Clonorchis sinensis (C. sinensis), commonly known as liver fluke, is a parasite that poses a significant health threat (1). C. sinensis is prevalent mainly in East Asian countries, including Japan, Korea, Vietnam and China, especially in certain geographical regions, approximately over 750 million people worldwide are at risk of liver fluke infection. Humans or other mammals are infected by consuming raw or undercooked freshwater fish containing metacercaria (2). The fluke’s infection can lead to serious health issues, including an increased risk of bile duct stones and cholangiocarcinoma (3, 4). Unfortunately, diagnosis of C. sinensis infection is often challenging, resulting in a high rate of missed diagnoses (5). Currently, the primary diagnostic method relies on microscopic examination of stool samples, which requires skilled personnel.
Echinostoma hortense (E. hortense) belongs to the class of Trematoda and genus of Echinostoma (6). Humans are mostly infected by eating fish, frogs and snails containing metacercariae. Loach has been proved to be the second intermediate host of E. hortense (7). E. hortense are similar to C. sinensis in that they are mainly distributed in East Asian countries and are mostly reported in the form of case reports, with the highest number of reports coming from South Korea (8). Most of patients have no obvious symptoms, so it is difficult to diagnose and delay treatment.
C. sinensis and E. hortense are two different types of trematodes, but they have some commonalities (9). First, they belong to the flatworm phylum and both possess a sucker structure, typically comprising a mouth sucker and a ventral sucker, for attaching to and extracting nutrients from the host. Second, they share similar life cycles and parasitism. Both of them belong to indirect developmental trematodes, with complex life histories that require multiple stages and intermediate hosts. Their infectious stage is usually metacercaria, which are transmitted to the final host such as humans and mammals. Last, both of them can parasitize the digestive tract of the host and cause a series of clinical symptoms, such as diarrhea, abdominal pain, and dyspepsia.
2 MethodsThe data in the medical records were collected as part of routine diagnosis and treatment, sharing data of the descriptive retrospective study anonymously was agreed by patients.
3 Results 3.1 Case 1A 54-year-old male patient presented with abdominal pain, prompting his admission to the hospital. Upon admission, blood biochemistry tests revealed elevated levels of total bilirubin at 37 μmol/L (normal range: 0–26 μmol/L), direct bilirubin at 11.6 μmol/L (normal range: 0–6.8 μmol/L), aspartate aminotransferase (AST) at 59.5 IU/L (normal range: 15–40 IU/L), alanine aminotransferase (ALT) at 304 U/L (normal range: 9–50 U/L), γ-glutamyltranspeptidase at 465 U/L (normal range: 10–60 U/L), and alkaline phosphatase (ALP) at 457 U/L (normal range: 40–129 U/L).The patient lived in the endemic area of C. sinensis and had the habit of eating freshwater fish for many times, but no parasitic eggs were detected in the patient’s stool upon admission. An abdominal computed tomography (CT) scan suggested the presence of stones at the terminal bile duct, leading to the decision to perform endoscopic retrograde cholangiopancreatography (ERCP)-guided bile duct lithotomy. During the procedure, following routine duodenal papilla intubation and sphincterotomy (Figure 1A), an unexpected discovery was made: worms emerging from the ampulla of Vater (Figure 1B). These worms exhibited a narrow, elongated, and twisted appearance, coupled with remarkable extensibility. The body exhibits a flat form, devoid of spiny epidermis, and the diameter of the oral sucker surpasses that of the ventral sucker (Figure 1C). Upon the worm’s emergence, the intricate branching of the ovaries became distinctly visible, as shown in Figure 1D. Ultimately, the worm fully navigated into the intestinal cavity (Figure 1E). Cholangiography analysis revealed a void in filling at the terminus of the common bile duct, indicative of a stone shadow (Figure 1F). Based on meticulous morphological assessment of the parasite’s body and repeated fecal egg examinations (Figure 2), a diagnosis of C. sinensis infection was confirmed. Subsequently, the patient was diagnosed with bile duct stones concurrently infected with C. sinensis. Following the removal of the stones, the patient underwent a three-day treatment with praziquantel at a dose of 210 mg/Kg body weight, resulting in a complete resolution of abdominal pain symptoms.
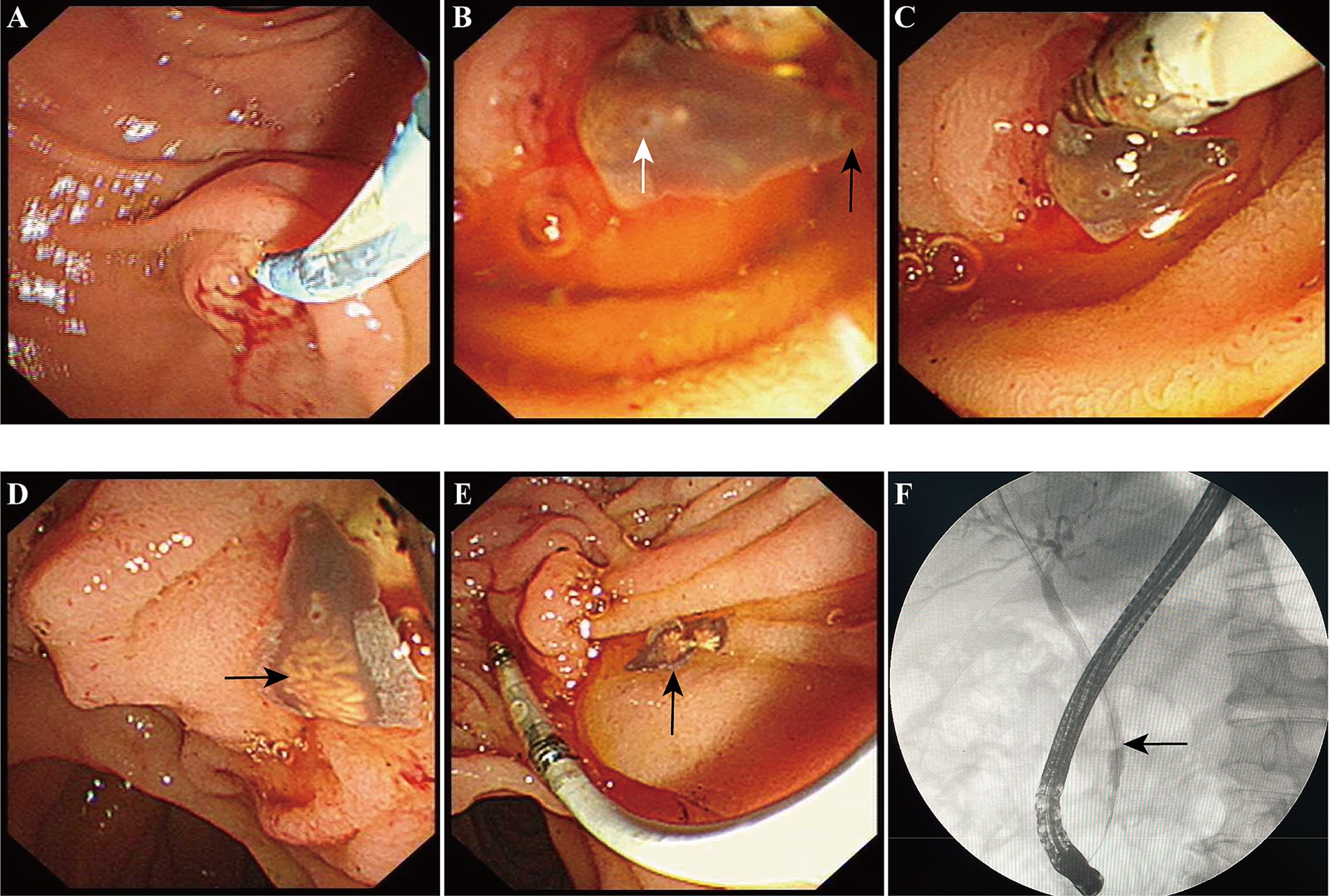
Figure 1. The diagnosis and migration of Clonorchis sinensis under direct endoscopic observation. (A) The conventional procedure of common bile duct intubation and nipple incision. (B) The anterior portion of C. sinensis is clearly visible as it emerges from the Vater’s ampulla. Black arrow pointed to the oral sucker, white arrow denoted the abdominal sucker. (C,D) The anterior and most of the body of C. sinensis have already entered the duodenal lumen, black arrow indicates the uterus of C. sinensis. (E) The worm had completely entered the duodenal cavity. (F) Cholangiography revealed the presence of a common bile duct stone shadow.
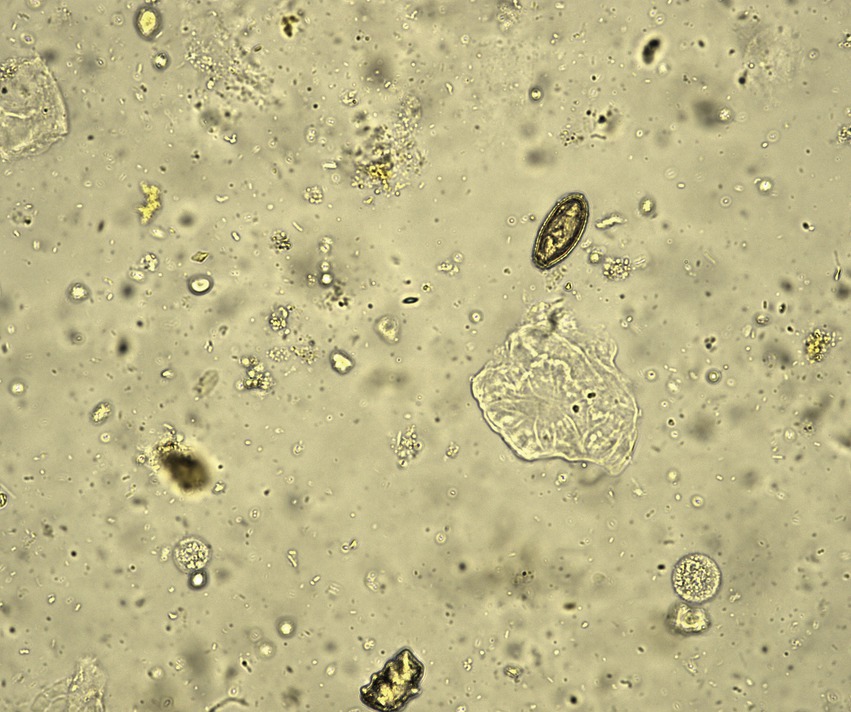
Figure 2. Multiple repeated fecal examinations finally identified the presence of C. sinensis eggs.
3.2 Case 2A 77-year-old female patient was admitted to a local hospital due to persistent upper abdominal pain and intermittent nausea that had persisted for 15 days. Laboratory indicators cannot be acquired. During a gastroduodenoscopy procedure, a significant finding emerged: multiple motile trematodes were observed adhering to the duodenal wall (Figure 3A). These worms exhibited a distinct, elongated leaf-like shape, approximately 8 mm in length, featuring red markings at their anterior ends. Their suckers were firmly entrenched on the intestinal wall. Two of these parasites were successfully extracted from the duodenum using endoscopic forceps. A detailed examination under a stereomicroscope revealed an opaque worm body, accompanied by prominently visible oral and abdominal suckers (Figure 3B). Concurrently, exhaustive microscopic analyses of the patient’s stool samples failed to detect any parasite eggs. Then eggs were obtained from the adult worms, their morphological characteristics mirrored those of Echinostoma (Figure 3C).
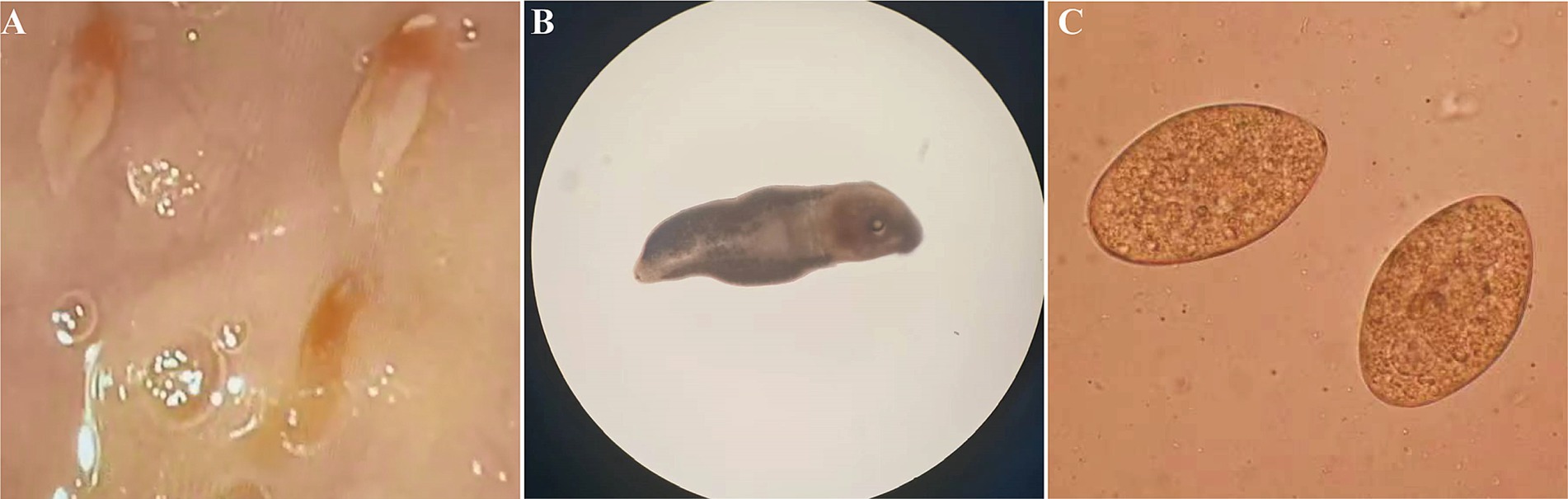
Figure 3. Morphological characterization of Echinostoma worms and their Eggs. (A) Gastro-duodenoscopic visualization of worms adhered to intestinal mucosa. (B) Microscopic examination of worm morphology in vitro. (C) Egg morphology obtained from worms.
To definitively identify the species, the worms’ DNA was extracted. The amplification conditions for PCR are as follows: pre-denaturation at 94°C for 5 min; enter the cycle: denaturation at 98°C for 30 s, annealing at 55°C for 30 s, extension at 72°C for 60 s, for a total of 35 cycles. The ribosomal internal transcribed spacer (ITS) sequence was amplified via PCR. The ITS forward primer sequence was 5′ -TTA GTT TCT TTT CCT CCG CT-3′, and the reverse primer sequence was 5′ -GTA GGT GAA CCT GCG GAA GGA TCA TT-3′. The gel image distinctly reveals a clear band at 1200 bp, corroborating the expected size of the target gene (Figure 4A). Then this target band was excised, purified, and subsequently sequenced. Homology analysis of the obtained sequence indicated a remarkable similarity with Echinostoma hortense and Isthmiophora hortense, achieving 98 and 93% sequence homology, respectively. To further substantiate our findings, we designed specific primers for the mitochondrial cyclooxygenase-1 (COX-1) gene and 18S ribosomal RNA gene. COX-1 forward primer: TAA GTC CTG TCG CTG CTA, and COX-1 reverse primer: CCT CCA CCA ACC TAA CCT. 18S forward primer: 5’-CTG GTT GAT CCT GCC AGT AGT C-3′, 18S reverse primer: 5’-ACG ACT TTT ACT TCC TCT AAA T-3′. The PCR product of 18S was sequenced, and the results showed that the product had two peaks, proving that the product is non-specific. The COX-1 sequences were successfully amplified (Figure 4B), and homology analysis unveiled a perfect match of 100% with the COX-1 gene of Echinostoma hortense, conclusively diagnosing the patient’s condition. Upon delving into the patient’s medical history, it emerged that she had consumed raw loach approximately 3 months prior to the onset of her symptoms. Following a three-day course of praziquantel therapy at a dose of 100 mg/Kg body weight, the abdominal discomfort notably subsided.
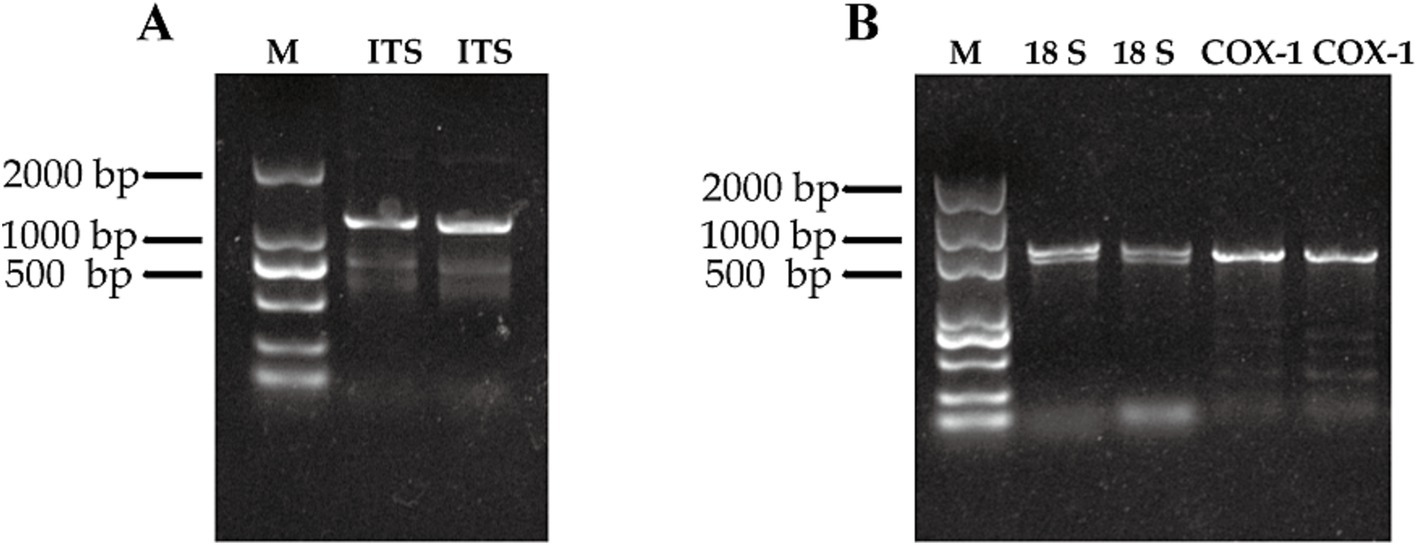
Figure 4. Image of nucleic acid gel after gene amplification by PCR. (A) Amplification results of ITS sequences of two E. hortense worms. (B) Amplification results of 18S and COX-1 sequences of two E. hortense worms.
4 DiscussionTwo studies that diagnose C. sinensis under direct endoscopy are presented, utilizing laparoscopic exploration and peroral cholangioscopy respectively, for both diagnosis and treatment purposes (10, 11). Liu et al. (10) reported 2 cases with pain and jaundice, who ate raw fish, found cholangiolithiasis and cholecystolithiasis. During laparoscopic cholecystectomy, adult worms of C. sinensis were found in the intrahepatic bile ducts, and a description of their morphology was provided. For another case (11), doctors conducted an endoscopic ultrasonography and ERCP-guided peroral cholangioscopy on an 80-year-old patient with biliary dilation. C. sinensis was found in both the common bile duct and intrahepatic bile ducts, and the worms were captured and removed using a basket.
The utilization of endoscopic direct observation for diagnosing C. sinensis infection boasts several pivotal advantages. Foremost among them is the unparalleled capacity to visually discern the parasite, thereby furnishing a conclusive and unambiguous diagnosis that significantly mitigates the likelihood of misdiagnosis or false negatives. Furthermore, this method exhibits high sensitivity, ensuring an accurate and reliable assessment of the infection status. Lastly, endoscopy presents a unique opportunity for a combined assessment, facilitating a simultaneous examination of various gastrointestinal pathologies and thereby enabling a comprehensive evaluation of the patient’s overall condition.
However, relying solely on morphological methods for the accurate diagnosis of Echinostoma poses a significant challenge, given the vast diversity of parasites encompassing over 600 species within 11 subfamilies and 51 genera (12–14). The precise identification of these parasites forms the cornerstone for the effective prevention, control, and scientific research pertaining to parasitic diseases. We summarized the cases diagnosed with E. hortense and published in English in the past and found that most of them relied solely on morphological diagnosis, which is not reliable for distinguishing a wide variety of Echinostoma (Table 1).

Table 1. Human cases infected with Echinostoma hortense.
Specifically, we employ two distinct and conserved sequences, ITS and COX-1, as invaluable tools for species identification. ITS, encompassing the internal transcribed spacer regions (ITS1, 5.8S rRNA, and ITS2), is situated between the large and small subunit rRNA genes within the ribosomal DNA (rDNA) (15). Its unique blend of high variability within species and marked differences between species, coupled with a sequence length typically ranging from 1,000 to 1,200 base pairs (bp), renders it exception-ally suitable for species-level identification and phylogenetic analyses (16). Notably, the ITS evolution rate surpasses that of 18S rDNA by tenfold, further underscoring its utility in these endeavors. By sequencing ITS and comparing it with established Echinostoma ITS sequences, we achieve accuracy in taxonomic classification, resolving controversies and elucidating both intraspecific and interspecific relationships. Additionally, we harness the power of COX-1, a highly conserved yet rapidly evolving and maternally inherited gene prevalent in various organisms, including parasites (17, 18). This gene, despite its length variability, is commonly analyzed in partial sequences due to its unique combination of conservatism and evolutionary dynamics. In our study, through PCR amplification and sequencing of both ITS and COX-1, we conclusively authenticated the species as E. hortense, demonstrating the efficacy of our approach in advancing Echinostoma taxonomy.
From a One Health perspective (19), these clinical findings are invaluable for several reasons: First, identifying infection sources. The diagnosis highlights the need to identify and manage the sources of infection. In the context of C. sinensis and E. hortense, this typically involves tracing the contamination of freshwater fish, which serve as the intermediate hosts for this parasite. By exploring the infection sources, public health authorities can implement control measures to prevent the spread of the parasite to humans through contaminated food. Second, assessing food habits and risk. The clinical cases also draw attention to the risks associated with specific food habits, particularly the consumption of raw or undercooked freshwater fish. From a One Health standpoint, understanding these food habits is crucial for developing targeted interventions to change behaviors that increase the risk of infection. This may involve educational campaigns, regulatory measures, or both, to inform the public about the risks and promote safe food practices. Last, promoting interdisciplinary collaboration. The One Health approach encourages collaboration between medical professionals, veterinarians, environmental scientists, and other stakeholders to address complex health issues. This collaboration can lead to a more comprehensive understanding of the parasite’s lifecycle, transmission routes, and impact on human and animal health. This, in turn, can facilitate the development of integrated control strategies that address the parasite at multiple levels (20).
5 ConclusionTraditional diagnosis of trematode infections heavily reliant on fecal microscopy, suffering from low sensitivity and a high incidence of missed diagnoses. As a complementary approach, endoscopy with PCR-based molecular diagnosis offering a more comprehensive and reliable method. This provides more accurate methods for the prevention, diagnosis, and treatment of diseases, and offers methodological support for the One Health system.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statementThe requirement of ethical approval was waived by Ethics Committee of the First Hospital of Jilin University for the studies involving humans because Ethics Committee of the First Hospital of Jilin University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the patients for the publication of all images and data included in this article.
Author contributionsLW: Conceptualization, Data curation, Formal analysis, Methodology, Software, Validation, Visualization, Writing – original draft. BW: Conceptualization, Methodology, Writing – review & editing. HZ: Conceptualization, Data curation, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Petney, TN, Andrews, RH, Saijuntha, W, Wenz-Mucke, A, and Sithithaworn, P. The zoonotic, fish-borne liver flukes Clonorchis sinensis, Opisthorchis felineus and Opisthorchis viverrini. Int J Parasitol. (2013) 43:1031–46. doi: 10.1016/j.ijpara.2013.07.007
PubMed Abstract | Crossref Full Text | Google Scholar
2. Torgerson, PR, Devleesschauwer, B, Praet, N, Speybroeck, N, Willingham, AL, Kasuga, F, et al. World health organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: a data synthesis. PLoS Med. (2015) 12:e1001920. doi: 10.1371/journal.pmed.1001920
PubMed Abstract | Crossref Full Text | Google Scholar
3. Sithithaworn, P, Yongvanit, P, Duenngai, K, Kiatsopit, N, and Pairojkul, C. Roles of liver fluke infection as risk factor for cholangiocarcinoma. J Hepatobiliary Pancreat Sci. (2014) 21:301–8. doi: 10.1002/jhbp.62
PubMed Abstract | Crossref Full Text | Google Scholar
4. Prueksapanich, P, Piyachaturawat, P, Aumpansub, P, Ridtitid, W, Chaiteerakij, R, and Rerknimitr, R. Liver fluke-associated biliary tract Cancer. Gut Liver. (2018) 12:236–45. doi: 10.5009/gnl17102
PubMed Abstract | Crossref Full Text | Google Scholar
5. Tang, ZL, Huang, Y, and Yu, XB. Current status and perspectives of Clonorchis sinensis and clonorchiasis: epidemiology, pathogenesis, omics, prevention and control. Infect Dis Poverty. (2016) 5:71. doi: 10.1186/s40249-016-0166-1
PubMed Abstract | Crossref Full Text | Google Scholar
7. Cho, YK, Ryang, YS, Kim, IS, Park, SK, Im, JA, and Lee, KJ. Differential immune profiles following experimental Echinostoma hortense infection in BALB/c and C3H/HeN mice. Parasitol Res. (2007) 100:1053–61. doi: 10.1007/s00436-006-0419-1
Crossref Full Text | Google Scholar
8. Sohn, WM, Na, BK, and Cho, SH. Echinostoma hortense and heterophyid metacercariae encysted in yellowfin goby, Acanthogobius flavimanus, from Shinan-gun and Muan-gun (Jeollanam-do). Korea Korean J Parasitol. (2009) 47:307–10. doi: 10.3347/kjp.2009.47.3.307
PubMed Abstract | Crossref Full Text | Google Scholar
9. Toledo, R, Conciancic, P, Fiallos, E, Esteban, JG, and Munoz-Antoli, C. Echinostomes and other intestinal trematode infections. Adv Exp Med Biol. (2024) 1454:285–322. doi: 10.1007/978-3-031-60121-7_8
PubMed Abstract | Crossref Full Text | Google Scholar
10. Liu, X, Zhu, G, Cai, C, Lv, Z, and Li, J. Clonorchiasis sinensis detected by laparoscopic exploration of biliary tracts in two patients with obstructive jaundice. BMC Infect Dis. (2019) 19:33. doi: 10.1186/s12879-019-3679-y
PubMed Abstract | Crossref Full Text | Google Scholar
11. Tao, LY, Wang, HG, Guo, QM, Liu, SZ, Guo, X, Yang, MY, et al. Peroral cholangioscopy-guided diagnosis and treatment of Clonorchis sinensis liver flukes. Endoscopy. (2024) 56:E498–9. doi: 10.1055/a-2333-9258
PubMed Abstract | Crossref Full Text | Google Scholar
12. Panich, W, Jaruboonyakorn, P, Raksaman, A, Tejangkura, T, and Chontananarth, T. Development and utilization of a visual loop-mediated isothermal amplification coupled with a lateral flow dipstick (LAMP-LFD) assay for rapid detection of Echinostomatidae metacercaria in edible snail samples. Int J Food Microbiol. (2024) 418:110732. doi: 10.1016/j.ijfoodmicro.2024.110732
PubMed Abstract | Crossref Full Text | Google Scholar
14. Ray, M, Trinidad, M, Francis, N, and Shamsi, SCharacterization of Echinostoma spp. (Trematoda: Echinostomatidae Looss, 1899) infecting ducks in South-Eastern Australia. Int J Food Microbiol. (2024) 421:110754. doi: 10.1016/j.ijfoodmicro.2024.110754
PubMed Abstract | Crossref Full Text | Google Scholar
15. Gu, W, Deng, X, Lee, M, Sucu, YD, Arevalo, S, Stryke, D, et al. Rapid pathogen detection by metagenomic next-generation sequencing of infected body fluids. Nat Med. (2021) 27:115–24. doi: 10.1038/s41591-020-1105-z
PubMed Abstract | Crossref Full Text | Google Scholar
16. Le, TH, Pham, L, Van Quyen, D, Nguyen, KT, Doan, H, Saijuntha, W, et al. The ribosomal transcription units of five echinostomes and their taxonomic implications for the suborder Echinostomata (Trematoda: Platyhelminthes). Parasitol Res. (2024) 123:103. doi: 10.1007/s00436-023-08110-z
PubMed Abstract | Crossref Full Text | Google Scholar
17. Marascio, N, Loria, MT, Lamberti, AG, Pavia, G, Adams, NJ, Quirino, A, et al. Molecular characterization of Schistosoma infections in African migrants: identification of a Schistosoma haematobium-bovis hybrid in bladder biopsies. J Travel Med. (2022) 29:29. doi: 10.1093/jtm/taab194
PubMed Abstract | Crossref Full Text | Google Scholar
18. Sudan, V, Shanker, D, Paliwal, S, Kumar, R, and Singh, A. Phylogenetics of Sarcocystis fusiformis isolates based on 18S rRNA and cox 1 genes. Microb Pathog. (2021) 159:105144. doi: 10.1016/j.micpath.2021.105144
PubMed Abstract | Crossref Full Text | Google Scholar
19. Liu, K, Tan, J, Xiao, L, Pan, R, Yao, X, Shi, F, et al. Spatio-temporal disparities of Clonorchis sinensis infection in animal hosts in China: a systematic review and meta-analysis. Infect Dis Poverty. (2023) 12:97. doi: 10.1186/s40249-023-01146-4
PubMed Abstract | Crossref Full Text | Google Scholar
21. Seo, BS, Hong, ST, Chai, JY, and Lee, SH. Studies on intestinal trematodes in Korea: VIII. A human case of Echinostoma hortense infection. Kisaengchunghak Chapchi. (1983) 21:219–23. doi: 10.3347/kjp.1983.21.2.219
PubMed Abstract | Crossref Full Text | Google Scholar
22. Chai, JY, Hong, ST, Lee, SH, Lee, GC, and Min, YI. A case of echinostomiasis with ulcerative lesions in the duodenum. Korean J Parasitol. (1994) 32:201–4. doi: 10.3347/kjp.1994.32.3.201
PubMed Abstract | Crossref Full Text | Google Scholar
23. Ryang, YS, Ahn, YK, Lee, KW, Kim, TS, and Han, MH. Two cases of natural human infection by Echinostoma hortense and its second intermediate host in Wonju area. Kisaengchunghak Chapchi. (1985) 23:33–40. doi: 10.3347/kjp.1985.23.1.33
PubMed Abstract | Crossref Full Text | Google Scholar
24. Cho, CM, Tak, WY, Kweon, YO, Kim, SK, Choi, YH, Kong, HH, et al. A human case of Echinostoma hortense (Trematoda: Echinostomatidae) infection diagnosed by gastroduodenal endoscopy in Korea. Korean J Parasitol. (2003) 41:117–20. doi: 10.3347/kjp.2003.41.2.117
PubMed Abstract | Crossref Full Text | Google Scholar
25. Chang, YD, Sohn, WM, Ryu, JH, Kang, SY, and Hong, SJ. A human infection of Echinostoma hortense in duodenal bulb diagnosed by endoscopy. Korean J Parasitol. (2005) 43:57–60. doi: 10.3347/kjp.2005.43.2.57
PubMed Abstract | Crossref Full Text | Google Scholar
26. Tanaka, T, Kamada, T, Koga, H, Tanaka, A, Manabe, N, Hata, J, et al. Echinostoma hortense Asada infection in the duodenum: incidental findings during routine gastrointestinal endoscopy. Dig Endosc. (2008) 20:87–9. doi: 10.1111/j.1443-1661.2008.00774.x
留言 (0)