Ovarian masses are occasionally found incidentally, and distinguishing between benign and malignant ovarian masses is crucial for determining the best course of treatment. Ovarian cancer accounts for 3.7% of all cancers in women and is the seventh most prevalent type (1). Five to ten percent of women will have surgery for a suspected ovarian tumor at some point in their lives (2). The age-adjusted incidence rate in Europe in 2012 was 13.1 per 100,000 women, with a total of 65,538 newly reported cases. Because it can be fatal, ovarian cancer is a significant health issue in gynecology, accounting for 42,700 fatalities in Europe in 2012 (mortality rate, 7.6 per 100,000) (3).
Why is the early detection of ovarian cancer crucial? The majority of ovarian malignancies are epithelial ovarian cancer (EOC) that develops rapidly, so early diagnosis is urgent. Immediate referral to a gynecologic oncologist for a prompt diagnosis of the mass’s nature can ensure more effective treatment (4). Treatment strategy can be altered by determining preoperatively whether an adnexal mass is benign or malignant, but this is often the most challenging step because currently accessible diagnostic tests are not entirely reliable. Radiological imaging and tumor markers are the most frequently used procedures, but the need for improvement cannot be overstated.
Previous studies have reported that between 5% and 40% of adnexal cysts are malignant, meaning that other patients whose cysts are classified as benign or ambiguous may wait longer for treatment rather than being referred for unneeded surgery (5, 6). The International Ovarian Tumor Analysis (IOTA) group suggested modifying the simple rules risk (SRR) technique to increase its predictive value (7). All kinds of adnexal masses can have the likelihood of their malignancy calculated using this logistic regression model.
Imaging methods, such as ultrasonography (USG), computed tomography (CT), and magnetic resonance imaging (MRI) can be used to assess soft tissue structure, development, and lymph nodes. The first-line examination before surgery for ovarian masses is ultrasound—either abdominal or transvaginal—the most commonly used tool for detecting pelvic and abdominal pathology. This non-invasive imaging technique can accurately distinguish between normal and malignant adnexal masses (8). Therefore, several recommendations and recognized reports relying on sonographic characterization have been proposed to determine the likelihood of ovarian masses being malignant. The IOTA group’s Simple Rules (IOTA-SR), developed in 2008, is one such recommendation widely used in clinical practice (9). The IOTA-SR includes five ultrasonography characteristics that can differentiate benign from malignant tumors. Benign tumor traits (B-features) include B1 = unilocular, B2 = presence of solid portions with the greatest solid component < 7mm in diameter, B3 = presence of sonic shadows, B4 = smooth multilocular tumor with ≤ 10 cm its largest diameter, and B5 = no blood supply (color score 1). Malignant tumor traits (M-features) include M1 = irregular solid tumor, M2 = presence of ascites, M3 = presence of four or more papillary structures, M4 = irregular multilocular tumor with ≥10 cm maximum diameter, and M5 = very strong blood flow (color score 4) (1).
Subsequently, a CT scan and an MRI are performed to check for nodal involvement, the breadth of the disease in the upper abdominal region, the structure of the masses, and whether there is any uncertainty regarding the origin of the mass, such as the gastrointestinal tract, urinary system, or retroperitoneum. Subjective pattern recognition assessment using USG is operator-dependent because it relies on the operator’s experience. Furthermore, there needs to be more in the USG-based explanation of the adnexal masses’ terminology and classification standards. Different classification systems have been developed to overcome these limitations that use USG findings and other modalities to distinguish adnexal masses. These prompted the development of various scoring systems for differentiating benign from malignant adnexal tumors, including the IOTA-SR (10, 11).
Several studies conducted in the literature mentioned that the smaller sample size limit the generalizability of diagnostic performance of IOTA-SR in diagnosing ovarian tumors, by pooling the data from several studies, meta-analysis affords a more robust estimate of sensitivity, specificity and diagnostic accuracy.
The authors note that few studies have been conducted in the Gulf and low-income countries, pointing to a gap in knowledge and practice using IOTA-SR in the diagnosis and differentiation of ovarian masses. Filling this gap in knowledge and practice is vital. This study was conducted to evaluate the efficacy of IOTA strategies for diagnosing ovarian masses using a larger sample size. In the view of the authors, it was effective, simple, and easy to apply in clinical practice with excellent performance.
2 Methodology2.1 Sources of information and search strategyA systematic search was performed in relevant databases, including PubMed, Scopus, Google Scholar, Web of Science, Embase, and MEDLINE, for articles that evaluated the accuracy of IOTA-SR in diagnosing ovarian masses. The following terms were searched: “IOTA”, “adnexal tumor”, and “ovarian cancer scoring”. The initial search strategy’s results were filtered using a title and abstract screening process. The titles of 1,172 studies were extracted. Only 228 were identified for further screening. Of those, 187 were excluded for various reasons, and 27 were included in the meta-analysis. Figure 1 illustrates the results of our systematic search and meta-analysis.

Figure 1. Flowchart of selection of studies for the meta-analysis to assess the performance of IOTA-SR in discriminating malignant ovarian tumors. IOTA-SR, International Ovarian Tumor Analysis Simple Rules.
2.2 Eligibility criteriaTwo authors read and examined all relevant articles to determine those meeting the inclusion and exclusion criteria for this meta-analysis. The inclusion criteria were 1) women with ovarian tumors; 2) ovarian cancer was diagnosed via IOTA and histopathology; 3) outcome indicated performance quality of testing, including specificity and sensitivity; and 4) rates of true positive (TP), false positive (FP), false negative (FN), and true negative (TN) could be inferred from the study. The exclusion criteria were case reports, systematic reviews, lack of original data, duplicate data, and conflicting outcome indicators.
2.3 Data extractionFrom the selected studies, the extracted data were entered separately by the same two authors. These consisted of the first author’s name, publication year, study design (prospective vs. retrospective), blind method (blind from the reference standard vs. unreported), number of patients, number of malignant tumors, number of benign tumors, sensitivity, specificity, TP, FP, FN, and TN, which were gathered and tabulated.
2.4 Quality assessment2.4.1 Assessment of risk biasThe included studies’ risk of bias and applicability were evaluated by two authors (AG and AA) using the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) instrument (12). This instrument uses a series of questions across four domains, each of which can be answered yes, no, or unclear, to assess the whole range of bias present in the included studies’ design and execution. The following domains are represented in QUADAS-2 items. 1) Patient selection: This domain assesses the risk to diagnostic accuracy of bias in the selection of participants for the study, including the methods used to recruit and enroll participants and the criteria used to include or exclude them. It included the following questions: a) Was the patient sample enrolled in sequential or random order? b) Is a case–control study avoided? c) Has the study been prevented from unnecessary exclusion? d) Were the patients chosen typical of the participant group to which the test index would apply?
2) Index test: This domain assesses the risk of bias in how the index test (the diagnostic test being evaluated) was performed, interpreted, and reported. It consisted of the following question: Were the outcome of the index test results evaluated without identifying the results of the gold standard?
3) Reference standard: This domain assesses the risk of bias in how the reference standard (the gold standard used to diagnose the condition of interest) was performed, interpreted, and reported and included two questions: a) Is the reference standard a good indicator to classify the target status correctly? b) Were the standard reference findings interpreted without identifying the findings of the index test?
4) Flow and timing: This domain assesses the risk of bias in the flow of patients during the study and the duration of the test index and gold standard relative to each other and included four questions: a) Was there a suitable interval between index test(s) and the gold standard? b) Were all patients provided a reference standard? c) Did all patients have access to the same gold standard? d) Were all participants included in the analysis?
The application assessment is conducted using three domains: a) the individuals’ selection domains (Is there a concern that the included patients do not match the review question)?, b) the index test domain (Does it matter if the index test, its conduct, or its interpretation differs from the review question)?, and c) the gold standard (Is there a concern that the target condition, as defined by the standard of reference, does not match the review question)? (see Figure 2).
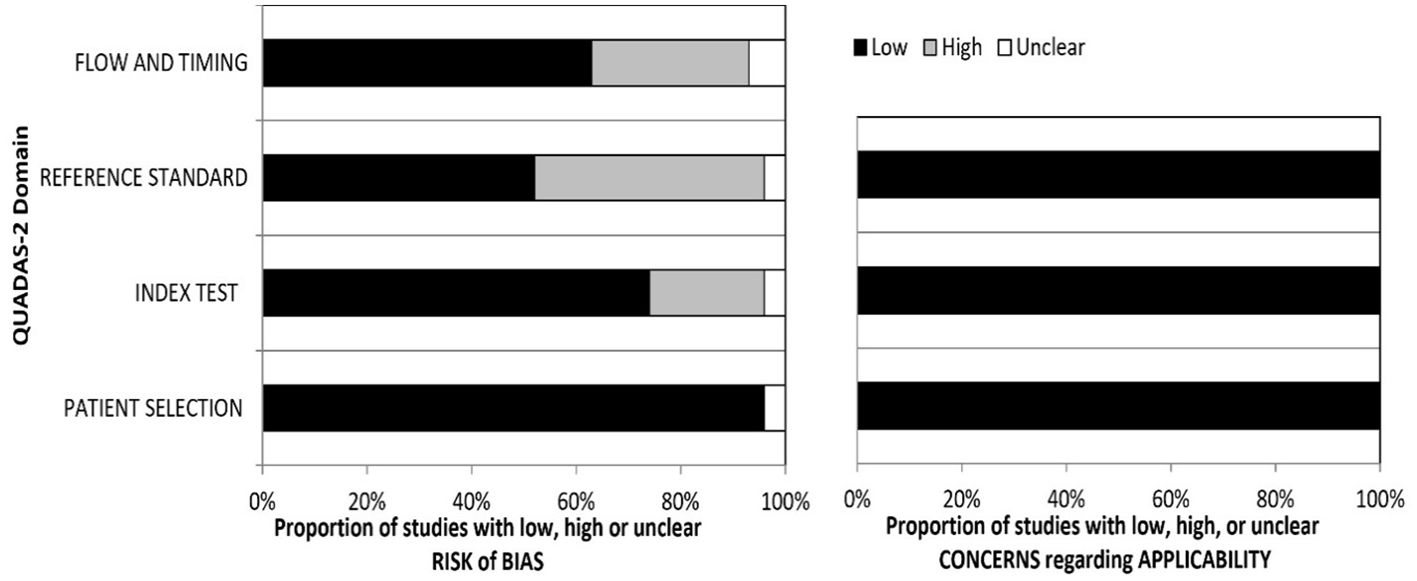
Figure 2. Summary graph of the QUADAS-2 assessment in included studies. QUADAS-2, Quality Assessment of Diagnostic Accuracy Studies 2.
2.5 Statistical analysisThe diagnostic performance of IOTA-SR in classifying benign or malignant ovarian tumors was evaluated. The analysis strategy used was similar to that described by Shim et al., in which the bivariate random effects model was used to estimate the pooled effect of sensitivity, specificity, and diagnostic odds ratio (DOR), followed by an estimation of the summary receiver operating characteristic (SROC) curve. The heterogeneity of the studies was quantitatively evaluated using Cochran’s Q statistics and Higgins’s inconsistency test through the I2 index for pooled analysis. If Cochran’s Q statistics p-value is <0.05 and the I2 is >50%, these are considered evidence of substantial heterogeneity. Possible publication bias was evaluated quantitatively and visually using the funnel plot and Egger’s test, respectively, with a p-value of <0.10 indicating significant asymmetry and the presence of publication bias. This analysis was conducted by employing the “Mada” and “meta” packages in the R statistical software (Version 1.4.1103, R Foundation for Statistical Computing, Vienna, Austria). Meta-regression was used to explain the heterogeneity in the results. The DOR was used to assess publication bias and heterogeneity, as it depends significantly on sensitivity and specificity.
3 ResultsThe study was conducted on 7,841 ovarian masses included in the publications reviewed, approximately 72.2% of which were benign and 27.8% were malignant on histopathology (Table 1).
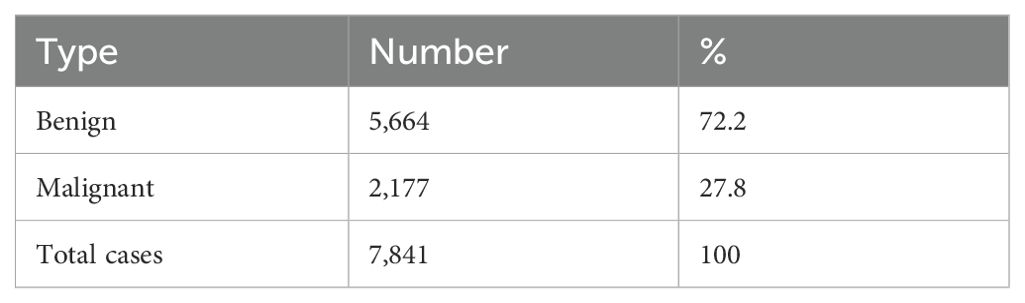
Table 1. Type of ovarian tumors according to histopathology results.
The total number of studies included was 27. Only one study was conducted on pregnant women (32). Four studies were included twice in the analysis, resulting in 31 total studies. Dakhly et al. perform two step analysis, one step with inconclusive masses were excluded and another step with inconclusive included and considered as malignant. de Gauna et al. analyzed two centers, A and B. Knafel et al. analyzed level 1 and 2 IOTA-SR studies based on examiner experience, and Hidalgo et al. used the simple rule descriptor in Steps 1 and 2) (21, 23, 26, 36).
IOTA-SR was applicable in 85.7% of the total cases included in the studies. The total number of malignant tumors was 1,971, and 4,889 were benign, based on histopathology. With regard to IOTA-SR, the number of TPs was 1,795, TNs 4,457, FPs 432, and FNs 176, as shown in Table 2 and Figure 3.
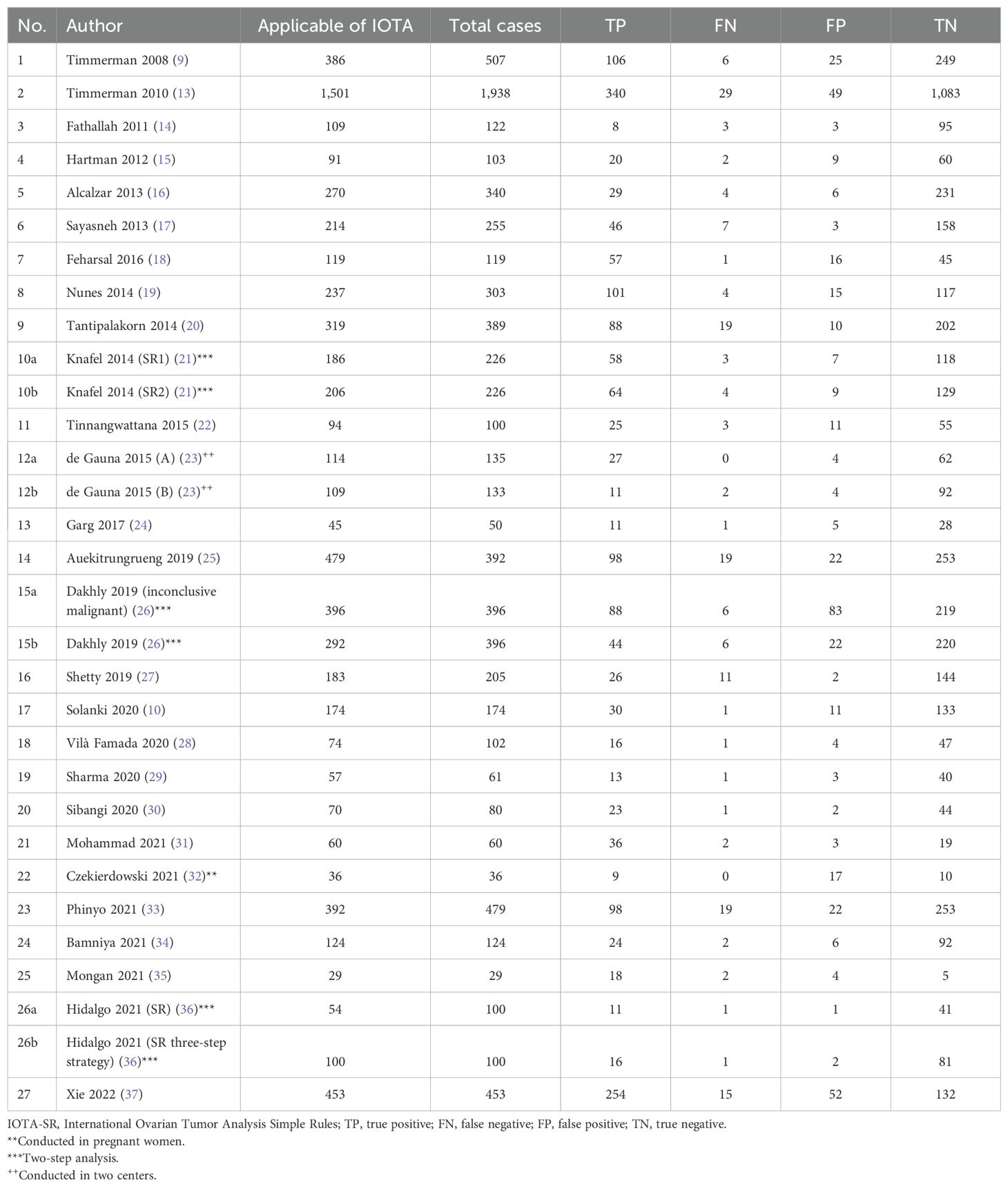
Table 2. Frequency of total and number of cases in which IOTA-SR was applied and the performance of IOTA-SR in the diagnosis of malignant tumors.
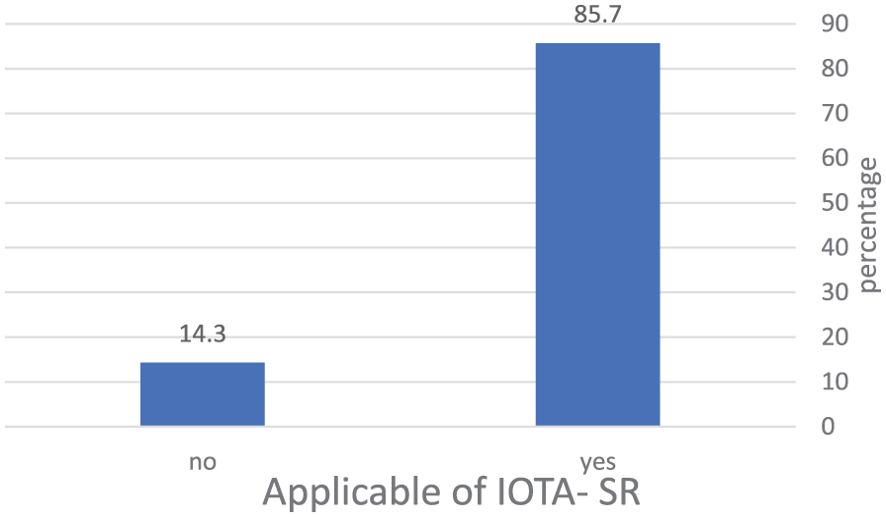
Figure 3. The percentage of masses included in the application of IOTA-SR in the diagnosis of ovarian tumors. IOTA-SR, International Ovarian Tumor Analysis Simple Rules.
3.1 Assessment of QUADAS-2The summary graph of the QUADAS-2 assessment for the selected publications is shown in Figure 2. The four areas (domains) depicted on the bias risk graph are the patient selection domain, the index test domain, the reference, and the domain of flow and timing. The concerns regarding applicability in the graph are represented in three domains: the gold standard, the index test, and patient selection. In both the risk of bias and the applicability of data, patient selection showed low concerns. However, in some studies, there are concerns regarding the reference standard and index test, as some classified borderline tumors as malignant in IOTA classifications, while one study considered borderline malignant in histopathology.
3.2 Results of diagnostic performance of IOTA- SR in diagnosing ovarian tumorsIn the meta-regression graph, Figure 4 shows the bubble plot, DOR on the y-axis, and prevalence on the x-axis. The bubble’s size represents the study weight. The gradient of the line was −0.02 [95% confidence interval (CI), −0.001 to −0.05], suggesting that the DOR decreased insignificantly by 0.02 per one unit of prevalence rate (p = 0.05).
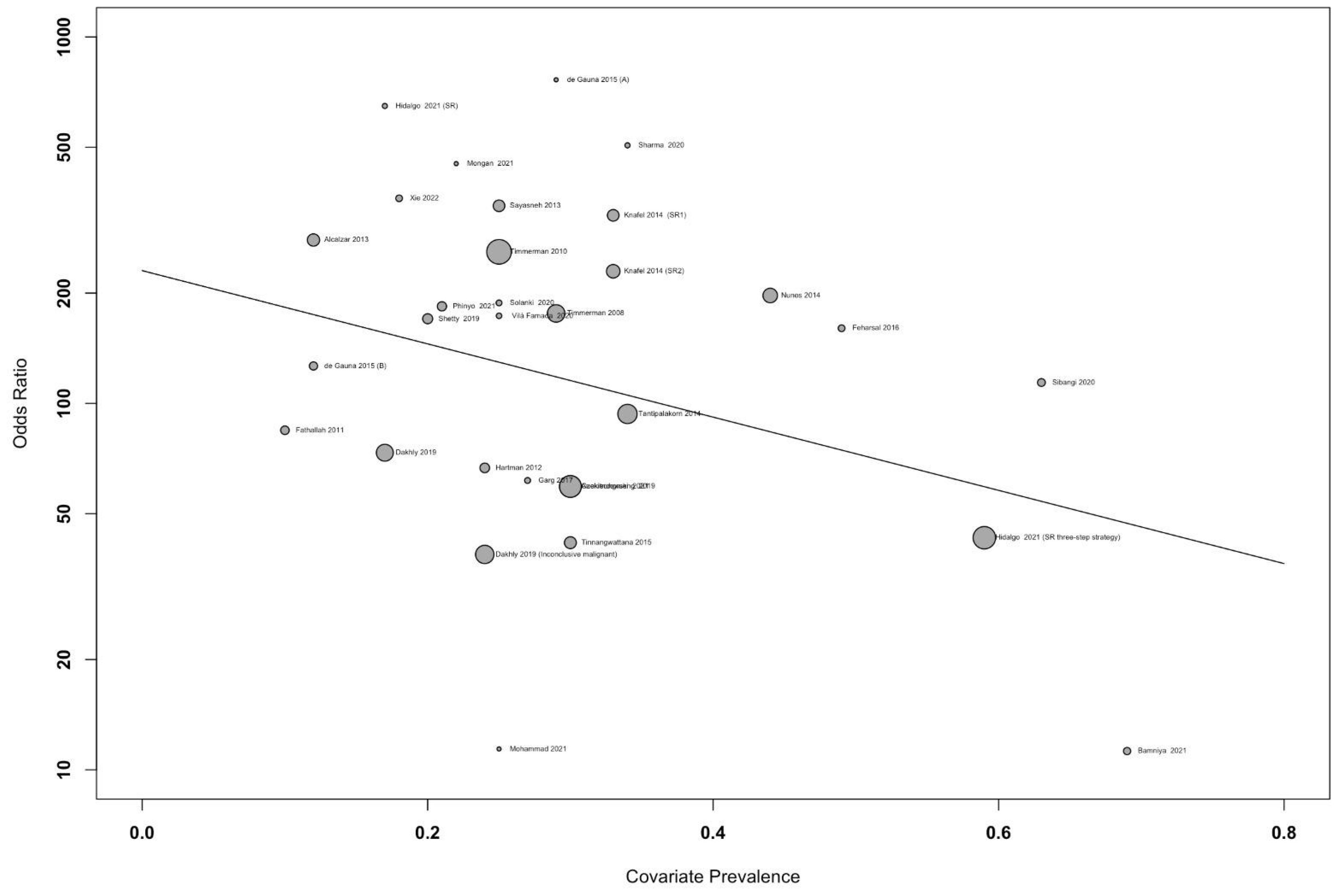
Figure 4. Meta-regression graph showing the diagnostic odds ratio (DOR) against accuracy of the study, with the size of bubble representing the study weight.
The pooled sensitivity for the diagnosis of ovarian malignancies was 0.92 (95% CI, 0.89–0.94), as shown in Figure 5, while the pooled specificity was 0.92 (95% CI, 0.89–0.94) (Figures 6, 7). The DOR was 115.59 (95% CI, 83.33–160.34), as shown in Figure 8. Figure 8 displays a ROC curve with an area under the curve (AUC) of 0.95 (I2 = 5.3% in the abstract). In the sensitivity, specificity, and DOR analyses, the I2 was 50.7%, 90.6%, and 52.9%, respectively, while Cochran’s Q statistics p value was <0.01 in all, indicating heterogeneity. The generated funnel plot in Figure 9 was symmetrical (Egger’s test, p = 0.19), indicating the absence of publication biases.
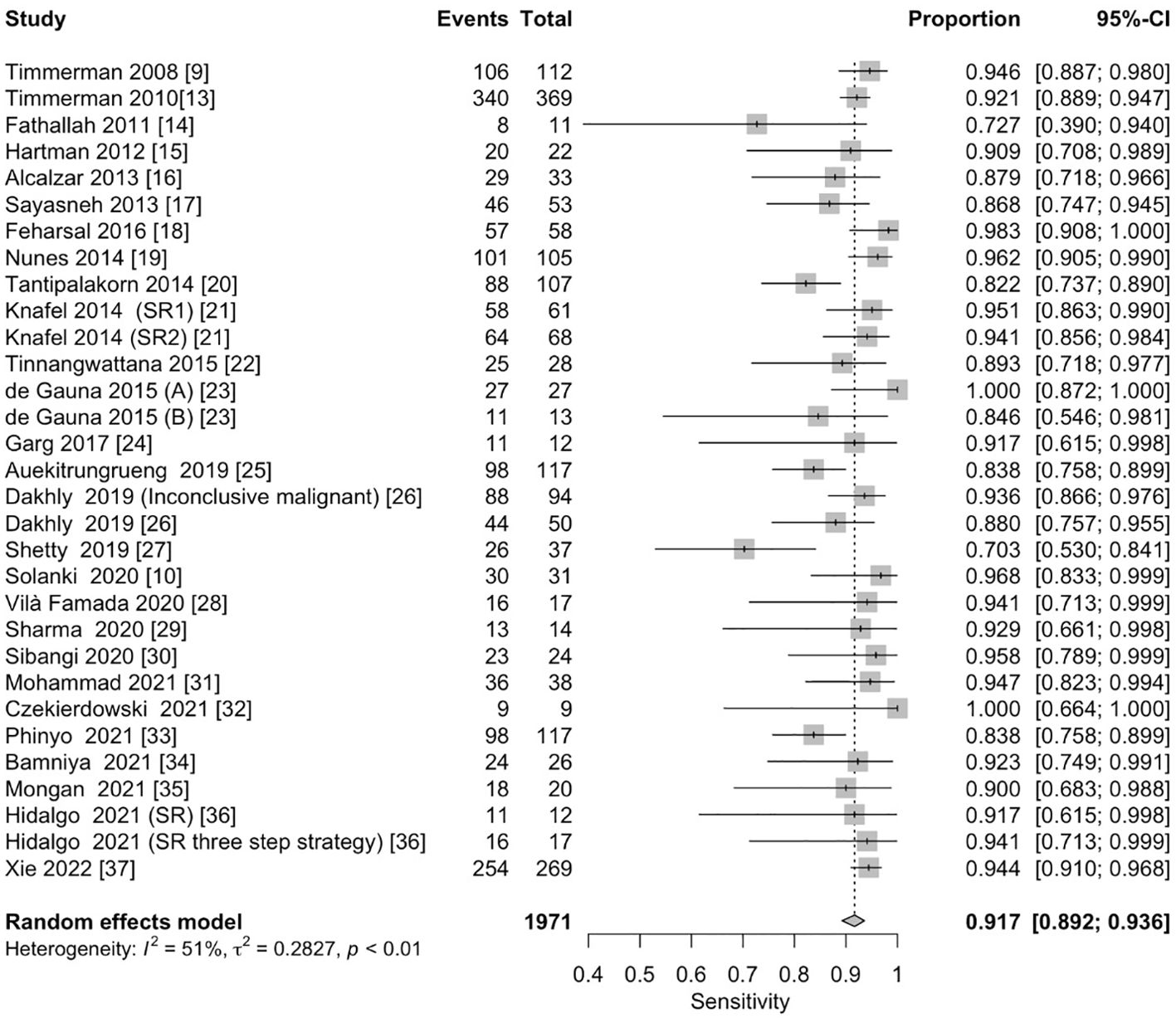
Figure 5. Forest plot of the pooled sensitivity of the IOTA Simple Rules in the diagnosis of ovarian cancer. The dotted vertical line represents the pooled effect size point where the effect size in individual studies has a very different distribution (heterogeneity) around this line. The diamond at the bottom represents the pooled effect and its 95% CI. CI, confidence interval; IOTA, International Ovarian Tumor Analysis.
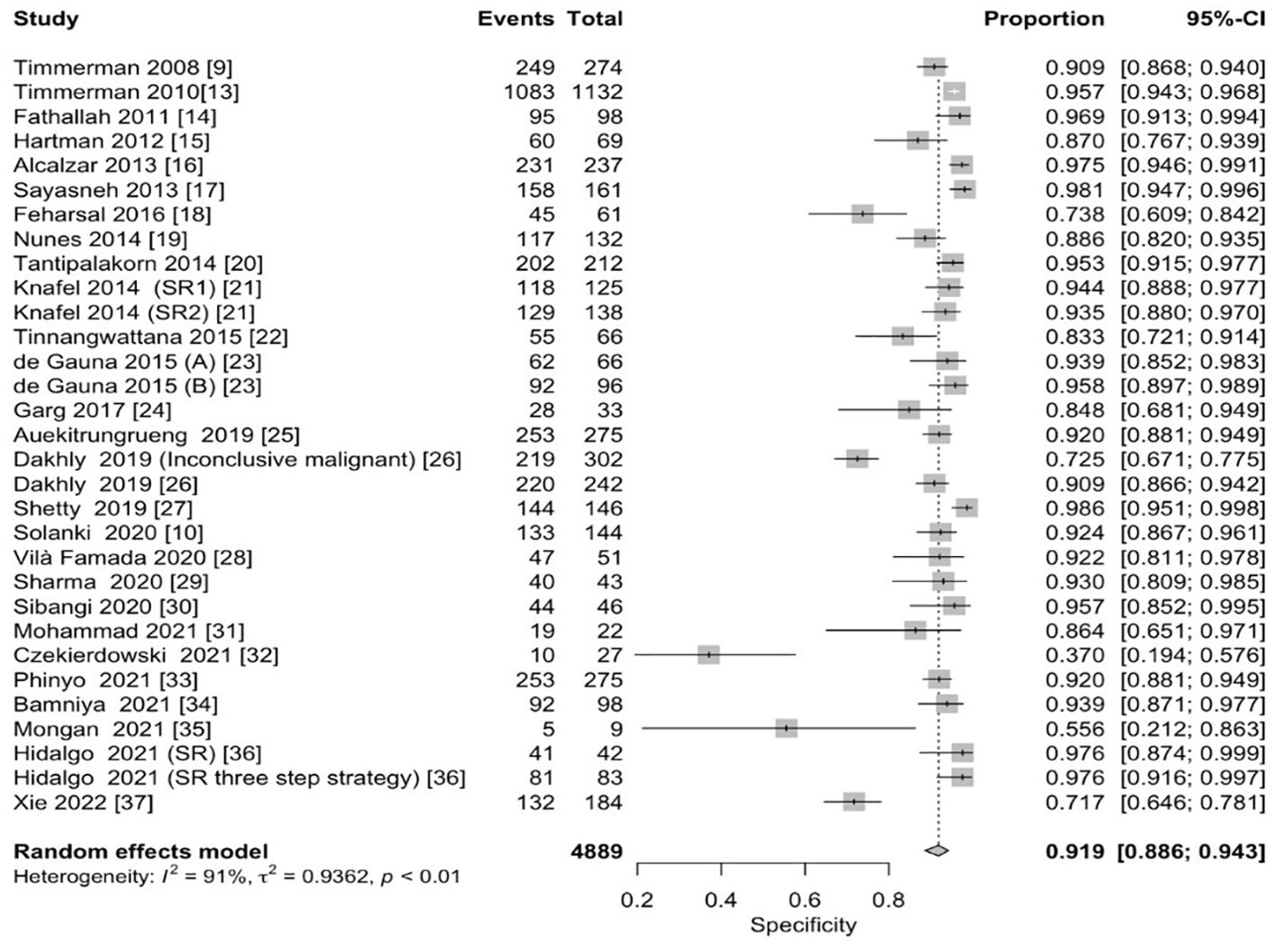
Figure 6. Forest plot of the pooled specificity of IOTA-SR in the diagnosis of ovarian cancer. The dotted vertical line represents the pooled effect size point where the effect size in individual studies has a very different distribution (heterogeneity) around this line. The diamond at the bottom represents the pooled effect and its 95% CI. CI, confidence interval; IOTA-SR, International Ovarian Tumor Analysis Simple Rules.
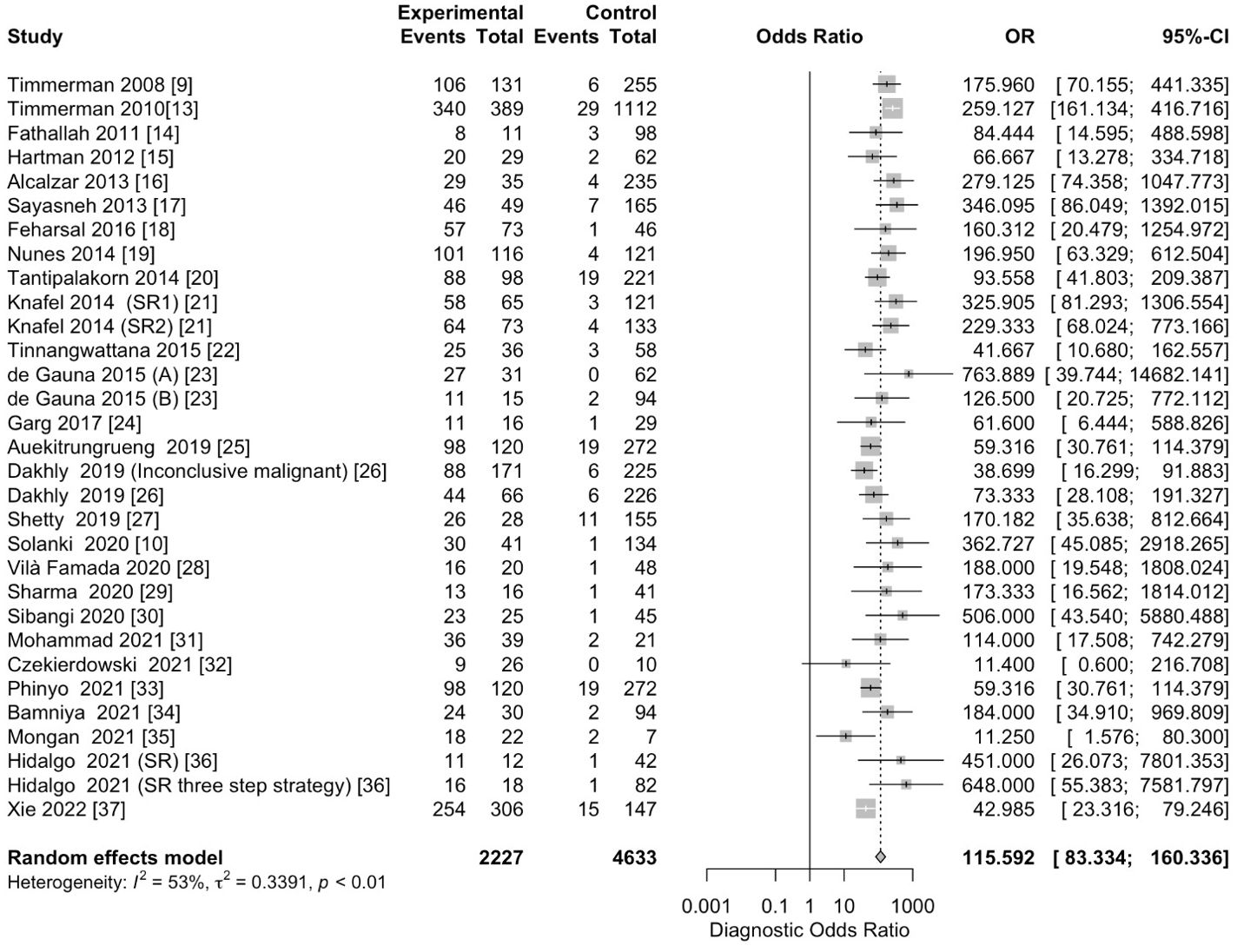
Figure 7. Forest plot of the pooled DOR of IOTA-SR in the diagnosis of ovarian cancer. The dotted vertical line represents the pooled effect size point where the effect size in individual studies has a very different distribution (heterogeneity) around this line. The diamond at the bottom represents the pooled effect and its 95% CI. OR, odds ratio; CI, confidence interval; DOR, diagnostic odds ratio; IOTA-SR, International Ovarian Tumor Analysis Simple Rules.
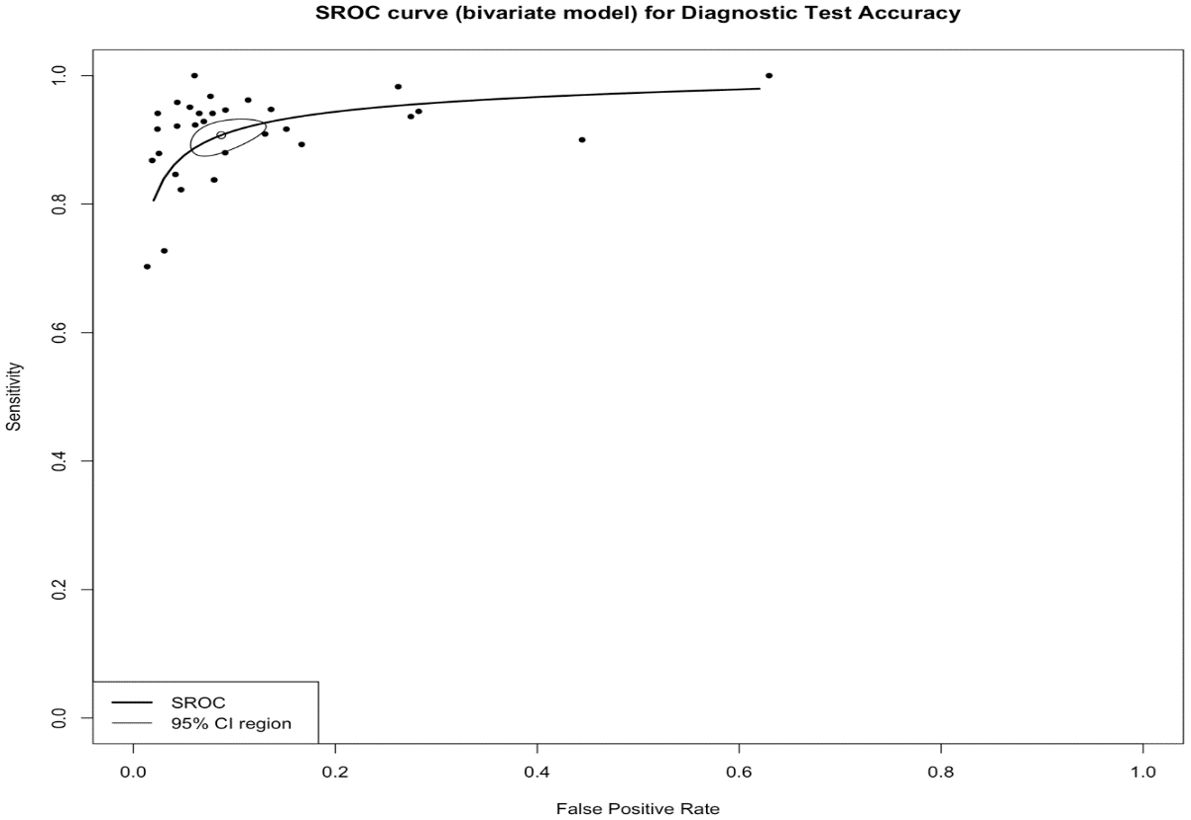
Figure 8. The summary receiver operating characteristic (SROC) curve of IOTA-SR in the diagnosis of ovarian cancer. CI, confidence interval; AUC, area under the curve; IOTA-SR, International Ovarian Tumor Analysis Simple Rules.
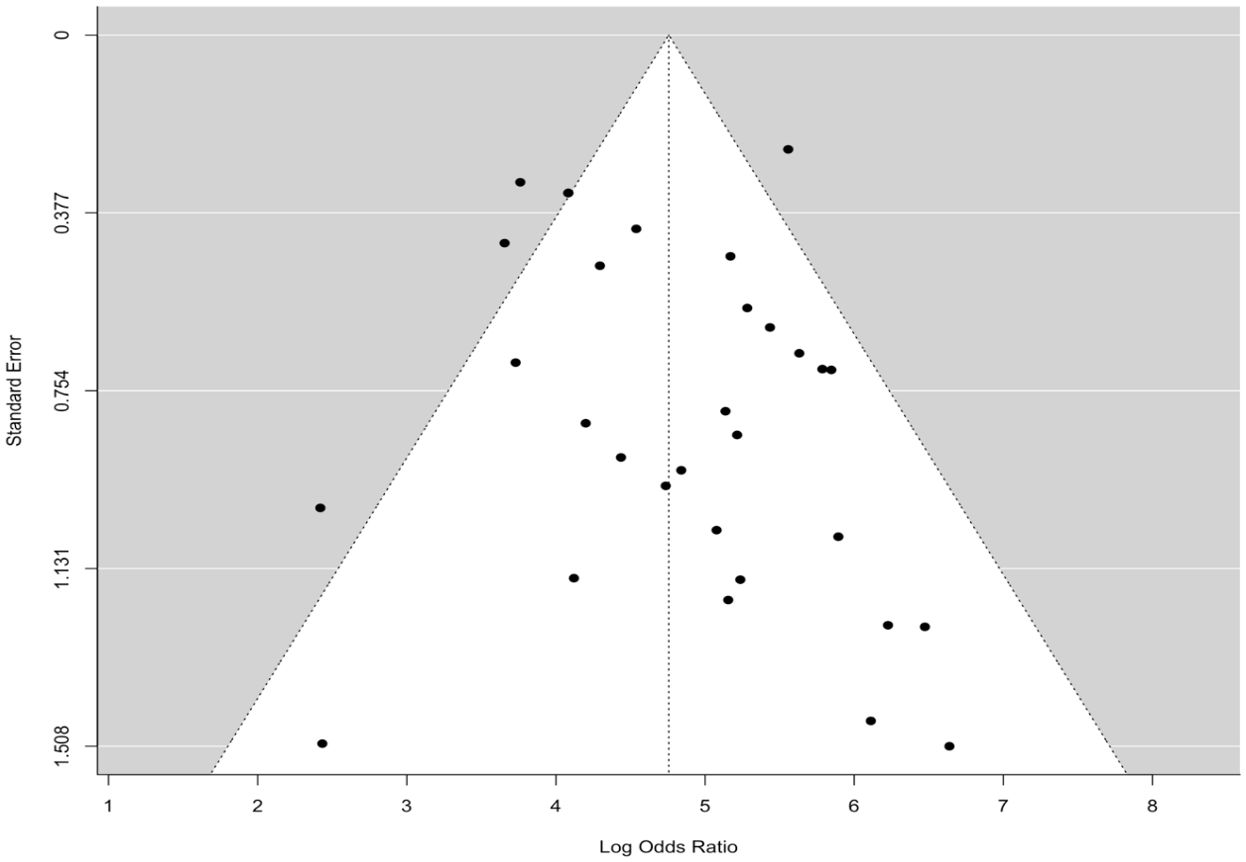
Figure 9. The funnel plot is symmetrical, showing the absence of publication bias.
4 DiscussionThe differential diagnosis of adnexal masses is a recognized problem in gynecological practice. To this end, statements on imaging, biomarkers, and prediction models for the preoperative diagnosis of ovarian cancers were collaboratively developed by the European Society of Gynaecological Oncology, the International Society of Ultrasound in Obstetrics and Gynecology, the IOTA group, and the European Society for Gynaecological Endoscopy (38). In this study, the efficacy of IOTA Simple Rules in differentiating benign from malignant adnexal tumors was investigated. The accuracy of IOTA in evaluating ovarian tumors was found to be relatively high.
Many USG technologies for the diagnosis of adnexal masses have been introduced worldwide. The introduction of imagistic exploration has hastened and enhanced USG diagnoses. The gynecologist must apply the IOTA to evaluate benign, borderline, and malignant adnexal masses (39, 40). The IOTA study is the most extensive work on the accuracy of USG in diagnosing ovarian tumors. Ovarian tumors have been studied to better understand their morphology and duplex characteristics. The IOTA group was founded 15 years ago to develop a reliable algorithm for diagnosing ovarian cancer. The IOTA-SR is the first scoring system to rely on a set of five USG features indicative of a malignant ovarian tumor (M-features) and five USG features indicative of a benign tumor (B-features) (1).
In this review, the diagnostic performance of IOTA-SR was assessed for classifying benign or malignant ovarian tumors. It was found that the pooled sensitivity was 92% (95% CI, 0.89–0.94) and that the pooled specificity was 92% (95% CI, 0.89–0.94). These results align with a previous meta-analysis by Nunes et al., who reported that the IOTA-SR is a reliable triage test for USG operators with varying experience levels and has a pooled sensitivity of 93% and a specificity of 95% when they are applicable (19). This finding is compatible with a previous meta-analysis with a large sample size of adnexal masses that reported 93% and 80% sensitivity and specificity for IOTA-SR, respectively (41). Another previous study on the external validation of IOTA-SR reported 94.3% and 94.9% overall sensitivity and specificity, respectively (42). A previous study reported that subjective assessment based on USG and IOTA may offer superior value to tumor markers, such as cancer antigen 125 (CA-125) and human epididymis protein 4 (HE4) assessment in complex adnexal masses (43). Furthermore, another study reported that the IOTA-SR had a high diagnostic accuracy compared to the risk of malignancy index (RMI) in distinguishing benign from malignant ovarian tumors (25).
Despite the excellent performance and simplicity of IOTA-SR, they have a significant limitation: they cannot be applied in all cases of adnexal masses (11). Tantipalakorn et al. reported that the IOTA-SR could be applicable in 80.1% of adnexal masses (20), and Lee et al. reported that IOTA-SR could be applicable in 80% of adnexal masses but was inconclusive (1). Nunes et al. reported that IOTA-SR was applicable in 78.2% with 96.2% sensitivity and 88.6% specificity (19). These meta-analysis results showed that IOTA-SR was applicable in 85.7% of adnexal masses in the included studies.
Ultimately, for accurate presurgical diagnosis of adnexal masses, the American College of Obstetricians and Gynecologists recommends “a multivariable approach by combining demographic, clinical, imaging, and laboratory parameters to perform the best diagnosis” (44).
Several studies have assessed the performance of IOTA-SR in combination with biomarkers to enhance the diagnostic accuracy of ovarian mass assessments. A study on 479 pre- and postmenopausal women reported that a combination of CA-125 and IOTA-SR models had better diagnostic value in differentiating malignant from benign ovarian tumors with AUCs of 0.94 and 0.98, respectively (33). Jha et al. found that the utilization of both IOTA Simple Rules and CA-125 exhibited exceptional diagnostic efficacy in distinguishing between benign and malignant ovarian tumors, surpassing the use of either CA-125 or IOTA Simple Rules individually (AUC of 0.94 for the combination tool; IOTA-SR + CA-125) (45). A study conducted by Xie et al. to assess the efficacy of the IOTA Simple Rules, Ovarian-Adnexal Reporting and Data System (O-RADS), and CA-125 in discerning between benign and malignant adnexal masses was examined. The findings indicated no notable variance in diagnostic precision when employing a combination of two approaches, specifically, IOTA-SR and CA-125 versus O-RADS and CA-125. As a result, the authors suggest using either IOTA-SR or O-RADS in conjunction with CA-125 to distinguish between benign and malignant lesions prior to surgery (37). Other studies have found that the integration of IOTA Simple Rules with established tumor markers (CA-125 and HE4) enhances the precision of diagnosing malignant ovarian masses before surgery (46).
The main strength of the current study is that it includes many previous studies without significant bias, as indicated in the analysis (Figure 9), thus improving diagnostic accuracy. However, the study must acknowledge a few limitations because heterogeneity was found among the studies selected, as evidenced by Cochran’s Q statistics; inconsistencies and disparities in the study population’s characteristics and variations in the percentage of malignancies across the selected population were the main contributors to this heterogeneity.
There is a lack of studies in the Gulf and low-income countries on the application of IOTA-SR in ovarian tumors, which underscores a gap in knowledge and practices on this topic. The IOTA-SR criteria significantly influence international literature; these criteria offer good sensitivity and specificity in distinguishing between benign and malignant ovarian tumors. They reduce the need for expensive diagnostic procedures, such as MRI or CT, guide patient care, and empower medical professionals to opt for surgery, monitoring, or further imaging, ultimately improving patient outcomes. The education and training of ultrasound practitioners on IOTA-SR reduce interobserver variability and encourage uniform diagnostic procedures across regions. The IOTA’s clinical significance is supported by this systematic review and meta-analysis, serving as a roadmap for future research in ovarian cancer, particularly in the Gulf and low-resource countries.
5 ConclusionIn conclusion, this meta-analysis demonstrated that IOTA-SR is highly effective in the presurgical differentiation of malignant from benign adnexal masses. The IOTA Simple Rules is an effective test for detecting ovarian malignancies with a pooled sensitivity of 92% (95% CI, 0.89–0.94) and a pooled specificity of 92% (95% CI, 0.89–0.94).
Author contributionsAG: Funding acquisition, Writing – original draft, Writing – review & editing, Conceptualization, Formal analysis, Methodology. MG: Conceptualization, Formal analysis, Funding acquisition, Methodology, Supervision, Writing – original draft, Writing – review & editing. SAA: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. AA: Conceptualization, Formal analysis, Funding acquisition, Validation, Visualization, Writing – original draft, Writing – review & editing. ME: Funding acquisition, Methodology, Supervision, Writing – original draft, Writing – review & editing. WA: Funding acquisition, Investigation, Methodology, Software, Writing – review & editing. ID: Conceptualization, Data curation, Funding acquisition, Project administration, Resources, Writing – original draft. SA: Data curation, Funding acquisition, Methodology, Project administration, Writing – original draft, Writing – review & editing. MAl: Funding acquisition, Project administration, Software, Writing – original draft. EA: Data curation, Funding acquisition, Investigation, Resources, Visualization, Writing – review & editing. FHA: Conceptualization, Funding acquisition, Investigation, Software, Writing – review & editing. RAA: Data curation, Funding acquisition, Investigation, Project administration, Software, Writing – review & editing. MK: Supervision, Validation, Visualization, Writing – review & editing. ZYH: Funding acquisition, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MAd: Supervision, Validation, Writing – review & editing, Conceptualization.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this work through Large Research Project under grant number RGP2/431/45.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Lee SJ, Oh HR, Na S, Hwang HS, Lee SM. Ultrasonographic ovarian mass scoring system for predicting Malignancy in pregnant women with ovarian mass. Obstet Gynecol Sci. (2022) 65:1–13. doi: 10.5468/ogs.21212
PubMed Abstract | Crossref Full Text | Google Scholar
3. Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer. (2013) 49:1374–403. doi: 10.1016/j.ejca.2012.12.027
PubMed Abstract | Crossref Full Text | Google Scholar
4. Abramowicz JS, Timmerman D. Ovarian mass–differentiating benign from Malignant: The value of the International Ovarian Tumor Analysis ultrasound rules. Am J Obstet Gynecol. (2017) 217:652–60. doi: 10.1016/j.ajog.2017.07.019
PubMed Abstract | Crossref Full Text | Google Scholar
5. Sadowski EA, Paroder V, Patel-Lippmann K, Robbins JB, Barroilhet L, Maddox E, et al. Indeterminate adnexal cysts at US: prevalence and characteristics of ovarian cancer. Radiology. (2018) 287:1041–9. doi: 10.1148/radiol.2018172271
PubMed Abstract | Crossref Full Text | Google Scholar
6. Froyman W, Landolfo C, De Cock B, Wynants L, Sladkevicius P, Testa AC, et al. Risk of complications in patients with conservatively managed ovarian tumours (IOTA5): A 2-year interim analysis of a multicentre, prospective, cohort study. Lancet Oncol. (2019) 20:448–58. doi: 10.1016/S1470-2045(18)30837-4
PubMed Abstract | Crossref Full Text | Google Scholar
7. Timmerman D, Van Calster B, Testa A, Savelli L, Fischerova D, Froyman W, et al. Predicting the risk of Malignancy in adnexal masses based on the Simple Rules from the International Ovarian Tumor Analysis group. Am J Obstet Gynecol. (2016) 214:424–37. doi: 10.1016/j.ajog.2016.01.007
PubMed Abstract | Crossref Full Text | Google Scholar
8. Suh-Burgmann E, Brasic N, Jha P, Hung YY, Goldstein RB. Ultrasound characteristics of early-stage high-grade serous ovarian cancer. Am J Obstet Gynecol. (2021) 225(4):409.e1–409.e8. doi: 10.1016/j.ajog.2021.04.262
PubMed Abstract | Crossref Full Text | Google Scholar
9. Timmerman D, Testa AC, Bourne T, Ameye L, Jurkovic D, Van Holsbeke C, et al. Simple ultrasound-based rules for the diagnosis of ovarian cancer. Ultrasound Obstet Gynecol. (2008) 31:681–90. doi: 10.1002/uog.v31:6
Crossref Full Text | Google Scholar
10. Solanki V, Singh P, Sharma C, Ghuman N, Sureka B, Shekhar S, et al. Predicting Malignancy in adnexal masses by the international ovarian tumor analysis-simple rules. J Mid-life Health. (2020) 11:217–23. doi: 10.4103/jmh.JMH_103_20
PubMed Abstract | Crossref Full Text | Google Scholar
11. Kaijser J. Towards an evidence-based approach for diagnosis and management of adnexal masses: findings of the International Ovarian Tumour Analysis (IOTA) studies. Facts Views Vis Obgyn. (2015) 7:42–59.
PubMed Abstract | Google Scholar
12. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2 Group QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
PubMed Abstract | Crossref Full Text | Google Scholar
13. Timmerman D, Ameye L, Fischerova D, Epstein E, Melis GB, Guerriero S, et al. Simple ultrasound rules to distinguish between benign and Malignant adnexal masses before surgery: prospective validation by IOTA group. Bmj. (2010) 341:c6839. doi: 10.1136/bmj.c6839
PubMed Abstract | Crossref Full Text | Google Scholar
14. Fathallah K, Huchon C, Bats AS, Metzger U, Lefrere-Belda MA, Bensaid C, et al. External validation of simple ultrasound rules of Timmerman on 122 ovarian tumors. Gynecol Obstetrique Fertilite. (2011) 39:477–81. doi: 10.1016/j.gyobfe.2011.05.007
PubMed Abstract | Crossref Full Text | Google Scholar
15. Hartman CA, Juliato CR, Sarian LO, Toledo MC, Jales RM, Morais SS, et al. Ultrasound criteria and CA 125 as predictive variables of ovarian cancer in women with adnexal tumors. Ultrasound Obstetrics Gynecol. (2012) 40:360–6. doi: 10.1002/uog.11201
PubMed Abstract | Crossref Full Text | Google Scholar
16. Alcázar JL, Pascual MA, Olartecoechea B, Graupera B, Auba M, Ajossa S, et al. IOTA simple rules for discriminating between benign and Malignant adnexal masses: prospective external validation. Ultrasound Obstetrics Gynecol. (2013) 42:467–71. doi: 10.1002/uog.12485
PubMed Abstract | Crossref Full Text | Google Scholar
17. Sayasneh A, Wynants L, Preisler J, Kaijser J, Johnson S, Stalder C, et al. Multicentre external validation of IOTA prediction models and RMI by operators with varied training. Br J Cancer. (2013) 108:2448–54. doi: 10.1038/bjc.2013.224
PubMed Abstract | Crossref Full Text | Google Scholar
18. Feharsal Y, Putra AD. International ovarian tumor analysis (IOTA) scoring system to predict ovarian Malignancy preoperatively. Indonesian J Obstetrics Gynecol. (2016) 14:42–6. doi: 10.32771/inajog.v4i1.71
Crossref Full Text | Google Scholar
19. Nunes N, Ambler G, Foo X, Naftalin J, Widschwendter M, Jurkovic D. Use of IOTA simple rules for diagnosis of ovarian cancer: meta-analysis. Ultrasound Obstetrics Gynecol. (2014) 44:503–14. doi: 10.1002/uog.13437
PubMed Abstract | Crossref Full Text | Google Scholar
20. Tantipalakorn C, Wanapirak C, Khunamornpong S, Sukpan K, Tongsong T. IOTA simple rules in differentiating between benign and Malignant ovarian tumors. Asian Pac J Cancer Prev. (2014) 15:5123–6. doi: 10.7314/apjcp.2014.15.13.5123
PubMed Abstract | Crossref Full Text | Google Scholar
21. Knafel A, Banas T, Nocun A, Wiechec M, Jach R, Ludwin A, et al. The prospective external validation of international ovarian tumor analysis (IOTA) simple rules in the hands of level I and II examiners. Ultraschall Der Medizin-European J Ultrasound. (2016) 37:516–23. doi: 10.1055/s-0034-1398773
PubMed Abstract | Crossref Full Text | Google Scholar
22. Tinnangwattana D, Vichak-Ururote L, Tontivuthikul P, Charoenratana C, Lerthiranwong T, Tongsong T. IOTA simple rules in differentiating between benign and Malignant adnexal masses by non-expert examiners. Asian Pacific J Cancer Prev. (2015) 16:3835–8. doi: 10.7314/APJCP.2015.16.9.3835
留言 (0)