A recent case report by Sahota and colleagues has provided new insights into treatment of dysglycemia via dopamine (DA) receptor stimulation in the setting of autoimmune diabetes (1). Briefly, a patient with autoimmune diabetes was diagnosed with a pituitary prolactinoma, resulting in treatment with cabergoline, an agonist of DA D2-like receptors, alongside preexisting diabetes medications. Over time, the patient was switched to cabergoline monotherapy which reversed his insulin requirement. This led to significantly improved glycemic control and a revised diagnosis of latent autoimmune diabetes of adults (LADA). Ultimately, however, the patient was restarted on insulin therapy in the setting of progressively increased blood glucose.
Patients with LADA often achieve adequate glycemic control soon after the initiation of antihyperglycemic treatment, including non-insulin agents (2). Consistent with this, recent studies in LADA patients with non-insulin agents like dipeptidyl peptidase 4 inhibitors (e.g., saxagliptin), or glucagon‐like peptide 1 receptor agonists (e.g., dulaglutide) showed improved glycemic control for months and delayed progression to insulin requirement (2–5). Importantly, in contrast to the more commonly used drug classes above, this case represents the first description of DA receptor agonist monotherapy for autoimmune diabetes (1). These findings have raised important questions concerning the biological mechanisms by which D2-like receptor agonists can effectively treat dysglycemia, particularly in the setting of diabetes.
2 Discussion2.1 CNS targetsD2-like receptor agonists such as cabergoline and bromocriptine have been used for decades to control CNS prolactinoma size and secretion given their expression of the DA D2 receptor (D2R) (6). There is much evidence that these agonists are associated with improved glycemic control (7). Moreover, bromocriptine was approved by the United States Food & Drug Administration as a novel treatment for dysglycemia in type 2 diabetes mellitus (T2DM) (8, 9). While mechanisms by which D2-like receptor agonists improve glycemic control have remained unclear, most attention has been devoted to these drugs’ actions on neuroendocrine targets within the central nervous system (CNS) (8).
Sahota et al. suggested that drug-induced reduction of pathological prolactin levels led to the patient’s metabolic improvements (1). CNS D2R agonism via cabergoline therapy could therefore modify prolactin-induced disruptions in lipid and glucose metabolism in insulin-responsive tissues including adipose tissue and skeletal muscle (1, 10, 11). These prolactin reductions also likely contributed to improved testosterone levels, which in turn reversed the patient’s hypogonadism. This is consistent with evidence showing that testosterone restoration contributes to significant weight loss as well as improved insulin resistance and overall glycemic control (12, 13). Cabergoline-induced normalization of prolactin may therefore lead to restored total and free testosterone levels to improve glycemic control via a wide range of mechanisms including via reductions in inflammation and weight gain – factors that further drive insulin resistance (11, 14). Moreover, D2R is also expressed in the hypothalamus and is implicated in centrally-mediated metabolic regulation, including through control over satiety (15, 16). Therefore, it is possible that D2R agonists may improve glycemic control via these CNS pathways, in addition to its actions in the pituitary (Figure 1A).
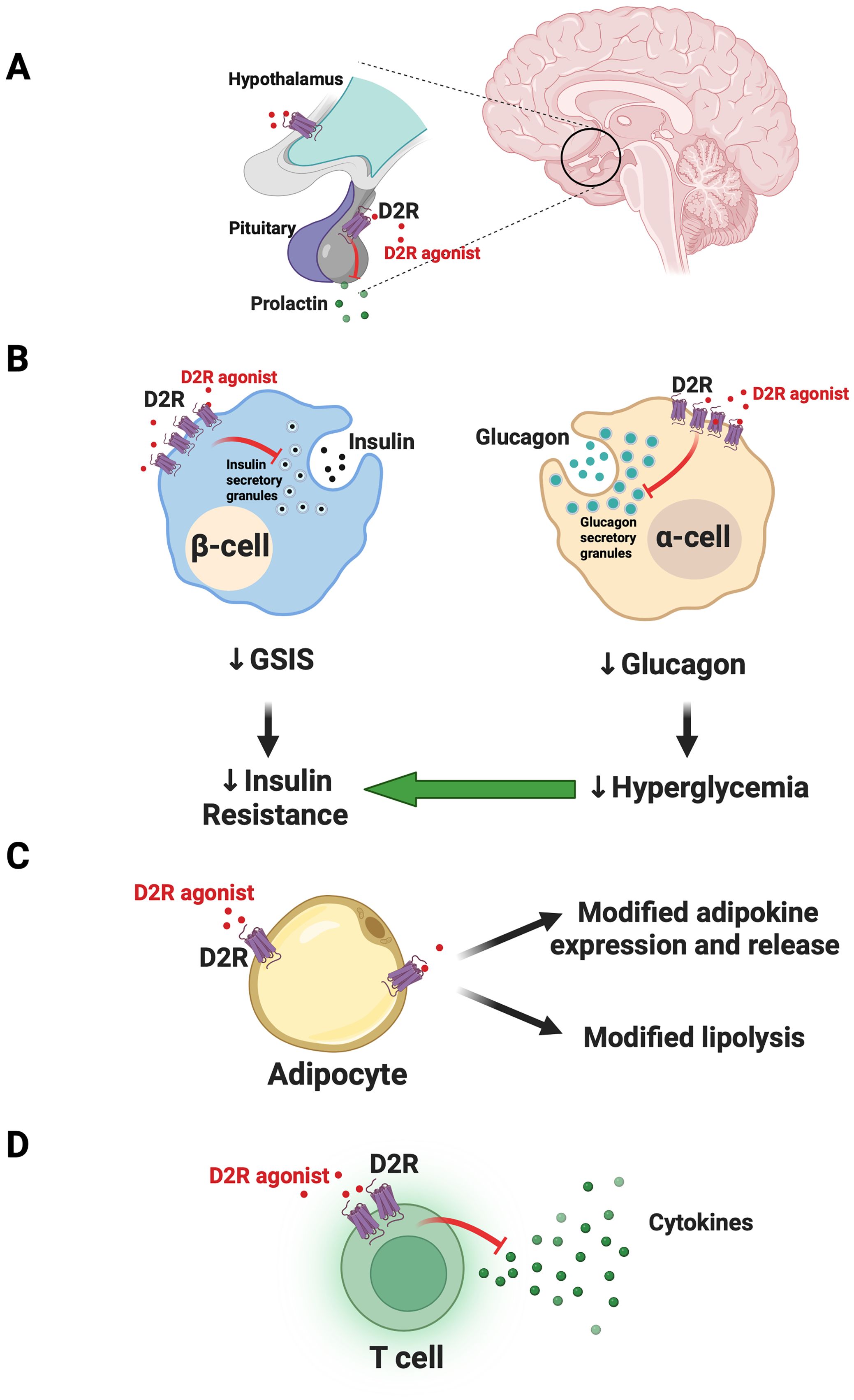
Figure 1. Model for joint actions of dopamine D2-like receptor agonist actions on CNS and peripheral targets to improve glycemic control. (A) In the CNS, dopamine D2-like receptor agonists like bromocriptine and cabergoline act on targets including the dopamine D2 receptor (D2R) in the pituitary to limit prolactin release. Targeting of additional hypothalamic targets may further modulate satiety and central metabolic circuitry to improve glycemic control. (B) Outside the CNS, in the endocrine pancreas, D2-like receptor agonists act on D2R expressed in beta-cells. The resulting inhibition of glucose-stimulated insulin secretion (GSIS) ultimately leads to therapeutic reductions in insulin resistance. In parallel, agonism of D2R in alpha-cells diminishes glucagon secretion to reduce hyperglycemia and further improve insulin sensitivity. (C) In adipose tissue, D2-like receptor agonists can act on D2R in adipocytes to modify release of adipokines and possibly lipolysis, improving insulin sensitivity. (D) D2-like receptor agonists also act on T cells in the endocrine pancreas to reduce cytokine release. This may reduce local inflammatory processes to improve islet function. Panel (B) was adapted from Aslanoglou et al. (2022) iScience 25 (2022) 104771. Created with Biorender.com.
Though CNS DA receptor agonism was proposed by Sahota et al. as a primary driver of improved glycemic control (1), additional factors likely play key roles. A leading determinant of improved glucose control is the “honeymoon” effect where patients present with temporary remission after symptomatic onset. The honeymoon period in LADA typically lasts weeks to months and may reflect reduced stress on remaining islet beta-cells (17–19). Body weight loss similarly improves glycemic control, which in turn lowers cell stress to help preserve beta-cell function (e.g., insulin synthesis and release) (20).
2.2 Endocrine pancreasIn addition to CNS targets, we posit that the ability of D2-like receptor agonists to effectively treat dysglycemia in diabetes is at least in part via their actions on metabolically relevant peripheral targets including the endocrine pancreas. We and others demonstrated that pancreatic islet cells express D2-like receptors (21–26). Moreover, alpha-cells and beta-cells produce their own DA which signals locally via D2-like receptors as an autocrine/paracrine negative modulator of insulin and glucagon secretion (21, 22, 26, 27). More recently, we found that bromocriptine acts directly on peripheral D2R to inhibit islet insulin and glucagon secretion (28). It is possible that D2-like receptor agonist inhibition of glucose-stimulated insulin secretion (GSIS) therefore leads to “beta-cell rest.” Lowering excessive insulin release may reduce cytotoxic beta-cell stress and re-sensitize insulin-resistant tissues like skeletal muscle, adipose tissue, and liver to improve dysglycemia (26). Interestingly, besides DA receptors, beta-cells also express inhibitory adrenergic receptors including alpha2A adrenergic receptors which can similarly be stimulated by local DA or D2-like receptor agonists like bromocriptine (22, 28, 29). This results in further inhibition of GSIS (22, 28, 29). Likewise, diminishing alpha-cell glucagon secretion via D2R agonism may concurrently lower hyperglycemia and improve both insulin resistance and overall glycemic control (26) (Figure 1B).
2.3 Adipose tissueD2-like receptors are expressed in adipose tissue (30, 31). Increasing evidence suggests that dopaminergic signaling in adipocytes modulates expression of adipokines including leptin (32–34). Consistent with this, recent work showed that D2R expression was upregulated in human subcutaneous adipose tissue in response to hyperglycemia and T2DM (34). The DA D4 receptor (DRD4), another D2-like receptor, was also upregulated in adipose tissue of patients with prediabetes (35). Moreover, bromocriptine treatment inhibited lipolysis in response to beta-adrenergic receptor stimulation, suggesting that D2-like receptor agonists may be acting directly on adipocytes to modify their function (34). Despite this, the same study reported that physiological concentrations of DA did not modify either adipocyte glucose uptake or lipolysis (34). This raises the possibility that D2-like receptor agonists achieve their therapeutic effects via actions at additional non-dopaminergic adipocyte receptors. It is also possible that at least some of the therapeutic effects of D2-like receptor agonists on peripheral insulin resistance are due to pleotropic, combined actions at multiple peripheral sites which include adipocytes, but which also include other sites such as liver. Indeed, earlier work demonstrated that bromocriptine treatment led to remodeling of adipose tissue with increases in fasting insulin signaling in brown adipose tissue (35). In parallel, bromocriptine may also act on liver (e.g., diminished liver triglyceride content) (35). Ultimately, more work is clearly needed to investigate direct and indirect therapeutic actions of D2-like receptor agonists on adipocyte function (Figure 1C).
2.4 Skeletal muscleIn addition to adipose tissue, skeletal muscle also plays a key role in maintaining adequate peripheral insulin sensitivity and optimal glucose control. However, effects of D2-like receptor agonists on skeletal muscle are mixed. Limited preclinical evidence in rodents showed that bromocriptine increased phosphorylation of skeletal muscle AMP-activated protein kinase (AMPK), an energy-sensing enzyme and therapeutic target in diabetes (36, 37). In contrast, other preclinical and clinical studies showed no significant effects of bromocriptine on skeletal muscle, including on insulin sensitivity (35, 38). Nevertheless, in the case of the patient described by Sahota and colleagues (1), irrespective of potential direct actions of a D2-like receptor agonist on skeletal muscle, drug-induced restoration of serum levels of testosterone may lead to improved skeletal muscle mass and strength and improve insulin sensitivity (12, 39).
2.5 T cellsImmune T cells that have infiltrated pancreatic islets represent another possible peripheral therapeutic target for D2-like receptor agonists. Immune cells express D2-like receptors and stimulation of these receptors can decrease cytokine secretion, potentially suppressing activated actions of islet T cells (40). We therefore posit that resulting decreases in islet inflammation can improve islet function and glycemic control (Figure 1D).
2.6 Tandem CNS and peripheral dopaminergic actionsWe recently found that D2-like receptor agonists required access to both CNS and peripheral targets to treat dysglycemia. Importantly, restricting access to one compartment or the other eliminated the therapeutic efficacy of the agonist drugs in reducing dysglycemia (41). Overall, we conclude that tandem actions of D2-like receptor agonists on CNS and peripheral targets offer a novel mechanism for dysglycemia treatment of autoimmune diabetes and T2DM.
Author contributionsZF: Conceptualization, Writing – original draft, Writing – review & editing. RC: Conceptualization, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the National Institutes of Health (R01DK124219), the Department of Defense (PR210207) and the Baszucki Group to ZF.
Conflict of interestZF is funded by UPMC Enterprises for investigator-initiated studies.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References2. Buzzetti R, Tuomi T, Mauricio D, Pietropaolo M, Zhou Z, Pozzilli P, et al. Management of latent autoimmune diabetes in adults: A consensus statement from an international expert panel. Diabetes. (2020) 69:2037–47. doi: 10.2337/dbi20-0017
PubMed Abstract | Crossref Full Text | Google Scholar
3. Pozzilli P, Di Mario U. Autoimmune diabetes not requiring insulin at diagnosis (latent autoimmune diabetes of the adult): definition, characterization, and potential prevention. Diabetes Care. (2001) 24:1460–7. doi: 10.2337/diacare.24.8.1460
PubMed Abstract | Crossref Full Text | Google Scholar
4. Zhang Z, Yan X, Wu C, Pei X, Li X, Wang X, et al. Adding vitamin D3 to the dipeptidyl peptidase-4 inhibitor saxagliptin has the potential to protect β-cell function in LADA patients: A 1-year pilot study. Diabetes/metabolism Res Rev. (2020) 36:e3298. doi: 10.1002/dmrr.v36.5
PubMed Abstract | Crossref Full Text | Google Scholar
5. Pozzilli P, Leslie RD, Peters AL, Buzzetti R, Shankar SS, Milicevic Z, et al. Dulaglutide treatment results in effective glycaemic control in latent autoimmune diabetes in adults (LADA): A post-hoc analysis of the AWARD-2, -4 and -5 Trials. Diabetes Obes Metab. (2018) 20:1490–8. doi: 10.1111/dom.2018.20.issue-6
PubMed Abstract | Crossref Full Text | Google Scholar
6. Wexler TL, Page-Wilson G. Dopamine agonists for the treatment of pituitary tumours: From ergot extracts to next generation therapies. Br J Clin Pharmacol. (2023) 89:1304–17. doi: 10.1111/bcp.15660
PubMed Abstract | Crossref Full Text | Google Scholar
7. Lamos EM, Levitt DL, Munir KM. A review of dopamine agonist therapy in type 2 diabetes and effects on cardio-metabolic parameters. Primary Care Diabetes. (2016) 10:60–5. doi: 10.1016/j.pcd.2015.10.008
PubMed Abstract | Crossref Full Text | Google Scholar
10. Posawetz AS, Trummer C, Pandis M, Aberer F, Pieber TR, Obermayer-Pietsch B, et al. Adverse body composition and lipid parameters in patients with prolactinoma: a case-control study. BMC endocrine Disord. (2021) 21:81. doi: 10.1186/s12902-021-00733-6
PubMed Abstract | Crossref Full Text | Google Scholar
14. Auriemma RS, Granieri L, Galdiero M, Simeoli C, Perone Y, Vitale P, et al. Effect of cabergoline on metabolism in prolactinomas. Neuroendocrinology. (2013) 98:299–310. doi: 10.1159/000357810
PubMed Abstract | Crossref Full Text | Google Scholar
15. Garcia-Tornadu I, Perez-Millan MI, Recouvreux V, Ramirez MC, Luque G, Risso GS, et al. New insights into the endocrine and metabolic roles of dopamine D2 receptors gained from the Drd2 mouse. Neuroendocrinology. (2010) 92:207–14. doi: 10.1159/000321395
PubMed Abstract | Crossref Full Text | Google Scholar
16. Romanova IV, Derkach KV, Mikhrina AL, Sukhov IB, Mikhailova EV, Shpakov AO, et al. Dopamine and serotonin receptors in hypothalamic POMC-neurons of normal and obese rodents. Neurochemical Res. (2018) 43:821–37. doi: 10.1007/s11064-018-2485-z
PubMed Abstract | Crossref Full Text | Google Scholar
17. Creusot RJ, Battaglia M, Roncarolo MG, Fathman CG. Concise review: cell-based therapies and other non-traditional approaches for type 1 diabetes. Stem Cells (Dayton Ohio). (2016) 34:809–19. doi: 10.1002/stem.2290
PubMed Abstract | Crossref Full Text | Google Scholar
18. Marcon LMR, Fanelli CG, Calafiore R. Type 1 diabetes (T1D) and latent autoimmune diabetes in adults (LADA): the difference between a honeymoon and a holiday. Case Rep Endocrinol. (2022) 2022:9363543. doi: 10.1155/2022/9363543
PubMed Abstract | Crossref Full Text | Google Scholar
19. Zhong T, Tang R, Gong S, Li J, Li X, Zhou Z. The remission phase in type 1 diabetes: Changing epidemiology, definitions, and emerging immuno-metabolic mechanisms. Diabetes/metabolism Res Rev. (2020) 36:e3207. doi: 10.1002/dmrr.v36.2
PubMed Abstract | Crossref Full Text | Google Scholar
21. Farino ZJ, Morgenstern TJ, Maffei A, Quick M, De Solis AJ, Wiriyasermkul P, et al. New roles for dopamine D(2) and D(3) receptors in pancreatic beta cell insulin secretion. Mol Psychiatry. (2020) 25:2070–85. doi: 10.1038/s41380-018-0344-6
PubMed Abstract | Crossref Full Text | Google Scholar
22. Aslanoglou D, Bertera S, Sánchez-Soto M, Benjamin Free R, Lee J, Zong W, et al. Dopamine regulates pancreatic glucagon and insulin secretion via adrenergic and dopaminergic receptors. Trans Psychiatry. (2021) 11:59. doi: 10.1038/s41398-020-01171-z
PubMed Abstract | Crossref Full Text | Google Scholar
23. Simpson N, Maffei A, Freeby M, Burroughs S, Freyberg Z, Javitch J, et al. Dopamine-mediated autocrine inhibitory circuit regulating human insulin secretion in vitro. Mol Endocrinol. (2012) 26:1757–72. doi: 10.1210/me.2012-1101
PubMed Abstract | Crossref Full Text | Google Scholar
24. Ustione A, Piston DW. Dopamine synthesis and D3 receptor activation in pancreatic beta-cells regulates insulin secretion and intracellular [Ca(2+)] oscillations. Mol Endocrinol. (2012) 26:1928–40. doi: 10.1210/me.2012-1226
PubMed Abstract | Crossref Full Text | Google Scholar
25. Rubi B, Ljubicic S, Pournourmohammadi S, Carobbio S, Armanet M, Bartley C, et al. Dopamine D2-like receptors are expressed in pancreatic beta cells and mediate inhibition of insulin secretion. J Biol Chem. (2005) 280:36824–32. doi: 10.1074/jbc.M505560200
PubMed Abstract | Crossref Full Text | Google Scholar
26. Freyberg Z, Gittes GK. Roles of pancreatic islet catecholamine neurotransmitters in glycemic control and in antipsychotic drug-induced dysglycemia. Diabetes. (2023) 72:3–15. doi: 10.2337/db22-0522
PubMed Abstract | Crossref Full Text | Google Scholar
27. Farino ZJ, Morgenstern TJ, Vallaghe J, Gregor N, Donthamsetti P, Harris PE, et al. Development of a rapid insulin assay by homogenous time-resolved fluorescence. PloS One. (2016) 11:e0148684. doi: 10.1371/journal.pone.0148684
PubMed Abstract | Crossref Full Text | Google Scholar
28. Aslanoglou D, Bertera S, Friggeri L, Sánchez-Soto M, Lee J, Xue X, et al. Dual pancreatic adrenergic and dopaminergic signaling as a therapeutic target of bromocriptine. iScience. (2022) 25:104771. doi: 10.1016/j.isci.2022.104771
PubMed Abstract | Crossref Full Text | Google Scholar
29. de Leeuw van Weenen JE, Parlevliet ET, Maechler P, Havekes LM, Romijn JA, Ouwens DM, et al. The dopamine receptor D2 agonist bromocriptine inhibits glucose-stimulated insulin secretion by direct activation of the alpha2-adrenergic receptors in beta cells. Biochem Pharmacol. (2010) 79:1827–36. doi: 10.1016/j.bcp.2010.01.029
PubMed Abstract | Crossref Full Text | Google Scholar
30. Freyberg Z, Aslanoglou D, Shah R, Ballon JS. Intrinsic and antipsychotic drug-induced metabolic dysfunction in schizophrenia. Front Neurosci. (2017) 11:432. doi: 10.3389/fnins.2017.00432
PubMed Abstract | Crossref Full Text | Google Scholar
31. Ballon JS, Pajvani U, Freyberg Z, Leibel RL, Lieberman JA. Molecular pathophysiology of metabolic effects of antipsychotic medications. Trends Endocrinol metabolism: TEM. (2014) 25:593–600. doi: 10.1016/j.tem.2014.07.004
PubMed Abstract | Crossref Full Text | Google Scholar
32. Borcherding DC, Hugo ER, Idelman G, De Silva A, Richtand NW, Loftus J, et al. Dopamine receptors in human adipocytes: expression and functions. PloS One. (2011) 6:e25537. doi: 10.1371/journal.pone.0025537
PubMed Abstract | Crossref Full Text | Google Scholar
34. Vranic M, Ahmed F, Kristófi R, Hetty S, Mokhtari D, Svensson MK, et al. Subcutaneous adipose tissue dopamine D2 receptor is increased in prediabetes and T2D. Endocrine. (2024) 83:378–91. doi: 10.1007/s12020-023-03525-1
PubMed Abstract | Crossref Full Text | Google Scholar
35. Tavares G, Marques D, Barra C, Rosendo-Silva D, Costa A, Rodrigues T, et al. Dopamine D2 receptor agonist, bromocriptine, remodels adipose tissue dopaminergic signalling and upregulates catabolic pathways, improving metabolic profile in type 2 diabetes. Mol Metab. (2021) 51:101241. doi: 10.1016/j.molmet.2021.101241
PubMed Abstract | Crossref Full Text | Google Scholar
36. Kjøbsted R, Hingst JR, Fentz J, Foretz M, Sanz MN, Pehmøller C, et al. AMPK in skeletal muscle function and metabolism. FASEB J. (2018) 32:1741–77. doi: 10.1096/fj.201700442R
PubMed Abstract | Crossref Full Text | Google Scholar
37. Tavares G, Melo B, Martins F, Matafome P, Conde S. Dopamine acts through distinct mechanisms in liver, adipose tissue and skeletal muscle regulating glucose uptake and insulin receptor and AMPK phosphorylation. Diabetologia. (2020) 63(SUPPL 1):S239–9.
40. Ghosh MC, Mondal AC, Basu S, Banerjee S, Majumder J, Bhattacharya D, et al. Dopamine inhibits cytokine release and expression of tyrosine kinases, Lck and Fyn in activated T cells. Int Immunopharmacol. (2003) 3:1019–26. doi: 10.1016/S1567-5769(03)00100-0
PubMed Abstract | Crossref Full Text | Google Scholar
41. Bonifazi A, Ellenberger M, Farino ZJ, Aslanoglou D, Rais R, Pereira S, et al. Development of novel tools for dissection of central versus peripheral dopamine D2-like receptor signaling in dysglycemia. Diabetes. (2024) 73:1411–25. doi: 10.2337/db24-0175
留言 (0)