Hypercalcemia is a relatively common clinical condition, affecting approximately 1% of worldwide population (1). It can be classified as mild (10.5 to 11.9 mg/dL, or 2.62 to 2.97 mmol/L), moderate (12.0 to 13.9 mg/dL, or 3.0 to 3.47 mmol/L), or severe, i.e., hypercalcemic crisis (≥ 14.0 mg/dL, or ≥ 3.5 mmol/L) (2). Hypercalcemia generally underlies a clinically-significant disorder; it therefore requires investigation of potential etiologies. Approximately 90% of cases of hypercalcemia are due to primary hyperparathyroidism (PHPT) or malignancies (3). Hyperthyroidism is known to be a cause of non-parathyroid mediated hypercalcemia, with asymptomatic hypercalcemia reported in around 20% of cases of hyperthyroidism (4). Adjusted calcium levels rarely exceed 3.0 mmol/L in hyperthyroidism-related hypercalcemia and severe hypercalcemia is quite rare. To date there are only a few cases of hypercalcemia secondary to hyperthyroidism reported in literature (5–8). The mechanism of hyperthyroidism-associated hypercalcemia is not fully understood, but it has been suggested that an increase in bone turnover mediated by free triiodothyronine (FT3) may play a key role (9). Vitamin D derangements are other uncommon but important causes of hypercalcemia. Granulomatous diseases (i.e., sarcoidosis, tuberculosis, or foreign bodies granulomas) are characterized by abnormally-elevated 1,25-dihydroxy vitamin D3 levels [1,25-(OH)2-D3], which lead to an increase in calcium and phosphate absorption, primarily from the gut (10). We describe a case of hypercalcemia with suppressed parathyroid hormone (PTH) presenting in a patient with new-onset autoimmune hyperthyroidism and concomitant foreign-body granulomas secondary to silicone injections.
Case presentationA 61-year-old, assigned male at birth transgender Hispanic patient, with a history of human immunodeficiency virus (HIV) on antiretroviral therapy from 1999 (in stable control and on single tablet regimen of bictegravir, emtricitabina, tenofovir alafenamide therapy from October 2019), presented to the infectious disease clinic in July 2023. He was complaining generalized malaise, asthenia, dyspnea and dysphagia associated with weight loss of 14 kilograms over 45 days and recurrent panic attacks. He was then admitted to in-stay in the infectious disease department for further investigations. On physical examination, blood pressure was 110/65 mmHg with a heart rate of 91 beats per minute. He was afebrile and his capillary oxygen saturation was 96% in room air. Cardiovascular, respiratory, abdominal and neurological examination was unremarkable, except from some gait uncertainty. On the other hand, serum and plasma laboratory exam revealed markedly elevated adjusted total calcium levels 13.1 mg/dL, or 3.27 mmol/L (normal range: 8.5-10.5 mg/dL, or 2.12-2.62 mmol/L), suppressed TSH <0.01 mIU/mL (normal range: 0.35-4.94 mIU/mL) and significantly high free T4 (FT4) of 54.7 pmol/L (normal range: 9-19 pmol/L) and free T3 (FT3) of 26.8 pmol/L (2.4-6 pmol/L). Renal function was normal [serum creatinine 0.61 mg/dL (0.7-1.2 mg/dL), with an estimated glomerular filtration rate (eGFR) of 107.8 mL/min/1.73 m2], serum albumin was low (30 g/L) and liver enzymes were mildly elevated [aspartate amino transferase 45 U/L (11-34 U/L), alanine amino transferase 52 U/L (≤ 49 U/L), gamma glutamyl transferase 89 U/L (12-68 U/L)]. Alkaline phosphatase was 110 U/L (43-115), C-reactive protein was 4.5 mg/L (normal up to 10 mg/L), and blood cell count and electrolytes (sodium, potassium and chloride) were within the reference ranges. Endocrinological consultancy was asked for and hydration with saline solution (0.9% NaCl, 100 mL/h continuously) simultaneously with intravenous loop diuretic (furosemide 20 mg, twice a day) was started immediately. In order to clarify the etiology of the hypercalcemia, we recommended further biochemical and imaging exams; at the same time considering evidence of hyperthyroidism, we suggested starting with methimazole 10 mg twice a day. It has been described that anti-retroviral therapy can be associated to Grave’s Disease occurrence even several months after starting, but the patient was on stable therapy regimen for about 5 years and he never showed signs of thyroid dysfunction although he underwent regular controls in the clinic. First of all, Tc-99m thyroid scintigraphy showed an intense and homogeneous uptake throughout the entire thyroid parenchyma. Thyroid ultrasound further described an increase in thyroid volume, diffusely non-uniform structure and markedly accentuated vascularization, and a 17-mm round solid iso-hyperechoic nodule with well defined margins and predominantly peripheral vascularization in the left lobe (Figure 1) (11). The day after admission, total serum calcium was 11.1 mg/dL, or 2.77 mmol/L (8.5-10.5 mg/dl, or 2.12-2.62 mmol/L), ionized calcium 6.0 mg/dL (4.7-5.2 mg/dL), serum phosphate 3.0 mg/dL, or 0.97 mmol/L (2.5-4.5 mg/dL, or 0.8-1.5 mmol/L), magnesium 1.5 mg/dL (1.4-2.4 mg/dL), 25-hydroxy vitamin D3 (25-OH-D3) 50 ng/mL (20–100 ng/mL), 1,25-dihydroxy vitamin D3 [1,25-(OH)2-D3] 56 ng/L (20-80 ng/L), alkaline phosphatase (ALP) 101 U/L (43-115 U/L), C-terminal telopeptides of type I collagen (CTX) 1900 ng/L (120-630 ng/L), serum creatinine 0.53 mg/dL (0.7-1.2 mg/dL), 24-hour urinary calcium 20.2 mg/dL (8.3-26.6 mg/dL), and PTH was significantly low (< 5.5 ng/L; normal range 14.9-56.9 ng/L). Of note, 24-hour urinary calcium was within normal range despite commencement of diuretic treatment. In addition, anti-thyroglobulin antibodies were 69 IU/mL (TGAb, normal range: 0–115 IU/mL), anti-thyroperoxidase antibodies 476 IU/mL (TPOAb, normal range: 0–34 IU/mL), anti-TSH-receptor antibodies 20.9 IU/L (TRAb, normal range: 0–1.22 IU/L), and unstimulated calcitonin was 0.7 ng/L (up to 9.5 ng/L). Finally, serum electrophoresis revealed a IgGλ monoclonal component of 2.8 g/L. Autoimmune hyperthyroidism, or Graves’ disease, was diagnosed and methimazole was increased in dosage at 10 mg three times a day. Suppressed parathyroid hormone (PTH) levels ruled out PTH-mediated hypercalcemia (e.g., primary hyperparathyroidism) as a cause of his hypercalcemia. 25-hydroxy vitamin D values excluded vitamin D deficiency or intoxication, while 1,25-dihydroxy vitamin D levels within reference ranges made granulomatous disease-driven hypercalcemia (e.g., sarcoidosis) unlikely. Normal urinary calcium excretion was inconsistent with familial hypocalciuric hypercalcemia (FHH); at the same time, in-range alkaline phosphatase (ALP) levels were inconsistent with high-turnover bone diseases like Paget’s disease. However, the presence of monoclonal gammopathy together with low PTH suggested the presence of tumor-mediated hypercalcemia, and in particular multiple myeloma was suspected. He was therefore prescribed a whole-body computed tomography (CT) scan, with particular regard to bone segments, and additional blood analyses. No further investigations were asked for the thyroid nodule, considering its low-risk ultrasound characteristics and low calcitonin value. In the meantime, calcium levels were decreasing with hydration, diuretics combined with thyrostatic treatment (Figure 2; Supplementary Table 1), with patient’s subjective improvement in anxiety, asthenia and dysphagia. Neither bone nor ocular pain or discomfort were reported by the patient and at physical examination clinical activity score for orbitopathy was 0. Otolaryngologist examination for dysphagia was performed but normal. Contrast-enhanced total-body CT scan showed an enlarged thyroid gland, with the known nodular lesion in the left lobe but no sign of tracheal compression, some small gallstones and prostatic hypertrophy. Conversely, lungs and heart had no alterations, no pathological lymph nodes and no structural bone lesions were found. Considering the CT scan results, the modest amount of the monoclonal component (2.8 mg/dL), the absence of anemia, renal insufficiency or Bence Jones protein, and the finding of kappa free light chains (k-FLC) 44 mg/L, lambda free light chains (λ-FLC) 38.9 mg/L, with a ratio of 1.13 (0.26-1.65), lactate dehydrogenase (LDH) 122 U/L (125-220 U/L), beta-2-microglobulin 4.3 mg/L (up to 3.5 mg/L), total serum proteins 5.0 g/L (6.5-8.5 g/L), plasmatic IgG 10.45 g/L (7-16 g/L), IgA 1.93 g/L (0.7-4 g/L), IgM 0.95 g/L (0.4-2.3 g/L), the presence of multiple myeloma was excluded and Monoclonal Gammopathy of Uncertain Significance (MGUS) was diagnosed. A consultation with a hematologist was performed but no further hematological investigations were deemed necessary. Prostate cancer was also ruled out according to prostate CT characteristics and prostatic specific antigen (PSA) value within the normal range. After a week of thyrostatic treatment, intravenous hydration and diuretics, calcium levels stabilized at a level of <12 mg/dL, or <3.0 mmol/L (Figure 2), and thyroid function significantly improved with TSH <0.01 mIU/mL, FT4 14.8 pmol/L (9-19 pmol/L) and FT3 6.8 pmol/L (2.4-6 pmol/L). No alterations in blood count, liver enzymes and phosphate levels were observed. Heart rate (HR) and corrected QT interval (c-QT) always remained within normal limits [HR 60-100 beats per minute (bpm); c-QT 300-440 ms]. Methimazole was therefore reduced from 10 mg three times a day to 10 mg twice daily, with a parallel reduction in hydration (from saline solution 100 ml/h to saline solution 50 ml/h) and diuretics (from intravenous furosemide 20 mg twice a day to oral furosemide 25 mg daily). To complete the clinical framework, a total body 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/TC) was performed. The exam highlighted homogeneous 18F-FDG uptake in the thyroid lodge and, interestingly, moderate 18F-FDG accumulation in the thoracic retro-mammary area and extensive bilateral accumulation in the gluteal regions, as per inflammatory response. The patient revealed that in the past he had silicone injections performed in those sites as part of gender transition: as a matter of fact, upon revision of previously performed CT scan sections, compatible images were visible at mammary and gluteal level (12) (Figure 3). No other areas of altered uptake were observed in the remaining body segments examined. Of note, no gender-affirming hormonal therapy was taken by our patient. In the following days, calcium levels continued to decrease, reaching 10.3 mg/dL (or 2.57 mmol/L) two weeks after admission; at the same time, TSH levels started to increase with a parallel decrease in free thyroid hormone fractions (TSH 0.05 mIU/mL, FT4 12.8 pmol/L, FT3 4.1 pmol/L). Intravenous hydration was suspended and replaced by oral hydration, while furosemide (25 mg daily) and methimazole (10 mg twice daily) were continued and the patient was discharged from hospital. At discharge patient was not complaining dysphagia or dyspnea any more, he referred amelioration of weakness and anxiety and a general sense of improved well-being. However, PTH levels still remained suppressed (<5.5 ng/L), thus, at the time of discharge, it was not possible to distinguish between hyperthyroidism-related hypercalcemia and chronic granulomatosis-related hypercalcemia due to foreign-body (silicone injections). PTH trend after thyroid function stabilization to normal ranges was particularly helpful to clarify the etiology of hypercalcemia. In fact, three weeks after hospital discharge, both thyroid function and calcium serum levers further improved (TSH <0.01 mIU/mL, FT4 10 pmol/L, FT3 5.4 pmol/L, adjusted total serum calcium 10.0 mg/dL or 2.5 mmol/L) and PTH levels re-established to normal values (PTH 21 ng/L; normal range 14.9-56.9 ng/L), which made hypercalcemia secondary to hyperthyroidism the most likely diagnosis in the case of our patient. After four months of methimazole therapy anti-TSH-receptor antibodies were still high (37.9 IU/L, normal range: 0–1.22 IU/L), with free thyroid hormones in normal range and a still suppressed TSH. At follow up a bone mineral density study was performed and it excluded osteoporosis (Spine L1-L4 T-score -0.1, Femoral neck T-score -1.1, Femoral total T-score -0.7, performed with Lunar instrument) while CTX levels decreased to 430 ng/L (120-630 ng/L) despite no use of any anti-osteoporotic treatment.
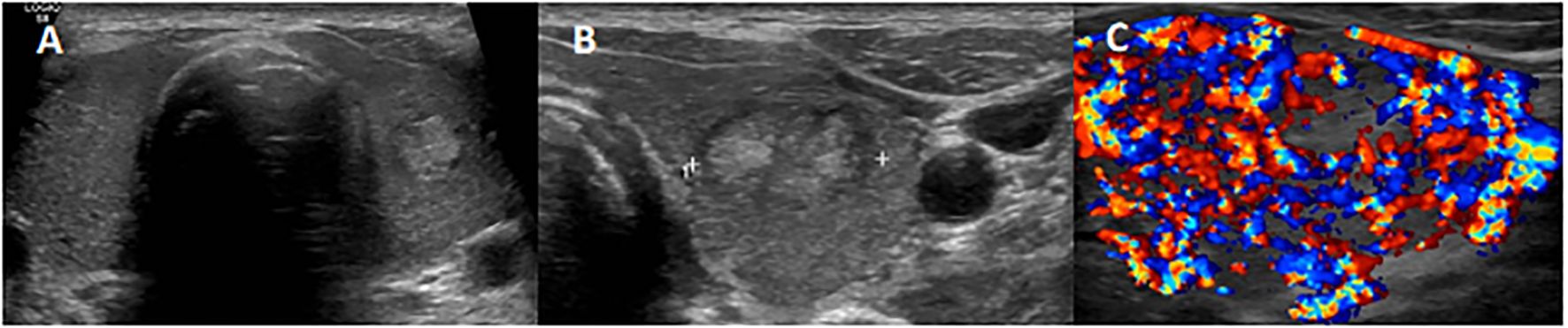
Figure 1. Thyroid ultrasonography (US) examination showing a non-uniform hypoechoic glandular parenchyma (A), with a 17-mm well-defined hyperechoic nodule in the left lobe (B) and a diffused markedly accentuated vascularization as described in thyroid storm (C).
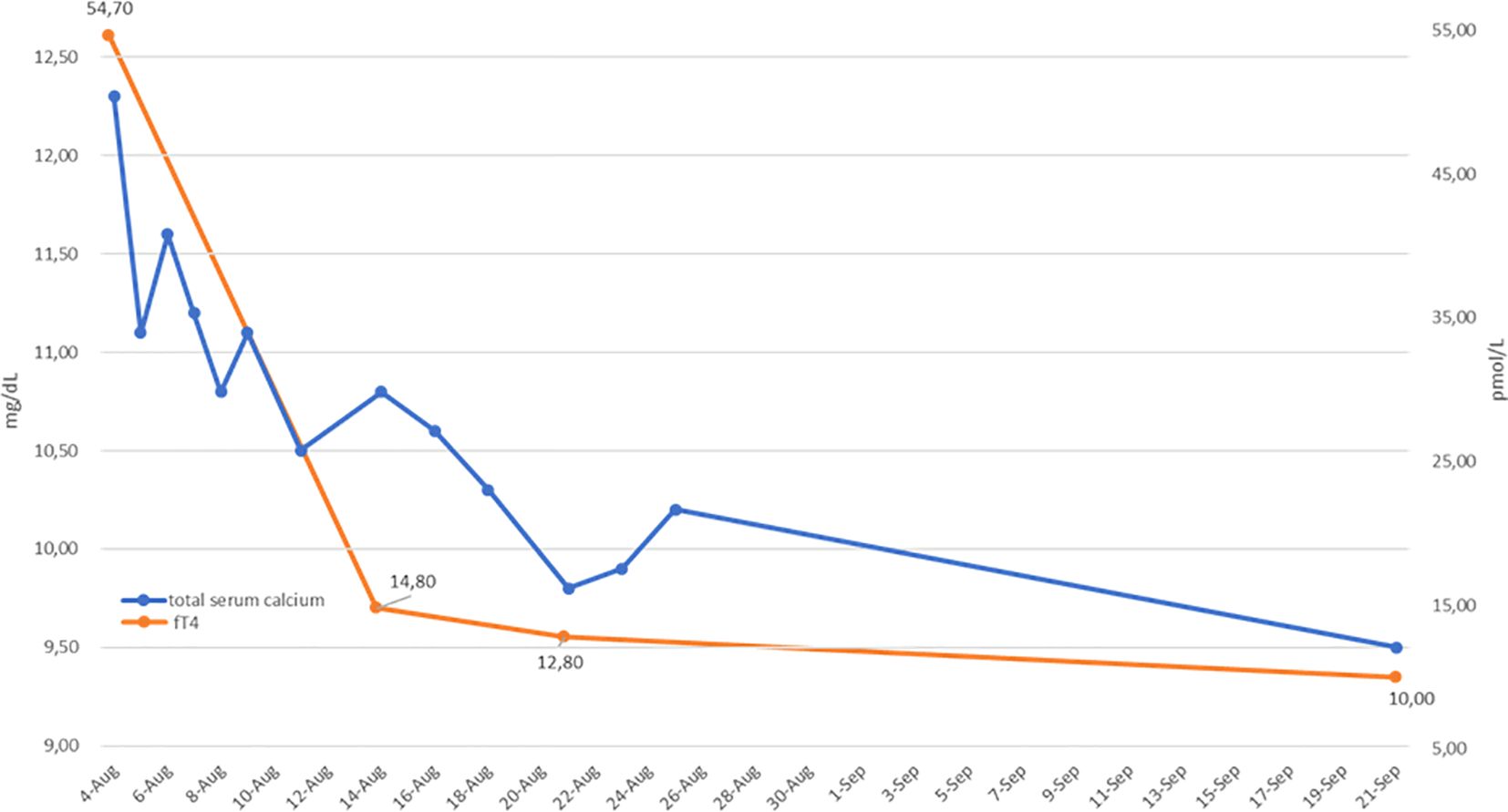
Figure 2. Serum adjusted total calcium (blue line) and free T4 (fT4; orange line) levels before and during hyperthyroidism treatment. A parallel reduction in fT4 and calcium values is shown.
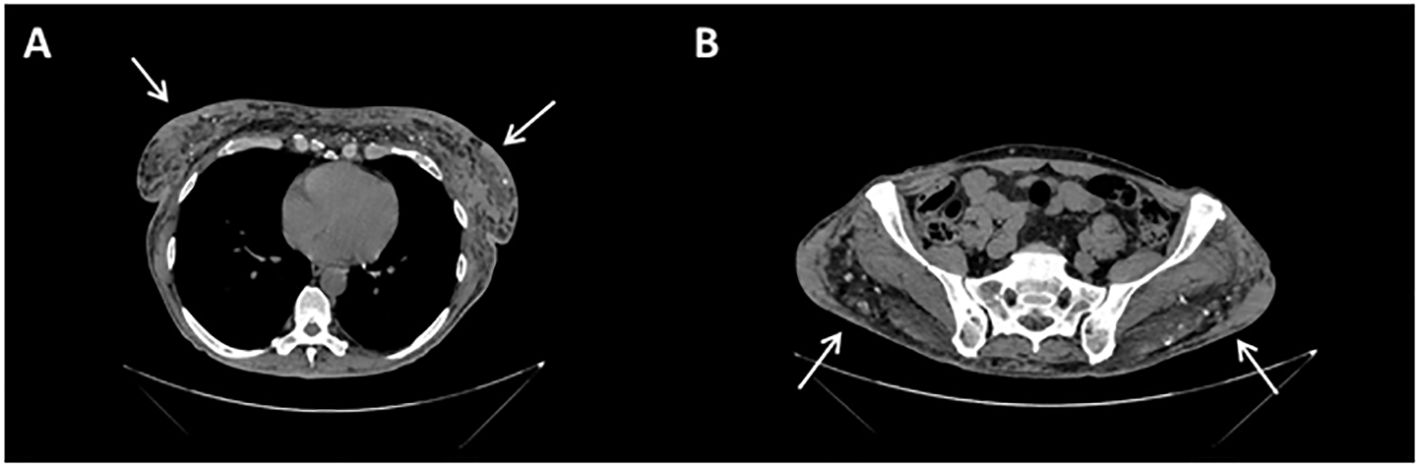
Figure 3. Contrast-enhanced CT scan sections: at mammary (A) and gluteal (B) level, images are compatible with patient history of silicone injections, in the form of soft-tissue densities with surrounding fat stranding (arrows) and peripheral calcifications.
DiscussionWe presented a case of moderate hypercalcemia in a patient affected by HIV-positivity with new-onset autoimmune hyperthyroidism as well as concomitant granulomas due to foreign bodies (silicone injections). In general, hypercalcemia can vary in presentation and severity, ranging from asymptomatic to a life-threatening emergency. A retrospective study by Nongnuch et al. (13) showed that most people living with HIV developing hypercalcemia were asymptomatic, and suggested that corrected serum calcium of ≥12 mg/dL should be investigated for underlying solid organ malignancy. In fact, in people living with HIV, major causes of hypercalcemia include solid organ malignancy, hematologic malignancy, and infections. In the general population the most common causes of hypercalcemia are primary hyperparathyroidism (PHPT) and malignancy; PTH is elevated in PHPT while suppressed in malignancy-related hypercalcemia (2). Other non-PTH-mediated etiologies of hypercalcemia include endocrine disorders, granulomatous diseases, and drugs (14). In the case we illustrated, hyperthyroidism was a possible cause of PTH-suppressed hypercalcemia, after exclusion of tumor- and granulomatosis-related hypercalcemia. As regards hypercalcemia secondary to neoplasm, underlying mechanisms vary by cancer type: in primary malignancies (e.g., lung, breast, kidney, skin) by producing parathyroid hormone-related protein (PTHrP) (14), in secondary malignancies (i.e., skeletal metastases) and myeloma by focal increases in bone resorption (15) in some instances of lymphomas by abnormal synthesis of 1,25-(OH)2-D3 in tumor-associated macrophages (16) and finally rarely by ectopic PTH secreted by certain malignant tumors (1). In the case we presented, solid malignancies were excluded basing on total-body imaging, and the presence of monoclonal gammopathy prompted further hematological investigations that ruled out multiple myeloma. With reference to granulomatosis-related hypercalcemia, the main pathological mechanism is the overexpression of 1-α-hydroxylase in macrophages, which are a main cell type involved in the granuloma reaction. Efficacy in the regulation of vitamin D metabolism, however, is impaired in macrophages, with production of 1-α-hydroxylase being unresponsive to feedback by activated vitamin D3, unlike in proximal kidney tubule cells, where 1-α-hydroxylase production is limited by levels of calcitriol. This leads to abnormally increased levels of 1,25-dihydroxy-vitamin D in granulomatous diseases, which in turn, increases blood calcium levels (17). Liquid or injectable silicone is a non-biodegradable material used in the past as an injectable filler for cosmetic body contouring. Although previously considered inert, silicone may generate inflammatory reactions, with granuloma formation being a rare but well-described complication. Thus, injectable silicone can lead to non-PTH mediated hypercalcemia through granuloma generation (3, 17–19). In our patient, a PET/TC scan revealed moderate 18F-FDG uptake in the retro-mammary area and extensive accumulation in the gluteal region, as per granulomatous inflammatory response to old silicone injections. However, 1,25-(OH)2-D3 levels within reference range and the rise in PTH values after discharge from hospital and thyroid function normalization made granulomatosis-related hypercalcemia unlikely. In people living with HIV other granulomatosis-related conditions inducing increased vitamin D hydroxylation such as tuberculosis should be excluded (13). Coming to hyperthyroidism-related hypercalcemia, the most supported explanation is increased bone turnover mediated by free thyroid hormones, with transfer of calcium from bone to serum. High levels of bone markers, such as C-terminal telopeptides of type I collagen (CTX) and alkaline phosphatase (ALP), were reported in hyperthyroid patients (20) similarly to what observed in our case report and their evaluation resulted helpful in characterizing the condition described. Although intestine and kidneys attempt to prevent calcium accumulation with decreased re-absorption and increased excretion respectively, fT3-stimulated osteoclast activation due to thyrotoxicosis results in overall increase of calcium serum levels (14). Histologic and morphometric bone changes described in bone biopsies before and after treatment for hyperthyroidism are consistent with sustained bone turnover (20). Another mechanism of hypercalcemia in Grave’s disease may involve PTHrP: a positive correlation between increased PTHrP levels and ionized calcium was observed in untreated hyperthyroid patients with simultaneously decreased levels of PTH (21). In our clinical case, we described how prompt initiation of methimazole treatment in hyperthyroidism-related hypercalcemia led to normalization of both serum calcium and PTH levels as soon as normal thyroid function was achieved. This probably also restored normal bone turnover, so that in our case there was no need for adjunctive therapies, such as bisphosphonates. Of course, diagnosis of hyperthyroidism-related hypercalcemia can only be reached after exclusion of other etiologies, but the excellent response to treatment strongly supports hyperthyroidism-related hypercalcemia as the most likely diagnosis.
The cornerstone of immediate management of hypercalcemia is intravenous rehydration, generally with normal saline solution, in order to increase glomerular filtration and calcium excretion (14). Diuretics can be added to prevent fluid overload, however their effect on calcium excretion is limited (22). Furthermore, bisphosphonates or calcitonin can be added to the treatment of moderate-severe hypercalcemia or in very symptomatic patients (2). It is likewise fundamental to target the cause of hypercalcemia (19, 22). For instance, thyrostatic agents such as thionamides (e.g., carbimazole, methimazole, propylthiouracil) are the primary treatment for hypercalcemia associated with hyperthyroidism (14). A literature search with PubMed research criteria: “hyperthyroidism AND Graves AND hypercalcemia AND case report” produces 34 results. Among those 13 papers have been published before 2000, 4 papers were not published in English or in international journals and 3 papers described different conditions (i.e. calcific uremic arteriopathy, membraneous nephropathy and polyuria) and excluded. The remaining 14 results of case report that already describedthe management and outcomes of Graves’ hyperthyroidism and hypercalcemia with or without other concomitant conditions are summarized in Table 1. In our case, intravenous hydration combined with diuretics and methimazole successfully controlled thyroid status as well as serum calcium levels.
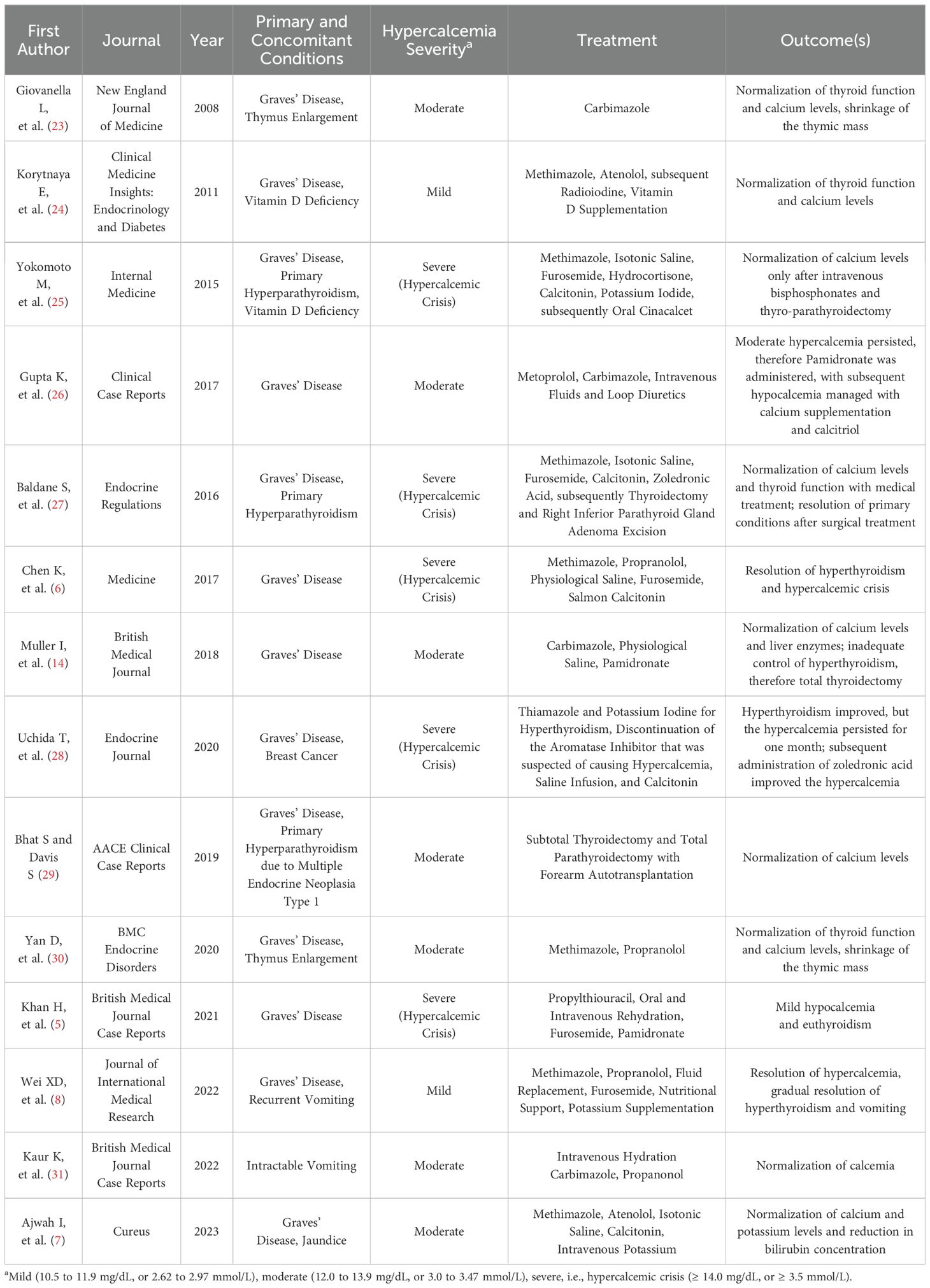
Table 1. Summary of case reports of Graves’ Disease and Hypercalcemia, with treatment and outcomes (PubMed research criteria: “hyperthyroidism AND Graves AND hypercalcemia AND case report”, inclusion criteria: published in English, in international journals, after the year 2000).
ConclusionIn summary, we presented a case of moderate hypercalcemia in a patient with new-onset autoimmune hyperthyroidism and concomitant granulomas secondary to foreign bodies (silicone injections). Differential diagnosis between the two likely etiologies (thyroid disease and granulomatosis) was endorsed by normal levels of 1,25-(OH)2-D3, the trend in PTH levels, and the improvement of calcemia in parallel with normalization of thyroid function. Such causes of hypercalcemia, although rare, need to be considered under particular circumstances, as proper etiological diagnosis is crucial to address the correct clinical management of hypercalcemia.
Patient perspective‘Before hospitalization I did not feel like myself anymore. I lost almost 15 kilograms in less than two months and gradually feeling worse and discombobulated I started developing terrifying panic attacks, which prevented me from living my life as I used to. My dear ones were very concerned. After starting medications, I regained my strength, appetite and my mood improved day after day. I really want to thank all the medical team for having promptly recognized the cause of my discomfort’.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statementEthical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributionsLM: Conceptualization, Writing – original draft, Writing – review & editing. GR: Writing – original draft. IPe: Writing – original draft. IPa: Writing – review & editing. PF: Supervision, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
AcknowledgmentsWe thank the “Fondazione Romeo e Enrica Invernizzi” for extraordinary support.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1447652/full#supplementary-material
References7. Ajwah I, Alshehri S, Alremthi F, Alahmari N. Rare presentation of a common disease: graves’ Thyrotoxicosis presented with non-parathyroid hypercalcemia and jaundice. Cureus. (2023) 15:e35206. doi: 10.7759/cureus.35206
PubMed Abstract | Crossref Full Text | Google Scholar
8. Wei X-D, Tan J-P, Chen X-M. An unusual case of hyperthyroidism with recurrent vomiting and hypercalcemia as the main manifestations. J Int Med Res. (2022) 50:30006052210991. doi: 10.1177/03000605221099120
PubMed Abstract | Crossref Full Text | Google Scholar
9. Wojcicka A, Bassett JHD, Williams GR. Mechanisms of action of thyroid hormones in the skeleton. Biochim Biophys Acta (BBA) - Gen Subj. (2013) 1830:3979–86. doi: 10.1016/j.bbagen.2012.05.005
PubMed Abstract | Crossref Full Text | Google Scholar
11. Durante C, Hegedüs L, Czarniecka A, Paschke R, Russ G, Schmitt F, et al. 2023 European Thyroid Association Clinical Practice Guidelines for thyroid nodule management. Eur Thyroid J. (2023) 12:e230067. doi: 10.1530/ETJ-23-0067
PubMed Abstract | Crossref Full Text | Google Scholar
12. Yahyavi-Firouz-Abadi N, Menias CO, Bhalla S, Siegel C, Gayer G, Katz DS. Imaging of cosmetic plastic procedures and implants in the body and their potential complications. Am J Roentgenology. (2015) 204:707–15. doi: 10.2214/AJR.14.13516
PubMed Abstract | Crossref Full Text | Google Scholar
13. Nongnuch A, Petcharut J, Suksuwan W, Davenport A, Phuphuakrat A. Causes of hypercalcemia in people living with HIV in the HAART era. HIV Res Clin Pract. (2020) 21:115–20. doi: 10.1080/25787489.2020.1836900
PubMed Abstract | Crossref Full Text | Google Scholar
16. Hewison M, Kantorovich V, Liker HR, Van Herle AJ, Cohan P, Zehnder D, et al. Vitamin D-mediated hypercalcemia in lymphoma: evidence for hormone production by tumor-adjacent macrophages. J Bone Mineral Res. (2003) 18:579–82. doi: 10.1359/jbmr.2003.18.3.579
PubMed Abstract | Crossref Full Text | Google Scholar
17. Pando BL, Goldsmith B, Webb AL, Kinger K, Helmly B. Free silicone-induced granulomatosis and hypercalcemia in a transgender female. HCA Healthcare J Med. (2022) 3:161–6. doi: 10.36518/2689-0216.1343
PubMed Abstract | Crossref Full Text | Google Scholar
18. K Jayathilaka DK, Gnanapragasam HP, Batra M. Silicone induced severe hypercalcemia. J Endocr Soc. (2021) 5:A222–3. doi: 10.1210/jendso/bvab048.452
Crossref Full Text | Google Scholar
19. Chin E, Cotter T, Harned L, Vemavarapu L, Harper R. ODP095 HYPERCALCEMIA FROM SILICONE BUTTOCK INJECTION INDUCED GRANULOMA. J Endocr Soc. (2022) 6:A164–5. doi: 10.1210/jendso/bvac150.338
Crossref Full Text | Google Scholar
20. Simonsen JK, Rejnmark L. Endocrine disorders with parathyroid hormone-independent hypercalcemia. Endocrinol Metab Clin North Am. (2021) 50:711–20. doi: 10.1016/j.ecl.2021.07.002
PubMed Abstract | Crossref Full Text | Google Scholar
21. Giovanella L, Suriano S, Keller F, Borretta G, Ceriani L. Evaluation of serum parathyroid hormone-related peptide in hyperthyroid patients. Eur J Clin Invest. (2011) 41:93–7. doi: 10.1111/j.1365-2362.2010.02385.x
PubMed Abstract | Crossref Full Text | Google Scholar
22. Tonon CR, Silva TAAL, Pereira FWL, Queiroz DAR, Junior ELF, Martins D, et al. A review of current clinical concepts in the pathophysiology, etiology, diagnosis, and management of hypercalcemia. Med Sci Monitor. (2022) 28:e935821. doi: 10.12659/MSM.935821
PubMed Abstract | Crossref Full Text | Google Scholar
24. Korytnaya E, Rao NG, Mayrin JV. An unusual case of hypercalcemia associated with graves’ Disease and vitamin D deficiency. Clin Med Insights Endocrinol Diabetes. (2011) 4: 25–8. doi: 10.4137/CMED.S7116
PubMed Abstract | Crossref Full Text | Google Scholar
25. Yokomoto M, Minamoto M, Utsunomiya D, Umakoshi H, Fukuoka T, Kondo S. Hypercalcemic crisis due to primary hyperparathyroidism occurring concomitantly with graves’ Disease. Internal Med. (2015) 54:813–8. doi: 10.2169/internalmedicine.54.2605
PubMed Abstract | Crossref Full Text | Google Scholar
26. Gupta K, Estrella J, Rajagopal R, Shanmugalingam P, Simmons D. An acute phase reaction with intravenous bisphosphonate use in a patient with recently diagnosed Graves’ disease. Clin Case Rep. (2017) 5:1226–9. doi: 10.1002/ccr3.1022
PubMed Abstract | Crossref Full Text | Google Scholar
27. Baldane S, Ipekci S, Evcen R, Gedik G, Guler I, Kebapcilar L. A case of hypercalcaemic crisis secondary to coexistence of primary hyperparathyroidism and Graves’ disease. Endocr Regul. (2016) 50:225–8. doi: 10.1515/enr-2016-0024
PubMed Abstract | Crossref Full Text | Google Scholar
28. Uchida T, Yamaguchi H, Kushima C, Yonekawa T, Nakazato M. Elevated levels of circulating fibroblast growth factor 23 with hypercalcemia following discontinuation of denosumab. Endocr J. (2020) 67:31–5. doi: 10.1507/endocrj.EJ19-0198
PubMed Abstract | Crossref Full Text | Google Scholar
29. Bhat S, Davis S. Co-existence of primary hyperparathyroidism due to multiple endocrine neoplasia 1 in a hypercalcemic patient with graves disease. AACE Clin Case Rep. (2019) 5:e13–5. doi: 10.4158/ACCR-2018-0217
PubMed Abstract | Crossref Full Text | Google Scholar
30. Yan D, Xu Y, Li LX. The coexistence of hypercalcemia, osteoporosis and thymic enlargement in graves’ disease: a case report. BMC Endocr Disord. (2020) 20:97. doi: 10.1186/s12902-020-00583-8
留言 (0)