Parkinson’s disease (PD) is a neurodegenerative disorder characterized by disruptions in motor function, e.g., delayed movement initiation, decreased movement speed, rest tremor, and increased joint rigidity (Jankovic, 2008). Non-motor symptoms, however, such as cognitive dysfunction, are also prevalent components of PD (Elgh et al., 2009; Michely et al., 2012). These cognitive impairments include changes in executive functions such as working memory, set shifting, and movement inhibition (Zgaljardic et al., 2006; Disbrow et al., 2013; Dujardin et al., 2013). Functional imaging studies have shown that the DLPFC, a critical node in the BGTC network involved in executive function, is impaired in PD (Owen, 2004; Leh et al., 2010; Cools and D’Esposito, 2011; Disbrow et al., 2013; Caspers et al., 2017). This impairment is likely secondary to the role of dopamine in mediating executive functions involving the DLPFC (Durstewitz et al., 2000; Dauer and Przedborski, 2003). While some functional imaging studies suggest parkinsonism results in hypoactivation in the prefrontal cortex (Lewis et al., 2003; Yu et al., 2007), consistent with classic models of basal ganglia function in which excessive inhibitory activity from the internal segment of the globus pallidus is hypothesized to result in reduced excitatory thalamo-cortical drive (Albin et al., 1989; DeLong, 1990), other studies have found hyperactivation in DLPFC in PD patients compared to controls (Disbrow et al., 2013; Martin et al., 2019; Ranchet et al., 2020). There are limited neuronal data at the single unit level characterizing changes in DLPFC activity in PD to support or refute either of these findings.
One task known to probe executive function in the context of DLPFC is the go/nogo task, which requires subjects to discern between two target types that indicate either taking or avoiding an action (Garavan et al., 1999; Menon et al., 2001). Additionally, the go/nogo paradigm has been useful to show alterations in executive function in PD, such as movement preparation and response inhibition (Garavan et al., 1999; Menon et al., 2001). In this study, a go/nogo touch screen task was used to engage DLPFC, and to test the hypothesis that neuronal processing in the DLPFC is abnormal in early PD through the induction of a mild parkinsonian state. We compared task-related single and multi-unit neuronal firing characteristics in the DLPFC of a nonhuman primate before and after induction of mild parkinsonism using the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP).
Methods Surgical proceduresAll procedures were approved by the University of Minnesota Institutional Animal Care and Use Committee and complied with US Public Health Service policy on the humane care and use of laboratory animals. One adult female rhesus macaque (Macaca mulatta, 20 years of age) was used in this study. Surgery was performed under isoflurane anesthesia using aseptic techniques. The animal was implanted in the right DLPFC with a 96-channel Utah microelectrode array (Pt-Ir, 1.5 mm depth, 400 um inter-electrode spacing, Blackrock Microsystems) using surgical methods described previously (Rousche and Normann, 1992; Maynard et al., 1997; Escobar Sanabria et al., 2017). DLPFC was identified based on sulcal landmarks during the array implantation surgery.
Go/nogo task and data collectionThe animal was trained to perform a visually cued go/nogo reaching task (Figure 1A). Trials were initiated when the animal placed its left hand on a capacitive touchpad (“start-pad”) and, after a 2 s delay, a cue appeared in one of three randomly selected locations. Two seconds following this cue a “go” target appeared at the selected location in 80% of the trials, and a “nogo” target would appear in 20% of the trials. A successful go trial required the primate to leave the start-pad within 1.5 s and touch the target within another 1.5 s. A successful nogo trial required the animal to hold on the start-pad for 1.5 s following target presentation. Successful trials resulted in a juice reward. Reaction time was defined as the time between presentation of the go target and reach initiation (the time when the animal’s hand left the start-pad). Reach duration was defined as the time between reach initiation and contact with the target. The animal initiated the next trial by voluntarily returning to the start-pad. Go and nogo target appearance timepoints were extracted from the task software and reach initiation timepoints were recorded from the start-pad. Raw neurophysiological data were collected using a TDT workstation (Tucker Davis Technologies) operating at ~25 kHz sampling rate. Activity of DLPFC units was recorded while the animal was seated, head fixed, in a primate chair performing the reaching task.
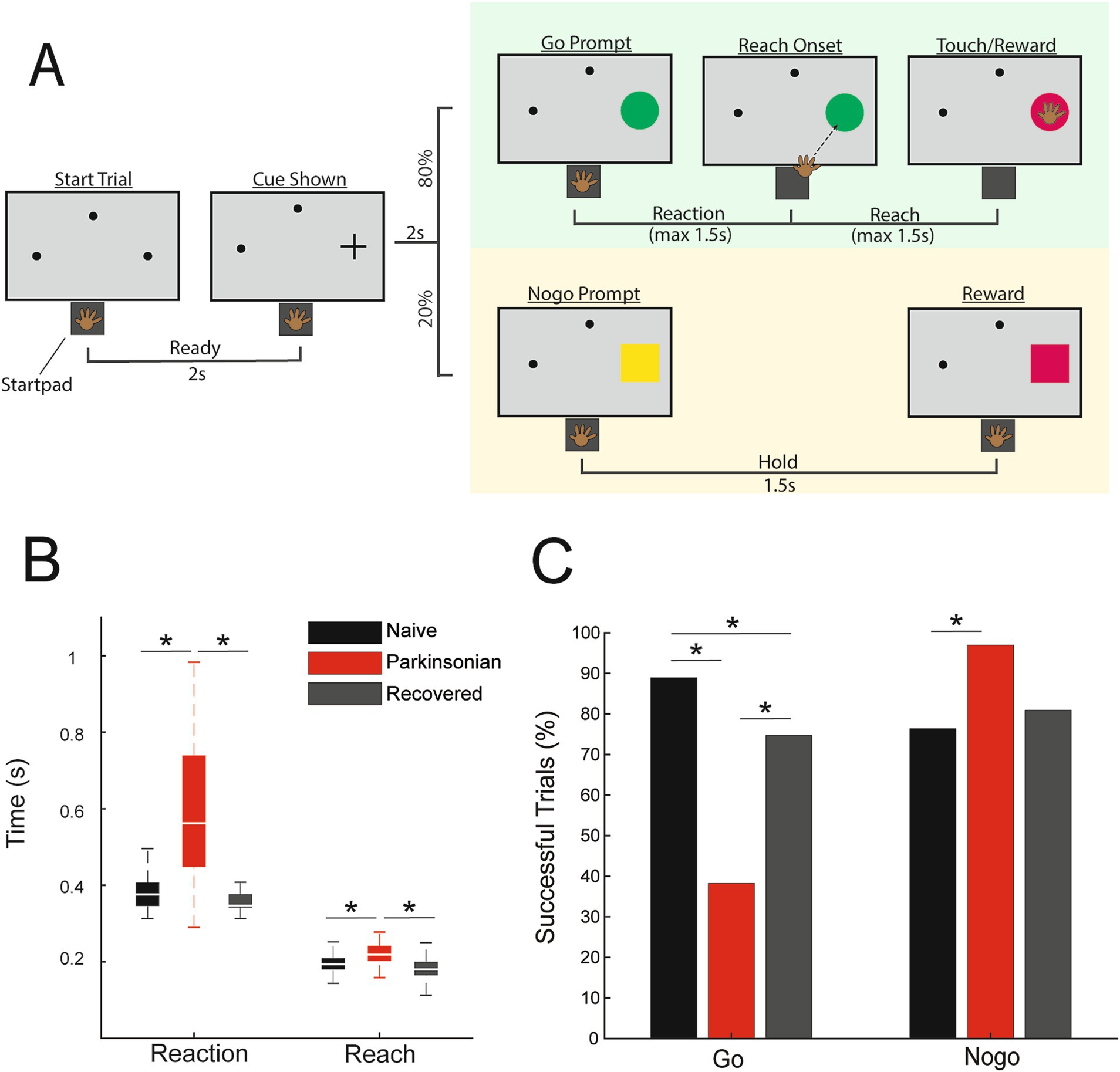
Figure 1. (A) Go/nogo task paradigm. (B) Reach and reaction times during successful go trials in naive (black), parkinsonian (red), and recovered (gray) (p < 0.05, WRS). (C) Percentage of successful go and nogo trials (p < 0.05, Χ2).
Once data were collected in the naive state (2 sessions, 360 trials), the animal was rendered parkinsonian by one intracarotid injection of MPTP (0.4 mg/kg, 0.1% solution in saline delivered over the course of 10 min) in the right side, ipsilateral to the neuronal recording site and contralateral to the limb used to perform the task (Bankiewicz et al., 1986). Overall parkinsonian severity was assessed using a modified Unified Parkinson’s Disease Rating Scale (mUPDRS), which rated appendicular motor symptoms (upper and lower limb rigidity, bradykinesia, akinesia, and tremor) on the hemi-body contralateral to neural recordings using a 0–3 scale (0 = naive, 3 = severe, maximum total score = 27) (Vitek et al., 2012). We observed mild parkinsonian signs (mUPDRS: 2.8 ± 1.14, 5 ratings) after MPTP injection; however motor signs improved over subsequent weeks and returned to a baseline mUPDRS score of 0 after 11 days (defined here as the “recovered” state). Post-MPTP neural data were obtained in both the mild parkinsonian (2 sessions, 154 trials) and recovered (2 sessions, 360 trials) states. Parkinsonian sessions were recorded 3 days after MPTP injection. Recovered sessions were recorded after the mUPDRS score had been stable at 0 for at least 1 week (~3 weeks after parkinsonian data collection).
Statistical analysis of neuronal dataNeuronal recordings were analyzed offline using custom software developed in MATLAB (Mathworks) and Offline Sorter (Plexon). Raw data were bandpass filtered 300–5,000 Hz, and single and multi-units were isolated and sorted using principal component and template-based methods in Offline Sorter (hereafter referred to collectively as “units”). Spike trains were aligned to go target appearance, nogo target appearance, or reach onset. A trial-averaged spike density function for each unit was generated by convolving each spike with a gaussian kernel (60 ms variance) and a time resolution of 10 ms. The baseline firing rate for each unit was defined as the mean of the spike density function during a 1.5 s period beginning 0.25 s after trial initiation, before cue presentation. Units with an extremely low firing rate were excluded from further analysis (less than 0.75 spikes per second). To investigate neuronal modulation during the reaction time period, spiking activity immediately following target appearance until the minimum reaction time across all trials (255 ms) was used. The same time frame was used for nogo trials. To investigate neuronal modulation during reach initiation, activity 50 ms prior to reach onset until the minimum reach duration across all trials (144 ms) was used. A one-sample 2-tailed t-test was used to compare the mean baseline firing rate of each unit to the firing rates during the reaction or reach periods. Units with a significant change in firing rate during the analysis window (p ≤ 0.002) compared to baseline were classified as modulated (Pasquereau et al., 2016). With a time resolution of 10 ms and a maximum window of 255 ms, the maximum number of comparisons was 25, so 0.002 (0.05/25) was chosen as a conservative threshold for determining whether a cell was modulated. Units with a significant increase in firing rate were further classified as activated, and those with a significant decrease as suppressed. Reaction times were compared across states using the Wilcoxon rank sum test (WRS), as were the reach durations. Chi-squared tests [Χ2(DoF, N)] were used to compare the percentage changes in the number of modulated, activated and suppressed units, the ratio of activated over suppressed units, and changes in task success rates, between the naïve, parkinsonian, and recovered conditions.
Results Effects of MPTP on task performanceMPTP administration induced a mild parkinsonian state based on clinical assessments (2.8 ± 1.14, 5 ratings). In the recovered state the mUPDRS score returned to zero. The NHP completed 288 go trials and 72 nogo trials in the naïve state and recovered state. In the parkinsonian condition the NHP completed 123 go trials and 31 nogo trials. As indicated in Figure 1B, reaction times increased in the parkinsonian condition compared to the naïve (WRS; z = −8.2862, p < 0.001, r = −0.48) and recovered states (WRS; z = 8.7851, p < 0.001 r = 0.53). Reach times were also longer in the parkinsonian condition compared to naïve (WRS; z = −5.4332, p < 0.001, r = −0.31) and recovered states (WRS; z = 6.1389, p < 0.001, r = 0.37). There was a decrease in task success rate during go trials from 89% in naïve to 36% in the parkinsonian condition (Figure 1C, left) [Χ2(1,411) = 85.1, p < 0.001]. Task success rate during go trials was higher in the recovered state compared to the parkinsonian condition [Χ2(1,411) = 38.0233, p < 0.001]. Nogo trial success rates were increased in the parkinsonian condition compared to the naïve state [Χ2(1,103) = 6.2442, p = 0.0125]. In the recovered state, nogo task performance returned to a level similar to that observed in the naïve state (Figure 1C, right).
Parkinsonism alters neuronal modulation in DLPFCA total of 410 units in DLPFC were recorded in this study (n = 149 naïve, n = 101 parkinsonian, and n = 160 recovered). Representative neurons (Figure 2B) illustrate our main finding that there is a significant reduction in task-related unit activity in DLPFC in the mild parkinsonian condition. We found no change in baseline firing rates between naive (median: 5.04; Q1: 2.816, Q3: 11.25), parkinsonian (median: 5.60; Q1: 1.729, Q3: 10.23) or recovered (median: 6.302; Q1: 2.487, Q3: 14.46) based on a Wilcoxon rank sum test. While 62.4% of units had significant firing rate modulation during the go reaction time period in the naïve state, in the parkinsonian condition only 24.8% were modulated ([Χ2(1,250) = 34.2638, p < 0.001]). Similarly, there was a reduction in the percent of units with significant modulation in the go reach period (53.7% in naïve compared to 31.7% in the parkinsonian condition, [Χ2(1, 250) = 11.7901, p < 0.001]) (Figure 2C). During the recovered state the percent of units with a significant firing rate modulation returned to levels similar to the naïve state for both go reaction (naive: 62.4%, parkinsonian: 24.8%, recovered: 61.9%) [Χ2(1,261) = 34.2148, p < 0.001] and go reach periods (naive: 53.7%, parkinsonian: 31.7%, recovered: 49.4%) [Χ2(1,261) = 7.9289, p = 0.005]. Modulation during the nogo reaction period decreased from 29.7% in the naïve state to 12.0% in the parkinsonian condition [Χ2(1, 250) = 10.7307, p = 0.001], but did not return to naïve levels in the recovered state [Χ2(1,261) = 0.9288, p = 0.3352].
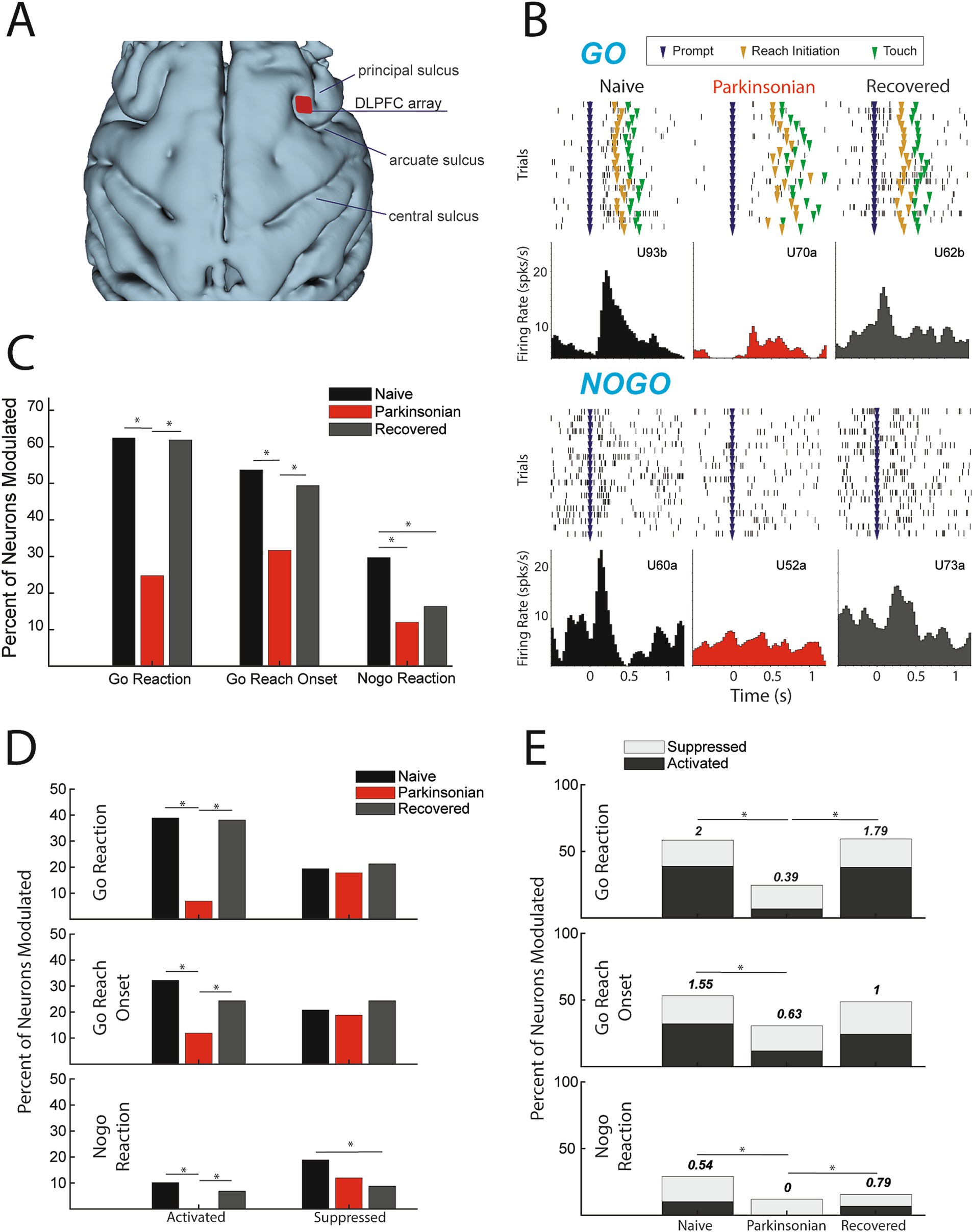
Figure 2. (A) 3D reconstruction of cortex with DLPFC array location. (B) Example cells during go (upper) and nogo (lower) trials from each condition (units labeled alphabetically following the channel number in the array). (C) Percentage of cells modulating during comparison window (p < 0.05, Χ2). (D) Percent of total cells activated (left) and suppressed (right) (p < 0.05, Χ2). (E) Ratio of percent activated over percent suppressed, indicated by number above the bars (p < 0.05, Χ2).
Parkinsonism decreases neuronal activationThe results presented in Figure 2C showed that fewer DLPFC cells were modulated in the mild parkinsonian condition, irrespective of whether that modulation was due to significant increases (activation) or decreases (suppression) in firing rate. We then examined how the parkinsonian state impacted the proportion of cells classified into these modulation subcategories (Figure 2D) As described below, we found that the reduction in modulation in the parkinsonian condition was driven predominantly by a loss of cells that were significantly activated during the go/nogo task.
ActivationThe percentage of cells activated during the go reaction period decreased from 38.9% in the naïve state to 6.9% in the parkinsonian condition [Χ2(1,250) = 32.0287, p < 0.001] and the percentage of cells activated during the reach period decreased from 32.2 to 11.9% [Χ2(1,250) = 10.0132, p < 0.001]. In addition, the percentage of cells activated during the nogo reaction period decreased to zero from naïve to the parkinsonian condition [Χ2(1,250) = 10.7876, p < 0.001]. In the recovered state the percentage of activated units increased back to naïve levels during go reaction (38.1%) [Χ2(1,261) = 31.2728, p < 0.001], reach (24.4%) [Χ2(1,261) = 6.1473, p = 0.0132], and nogo reaction (6.9%) [Χ2(1,261) = 7.2251, p = 0.0072].
SuppressionThere was no significant difference in the percentage of suppressed units during the go reaction period [Χ2(1,250) = 0.1062, p = 0.7445], reach [Χ2(1,250) = 0.1495, p = 0.699], or the nogo reaction period [Χ2(1,250) = 2.1119, p = 0.1462] between naïve and the parkinsonian condition. Similarly, there was no significant difference in the percentage of suppressed units between the parkinsonian condition and recovered state during the go reaction period [Χ2(1,261) = 0.4561, p = 0.4994], reach period [Χ2(1,261) = 1.1087, p = 0.2924], or nogo reaction period [Χ2(1,261) = 0.6939, p = 0.4048]. During the nogo reaction period there was a significant decrease in suppression between the naïve and recovered states [Χ2(1,309) = 6.6395, p = 0.01] (Figure 2D).
Ratio of activation to suppressionThe loss of activation without a significant change in suppression resulted in a decrease in the ratio of activation to suppression from naïve to the parkinsonian state during all three analysis periods (Figure 2E). This ratio decreased from 2 to 0.39 during go reaction [Χ2(1,118) = 13.6973, p < 0.001], 1.55 to 0.63 during reach [Χ2(1,112) = 6.7274, p = 0.01], and 0.54 to 0 during nogo reaction [Χ2(1,56) = 6.1091, p = 0.0134]. In the recovered state, the ratio of activated to suppressed cells increased during go reaction [Χ2(1,124) = 11.6236, p < 0.001] and nogo reaction [Χ2(1,38) = 8.0947, p = 0.004], returning closer to that observed in the naïve state.
DiscussionThe present study investigated the effects of MPTP-induced parkinsonism on neuronal activity in the DLPFC of a nonhuman primate. A unique advantage of this animal model not feasible in human studies is that it allows for a within-subject comparison of changes in activity of neuronal cell populations between healthy and parkinsonian conditions. Induction of the parkinsonian state was associated with a decrease in task-dependent neuronal modulation of firing rates. This reduced modulation was driven by a decrease in the number of activated neurons, leading to a decrease in the ratio of activated to suppressed neurons. The recovered state was associated with an increase in task-dependent modulation, driven by increased activation, compared to the parkinsonian condition. Importantly, these changes in neural activity occurred even in a mild state of parkinsonism, suggesting that the mechanisms involved in cognitive dysfunction may be initiated in the early stages of PD.
Comparison to previous studies of DLPFC in PDOur findings of a reduced proportion of cells with significant activation in response to task events suggest DLPFC is hypoactive during motor preparation and execution in mild parkinsonism. Consistent with this observation, studies utilizing PET and fMRI have found decreased activation during both self-initiated and externally cued timing tasks in the right DLPFC in PD patients compared to healthy control subjects (Jahanshahi et al., 1995; Lewis et al., 2003; Yu et al., 2007). These groups hypothesized that the decrease in DLPFC activation is a result of reduced thalamic output to the cortex as a result of decreased dopamine in the basal ganglia, consistent with the classical model of PD pathophysiology (DeLong, 1990). Nevertheless, there are multiple imaging studies that have identified increased rather than decreased activation in the DLPFC during motor tasks (Disbrow et al., 2013; Martin et al., 2019; Ranchet et al., 2020). Martin et al. used fMRI to identify increased activation in the DLPFC during motor planning in early-stage PD patients performing a finger tapping task, but found no change in DLPFC activity during movement execution (Martin et al., 2019). Similarly, Disbrow et al. found increased BOLD signal in DLPFC bilaterally prior to un-cued movement (Disbrow et al., 2013). Some suggest that this relative hyperactivity in DLPFC could be a compensatory mechanism to accommodate for disrupted function of motor areas in PD (Martin et al., 2019; Ranchet et al., 2020). Evidence of compensatory mechanisms in these studies were supported by a lack of change in task performance despite increased motor symptoms based on UPDRS-III motor signs, though this was not a phenomenon observed in the present study (i.e., motor signs were observed and quantified by clinical exam as well as during the task).
While our findings are consistent with the classical model of PD, it is also possible that though our task clearly engaged DLPFC, it may not have required the conceptualization of movement prior to target appearance that may involve compensatory mechanisms, as suggested by Martin et al. (2019). Furthermore, the high success rate in the parkinsonian condition suggests that the primate may not have been habituated to the go condition, and therefore may have been simply waiting for the go cue to appear before planning movement (Casey et al., 1997). There is also the possibility that the parkinsonian condition obtained was too mild to have induced any compensatory effects. Some studies, however, finding hyperactivation in DLPFC suggested compensatory effects were present in mild PD patients (Martin et al., 2019; Ranchet et al., 2020). While we did not find evidence of an overactive DLPFC in the PD state as might be hypothesized based on these imaging studies, future studies are necessary to fully probe the hypothesis of compensatory mechanisms triggered in the frontal cortex in PD. For example, while our data was collected in a mild PD state, more severe PD states should be investigated to determine whether hyperactivity develops when motor signs are more severe, and compensation is necessary to perform the task.
Recovery from MPTP injectionRecovery from a mild state of parkinsonism following MPTP administration has been previously documented (Taylor et al., 1997; Blesa et al., 2010). The mechanisms of this recovery, however, are not fully understood. Hypotheses include reactive synaptogenesis (temporary, quick onset synapse formation) and denervation hypersensitivity (increased sensitivity to a neurotransmitter after loss of synapses), and uptake of excess dopamine in the nigrostriatal circuit (Bogerts et al., 1983; Eidelberg et al., 1986; Franke et al., 2015). The most likely mechanism underlying this recovery however, is that MPTP administration causes cell injury, but some cells recover over time (Bogerts et al., 1983). By including the recovered condition in this study we were able to show a possible correlation between DLPFC activity and go/nogo task behaviors such as success rate and reaction time. Although the changes in DLPFC activity in the parkinsonian condition that resolved following recovery provide compelling evidence is support of the role of DLPFC deficits in the observed motor dysfunction, whether the change in behavior was the cause or the result of the change in DLPFC activity needs to be determined with additional studies.
Limitations and future directionsThis study included only one NHP, but represents our early findings that are part of a larger study where multiple animals are being enrolled to validate these findings. Another potential limitation is that the task may not have probed the response inhibition aspects of the DLPFC that we had intended, which may be reflected in the increased success rate of nogo trials in the parkinsonian condition. Future studies using go/nogo paradigms in MPTP treated NHPs will need to account for this potential difference in motivation in the design and interpretation of such studies. For example, to better study the neural mechanisms of response inhibition in PD states, task parameters may need to be adjusted such that behavioral performance can be titrated to a level with a greater proportion of nogo trials. It must be recognized that the behavioral task may be more a reflection of apathy, motivation and/or vigilance in MPTP treated NHPs than response inhibition. Nevertheless, characteristics like apathy are a known and significant non-motor symptoms in PD patients (Pagonabarraga et al., 2015) and task paradigms and frontal cortex neural recordings as employed here can be an important step towards better understanding and treatment of these issues. In the future we will modify the task paradigm to induce response inhibition while allowing us to investigate the changes in the DLPFC in early PD where the animals are still able to perform the task. There are challenges, however, to designing tasks that are both cognitively complex and feasible for a parkinsonian animal given their impaired cognitive and motor functions. Regardless, this study provided data to support the finding that even in a mild disease state there are salient changes to neural activity in the DLPFC. Furthermore, DLPFC has been implicated in functions other than response inhibition that have also been shown to be disrupted in PD such as working memory, set shifting, and action planning (Curtis and D’Esposito, 2003; Zgaljardic et al., 2006; Tanji et al., 2007; Bissonette et al., 2013; Disbrow et al., 2013). Future studies could employ additional paradigms such as the Wisconsin card sorting task or n-back to probe set-shifting or working memory, respectively (Zgaljardic et al., 2006). We may also look to quantify cognitive performance of the primate while parkinsonian to characterize neurophysiological changes related specifically to cognitive disruption and identify how dopamine replacement therapy and DBS alter pre-frontal cortical activity. While deep brain stimulation is effective at modulating motor cortex activity in PD patients, its effect on frontal cortical regions is less well understood (Neumann et al., 2023). We are recording from area 9/46d, medial to the principal sulcus and lateral to the arcuate sulcus. This area receives input from the medial dorsal nucleus of the thalamus and projects to other cortical areas and back to the thalamus. A developed macaque has a prefrontal cortical thickness of ~1.7 mm, and the DLPFC in area 9/46d has a prominent layer V (Petrides and Pandya, 1999; Xia et al., 2023). Therefore, it is likely that our 1.5 mm electrodes were recording from layer V, the internal pyramidal layer. This layer includes cells projecting to the basal ganglia along the hyperdirect pathway from cortical regions such as DLPFC to the subthalamic nucleus (STN) (Dagher, 2020). The hyperdirect pathway could be antidromically modulated during DBS and provide an approach to modulate neuronal activity in DLPFC, similar to what has been reported in primary motor cortex using the same type of cortical array (Johnson et al., 2020). STN stimulation could also modulate DLPFC via the later portions of the indirect pathway, due to STN projections through globus pallidus internus and thalamus back to the cortex (Hashimoto et al., 2003; Xu et al., 2008). Understanding the neural mechanisms underlying frontal cortical dysfunction in PD will help motivate and inform neuromodulation techniques that would allow us to improve neural function for both parkinsonian motor and cognitive behaviors.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe animal study was approved by Institutional Animal Care and Use Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributionsNH: Data curation, Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. BM: Formal analysis, Investigation, Supervision, Writing – review & editing. TH: Data curation, Writing – review & editing. MJ: Writing – review & editing. JW: Conceptualization, Methodology, Writing – review & editing. LJ: Conceptualization, Methodology, Supervision, Writing – review & editing. JV: Conceptualization, Funding acquisition, Methodology, Resources, Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Institutes of Health, National Institute of Neurological Disorders and Stroke (NINDS) R01-NS058945, R01-NS037019, R37-NS077657, R01NS117822, R01NS110613, P50-NS123109, MnDRIVE (Minnesota’s Discovery Research and Innovation Economy) Brain Conditions Program, and the Engdahl Family Foundation.
AcknowledgmentsWe would like to thank our colleagues in the Neuromodulation Research Center for helpful comments and critiques related to this study and especially thank our animal core team of Claudia Hendrix, Hannah Baker, Adele DeNicola, and Elizabeth McDuell as well as our veterinary and animal care colleagues at the University of Minnesota Research Animal Resources (RAR).
Conflict of interestJerrold L. Vitek serves as a consultant for Medtronic, Boston Scientific, and Abbott. He also serves on the Executive Advisory Board for Abbott and is a member of the scientific advisory board for Surgical Information Sciences.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ReferencesBankiewicz, K. S., Oldfield, E. H., Chiueh, C. C., Doppman, J. L., Jacobowitz, D. M., and Kopin, I. J. (1986). Hemiparkinsonism in monkeys after unilateral internal carotid artery infusion of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Life Sci. 39, 7–16. doi: 10.1016/0024-3205(86)90431-5
PubMed Abstract | Crossref Full Text | Google Scholar
Bissonette, G. B., Powell, E. M., and Roesch, M. R. (2013). Neural structures underlying set-shifting: roles of medial prefrontal cortex and anterior cingulate cortex. Behav. Brain Res. 250, 91–101. doi: 10.1016/j.bbr.2013.04.037
PubMed Abstract | Crossref Full Text | Google Scholar
Blesa, J., Juri, C., Collantes, M., Peñuelas, I., Prieto, E., Iglesias, E., et al. (2010). Progression of dopaminergic depletion in a model of MPTP-induced parkinsonism in non-human primates. An (18)F-DOPA and (11)C-DTBZ PET study. Neurobiol. Dis. 38, 456–463. doi: 10.1016/j.nbd.2010.03.006
PubMed Abstract | Crossref Full Text | Google Scholar
Bogerts, B., Häntsch, J., and Herzer, M. (1983). A morphometric study of the dopamine-containing cell groups in the mesencephalon of normals, Parkinson patients, and schizophrenics. Biol. Psychiatry 18, 951–969
PubMed Abstract | Google Scholar
Casey, B. J., Trainor, R. J., Orendi, J. L., Schubert, A. B., Nystrom, L. E., Giedd, J. N., et al. (1997). A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. J. Cogn. Neurosci. 9, 835–847. doi: 10.1162/jocn.1997.9.6.835
PubMed Abstract | Crossref Full Text | Google Scholar
Caspers, J., Mathys, C., Hoffstaedter, F., Südmeyer, M., Cieslik, E. C., Rubbert, C., et al. (2017). Differential functional connectivity alterations of two subdivisions within the right dlPFC in Parkinson’s disease. Front. Hum. Neurosci. 11:288. doi: 10.3389/fnhum.2017.00288
PubMed Abstract | Crossref Full Text | Google Scholar
Cools, R., and D’Esposito, M. (2011). Inverted-U shaped dopamine actions on human working memory and cognitive control. Biol. Psychiatry 69, e113–e125. doi: 10.1016/j.biopsych.2011.03.028
PubMed Abstract | Crossref Full Text | Google Scholar
Disbrow, E. A., Sigvardt, K. A., Franz, E. A., Turner, R. S., Russo, K. A., Hinkley, L. B., et al. (2013). Movement activation and inhibition in Parkinson’s disease: a functional imaging study. J. Parkinsons Dis. 3, 181–192. doi: 10.3233/JPD-130181
PubMed Abstract | Crossref Full Text | Google Scholar
Dujardin, K., Leentjens, A. F. G., Langlois, C., Moonen, A. J. H., Duits, A. A., Carette, A.-S., et al. (2013). The spectrum of cognitive disorders in Parkinson’s disease: A data-driven approach. Mov. Disord. 28, 183–189. doi: 10.1002/mds.25311
PubMed Abstract | Crossref Full Text | Google Scholar
Durstewitz, D., Seamans, J. K., and Sejnowski, T. J. (2000). Dopamine-mediated stabilization of delay-period activity in a network model of prefrontal cortex. J. Neurophysiol. 83, 1733–1750. doi: 10.1152/jn.2000.83.3.1733
PubMed Abstract | Crossref Full Text | Google Scholar
Eidelberg, E., Brooks, B. A., Morgan, W. W., Walden, J. G., and Kokemoor, R. H. (1986). Variability and functional recovery in the N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of parkinsonism in monkeys. Neuroscience 18, 817–822. doi: 10.1016/0306-4522(86)90102-8
PubMed Abstract | Crossref Full Text | Google Scholar
Elgh, E., Domellöf, M., Linder, J., Edström, M., Stenlund, H., and Forsgren, L. (2009). Cognitive function in early Parkinson’s disease: a population-based study. Eur. J. Neurol. 16, 1278–1284. doi: 10.1111/j.1468-1331.2009.02707.x
PubMed Abstract | Crossref Full Text | Google Scholar
Escobar Sanabria, D., Johnson, L. A., Nebeck, S. D., Zhang, J., Johnson, M. D., Baker, K. B., et al. (2017). Parkinsonism and vigilance: alteration in neural oscillatory activity and phase-amplitude coupling in the basal ganglia and motor cortex. J. Neurophysiol. 118, 2654–2669. doi: 10.1152/jn.00388.2017
PubMed Abstract | Crossref Full Text | Google Scholar
Franke, F., Quian Quiroga, R., Hierlemann, A., and Obermayer, K. (2015). Bayes optimal template matching for spike sorting – combining fisher discriminant analysis with optimal filtering. J. Comput. Neurosci. 38, 439–459. doi: 10.1007/s10827-015-0547-7
PubMed Abstract | Crossref Full Text | Google Scholar
Garavan, H., Ross, T. J., and Stein, E. A. (1999). Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc. Natl. Acad. Sci. USA 96, 8301–8306. doi: 10.1073/pnas.96.14.8301
PubMed Abstract | Crossref Full Text | Google Scholar
Hashimoto, T., Elder, C. M., Okun, M. S., Patrick, S. K., and Vitek, J. L. (2003). Stimulation of the subthalamic nucleus changes the firing pattern of Pallidal neurons. J. Neurosci. 23, 1916–1923. doi: 10.1523/JNEUROSCI.23-05-01916.2003
PubMed Abstract | Crossref Full Text | Google Scholar
Jahanshahi, M., Jenkins, I. H., Brown, R. G., Marsden, C. D., Passingham, R. E., and Brooks, D. J. (1995). Self-initiated versus externally triggered movements: I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson’s disease subjects. Brain 118, 913–933. doi: 10.1093/brain/118.4.913
PubMed Abstract | Crossref Full Text | Google Scholar
Johnson, L. A., Wang, J., Nebeck, S. D., Zhang, J., Johnson, M. D., and Vitek, J. L. (2020). Direct activation of primary motor cortex during subthalamic but not Pallidal deep brain stimulation. J. Neurosci. 40, 2166–2177. doi: 10.1523/JNEUROSCI.2480-19.2020
PubMed Abstract | Crossref Full Text | Google Scholar
Leh, S. E., Petrides, M., and Strafella, A. P. (2010). The neural circuitry of executive functions in healthy subjects and Parkinson’s disease. Neuropsychopharmacology 35, 70–85. doi: 10.1038/npp.2009.88
PubMed Abstract | Crossref Full Text | Google Scholar
Lewis, S. J. G., Dove, A., Robbins, T. W., Barker, R. A., and Owen, A. M. (2003). Cognitive impairments in early Parkinson’s disease are accompanied by reductions in activity in Frontostriatal neural circuitry. J. Neurosci. 23, 6351–6356. doi: 10.1523/JNEUROSCI.23-15-06351.2003
PubMed Abstract | Crossref Full Text | Google Scholar
Martin, J. A., Zimmermann, N., Scheef, L., Jankowski, J., Paus, S., Schild, H. H., et al. (2019). Disentangling motor planning and motor execution in unmedicated de novo Parkinson’s disease patients: an fMRI study. Neuroimage Clin 22:101784. doi: 10.1016/j.nicl.2019.101784
PubMed Abstract | Crossref Full Text | Google Scholar
Maynard, E. M., Nordhausen, C. T., and Normann, R. A. (1997). The Utah Intracortical electrode Array: A recording structure for potential brain-computer interfaces. Electroencephalogr. Clin. Neurophysiol. 102, 228–239. doi: 10.1016/S0013-4694(96)95176-0
PubMed Abstract | Crossref Full Text | Google Scholar
Menon, V., Adleman, N. E., White, C. D., Glover, G. H., and Reiss, A. L. (2001). Error-related brain activation during a go/NoGo response inhibition task. Hum. Brain Mapp. 12, 131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C
PubMed Abstract | Crossref Full Text | Google Scholar
Michely, J., Barbe, M. T., Hoffstaedter, F., Timmermann, L., Eickhoff, S. B., Fink, G. R., et al. (2012). Differential effects of dopaminergic medication on basic motor performance and executive functions in Parkinson’s disease. Neuropsychologia 50, 2506–2514. doi: 10.1016/j.neuropsychologia.2012.06.023
PubMed Abstract | Crossref Full Text | Google Scholar
Neumann, W.-J., Steiner, L. A., and Milosevic, L. (2023). Neurophysiological mechanisms of deep brain stimulation across spatiotemporal resolutions. Brain 146, 4456–4468. doi: 10.1093/brain/awad239
PubMed Abstract | Crossref Full Text | Google Scholar
Pagonabarraga, J., Kulisevsky, J., Strafella, A. P., and Krack, P. (2015). Apathy in Parkinson’s disease: clinical features, neural substrates, diagnosis, and treatment. Lancet Neurol. 14, 518–531. doi: 10.1016/S1474-4422(15)00019-8
PubMed Abstract | Crossref Full Text | Google Scholar
Pasquereau, B., DeLong, M. R., and Turner, R. S. (2016). Primary motor cortex of the parkinsonian monkey: altered encoding of active movement. Brain 139, 127–143. doi: 10.1093/brain/awv312
PubMed Abstract | Crossref Full Text | Google Scholar
Petrides, M., and Pandya, D. N. (1999). Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur. J. Neurosci. 11, 1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x
PubMed Abstract | Crossref Full Text | Google Scholar
Ranchet, M., Hoang, I., Cheminon, M., Derollepot, R., Devos, H., Perrey, S., et al. (2020). Changes in prefrontal cortical activity during walking and cognitive functions among patients with Parkinson’s disease. Front. Neurol. 11:601686. doi: 10.3389/fneur.2020.601686
PubMed Abstract | Crossref Full Text | Google Scholar
Rousche, P. J., and Normann, R. A. (1992). A method for pneumatically inserting an array of penetrating electrodes into cortical tissue. Ann. Biomed. Eng. 20, 413–422. doi: 10.1007/BF02368133
PubMed Abstract | Crossref Full Text | Google Scholar
Taylor, J. R., Elsworth, J. D., Roth, R. H., Sladek, J. R., and Redmond, D. E. (1997). Severe long-term 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in the vervet monkey (Cercopithecus aethiops sabaeus). Neuroscience 81, 745–755. doi: 10.1016/s0306-4522(97)00214-5
PubMed Abstract | Crossref Full Text | Google Scholar
Vitek, J. L., Zhang, J., Hashimoto, T., Russo, G. S., and Baker, K. B. (2012). External pallidal stimulation improves parkinsonian motor signs and modulates neuronal activity throughout the basal ganglia thalamic network. Exp. Neurol. 233, 581–586. doi: 10.1016/j.expneurol.2011.09.031
留言 (0)