In children with growth hormone (GH) deficiency, response to recombinant GH treatment is highly variable due to many factors, making correct dosing challenging (1–4). International guidelines recommend weight- or body surface-based dosing because of a lack of evidence to support alternative dosing strategies (5). A potential dosing approach involves titration of GH to the serum levels of insulin-like growth factor-1 (IGF-1).
IGF-1, a biomarker of GH status, could guide GH treatment but has some limitations. Serum IGF-1, which is measured clinically, is mainly derived from hepatic production mediated by GH and is negatively affected by catabolic conditions (6), and inter-assay variability adds complexity to the interpretation (7). Moreover, there is considerable individual variability in IGF-1 levels. In a pediatric cohort with individuals at different stages of maturation, using IGF-1 as a biomarker poses even greater challenges as growth rates and adult height also depend on birth length, bone age maturation, and nutritional status (8).
Short-term height benefits of GH treatment have been shown with an IGF-1 target defined as a standard deviation score (SDS) of 0 or +2 compared to weight-based dosing. Increasing the dose of GH to reach higher IGF-1 targets has yielded enhanced growth responses (4, 9). In a randomized controlled trial, Cohen et al. showed that GH dosing targeted to IGF-1 0 SDS could be dose-sparing compared to conventional weight-based dosing and theoretically safer as high IGF-1 levels were avoided (10). However, an optimal target for long-term IGF-1 levels has not yet been established to optimize height outcomes and address safety and cost concerns, which would be needed to make a recommendation regarding IGF-1 titration in GH dosing.
Currently, the recommended use of IGF-1 is as a long-term safety marker for patients treated with GH. It is recommended that levels of IGF-1 should not exceed 2 SDS due to concerns about increasing the potential risk of adverse events (5). Epidemiological studies in adults have shown an association between IGF-1 levels in the upper quartile of the normal range and an increased risk of colorectal cancer, breast cancer, and prostate cancer, while IGF-1 levels in the lower quartile of the normal range have been linked to ischemic heart disease (11, 12). Short-term elevation of IGF-1 has occasionally been acceptable and has not led to severe adverse effects, although supraphysiological doses have been shown to accelerate bone maturation (13). The national Swedish guidelines from 2020 recommend a standard GH dose of 33µg/kg/day adjusted for IGF-1 with an upper limit below 2 SDS, whereas in the first two years of treatment, an IGF-1 up to 3 SDS can be short-term tolerated if necessary. Compliance with these guidelines requires a reliable IGF-1 reference range.
IGF-1 levels gradually increase during childhood and appear to peak in mid-puberty, with high levels occurring two to four years after the pubertal growth spurt (14). Levels of IGF-1 peak during puberty, indicating a link between IGF-1 and endogenous sex steroids and, hence, sexual maturation (14–16). It has been suggested that the GH endogenous production pattern is modulated by estradiol as previous studies have shown that estrogen receptor blockers and aromatase inhibitors in males tend to decrease GH and IGF-1 levels (17, 18). In our clinic, we have occasionally seen that children treated with GH during the early pubertal years have increases in IGF-1 despite having no clinical pubertal signs according to Tanner staging nor changes in the GH dose. We hypothesized that this pattern could be due to an early rise in sex steroid levels before the appearance of clinical pubertal signs.
Whether IGF-1 will be used for GH dose titration or treatment monitoring, the correct interpretation of the levels is important. The objective of the current study is to assess a well-defined group of peripubertal children with short stature receiving GH treatment to investigate how pubertal maturation and sex steroid levels may impact the interpretation of IGF-1 and the characteristics of the children with higher or lower serum IGF-1 SDS.
2 Materials and methods2.1 Study population and designA total of 98 children (72 boys and 26 girls) who previously participated in a GH dosing clinical trial (n = 33 with GH-deficiency; n = 65 with non-GH-deficiency based on both a provocation test and a spontaneous GH-secretion profile) were considered eligible for the study. The trial was conducted in Sweden at five pediatric clinics in Gothenburg, Umeå, Uppsala, Malmö, and Halmstad (Swedish study number NRA 6280003 and ClinicalTrials.gov identifier NCT02879747). See previous publication for more details about the cohort (19).
The time frame of our study spanned 2 years before pubertal start and 3 years after pubertal start, thus, up to 6 years of follow-up data were available. Pubertal starts were defined as Tanner breast stage >1 in girls, and at least one testis >3 mL in boys. We collected clinical data from a trial database, including age, pubertal stage, weight, height, GH dose, IGF-1, and IGF-1 SDS. Blood samples were taken at least yearly and stored in a freezer at -80 degrees Celsius. We assembled a set of frozen samples obtained in the morning between 0800-1000 am closest in time to clinical pubertal start, one year and two years prior, and one, two, and three years after the start of puberty. For the girls, we analyzed estradiol levels, and for the boys, testosterone levels (20). Five boys were excluded because of a lack of clinical data or lack of a series of stored blood samples. A total of 93 children were hence included (67 boys and 26 girls).
Of a total of 475 IGF-1 measurements, 31 were missing for the dates when data on sex steroids were available, and thus, these missing IGF-1 data were analyzed later from frozen samples. Weight and height data were collected +/- 30 days from IGF-1 and sex steroid sampling dates.
2.2 Hormonal analysesSerum IGF-I concentrations were measured using a specific radioimmunoassay (RIA) (Mediagnost GmbH. Tübingen, Germany) (21). The total coefficient of variation (CV) was 18% at 40 µg/L and 11% at 225 µg/L. For IGF-1 analyses of the 31 frozen samples, we used the chemiluminescent immunoassay IDS iSYS (Immunodiagnostic Systems Holdings, Tyne and Wear, UK). The two methods correlate with r=0.98, and no conversion was needed.
Serum estradiol and testosterone levels were simultaneously determined by gas chromatography-tandem mass spectrometry (GC-MS/MS), as described in detail elsewhere (20). The lower limit of detection (LOD) for estradiol was 2 pmol/L, and for testosterone, 0.1 nmol/L. Total CV for estradiol was 19% at 8 pmol/L and 6% at ≥36 pmol/L, and for testosterone, 16% at 0.3 nmol/L and <10% at >1.5 nmol/L.
Biological reference range for serum estradiol and testosterone in children have previously been published (22). Replacing (extraction-) RIAs with the newer GC-MS/MS provides a high degree of agreement between the methods (20), and therefore, previously established reference ranges could be adopted. In girls with Tanner breast stage 2, 90% of the serum estradiol concentrations ranged between 7 and 77 pmol/L. However, estradiol concentrations ≤24 pmol/L were seen in both prepubertal girls and in girls with Tanner breast stage 2. Therefore, we assigned an estradiol concentration of ≥25 pmol/L as a cut-off value for pubertal levels in girls. Following similar reasoning, we assigned a testosterone concentration of ≥0.47 nmol/L as a cut-off value for pubertal levels in boys. For evaluation purposes, the results of < LOD were assigned the values of LOD/2.
IGF-1 levels were converted to SDS values according to the reference range of Löfqvist et al. (15). The reference range is sex- and age-specific and is also divided into four pubertal stages: prepubertal, early puberty, mid-puberty, and late puberty.
BMI SDS was calculated according to Karlberg et al. (23).
2.3 Statistical methodsData are expressed as medians or means (range). Linear mixed effect models were used for the comparison of sample groups. P-values <0.05 are considered statistically significant.
2.4 EthicsThe research protocol was approved by the local Research Ethics Committee with ref nr 320-03, 449-16 and the Medical Product Agency of Sweden study no., NRA 6280003. Parents signed informed consent, and assent was obtained from the child when age-appropriate. The study was performed according to the Helsinki Declaration.
3 Results3.1 Patient characteristics and pubertal startDuring the study period, the average number of samples analyzed for sex steroids per child was 4.8 (2-6) for girls and 5.2 (3-6) for boys. In girls with a clinical pubertal stage of Tanner breast stage 1, a total of 58 samples were analyzed to quantify estradiol concentrations, and for boys with testes size <4 mL, a total of 153 samples were analyzed to quantify testosterone concentrations. Characteristics of the children included in the study are described in Table 1. Puberty started at a normal age, at a median of 11.0 (9.5-12.5) years for girls and 12.5 (10.4-14.7) years for boys. At the start of puberty, IGF-I values were increased for most children, as expected.
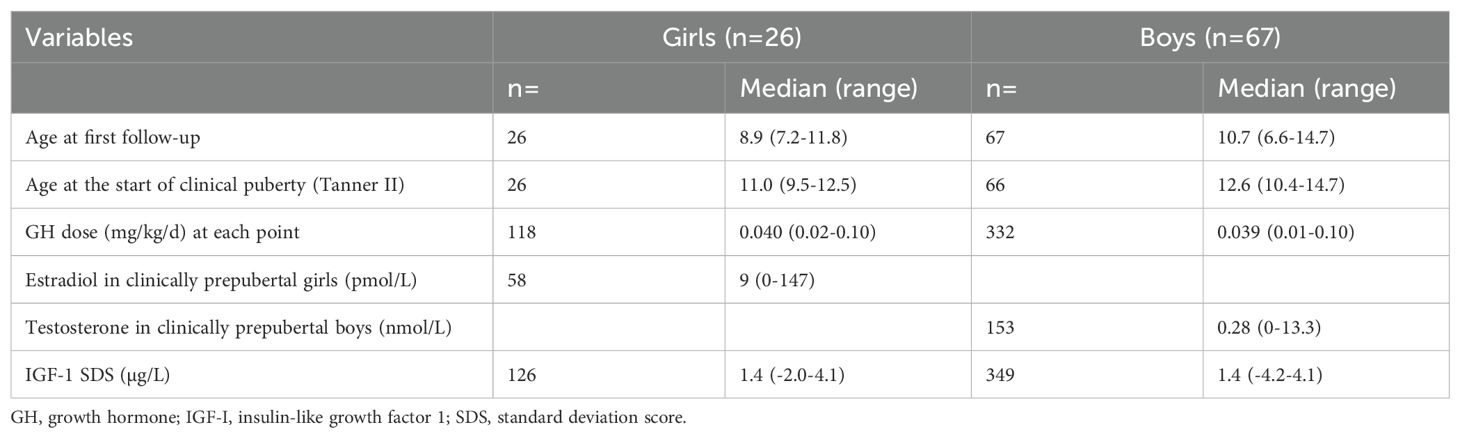
Table 1. Patient characteristics.
3.2 Sex steroids and IGF-1 SDSThe hormonal start of puberty in girls was defined using an estradiol concentration of ≥25 pmol/L as a cut-off value. Nine of 58 (15.5%) samples from girls with clinically-defined Tanner breast stage 1 had levels above this cut-off. When recalculating IGF-1 SDS for these samples using the IGF-1 reference range corresponding to early puberty instead of prepuberty, the IGF-1 SDS decreased from a median of 2.04 (1.29-3.62) to 1.44 (0.63-2.98), p<0.05. Four out of nine IGF-1 SDS levels decreased below the clinical dose-adjusting upper limit of 2 SDS recommended by guidelines (5). See Figure 1.
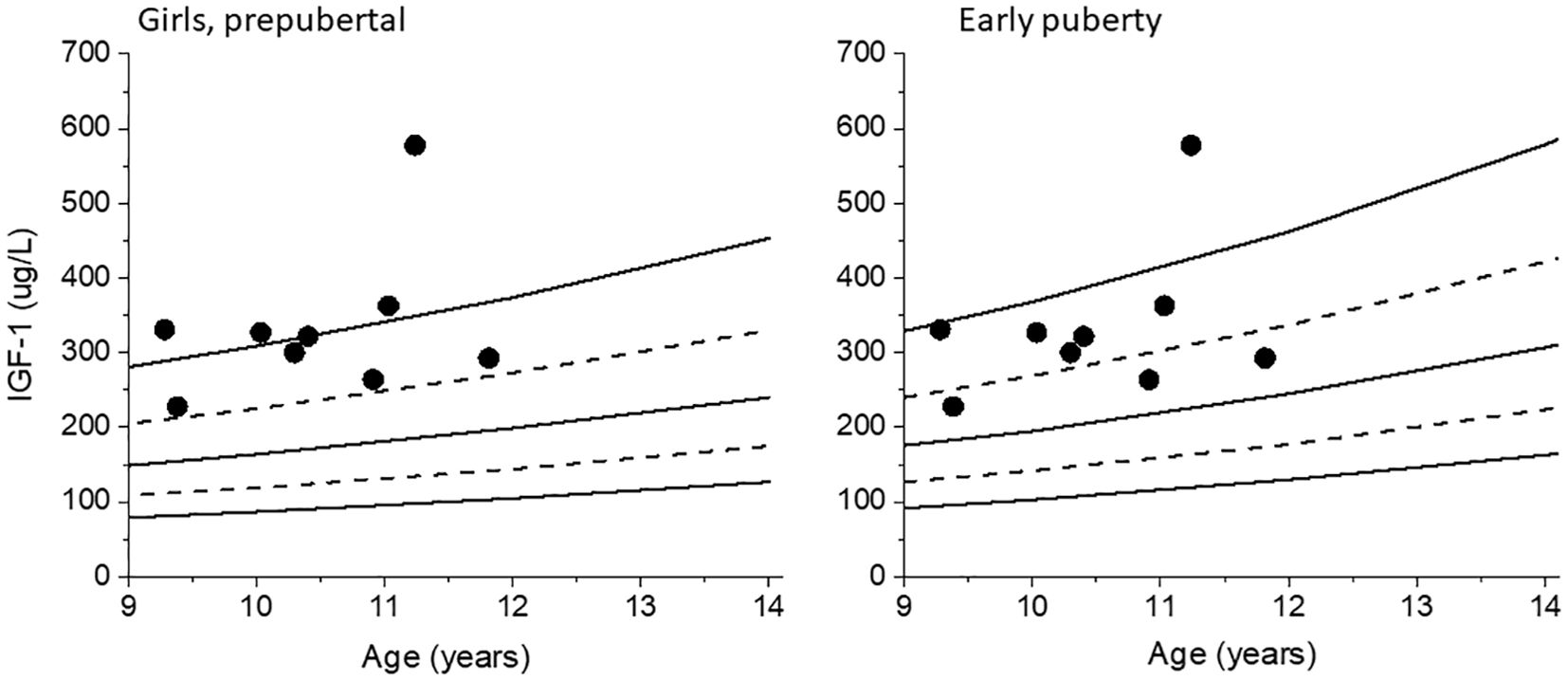
Figure 1. IGF-1 values for the girls with Tanner breast stage 1 but with pubertal levels of estradiol are plotted against the prepubertal reference range for girls, left. The same IGF-1 values are plotted against the early puberty reference range, right. Dashed lines +/- 1 SDS, solid lines mean and +/- 2 SDS.
For the boys with testes size <4 mL, 24 out of the 153 samples (15.7%) had pubertal levels of testosterone above the cut-off value of ≥0.47 nmol/L. Of these 24 samples with pubertal testosterone levels, 16 coincided with a testes size of 3 mL. Recalculating IGF-1 SDS for these 24 samples using the early puberty reference range instead of the prepubertal reference range decreased the median IGF-1 SDS from 1.96 (-0.66-3.75) to 1.29 (-1.42-3.10), p=0.02. Five out of the 24 IGF-1 SDS levels decreased below the clinical dose- adjusting upper limit of 2 SDS. See Figure 2.
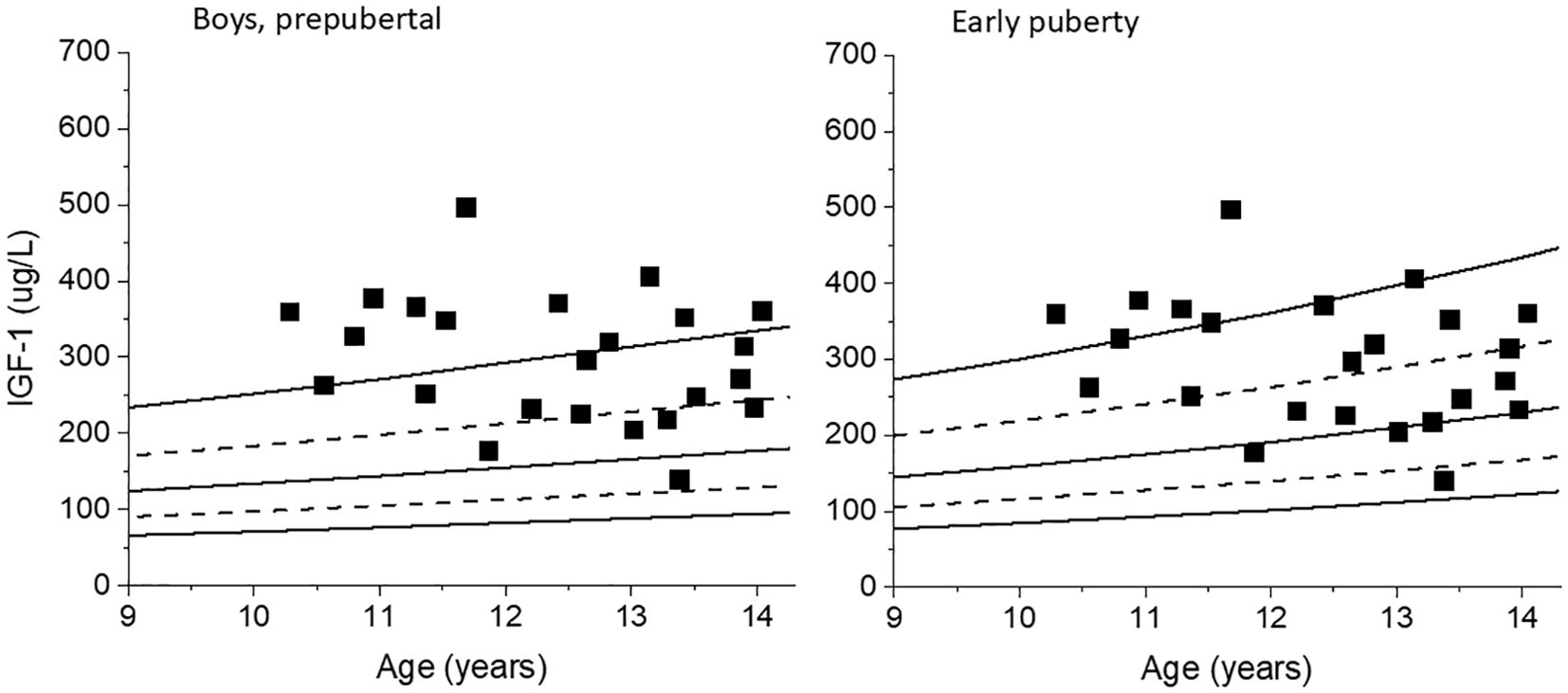
Figure 2. IGF-1 values for boys with testes size <4 mL but with pubertal levels of testosterone are plotted against the prepubertal reference range for boys, left. The same IGF-1 values are plotted against the early puberty reference range, right.
3.3 Comparing IGF-1 groupsAssessing all the IGF-1 data independent of pubertal staging, 166 of 475 (35%) samples for IGF-1 were ≥2 SDS, and the rest were <2 SDS. Characteristics of children with IGF-1 ≥2 SDS and <2 SDS were compared, and significant differences were found, see Table 2. The IGF-1 ≥2 SDS samples had a higher median GH dose, compared with the IGF-1 <2 SDS samples. In the IGF-1 ≥2 SDS samples vs the IGF <2 SDS samples, estradiol levels were lower among girls, and testosterone levels were lower among boys. Further stratification of the data by diagnosis yielded consistent results for the non-GHD, with same significant differences observed as prior to stratification. The GHD group was very small and hence the results not conclusive with Table 2 (see Supplementary Table S1).
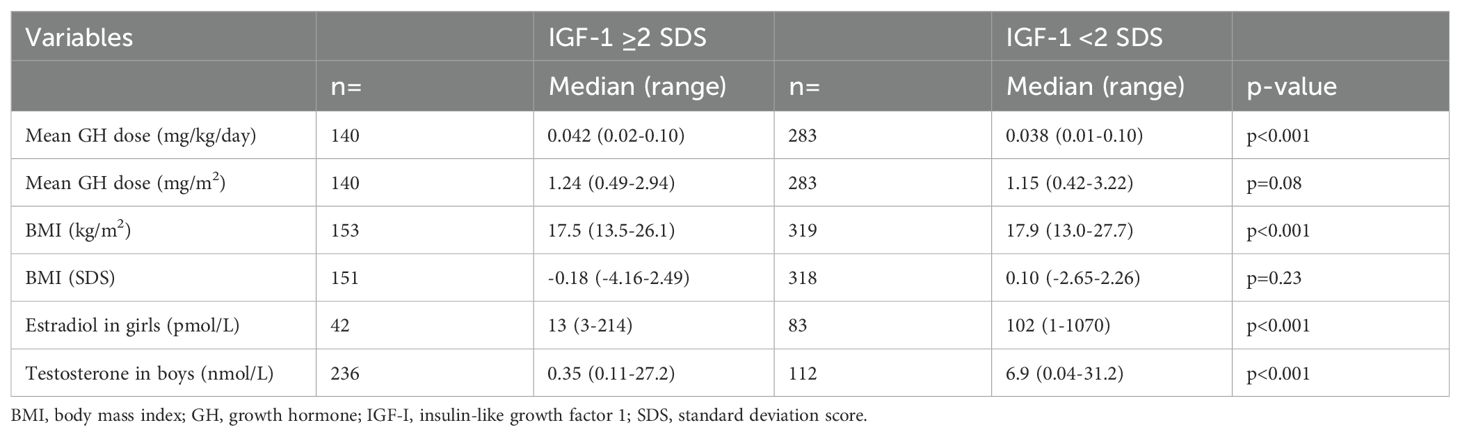
Table 2. Characteristics of the children at samples of either high or low IGF-1 SDS.
4 DiscussionGH dosing is challenging due to individual variability in sensitivity. Based on our clinical experience, we hypothesized that children treated with GH during the early pubertal years could have increases in IGF-1 due to an early rise in sex steroid levels before the appearance of clinical pubertal signs. The present retrospective study showed that as many as 15% of children undergoing GH treatment had pubertal levels of sex steroids in the absence of pubertal clinical signs. This proportion exceeds the expected 5% corresponding to children in the 90th percentile of the pubertal cut-off values for the sex steroids. The median estradiol for the clinically prepubertal girls in the cohort was 9 pmol/L and for the prepubertal boys 0.28 nmol/L, however one girl had a surprisingly high level of estradiol of 147 pmol/L, and one boy had surprisingly high testosterone of 13.3 nmol/L. Both samples were reanalyzed showing the same results. The girl attained menarche at the age 12 and the boy only attained 12 mL in testicle size by the age 18.
In clinical practice, IGF-1 titration is one of several tools to facilitate GH dosing, given that there is a reliable reference range. Some clinics use reference ranges that are age- and sex-dependent but lack pubertal staging. In a GH-naïve cohort of peripubertal children, Inoue-Lima et al. showed that IGF-1 reference ranges adjusted for the pubertal stage have the best positive predictive value of GH deficiency (24). The present study used the IGF-1 reference range by Löfqvist et al. (15), which includes age and gender, but also clinical pubertal staging expressing IGF-1 levels as SDS, which we believe is needed to accurately interpret IGF-1 SDS as sex steroids are such an important factor affecting IGF-1 levels.
An upper limit of 2 SDS for IGF-1 levels is often recommended in international guidelines when treating children with GH for safety reasons (5). In our study, nine of the 58 samples in clinically prepubertal girls had pubertal levels of estradiol, and when IGF-1 SDS was recalculated using the pubertal reference range, 4 had IGF-1 <2 SDS. Similarly, five out of 24 pubertal samples in clinically prepubertal boys had IGF-1 <2 SDS. In these patients, an unnecessary decrease in GH dose would have been applied outside the clinical trial according to guidelines.
When we divided the cohort based on IGF-1 SDS values of ≥2 or <2, we found a remarkable difference in sex steroid expression. Girls with IGF-1 ≥2 SDS had much lower estradiol levels than those with <2 SDS (13 [3-214] pmol/L vs 102 [1-1070] pmol/L, p<0.001). In a similar vein, boys with IGF-1 ≥2 SDS had lower testosterone levels than those with <2 SDS (0.35 [0.11-27.2] nmol/L vs 6.9 [0.04-31.2] nmol/L, p<0.001). These results indicate that children in the early pubertal years tend to be labeled with higher IGF-1 SDS, possibly due to an overestimation of IGF-1 SDS by the reference range used clinically. This finding aligns well with what has been described clinically, where a rise in IGF-1 SDS is seen during the early pubertal years in clinically prepubertal children. However when stratified by diagnosis, the GHD group showed early pubertal estradiol levels for both high and low IGF-1 SDS, possibly due to the small number of data in each group.
In further analyzing the differences between the groups with higher and lower IGF-1 SDS, we could see that the IGF-1 ≥2 SDS samples corresponded to a significantly higher GH dose per body weight, as expected. However, this was not the case when expressing the GH dose per body surface area and is in line with the finding of a slightly lower BMI in the IGF-1 ≥2 SDS samples, 17.5 (13.5-26.1) vs 17.9 (13.0-27.7) kg/m2 p<0.001.
While most recommendations for GH dosing are based on body weight, some studies have evaluated GH dosing by IGF-1 titration. Cohen et al. showed in a randomized controlled trial that titrating GH doses to achieve higher IGF-1 targets resulted in augmented growth responses compared to conventional dosing in pre-pubertal children with short stature, although using higher GH doses (9). Titrating IGF-1 targeted to the age- and gender-adjusted mean seemed to be dose-sparing and theoretically safer as higher IGF-1 levels were avoided (10). This shows the benefits of IGF-1 titration and we believe that by addressing the pitfalls of IGF-1 interpretation we can come closer to understanding IGF-1 and possibly have greater use to it clinically.
However, in patients with relative IGF-1 resistance, such as the case with children born small for gestational age or girls with Turner syndrome, seem not to benefit from IGF-I titration within the normal range (25, 26). These children would have required higher GH doses and had higher IGF-1 levels during GH treatment to achieve an adult height within the normal range.
A strength of the study is that the cohort is from a previous randomized controlled clinical study, and thus, the data are controlled and reliable, although the size of the cohort is limited. Moreover, we used stringent cut-off levels for the sex steroid levels to ensure that children had definitively reached puberty clinically as well as biochemically, albeit with a possibility of missing a few pubertal children with low sex steroid levels. Adherence data were collected thoroughly during the prepubertal and early pubertal years by counting empty syringes, although digital data from accurate devices were lacking. Moreover, the longitudinal approach with sampling at least every twelve months for six consecutive years is also an advantage in discovering changes over time. GH dosing was per protocol and, thus, was not altered depending on the IGF-1 levels, resulting in IGF-1 levels that reached 35% above international recommendations of +2 SDS. This made it possible for us to analyze and identify the differences between children with lower and higher IGF-1 SDS in the study cohort. Although we believe that IGF-1 SDS is overestimated in some children in their early pubertal years, this notion is hard to prove. To our knowledge, there is no IGF-1 reference range based on sex steroid levels rather than clinical pubertal signs, apart from sex and age.
Based on the results of this study, we encourage further analyses of sex steroid levels when an increase in IGF-1 SDS is detected in prepubertal children where GH dosing has not been altered. If the sex steroid levels are above the pubertal cut-off values, we believe it is beneficial to use the early puberty reference ranges for these children rather than decreasing the GH dose and practicing close monitoring. We are convinced that IGF-1 is an important and useful tool in GH dosing, but more accurate reference ranges, preferably those including sex steroid levels, are needed. There is also a need to conduct further research on IGF-1 to investigate factors that influence levels during GH treatment to be able to fully utilize its potential as a useful biomarker.
5 ConclusionThis work highlights challenges in interpreting IGF-1 levels during the early pubertal years due to the influence of sex steroids. A substantial number of patients undergoing GH treatment may have IGF-1 SDS levels that are overestimated around the onset of puberty. Based on the present results, we stress the importance of sex steroid analyses in IGF-1 reference range to be able to better interpret results in peripubertal children.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe studies involving humans were approved by Gothenburg Research Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from primarily isolated as part of your previous study for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributionsHL: Data curation, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing, Conceptualization. CL: Conceptualization, Methodology, Validation, Writing – review & editing. HF: Conceptualization, Supervision, Writing – review & editing. SN: Formal analysis, Writing – review & editing. JD: Conceptualization, Resources, Supervision, Validation, Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from ALF Gothenburg, Sweden (ALFGBG-719711 and 965041), The Gothenburg Society of Medicine, and The Swedish Order of Freemasons. The providers of funding were not involved in the study design, data collection, data analysis, writing of the report, or submission of the article.
AcknowledgmentsWe thank the regional study teams and colleagues at the University Pediatric Endocrinology centers in Sweden, pediatricians and nurses at the county hospitals responsible for caring for the children close to home, and Lillemor Ljungberg for data management and monitoring. We also thank the GP-GRC laboratory for performing hormone analyses. Pfizer supplied Genotropin until the start of puberty for free for children 25% of the study population who were non-GH deficient in this investigator-initiated study.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1514935/full#supplementary-material
References1. Reiter EO, Price DA, Wilton P, Albertsson-Wikland K, Ranke MB. Effect of growth hormone (GH) treatment on the near-final height of 1258 patients with idiopathic GH deficiency: analysis of a large international database. J Clin Endocrinol Metab. (2006) 91:2047–54. doi: 10.1210/jc.2005-2284
PubMed Abstract | Crossref Full Text | Google Scholar
2. Kristrom B, Karlberg J, Albertsson-Wikland K. Prediction of the growth response of short prepubertal children treated with growth hormone. Swedish Paediatric Study Group for GH treatment. Acta Paediatr. (1995) 84:51–7. doi: 10.1111/j.1651-2227.1995.tb13483.x
PubMed Abstract | Crossref Full Text | Google Scholar
3. Wit JM, Rekers-Mombarg LT, Cutler GB, Crowe B, Beck TJ, Roberts K, et al. Growth hormone (GH) treatment to final height in children with idiopathic short stature: evidence for a dose effect. J Pediatr. (2005) 146:45–53. doi: 10.1016/j.jpeds.2004.08.055
PubMed Abstract | Crossref Full Text | Google Scholar
4. Cohen P, Germak J, Rogol AD, Weng W, Kappelgaard AM, Rosenfeld RG, et al. Variable degree of growth hormone (GH) and insulin-like growth factor (IGF) sensitivity in children with idiopathic short stature compared with GH-deficient patients: evidence from an IGF-based dosing study of short children. J Clin Endocrinol Metab. (2010) 95:2089–98. doi: 10.1210/jc.2009-2139
PubMed Abstract | Crossref Full Text | Google Scholar
5. Grimberg A, DiVall SA, Polychronakos C, Allen DB, Cohen LE, Quintos JB, et al. Guidelines for growth hormone and insulin-like growth factor-I treatment in children and adolescents: growth hormone deficiency, idiopathic short stature, and primary insulin-like growth factor-I deficiency. Horm Res Paediatr. (2016) 86:361–97. doi: 10.1159/000452150
PubMed Abstract | Crossref Full Text | Google Scholar
7. Clemmons DR. Consensus statement on the standardization and evaluation of growth hormone and insulin-like growth factor assays. Clin Chem. (2011) 57:555–9. doi: 10.1373/clinchem.2010.150631
PubMed Abstract | Crossref Full Text | Google Scholar
8. Kristrom B, Lundberg E, Jonsson B, Albertsson-Wikland K, study g. IGF-1 and growth response to adult height in a randomized GH treatment trial in short non-GH-deficient children. J Clin Endocrinol Metab. (2014) 99:2917–24. doi: 10.1210/jc.2014-1101
PubMed Abstract | Crossref Full Text | Google Scholar
9. Cohen P, Rogol AD, Howard CP, Bright GM, Kappelgaard AM, Rosenfeld RG, et al. Insulin growth factor-based dosing of growth hormone therapy in children: a randomized, controlled study. J Clin Endocrinol Metab. (2007) 92:2480–6. doi: 10.1210/jc.2007-0204
PubMed Abstract | Crossref Full Text | Google Scholar
10. Cohen P, Weng W, Rogol AD, Rosenfeld RG, Kappelgaard AM, Germak J. Dose-sparing and safety-enhancing effects of an IGF-I-based dosing regimen in short children treated with growth hormone in a 2-year randomized controlled trial: therapeutic and pharmacoeconomic considerations. Clin Endocrinol (Oxf). (2014) 81:71–6. doi: 10.1111/cen.2014.81.issue-1
PubMed Abstract | Crossref Full Text | Google Scholar
11. Gibney J, Johannsson G. Long-term monitoring of insulin-like growth factor I in adult growth hormone deficiency: a critical appraisal. Horm Res. (2004) 62 Suppl 1:66–72. doi: 10.1159/000080761
PubMed Abstract | Crossref Full Text | Google Scholar
12. Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. (2004) 363:1346–53. doi: 10.1016/S0140-6736(04)16044-3
PubMed Abstract | Crossref Full Text | Google Scholar
13. Guevara-Aguirre J, Rosenbloom AL, Guevara-Aguirre M, Saavedra J, Procel P. Recommended IGF-I dosage causes greater fat accumulation and osseous maturation than lower dosage and may compromise long-term growth effects. J Clin Endocrinol Metab. (2013) 98:839–45. doi: 10.1210/jc.2012-3704
PubMed Abstract | Crossref Full Text | Google Scholar
14. Juul A, Bang P, Hertel NT, Main K, Dalgaard P, Jorgensen K, et al. Serum insulin-like growth factor-I in 1030 healthy children, adolescents, and adults: relation to age, sex, stage of puberty, testicular size, and body mass index. J Clin Endocrinol Metab. (1994) 78:744–52. doi: 10.1210/jcem.78.3.8126152
PubMed Abstract | Crossref Full Text | Google Scholar
15. Lofqvist C, Andersson E, Gelander L, Rosberg S, Blum WF, Albertsson Wikland K. Reference values for IGF-I throughout childhood and adolescence: a model that accounts simultaneously for the effect of gender, age, and puberty. J Clin Endocrinol Metab. (2001) 86:5870–6. doi: 10.1210/jcem.86.12.8117
PubMed Abstract | Crossref Full Text | Google Scholar
16. Upners EN, Busch AS, Almstrup K, Petersen JH, Assens M, Main KM, et al. Does height and IGF-I determine pubertal timing in girls? Pediatr Res. (2021) 90:176–83. doi: 10.1038/s41390-020-01215-6
PubMed Abstract | Crossref Full Text | Google Scholar
17. Metzger DL, Kerrigan JR. Estrogen receptor blockade with tamoxifen diminishes growth hormone secretion in boys: evidence for a stimulatory role of endogenous estrogens during male adolescence. J Clin Endocrinol Metab. (1994) 79:513–8. doi: 10.1210/jcem.79.2.8045971
PubMed Abstract | Crossref Full Text | Google Scholar
18. Mauras N, O’Brien KO, Klein KO, Hayes V. Estrogen suppression in males: metabolic effects. J Clin Endocrinol Metab. (2000) 85:2370–7. doi: 10.1210/jc.85.7.2370
Crossref Full Text | Google Scholar
19. Decker R, Albertsson-Wikland K, Kristrom B, Halldin M, Gustafsson J, Nilsson NO, et al. GH dose reduction maintains normal prepubertal height velocity after initial catch-up growth in short children. J Clin Endocrinol Metab. (2019) 104:835–44. doi: 10.1210/jc.2018-01006
PubMed Abstract | Crossref Full Text | Google Scholar
20. Ankarberg-Lindgren C, Dahlgren J, Andersson MX. High-sensitivity quantification of serum androstenedione, testosterone, dihydrotestosterone, estrone and estradiol by gas chromatography-tandem mass spectrometry with sex- and puberty-specific reference intervals. J Steroid Biochem Mol Biol. (2018) 183:116–24. doi: 10.1016/j.jsbmb.2018.06.005
PubMed Abstract | Crossref Full Text | Google Scholar
21. Blum WF, Breier BH. Radioimmunoassays for IGFs and IGFBPs. Growth Regul. (1994) 4 Suppl 1:11–9.
22. Ankarberg-Lindgren CMB, Norjavaara E. Biological reference intervals for estradiol and testosterone in children. Horm Res Paediatr. (2013) 80:179.
23. Karlberg J, Luo Z, Albertsson-Wikland K. Body mass index reference values (mean and SD) for Swedish children. Acta Paediatr. (2001) 90:1427–34. doi: 10.1111/j.1651-2227.2001.tb01609.x
Crossref Full Text | Google Scholar
24. Inoue-Lima TH, Vasques GA, Scalco RC, Nakaguma M, Mendonca BB, Arnhold IJP, et al. IGF-1 assessed by pubertal status has the best positive predictive power for GH deficiency diagnosis in peripubertal children. J Pediatr Endocrinol Metab. (2019) 32:173–9. doi: 10.1515/jpem-2018-0435
PubMed Abstract | Crossref Full Text | Google Scholar
25. Jensen RB, Thankamony A, O’Connell SM, Kirk J, Donaldson M, Ivarsson SA, et al. A randomised controlled trial evaluating IGF1 titration in contrast to current GH dosing strategies in children born small for gestational age: the North European Small-for-Gestational-Age Study. Eur J Endocrinol. (2014) 171:509–18. doi: 10.1530/EJE-14-0419
PubMed Abstract | Crossref Full Text | Google Scholar
26. Jensen RB. Growth and adult height in girls with turner syndrome following IGF-1 titrated growth hormone treatment. J Clin Endocrinol Metab. (2020) 105(8):2566–74. doi: 10.1210/clinem/dgaa274
留言 (0)