It is well known that male factors contribute to infertility at approximately the same rate as female factors (1, 2). However, the exact causes of male infertility remain poorly understood. While traditional semen analysis focusing on sperm concentration, motility and morphology, has been commonly used to assess male fertility, accumulating research suggests that these parameters do not always correlate with the outcomes of assisted reproductive technology (ART) (3). Recently, sperm DNA fragmentation (SDF) assessment has gained attention as a potential indicator of male fertility, as reduced sperm DNA integrity has been observed in infertile patients across various diagnostic assays (4, 5).
Sperm DNA is highly organized, and the degree of chromatin organization can influence epigenetic changes and embryo development (2, 6). The extent of sperm DNA damage is typically measured by the sperm DNA fragmentation index (DFI). However, there is ongoing debate regarding the impact of DFI on assisted reproductive outcomes. Some studies suggest that an increased DFI adversely affects both natural conception (7) and ART outcomes (8, 9). High DFI can even disrupt normal physiological functions, leading to the transmission of incorrect genetic information to offspring, which routine semen analysis cannot assess (5). Two meta-analyses have shown that elevated sperm DFI is associated with lower rates of good-quality embryos, reduced clinical pregnancy rates, and increased miscarriage rates (10, 11). Nonetheless, other meta-analyses have concluded that sperm DFI does not predict IVF or intracytoplasmic sperm injection (ICSI) outcomes (12). Based on the available literature, the impact of sperm DFI on embryonic development, clinical outcomes, and particularly on perinatal and neonatal outcomes, remains to be fully understood.
The American Urological Association (AUA) and the European Association of Urology (EAU) have acknowledged the importance of SDF in their 2023 guidelines on male infertility (13, 14). To establish a definitive correlation, a rigorous investigation with a large sample size and extended study duration is essential. In our retrospective study, we explored the effects of sperm DFI on embryonic development, clinical outcomes, and the risk of adverse maternal and neonatal outcomes in singleton pregnancies.
Materials and methodsStudy design and participantsThis retrospective cohort study enrolled a total of 5271 infertile couples who underwent IVF for the first time at the Reproductive Medicine Center of Jiangxi Maternal and Child Health Hospital, affiliated with Nanchang Medical College from October 1, 2020 to July 31, 2023.
The study was approved by the Reproductive Medicine Ethics Committee of Jiangxi Maternal and Child Health Hospital (Approval No. 2024136) and adhered to the principles outlined in the Declaration of Helsinki. All patients provided written informed consent for data collection and the anonymous use of their information in scientific research.
The following patients were included in this study: (1) women aged 20–40 years; (2) patients with infertility attributed to female pelvic cavity or tubal factors, or male factors, who met the indications for IVF/ICSI treatment; (3) an antral follicle count (AFC) ≥ 5, a basal follicle stimulating hormone level ≤ 10mIU/ml or an anti-mullerian hormone (AMH) level ≥ 1.2 ng/ml; (4) normal chromosomes, reproductive organs, and sexual function in both partners. The exclusion criteria were as follows: (1) natural cycle pregnancies; (2) women with stage III or IV endometriosis; (3) males with azoospermia or undergoing thawing or testicular/epididymal aspiration; (4) males with testicular atrophy, genital tract malformation or urogenital infection; and (5) either partner with chromosomal abnormalities or contraindications to assisted reproduction. Based on the DFI values, the finally enrolled male participants were categorized into three groups: DFI < 15%, 15% ≤ DFI < 30%, and DFI ≥ 30%.
Semen collection and analysisSemen samples were collected by masturbation after 2 to 7 days of ejaculatory abstinence, following the guidelines outlined in the Laboratory Manual of the WHO for the Examination and Processing of Human Semen (6th edition) (15). Each semen sample was collected into a sterile plastic cup and incubated at 37°C until complete liquefaction was achieved. The semen analysis, including the DFI test values, were collected within one month prior to IVF/ICSI procedures.
Routine semen analysis was performed using the SAS Differential Version Sperm Quality Analyzer (SAS - II, SAS Medical, China). Sperm concentration and viability were automatically recorded. For morphological evaluation, seminal smears were prepared and stained using the Diff - Quik staining method (Sperm Morphology Staining Kit, Anhui ANKE Biotechnology, China). Approximately 10 μL of semen was spread into a thin, homogeneous layer on a clean glass slide and air-dried at room temperature for at least 10 minutes. The slides were then stained and examined under a microscope (BX41; Olympus, Tokyo, Japan). According to WHO guidelines, sperm with deformed heads, midpieces, or principal pieces were classified as abnormal. The Sperm Deformity Index (SDI) was calculated as the number of deformed sperm divided by the total number of sperm. For each semen sample, at least 200 sperm were counted using a double-blind method. The percentage of sperm with normal morphology was then calculated.
Sperm DFI assessmentSperm DFI was assessed using the SCSA method (Sperm Nuclear Integrity Staining Kit, XingBo Biotechnology) on a flow cytometer (DxFlex, Beckman Coulter, USA), strictly in accordance with the product instructions (16). In this method, the chromatin in sperm nuclei with damaged DNA forms a single-stranded structure after acid treatment, which binds to acridine orange and emits red or yellow fluorescence. In contrast, chromatin in normal sperm nuclei retains a double-stranded structure after acid treatment and emits green fluorescence when bound to acridine orange. The proportion of sperm with damaged DNA is recorded as the DFI.
Ovarian stimulation, embryo culture and fresh transferThe female patients underwent a regular long gonadotropin - releasing hormone agonist (GnRH - α) regimen for controlled ovarian stimulation. The follicle development was monitored by transvaginal ultrasound and serum estradiol (E2) levels. Once the leading follicle size reached 20 mm or ≥ 3 follicles reached 18 mm, human chorionic gonadotropin (HCG) was injected intravascularly for maturation. Oocyte retrieval was performed 36 hours later under ultrasound guidance, followed by insemination using either conventional IVF or ICSI, depending on semen quality. For first-time patients, ICSI is recommended only in cases of severe male-factor infertility, defined as a sperm concentration < 5 million/mL, forward motility (type a+b) < 10%, or the presence of specific types of teratozoospermia (15, 17, 18). In all other cases, IVF is performed, with the decision remaining independent of DFI values. Fertilization was assessed 16 - 18 h after insemination as recommended by ESHRE (19). Day 3 embryos were assessed at 68 ± 1 h post-insemination, and those graded I-II with 7 - 10 blastomeres were high - quality embryos according to the Cummins’ criteria (20). Day 5 - Day 6 blastocysts were graded using the Gardner method (21), with score ≥ 4BB considered high - quality embryos.
Fresh embryo transfer was scheduled after progesterone transformation. Up to two embryos were transferred 3 or 5 days later depending on the developmental stage. Daily luteal phase support was provided with vaginal progesterone gel (90 mg/d, Crinone, Merck Serono) and oral dydrogesterone (20 mg/d, Duphaston, Abbott), and continued until 10 gestational weeks.
Outcome parametersFor laboratory results, the oocyte retrieval rate was calculated by dividing the number of oocytes retrieved by the number of follicles ≥ 14 mm on trigger day. The ICSI mature oocytes (MII oocytes) rate and normal fertilization rate were determined by dividing the number of MII oocytes and 2 pronuclei (PN) zygotes by the total number of injected oocytes, respectively. The cleavage rate was obtained by dividing the number of day 3 cleavage-stage embryos produced from 2PN oocytes by the total number of 2PN oocytes. The good-quality embryo rate was calculated by dividing the number of good - quality embryos on day3 by the total number of embryos at the cleavage stage. The blastocyst formation rate was calculated by dividing the number of blastocysts by the number of day 3 embryos subjected to extended culture. Finally, the available blastocyst rate was calculated by dividing the number of available blastocysts by the total number of blastocysts on days 5 and 6.
Regarding pregnancy outcomes, biochemical pregnancy was defined as a serum Beta-human chorionic gonadotropin (β - hCG) level of ≥ 5 mIU/mL at 10–12 days after embryo transfer. Clinical pregnancy was determined by the identification of at least one gestational sac, with or without a fetal heartbeat, one month following transfer. The implantation rate was measured by dividing the number of gestational sacs by the number of embryos transferred. Miscarriage was defined as clinical pregnancy loss before the 24th gestational week, and live birth was defined as delivery of infants with viable signs after the 24th gestational week.
Obstetrical and neonatal outcomes were collected from couples by specially trained nurses using standardized questionnaires. Telephone surveys were conducted during each trimester of pregnancy and after delivery. Major obstetric complications assessed included hypertensive disorders of pregnancy (HDP), gestational diabetes mellitus (GDM), intrahepatic cholestasis of pregnancy (ICP), placenta previa, premature rupture of membranes, postpartum hemorrhage and cesarean delivery. Key neonatal outcomes included gender, gestational age, birthweight, Z - score, and major birth defects.
Statistical analysisContinuous variables were summarized as means with standard deviations and assessed for normality using the Shapiro - Wilk. Normally distributed data were compared using one-way analysis of variance (ANOVA), while skewed data were analyzed using the Kruskal-Wallis test. Categorical variables were presented as numbers and percentages, with comparisons were made using Pearson’s Chi - square test or Fisher’s exact test, as appropriate.
Multivariate logistic regression analyses were conducted to assess the independent effect of DFI. Adjusted variables included age, duration of infertility, type of infertility (primary or secondary), number of oocytes retrieved, endometrial thickness, presence of uterine scarring, number and type of embryos transferred, and fertilization method (IVF or ICSI). Using the DFI < 15% group as the reference, odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for pregnancy outcomes in the other DFI categories. The independent factors associated with low birth weight were further determined through multivariate stepwise regression analysis.
All statistical analyses were performed using SAS 9.4 (SAS Institute, USA). A two - tailed P value < 0.05 was considered statistically significant.
ResultsBaseline characteristics and semen parameters across DFI subgroupsThis study included 5271 couples, with 3313 couples in the low DFI group (DFI < 15%), 1585 couples in the medium DFI group (15% ≤ DFI < 30%), and 373 couples in the high DFI group (DFI ≥ 30%). Table 1 presents the baseline and clinical characteristics of patients stratified by DFI values. In the low DFI group, both male and female partners were younger compared to the medium and high DFI groups (P<0.05). The high DFI group had a higher proportion of primary infertility, which may be partly attributed to an increased incidence of male factor infertility. Other factors, including BMI, duration of infertility, ultrasonographic findings, and basal hormone levels, showed no statistically significant differences across the groups (P>0.05).
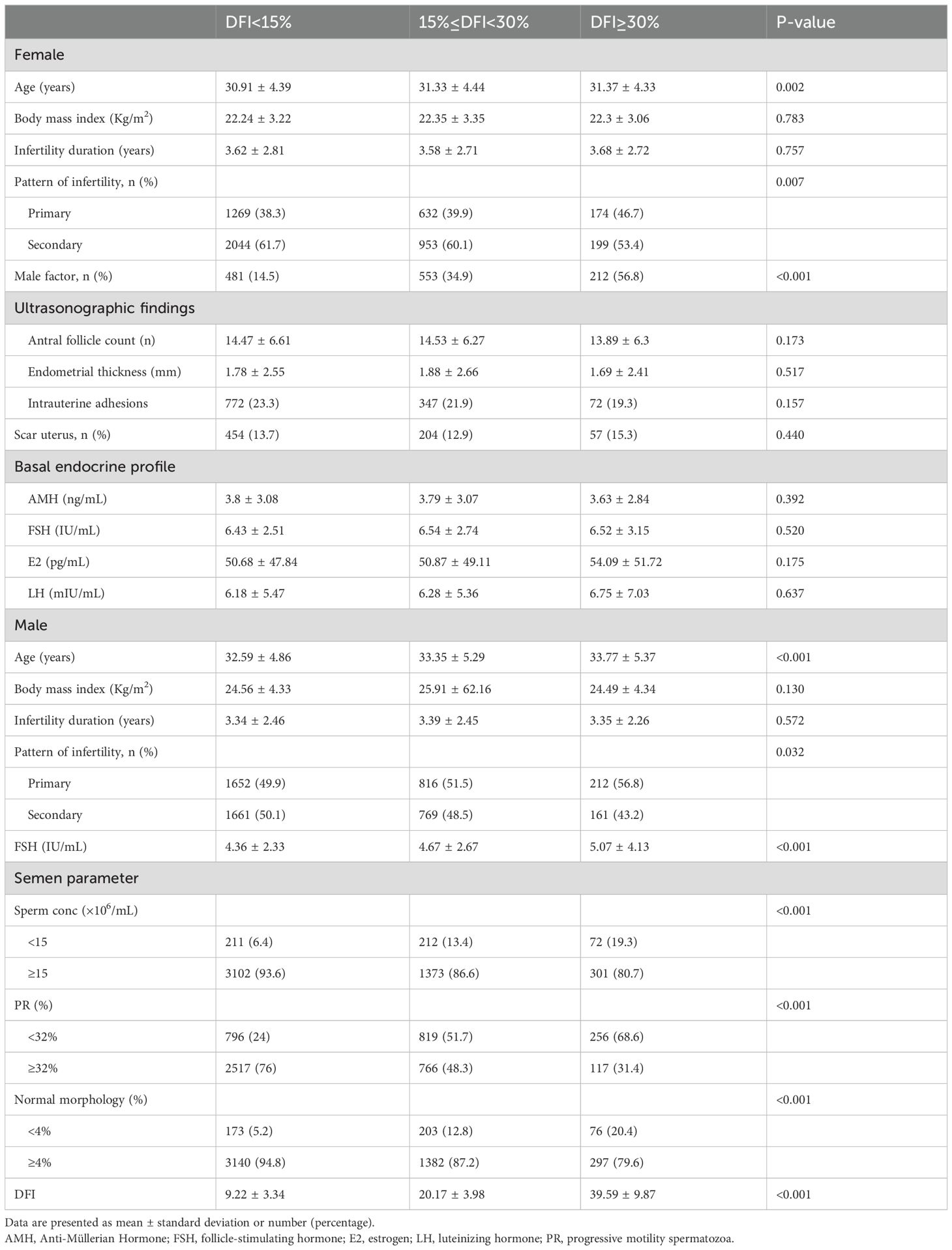
Table 1. Patient characteristics and male semen quality grouped by sperm DFI.
Sperm DFI was negatively correlated with progressive motility, sperm concentration, and the percentage of normal morphology. Additionally, male FSH levels were significantly elevated in the high DFI group. No significant differences were observed in male BMI or infertility duration among the three groups.
Cycle characteristics and laboratory outcomesCycle characteristics and laboratory outcomes grouped by the value of DFI are summarized in Table 2. No significant differences were observed in stimulation parameters across the three DFI groups (P>0.05). Regarding laboratory outcomes, compared to the low and medium DFI groups, the high DFI group had a lower proportion of IVF fertilization methods used (75.5%, 63.7%, 44.2%, respectively; P<0.001); however, there was no significant difference in the 2PN fertilization rate (64.83%, 61.25%, 61.29%, respectively; P=0.336) among the groups after IVF. The ICSI fertilization rate (80.94%, 79.92%, 76.26%, respectively; P<0.001), blastocyst formation rate (56.44%, 55.32%, 53.725%, respectively; P=0.045), and transplantable embryo rate (3.97 ± 2.71, 3.9 ± 2.70, 3.38 ± 2.40, respectively; P<0.001) were significantly lower in the high DFI group compared to the medium and low DFI groups (P<0.05).
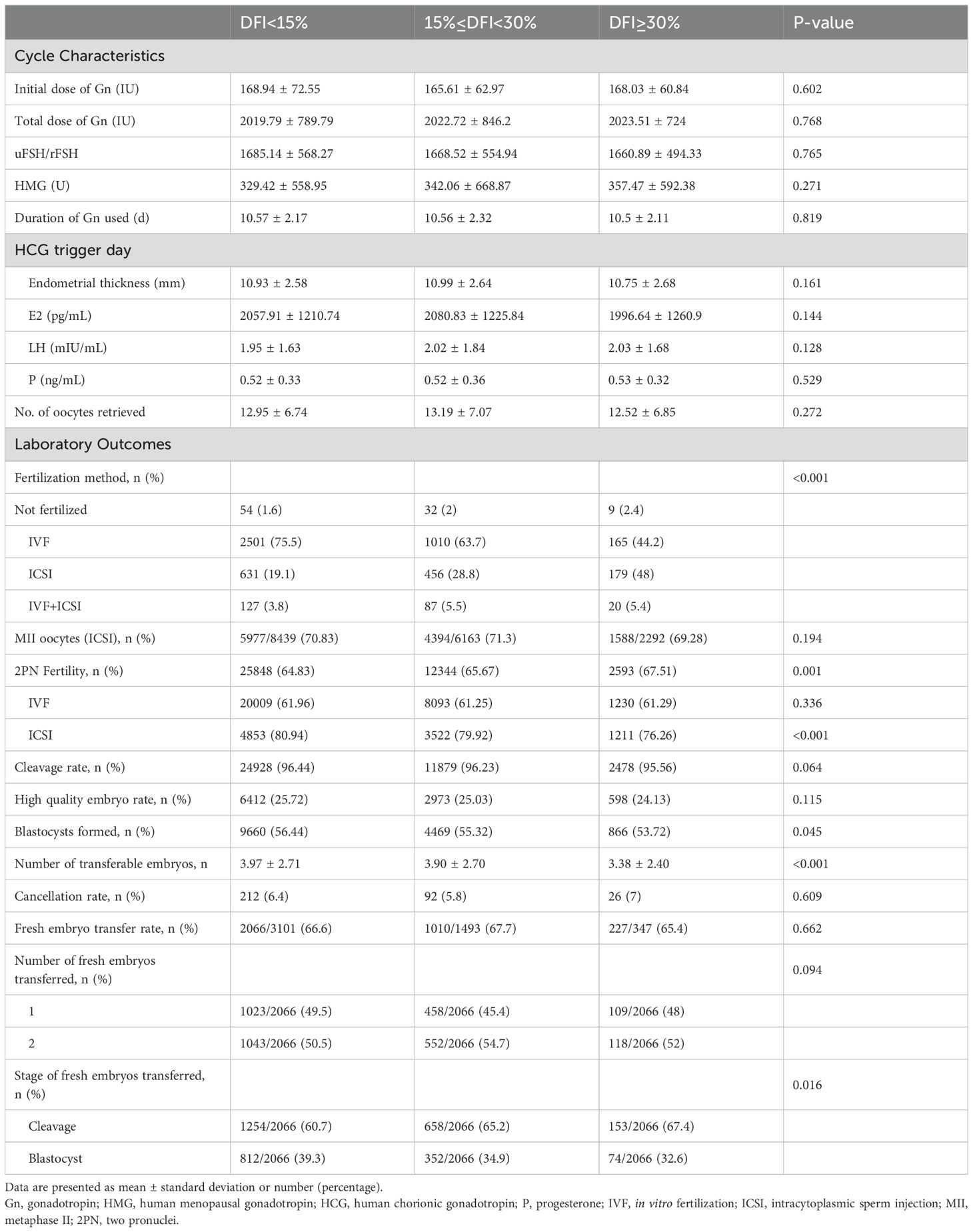
Table 2. Cycle characteristics and laboratory outcomes grouped by sperm DFI.
In fresh embryo transfer, the number of embryos transferred did not differ significantly among the three groups (P>0.05). However, the proportion of cleavage-stage embryo transfers was significantly higher in the high DFI group compared to the other groups (P<0.05). These findings are detailed in Table 2.
Clinical outcomes across DFI groupsTable 3 presents the pregnancy outcomes following fresh embryo transfer across the three DFI groups. No significant differences were observed in the positive hCG rate (76.6%, 75,3%, 74.9%, respectively; P=0.659), clinical pregnancy rate (68.1%, 67.3%, 66.5%, respectively; P= 0.849), multiple birth rate (20.1%, 21.5%, 20.8%, respectively; P=0.228), miscarriage rate (12.9%, 11.9%, 10.6%, respectively; P=0.616), or live birth rate (58.9%, 58.4%, 59.0%, respectively; P= 0.969) among the groups. These results remained consistent in both crude and adjusted analyses.
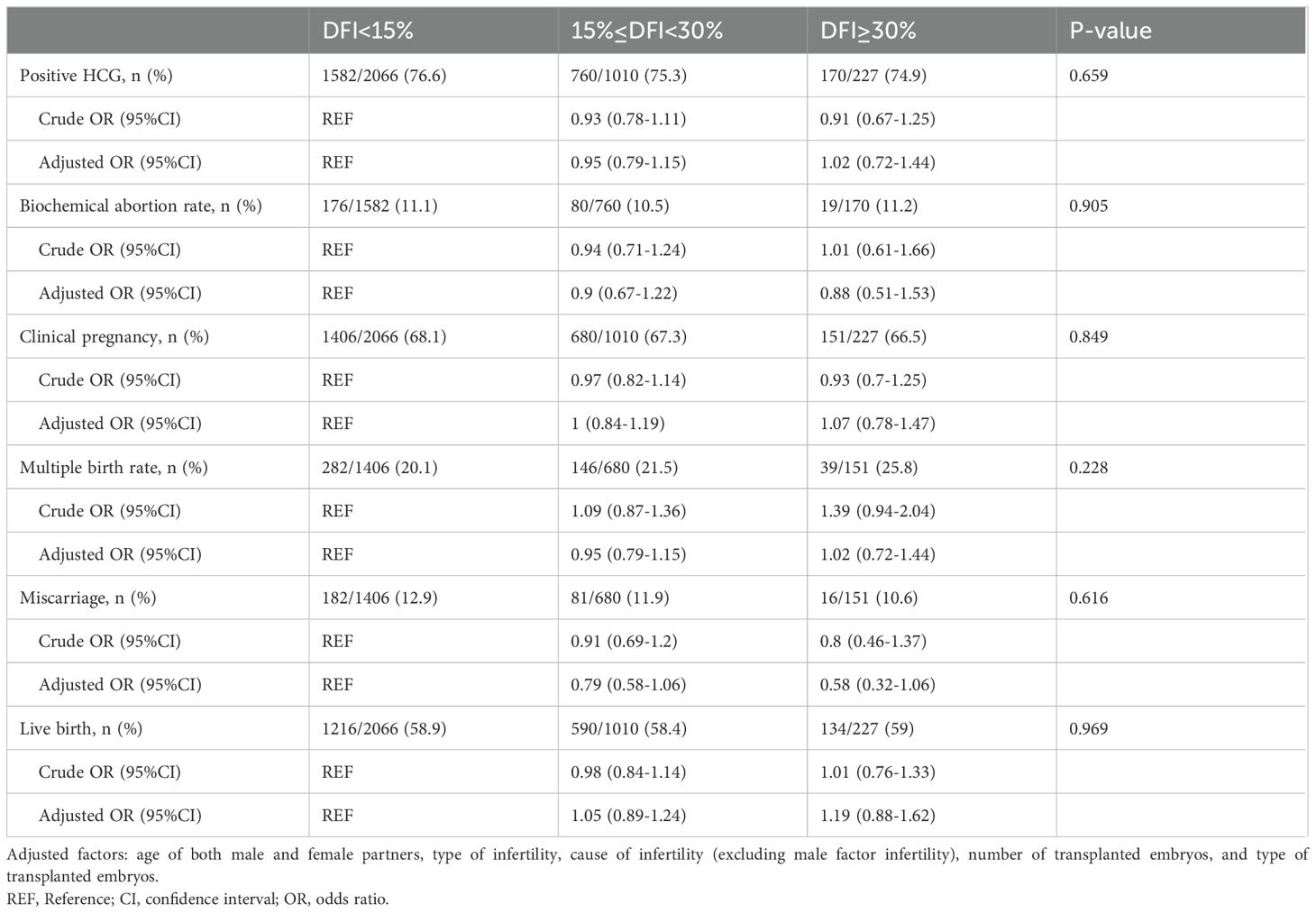
Table 3. Clinical outcomes and single/multiple factor analysis of fresh embryo transfer grouped by sperm DFI.
Maternal and neonatal outcomes in singleton pregnanciesAmong the 5271 couples included in the study, 1570 couples achieved a singleton live birth. The maternal and neonatal outcomes were analyzed across the three DFI groups (Table 4). Overall, no significant differences were observed in most outcomes among the groups (P>0.05). However, in the high DFI group, the proportion of low birth weight (LBW) newborns was significantly higher compared to the low and medium DFI groups (3.9%, 6.6%, 10.1%, respectively; P=0.006). In further stepwise multivariate regression analysis (Table 5), 15% ≤ DFI < 30% and DFI ≥ 30% were consistently associated with neonatal low birth weight (OR: 1.75, 95% CI 1.08–2.85, P= 0.023; OR: 2.78, 95% CI 1.34–5.75, P=0.006, respectively). Additionally, neonatal gender (OR: 1.67, 95% CI 1.06–2.62, P=0.028) and gestational age (OR: 0.33, 95% CI 0.27–0.39, P<0.001) were also significant risk factors for low birth weight.
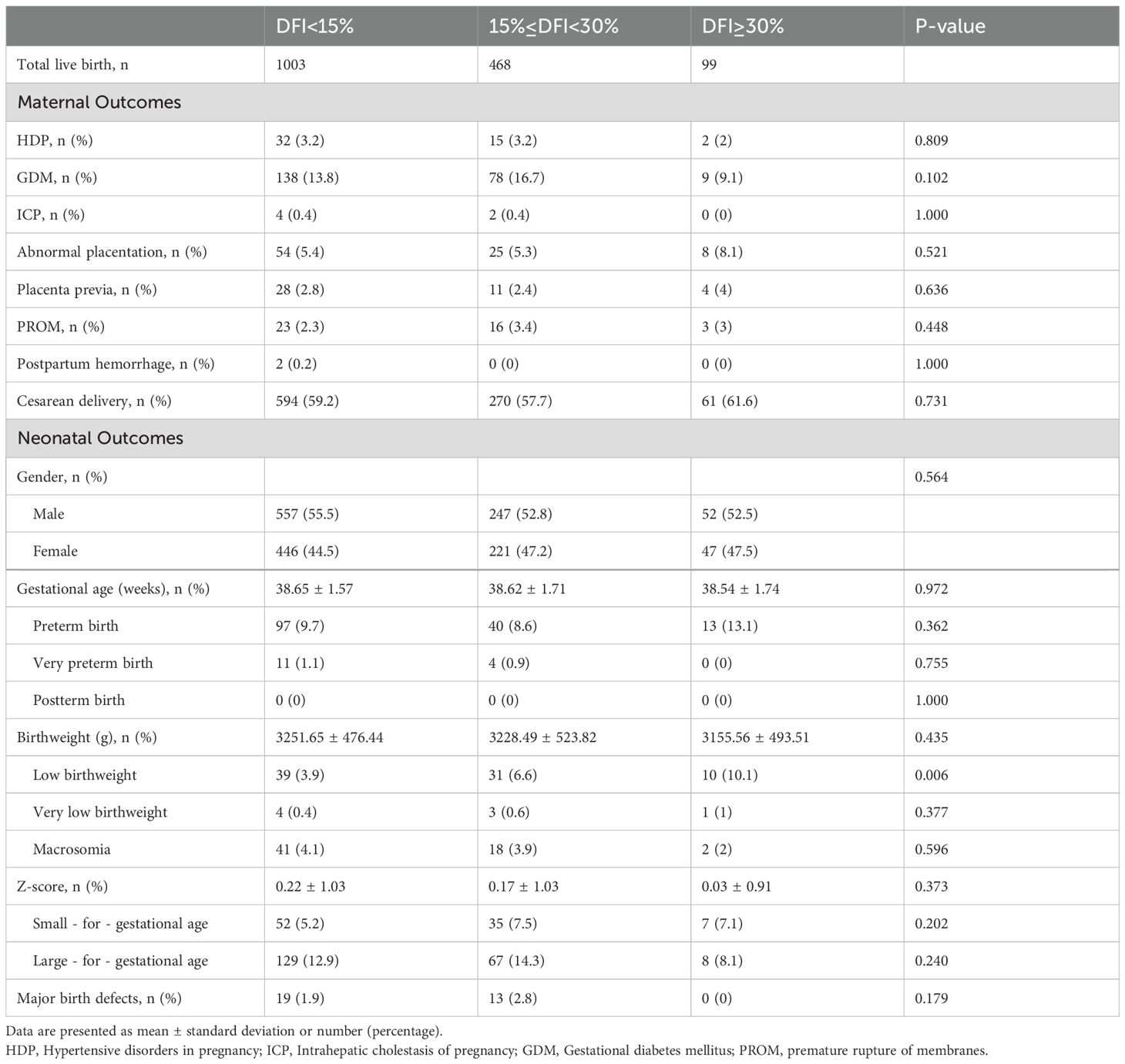
Table 4. Obstetric and perinatal outcomes in singleton pregnancies of couples grouped by sperm DFI.
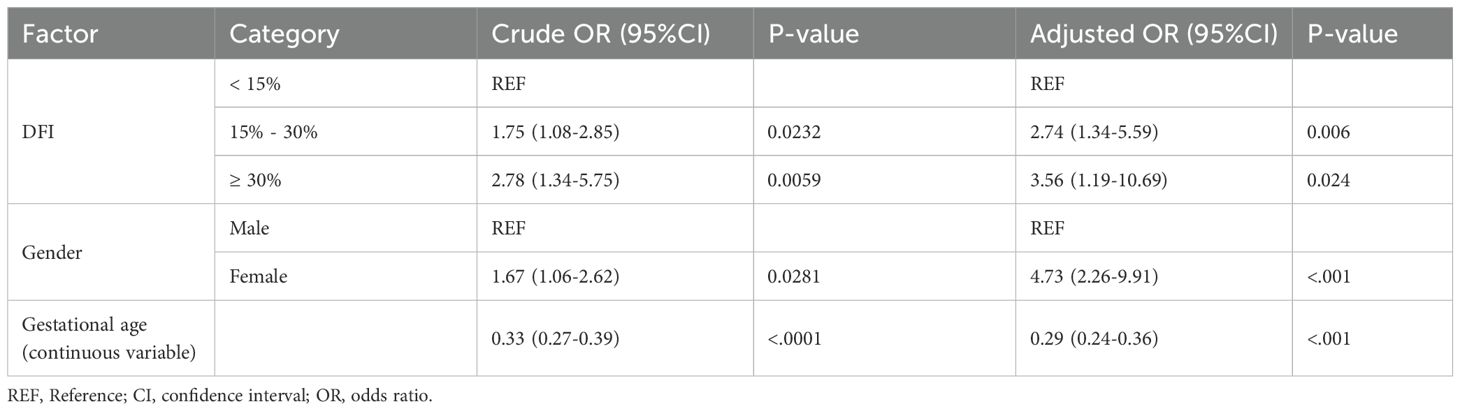
Table 5. Stepwise logistic regression on risk of low birth weight.
DiscussionBased on DFI values, our retrospective analysis demonstrated that patients with high DFI exhibited a significantly lower sperm quality. In addition, high DFI was found to impact embryonic development and increase the risk of low birth weight in offspring. However, no significant effects of DFI were observed on pregnancy, obstetrical or other neonatal outcomes.
Several studies have recommended incorporating sperm DNA fragmentation analysis as a routine complementary test in semen evaluation (6, 15, 22). In this study, we utilized the SCSA method due to its high sensitivity in detecting sperm DNA fragmentation (23, 24). Our findings reveal that high DFI is strongly associated with significant impairments in all seminal parameters. Additionally, couples with high DFI are more likely to seek assisted reproduction due to male factors, with a higher proportion opting for ICSI fertilization in subsequent treatments. These results are consistent with the majority of published studies (24–26), which emphasize that sperm DNA fragmentation—characterized by breaks or damage in the genetic material of spermatozoa - is a key contributor to male infertility. Such damage compromises the functional capacity of sperm, leading to difficulties in achieving natural conception or optimal outcomes in assisted reproduction.
Regarding the impact of DFI on embryonic development and clinical outcomes, our study found the fertilization rate in the ICSI group was negatively correlated with DFI values, whereas no significant differences were observed among the IVF groups. During ICSI procedure, sperm are subjectively selected based on morphological and motility criteria by operators, with little consideration for DNA integrity. This may explain the lower fertilization rates and poorer embryo quality observed with higher DFI (27, 28). In contrast, the IVF procedure involves a natural sperm selection process, potentially mitigating the impact of high DFI on fertilization outcomes. When observing the development of embryos, our findings is consistent with most published studies (29, 30), indicating that high DFI negatively affects blastocyst formation and the rate of available embryos. Given our center’s advocacy for single blastocyst transfer, fresh embryo transfer statistics revealed that patients with high DFI were more likely to undergo cleavage embryo transfer. This trend may stem from the reduced blastocyst formation rates and developmental arrest of embryos associated with high DFI (31–34). Recent studies have shown that Physiological Intracytoplasmic Sperm Injection (PICSI) can significantly improve both fertilization rates and embryo quality (35, 36), when hyaluronic acid bound sperm selection method is used, it generally selects a more genomic mature sperm with intact DNA, which can enhance embryological outcome (36). However, our center currently does not offer PICSI, and therefore, we are currently unable to implement such measures and make comparisons. This remains a promising area for future exploration among men with high DFI.
There is ongoing controversy regarding the impact of DFI on clinical outcomes, particularly its influence on miscarriage risk (30, 37). Our results indicate no significant differences among the three groups in terms of biochemical pregnancy rate, clinical pregnancy rate, miscarriage rate, multiple birth rate, or live birth rate. This may be attributed to the repair capacity of oocytes and early embryos, which can mitigate sperm DNA damage to a certain extent (38, 39). However, this repair mechanism is limited, and beyond a certain threshold, insufficient or aberrant repair may lead to mutations in the zygote genome, potentially resulting in pathologies in the offspring (40). In the present study, there was no significant difference in most obstetric and neonatal complications among the three groups. However, the proportion of low birth weight infants increased with higher DFI values, and this finding remained statistically significant even after adjusting for potential confounders. These results are consistent with Li et al.’s study (41). We further performed stepwise logistic regression analysis for risk of low birth weight, revealing that DFI, gestational age, and neonatal gender were all independent factors. However, there are some residual confounding factors, such as maternal nutrition and weight gain during pregnancy, that were not included in the study. Therefore, further prospective research is still needed to confirm our finding.
There are several limitations to this study that should be acknowledged. First, the retrospective design may have introduced bias. While the inclusion of a large dataset enabled us to account for critical confounding factors, our findings could still be influenced by some unmeasured confounders. Second, the lack of data linkage to electronic medical records may result in the underreport of maternal and neonatal outcomes, which may have influenced the statistical power. Lastly, due to time constraints, we were unable to complete live birth follow-up for the entire cohort; Lastly, due to time constraints, we were unable to complete live birth follow-up for the entire cohort. Furthermore, our analysis was limited to pregnancy outcomes following fresh embryo transfer, leaving the potential effects of frozen-thawed embryo transfers unexplored, warranting further investigation.
ConclusionOur study demonstrates that high sperm DFI is significantly associated with impairments in all semen parameters. Elevated DFI negatively impacts fertilization, the formation of usable blastocysts, and the rate of available embryos. While high DFI may contribute to an increased incidence of low birth weight in offspring, it does not significantly impact key clinical outcomes or other maternal and neonatal complications. Continued surveillance of offspring conceived through assisted reproduction in such cases is essential to monitor potential long-term health effects.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statementThe studies involving humans were approved by Reproductive Medicine Ethics Committee of Jiangxi Maternal and Child Health Hospital (No 202409). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributionsBY: Writing – original draft, Writing – review & editing. LX: Data curation, Formal analysis, Writing – review & editing. RD: Writing – original draft. LW: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. ZZ: Investigation, Methodology, Project administration, Writing – review & editing. XW: Conceptualization, Data curation, Formal analysis, Writing – review & editing. TD: Data curation, Methodology, Project administration, Writing – review & editing. YZ: Resources, Supervision, Validation, Writing – review & editing. JH: Funding acquisition, Project administration, Resources, Writing – review & editing. ZH: Formal analysis, Project administration, Supervision, Validation, Visualization, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Natural Science Foundation of Jiangxi Province (20224BAB216025, 20242BAB25446), and National Natural Science Foundation of China (82260315).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References2. Rotondo JC, Lanzillotti C, Mazziotta C, Tognon M, Martini F, et al. Epigenetics of male infertility: the role of DNA methylation. Front Cell Dev Biol. (2021) 9:689624. doi: 10.3389/fcell.2021.689624
PubMed Abstract | Crossref Full Text | Google Scholar
4. Simon L, Castillo J, Oliva R, Lewis SEM. Relationships between human sperm protamines, DNA damage and assisted reproduction outcomes. Reprod BioMed Online. (2011) 23:724–34. doi: 10.1016/j.rbmo.2011.08.010
PubMed Abstract | Crossref Full Text | Google Scholar
5. Alkhayal A, Gabriel San M, Zeidan K, Alrabeeah K, Noel D, McGraw R, et al. Sperm DNA and chromatin integrity in semen samples used for intrauterine insemination. J Assist Reprod Genet. (2013) 30:1519–24. doi: 10.1007/s10815-013-0101-3
PubMed Abstract | Crossref Full Text | Google Scholar
8. Simon L, Emery BR, Carrell DT. Review: Diagnosis and impact of sperm DNA alterations in assisted reproduction. Best Pract Res Clin Obstet Gynaecol. (2017) 44:38–56. doi: 10.1016/j.bpobgyn.2017.07.003
PubMed Abstract | Crossref Full Text | Google Scholar
9. Donald E, Regina W. Meta-analysis of sperm DNA fragmentation using the sperm chromatin structure assay. Reprod BioMed Online. (2006) 12:466–72. doi: 10.1016/s1472-6483(10)62000-7
PubMed Abstract | Crossref Full Text | Google Scholar
10. McQueen DB, Zhang J, Robins JC. Sperm DNA fragmentation and recurrent pregnancy loss: a systematic review and meta-analysis. Fertil Steril. (2019) 112(1):54–60 e3.
PubMed Abstract | Google Scholar
11. Gat I, Tang K, Quach K, Kuznyetsov V, Antes R, Filice M. Sperm DNA fragmentation index does not correlate with blastocyst aneuploidy or morphological grading. PloS one. (2017) 12(6):e0179002.
12. Cissen M, Wely M, Scholten I, Mansell S, Bruin J, Mol B, et al. Measuring sperm DNA fragmentation and clinical outcomes of medically assisted reproduction: A systematic review and meta-analysis. PloS One. (2016) 11:e0165125. doi: 10.1371/journal.pone.0165125
PubMed Abstract | Crossref Full Text | Google Scholar
13. Basar M, Kahraman S. Clinical utility of sperm DNA fragmentation testing: practice recommendations based on clinical scenarios. Trans Androl Urol. (2017) 6:S574–6. doi: 10.21037/tau.2017.04.40
PubMed Abstract | Crossref Full Text | Google Scholar
14. Bender Atik R, Christiansen OB, Elson J, Kolte AM, Lewis S, Middeldorp S, et al. ESHRE guideline: recurrent pregnancy loss: an update in 2022. Hum Reprod Open. (2023) 2023:e13739. doi: 10.1093/hropen/hoad002
PubMed Abstract | Crossref Full Text | Google Scholar
15. Lars B, Jackson KB. The sixth edition of the WHO Laboratory Manual for the Examination and Processing of Human Semen: ensuring quality and standardization in basic examination of human ejaculates. Fertil Steril. (2022) 117:246–51. doi: 10.1016/j.fertnstert.2021.12.012
PubMed Abstract | Crossref Full Text | Google Scholar
16. Donald P E, Kay K, Regina L W. Analysis of sperm DNA fragmentation using flow cytometry and other techniques. Soc Reprod Fertil Suppl. (2007) 65:93–113.
PubMed Abstract | Google Scholar
18. Zheng D, Zeng L, Yang R, Lian Y, Zhu YM, Liang X, et al. Intracytoplasmic sperm injection (ICSI) versus conventional in vitro fertilisation (IVF) in couples with non-severe male infertility (NSMI-ICSI): protocol for a multicentre randomised controlled trial. BMJ Open. (2019) 9:e030366. doi: 10.1136/bmjopen-2019-030366
PubMed Abstract | Crossref Full Text | Google Scholar
19. Maria José D, Susanna A, Giovanni C, Sophie D, Kersti L, Carlos PE, et al. Revised guidelines for good practice in IVF laboratories(2015). Hum Reprod. (2016) 31(4):685–6.
PubMed Abstract | Google Scholar
20. Cummins JM, Breen TM, Harrison KL, Shaw JM, Wilson LM, Hennessey JF. A formula for scoring human embryo growth rates in in vitro fertilization: its value in predicting pregnancy and in comparison with visual estimates of embryo quality. J In Vitro Fert Embryo Transf. (1986) 3:284–95. doi: 10.1007/BF01133388
PubMed Abstract | Crossref Full Text | Google Scholar
21. Ahmadi A, Ng S. Developmental capacity of damaged spermatozoa. Human Reprod (Oxford, England). (1999) 14(9):2279–85.
PubMed Abstract | Google Scholar
24. Evenson D, Djira G, Kasperson K, Christianson J. Relationships between the age of 25,445 men attending infertility clinics and sperm chromatin structure assay (SCSA®) defined sperm DNA and chromatin integrity. Fertil Steril. (2020) 114:311–20. doi: 10.1016/j.fertnstert.2020.03.028
PubMed Abstract | Crossref Full Text | Google Scholar
25. Campos LGA, Requejo LC, Minano CAR, Orrego JD, Loyaga EC, Cornejo LG. Correlation between sperm DNA fragmentation index and semen parameters in 418 men seen at a fertility center. JBRA Assist Reprod. (2021) 25:349–57. doi: 10.5935/1518-0557.20200079
PubMed Abstract | Crossref Full Text | Google Scholar
26. Stavros S, Potiris A, Molopodi E, Mavrogianni D, Zikopoulos A, Louis K, et al. Sperm DNA fragmentation: unraveling its imperative impact on male infertility based on recent evidence. Int J Mol Sci. (2024) 25. doi: 10.3390/ijms251810167
PubMed Abstract | Crossref Full Text | Google Scholar
27. Tarozzi N, Nadalini M, Borini A. Effect on sperm DNA quality following sperm selection for ART: new insights. Genet Damage Hum Spermatozoa. (2019), 169–87. doi: 10.1007/978-3-030-21664-1_10
PubMed Abstract | Crossref Full Text | Google Scholar
28. Asgari F, Nadalini M, Borini A. Risk of embryo aneuploidy is affected by the increase in sperm DNA damage in recurrent implantation failure patients under ICSI-CGH array cycles. Hum Fertil. (2021) 25:872–80. doi: 10.1080/14647273.2021.1920054
PubMed Abstract | Crossref Full Text | Google Scholar
29. Green KA, Patounakis G, Dougherty MP, Werner MD, Scott RT, Franasiak JM. Sperm DNA fragmentation on the day of fertilization is not associated with embryologic or clinical outcomes after IVF/ICSI. J Assisted Reprod Genet. (2019) 37:71–6. doi: 10.1007/s10815-019-01632-5
PubMed Abstract | Crossref Full Text | Google Scholar
30. Maghraby H, Elsuity MA, Adel N, Magdi Y, Abdelbadie AS, Rashwan MM, et al. Quantifying the association of sperm DNA fragmentation with assisted reproductive technology outcomes: An umbrella review. BJOG: Int J Obstet Gynaecol. (2024) 131:1181–96. doi: 10.1111/1471-0528.17796
PubMed Abstract | Crossref Full Text | Google Scholar
31. Newman H, Catt S, Vining B, Vollenhoven B, Horta F. DNA repair and response to sperm DNA damage in oocytes and embryos, and the potential consequences in ART: a systematic review. Mol Hum Reprod. (2022) 28. doi: 10.1093/molehr/gaab071
PubMed Abstract | Crossref Full Text | Google Scholar
33. Musson R, Gąsior Ł, Bisogno S, Ptak G. DNA damage in preimplantation embryos and gametes: specification, clinical relevance and repair strategies. Hum Reprod Update. (2022) 28:376–99. doi: 10.1093/humupd/dmab046
PubMed Abstract | Crossref Full Text | Google Scholar
34. Chapuis A, Gala A, Ferrieres-Hoa A, Mullet T, Bringer-Deutsch S, Vintejoux E, et al. Sperm quality and paternal age: effect on blastocyst formation and pregnancy rates. Basic Clin Androl. (2017) 27:2. doi: 10.1186/s12610-016-0045-4
PubMed Abstract | Crossref Full Text | Google Scholar
35. West R, Coomarasamy A, Frew L, Hutton R, Kirkman-Brown J, Lawlor M, et al. Sperm selection with hyaluronic acid improved live birth outcomes among older couples and was connected to sperm DNA quality, potentially affecting all treatment outcomes. Hum Reprod. (2022) 37:1106–25. doi: 10.1093/humrep/deac058
PubMed Abstract | Crossref Full Text | Google Scholar
36. Shivhare S, Karunakaran S, Bose AS, Goel R, Ananthakrishnan R. Does physiological intracytoplasmic sperm injection improve outcome in men with abnormal semen parameters: A retrospective cohort study. J Hum Reprod Sci. (2024) 17:200–6. doi: 10.4103/jhrs.jhrs_95_24
PubMed Abstract | Crossref Full Text | Google Scholar
37. Maria Christine K, Reinhardt Josefine N, Anna S, Vauvert Kathrine H, Marie Astrid K, David W, et al. Prospective reproductive outcomes according to sperm parameters, including DNA fragmentation, in recurrent pregnancy loss. Reprod BioMed Online. (2024) 49(2):103773. doi: 10.1016/j.rbmo.2023.103773
PubMed Abstract | Crossref Full Text | Google Scholar
38. Matsuda Y, Tobari I. Chromosomal analysis in mouse eggs fertilized in vitro with sperm exposed to ultraviolet light (UV) and methyl and ethyl methanesulfonate (MMS and EMS). Mutat Res. (1988) 198:131–44. doi: 10.1016/0027-5107(88)90048-6
PubMed Abstract | Crossref Full Text | Google Scholar
40. Saleh R, Agarwal A, Nada E, El-Tonsy M, Sharma R, Meyer A, et al. Negative effects of increased sperm DNA damage in relation to seminal oxidative stress in men with idiopathic and male factor infertility. Fertil Steril. (2003) p:1597–605. doi: 10.1016/S0015-0282(03)00337-6
PubMed Abstract | Crossref Full Text | Google Scholar
41. Li F, Duan X, Li M, Ma X. Sperm DNA fragmentation index affect pregnancy outcomes and offspring safety in assisted reproductive technology. Sci Rep. (2024) 14. doi: 10.1038/s41598-023-45091-6
留言 (0)