. The cerebrospinal fluid (CSF) metagenomic next-generation sequencing (mNGS) analysis revealed low reads of C. immitis-specific gene sequences, but the repeated CSF bacterial cultures showed no pathogens. To confirm the diagnosis, the Clinical Microbiology Laboratory in Huashan Hospital extended the CSF culture duration, based on the growth characteristics of Coccidioides reported in the literature (9, 10). Ultimately, 12 days after sampling, the fungal bottle containing the cerebrospinal fluid tested positive. Furthermore, the diagnosis of C. immitis was then confirmed by smear staining and the morphological characteristics of the colony, providing an important etiological basis for clinical diagnosis and treatment. This article will describe and discuss this case along with relevant insights.
Case presentationA 27-year-old male patient was admitted to the emergency department of Huashan Hospital due to a headache and fever on 11 August 2022. Around 2 months (25 June) earlier, he had been diagnosed with community-acquired pneumonia with symptoms including cough and irregular fever (up to 39.0°C) at the local hospital (Yangzhou, Jiangsu). After antibacterial treatment, the symptoms improved significantly. However, half a month earlier (31 July), the patient developed a persistent headache and fever, and the symptoms progressively worsened. For further diagnosis, he visited our hospital.
Upon admission, the chest CT scan showed scattered inflammation in the lower lobe of the left lung, but no abnormalities were observed in the head during the enhancement CT scan (Figure 1B). Except for the routine blood test showing increased leukocytosis and eosinophilia, all other laboratory exams, including urinalysis, blood routine, liver and kidney function, blood glucose, blood ketones, coagulation function, procalcitonin, and myocardial markers, showed no abnormalities. As headache was the most critical sign, lumbar puncture was performed on 13 August. The CSF analysis revealed positive Pandy test (protein 2,071 mg/L), intracranial pressure greater than 300 mmH2O, a white blood cell count (695 × 106/L) higher than the normal range, and glucose (1.2 mmol/L) and chloride(115 mmol/L) levels lower than the normal range (Figure 2). All the above results indicated the possibility of an infection in the central nervous system. Empiric combinatory therapy (levofloxacin 0.5 g ivgtt qd and ceftriaxone 2.0 g ivgtt bid) for bacterial meningitis was administered. However, there was no relief from the symptoms.
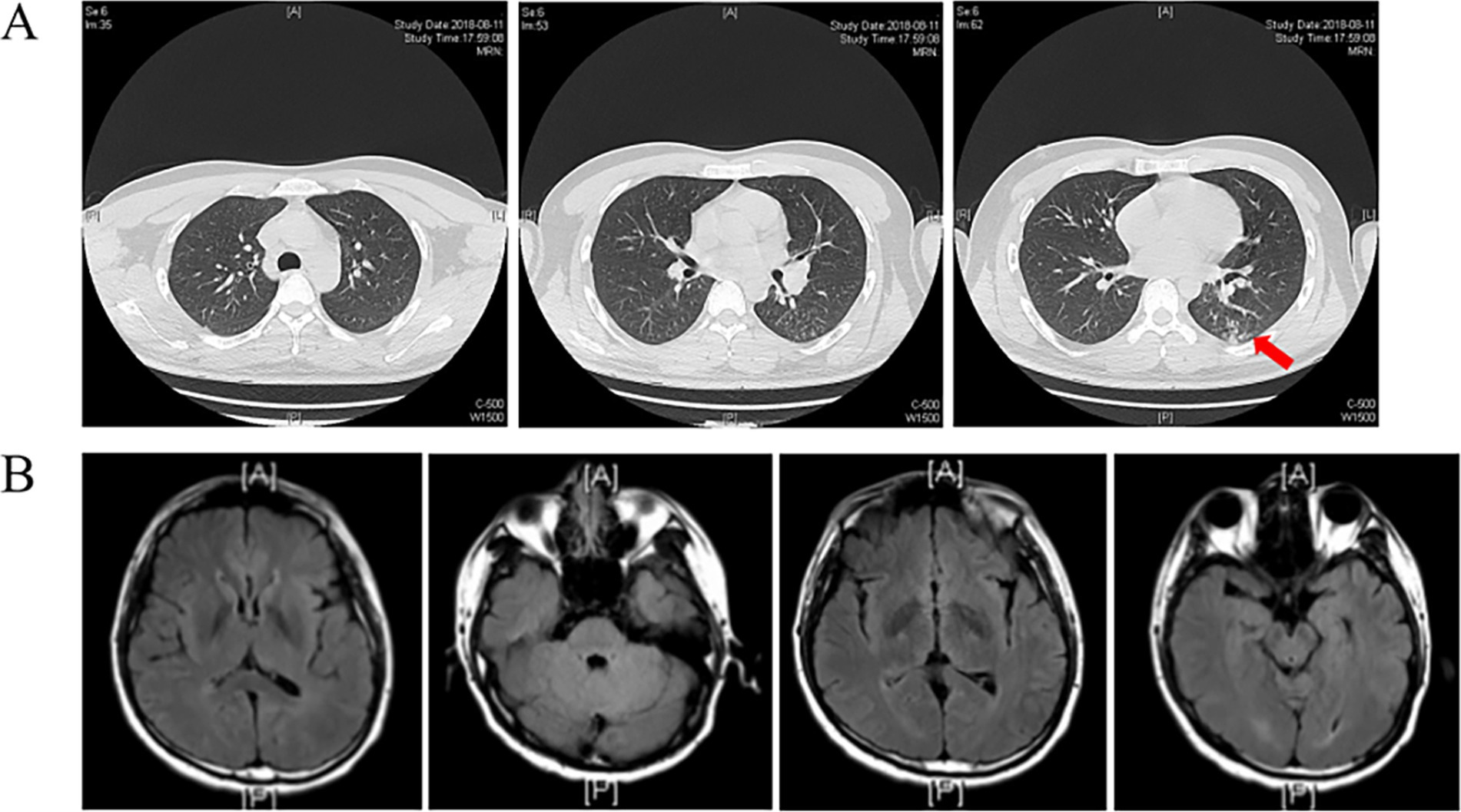
Figure 1. (A) Chest CT scan shows increased pulmonary markings in both lungs, with scattered patchy shadows in the left lower lobe (Arrow indicated). The trachea and main bronchi were patent. No enlarged lymph nodes were seen in the mediastinum or hilum; (B) Enhanced CT of the head shows no apparent abnormalities.
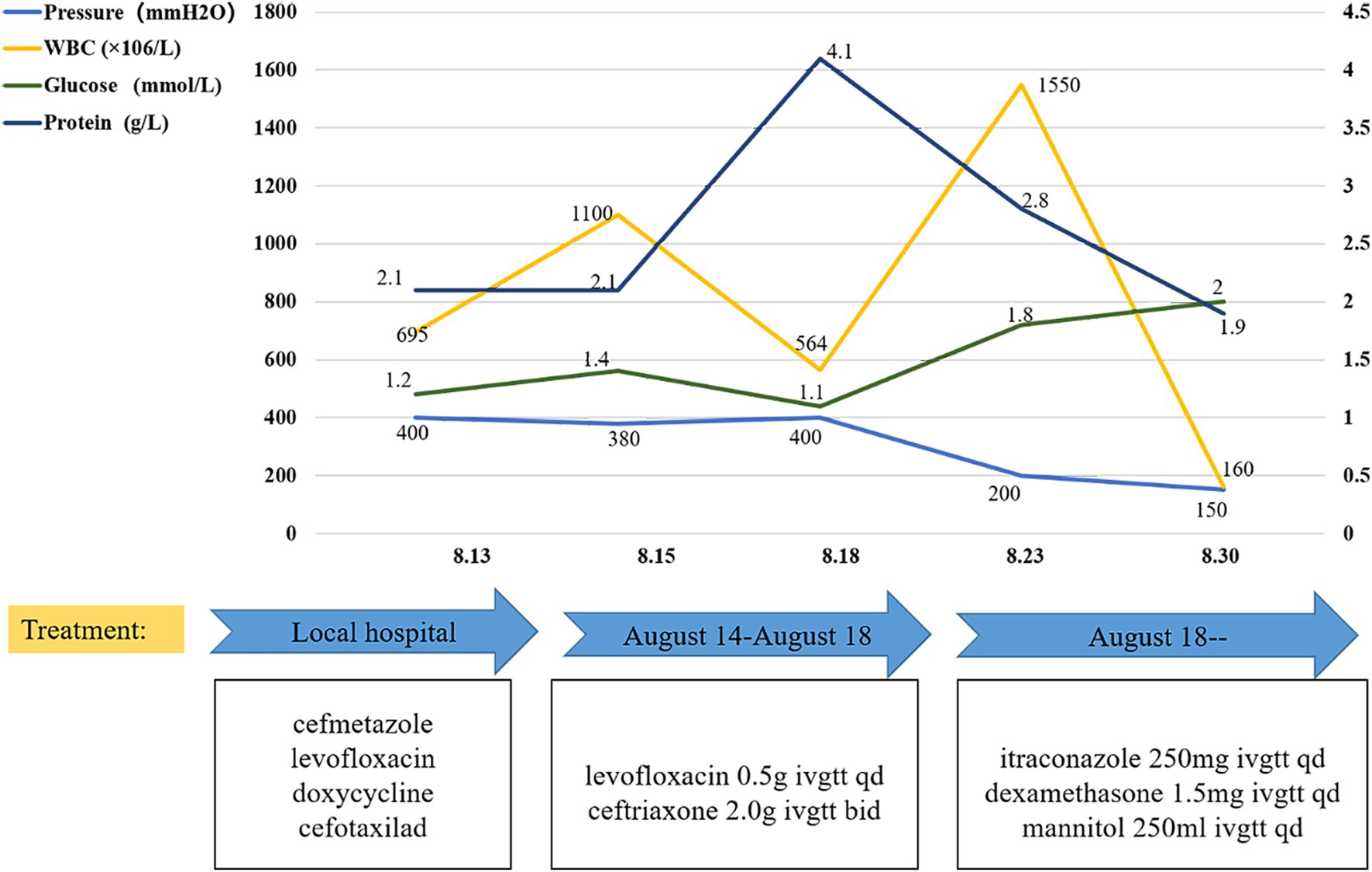
Figure 2. CSF investigation and antimicrobial drugs. After adjusting the treatment, the CSF factors, including glucose content, protein content, WBC, and intracranial pressure, gradually returned to normal.
As all etiological examinations showed negative results, CSF mNGS analysis was performed to further identify the pathogen. On 17 and 19 August, two CSF mNGS analyses suggested low reads of C. immitis (only four unique sequence reads matched). mNGS is a novel gene sequencing approach used to rapidly identify pathogens in clinical samples, but it cannot be used as a diagnosis method when only low reads of suspicious pathogens are detected (11). Due to the detection of C. immitis, which is generally considered to be limited to the Western Hemisphere, the patient’s travel history was further reviewed. The patient had lived in Los Angeles, USA, for approximately 3 months (March 2018 ~ June 2018) to support his wife with child-rearing. During this period, he grew scallions in his garden and had contact with the soil.
The subsequent second cerebrospinal fluid mNGS test also detected C. immitis. To verify the mNGS results, we improved the CSF culture condition according to references (7). A cerebrospinal fluid fungal culture was then conducted. Fortunately, 12 days after sampling (August 23), the fungus/mycobacteria culture flask showed positive results, and a cotton wool-like substance was produced in the flask (Figure 3A). Then, smear staining was performed, as shown in Figure 3, and thick-walled spheroids and segmented hyphae were observed using both Gram staining and Fluorescence staining. The culture material was stained with lactophenol cotton blue, revealing the characteristic microscopic features of Coccidioides, including barrel-shaped arthroconidia connected by thin-walled, empty-connected cells. The Gram staining and fluorescence staining revealed the presence of branching, septate hyphae, rather than thick-walled spherules, indicating a differential diagnosis of Malbranchea spp. At the same time, the products were transferred to Sabouraud dextrose agar (SDA) plates. The hyphal stage was observable on the SDA plates at 28°C, whereas the yeast phase appeared at 35°C, consistent with the traits of biphasic fungi (Figures 4A,B). Articular spores, a critical characteristic of C. immitis, were clearly identified through colony Medan staining (Figure 4C). Based on the growth and morphological characteristics of this fungus, we confirmed the mNGS results, identifying it as C. immitis. Subsequently, colony ITS sequencing and homology analysis were performed using BLASTp against the NCBI database to further confirm the species. Due to the lack of access to a laboratory capable of conducting serological testing, it was not performed in this study.
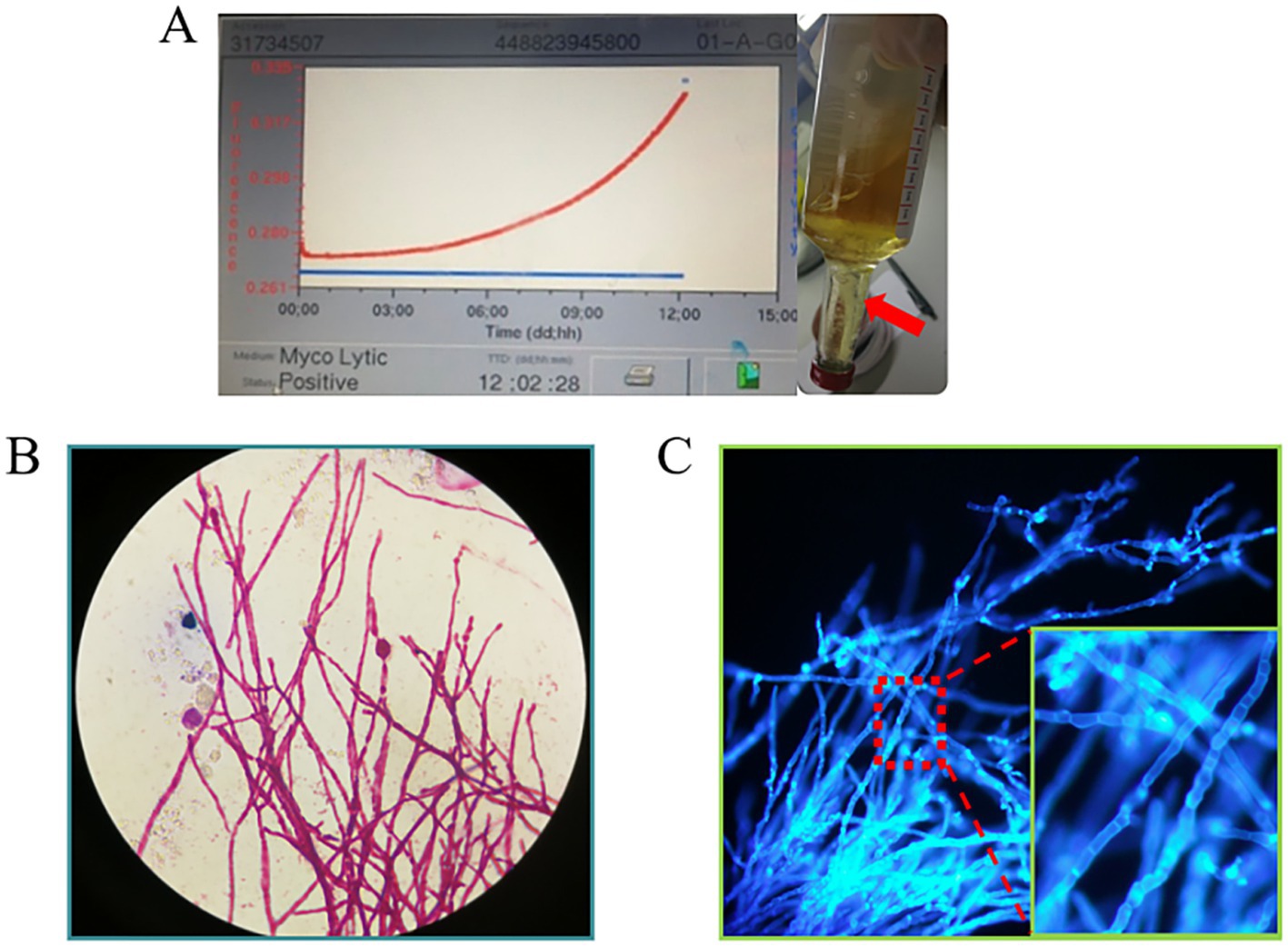
Figure 3. (A) Cerebrospinal fluid fungal culture flask annulation. After 12 days of continuous monitoring, the flask reported positivity (with an increasing growth curve). Cotton wool-like precipitation (indicated by red arrow) was visible in the bottle; (B,C) The cottony material was extracted and subjected to Gram staining and fluorescent staining separately. At high magnification (10×40), thick-walled spheroids and segmented hyphae were visible.
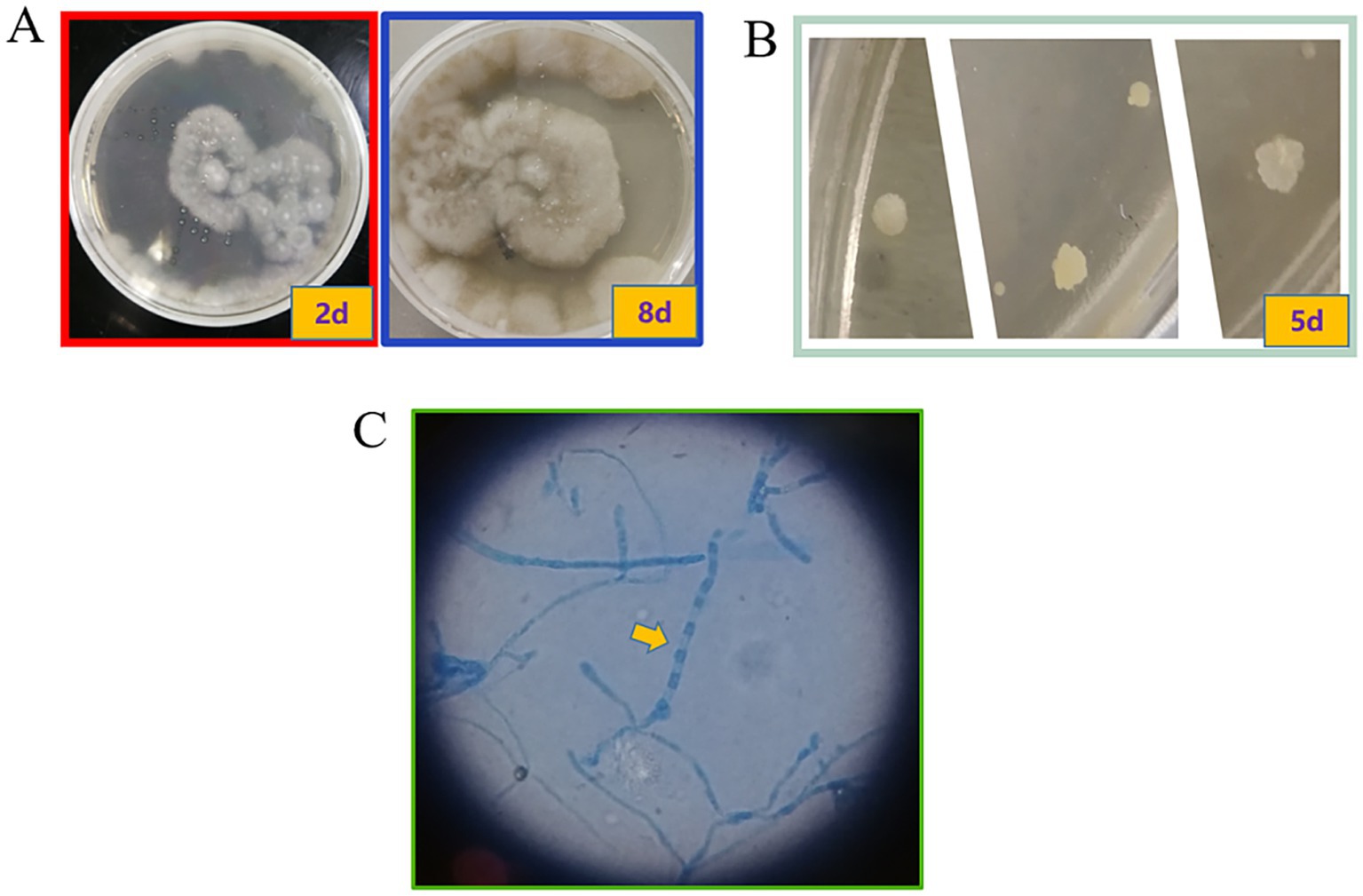
Figure 4. Colony on SDA plates after incubation at different temperatures. Coccidioides is a temperature-dependent dimorphic fungus that exhibits different colony morphologies at different temperatures. (A) The colony grown at 28°C had suede to villi, gray-white, and tan to brown; (B) The colony grown at 35°C was in the white yeast phase. (C) Through lactophenol cotton blue staining, arthroconidia and a large number of rectangular, barrel-shaped arthroconidia with septa were observed. These are thin-walled, connected cells. The arthroconidia and thin-walled connected cells alternate to form chains (10×100).
Therefore, the clinic changed his treatment from antibacterial to antifungal therapy. As the first-line drug for Coccidioides, Itraconazole was administered according to our identification results (12, 13). The detailed treatments are shown in Figure 2. With anti-coccidioidal treatment, the patient showed gradual improvement, which further corroborated that the patient’s symptoms were indeed caused by disseminated Coccidioides infection. By 30 August, there was a notable improvement in the patient’s CSF compared to the previous measurements and the symptoms also significantly improved. Subsequently, the patient was followed up at the infectious disease department of our hospital. After the diagnosis, the patient was treated with itraconazole, starting with a loading dose of 200 mg IV q12h for 2 days, followed by a maintenance dose of 200 mg IV qd. After 6 months, the treatment plan was changed from IV to oral administration: itraconazole oral solution 200 mg po q12h and prednisone 5 mg BID for treatment. The patient’s condition remained stable, and he continued to be followed up in the infectious disease department. For patients with Coccidioides meningitis, lifelong treatment with triazole medications is recommended.
DiscussionAs a result of population mobility, coccidioidomycosis cases are increasing in China (14). In addition, coccidioidal meningitis has a high mortality rate (90% within 1 year and 100% within 2 years). Therefore, early identification of the pathogen and prompt antifungal treatment are critical for a favorable prognosis. However, the etiology examination of coccidioidal meningitis remains a clinical challenge in non-endemic areas.
The diagnosis of coccidioidomycosis is mainly based on clinical manifestations, exposure history, and laboratory tests (15). In terms of laboratory tests, mNGS has emerged as one of the routine methods for detecting unknown pathogens in clinical microbiology laboratories, with the advantages of high sensitivity and specificity (15). mNGS is particularly recommended for diagnosing infections caused by new, rare, difficult-to-culture, or mixed pathogens (11). However, the cost of mNGS sequencing is high, and it cannot directly correlate suspected pathogens with clinical symptoms. Therefore, it is important to isolate pathogens using traditional cultural methods.
In this case, both the symptoms and the regular CSF analysis suggested an infection in the CNS; however, the standard CSF cultures showed negative results (no pathogen). This may be related to the fact that the culture was only observed for 3 days at 35°C on common bacterial media, which are not suitable for the growth of Coccidioides (which requires 28°C, using SDA, and can take 2–9 days for growth) (8). After adjusting the cultural conditions, we successfully observed the Coccidioides colony and its critical morphology. This finding highlights the need for microbiology laboratories to maintain close communication with clinics. When patients report a history of travel to endemic areas and exhibit symptoms such as headache, fever, and vomiting, along with CSF analysis revealing elevated opening pressure, increased protein levels, hypoglycorrhachia, and pleocytosis (16), we should consider changing the standard culture conditions to those suitable for the growth of Coccidioides.
This case highlights the potential of combining mNGS with traditional cultural methods to diagnose unknown pathogens quickly and accurately.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statementWritten informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributionsHL: Writing – original draft, Writing – review & editing. KZ: Writing – review & editing. CZ: Writing – original draft. MG: Writing – original draft, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Weida, L, and Zhao, LG. Current situation of coccidioidomycosis in China. Chin J Mycol. (2020) 15:321–4. doi: 10.3969/j.issn.1673-3827.2020.06.001
Crossref Full Text | Google Scholar
2. Nguyen, C, Barker, BM, Hoover, S, Nix, DE, Ampel, NM, Frelinger, JA, et al. Recent advances in our understanding of the environmental, epidemiological, immunological, and clinical dimensions of coccidioidomycosis. Clin Microbiol Rev. (2013) 26:505–25. doi: 10.1128/cmr.00005-13
PubMed Abstract | Crossref Full Text | Google Scholar
4. Liang, G, Shen, Y, Lv, G, Zheng, H, Mei, H, Zheng, X, et al. Coccidioidomycosis: imported and possible domestic cases in China: a case report and review, 1958-2017. Mycoses. (2018) 61:506–13. doi: 10.1111/myc.12750
PubMed Abstract | Crossref Full Text | Google Scholar
5. Yang, X, Song, Y, Liang, T, Wang, Q, Li, R, and Liu, W. Application of laser capture microdissection and PCR sequencing in the diagnosis of Coccidioides spp. infection: a case report and literature review in China. Emerg Microbes Infect. (2021) 10:331–41. doi: 10.1080/22221751.2021.1889931
PubMed Abstract | Crossref Full Text | Google Scholar
6. Ekeng, BE, Davies, AA, Osaigbovo, II, Warris, A, Oladele, RO, and Denning, DW. Pulmonary and extrapulmonary manifestations of fungal infections misdiagnosed as tuberculosis: the need for prompt diagnosis and management. J Fungi (Basel). (2022) 8:460. doi: 10.3390/jof8050460
PubMed Abstract | Crossref Full Text | Google Scholar
7. Kassis, C, Durkin, M, Holbrook, E, Myers, R, and Wheat, L. Advances in diagnosis of progressive pulmonary and disseminated Coccidioidomycosis. Clin Infect Dis. (2021) 72:968–75. doi: 10.1093/cid/ciaa188
PubMed Abstract | Crossref Full Text | Google Scholar
10. McHardy, IH, Barker, B, and Thompson, GR 3rd. Review of clinical and laboratory diagnostics for Coccidioidomycosis. J Clin Microbiol. (2023) 61:e0158122. doi: 10.1128/jcm.01581-22
PubMed Abstract | Crossref Full Text | Google Scholar
11. Yue, L, Yangyang, H, Xing, Z, Yadong, W, and Caiyan, Z. Interpretation of expert consensus on clinical application of Chinese metagenomics second-generation sequencing technology to detect infectious pathogens. J Hebei Med Univ. (2021) 42:745–9. doi: 10.3969/j.issn.1007-3205.2021.07.001
Crossref Full Text | Google Scholar
12. Galgiani, JN, Ampel, NM, Blair, JE, Catanzaro, A, Johnson, RH, Stevens, DA, et al. Coccidioidomycosis. Clin Infect Dis. (2005) 41:1217–23. doi: 10.1086/496991
PubMed Abstract | Crossref Full Text | Google Scholar
13. Ramani, R, and Chaturvedi, V. Antifungal susceptibility profiles of Coccidioides immitis and Coccidioides posadasii from endemic and non-endemic areas. Mycopathologia. (2007) 163:315–9. doi: 10.1007/s11046-007-9018-7
PubMed Abstract | Crossref Full Text | Google Scholar
14. Stevens, DA, Zhang, Y, Finkelman, MA, Pappagianis, D, Clemons, KV, and Martinez, M. Cerebrospinal fluid (1,3)-Beta-d-glucan testing is useful in diagnosis of Coccidioidal meningitis. J Clin Microbiol. (2016) 54:2707–10. doi: 10.1128/jcm.01224-16
PubMed Abstract | Crossref Full Text | Google Scholar
16. Guo, X, Ruan, Q, Jin, J, Zheng, J, Shao, L, Li, N, et al. Disseminated coccidioidomycosis in immunocompetent patients in non-endemic areas: a case series and literature review. Eur J Clin Microbiol Infect Dis. (2022) 41:925–39. doi: 10.1007/s10096-022-04447-y
留言 (0)