Intracerebral hemorrhage (ICH) is a prevalent cerebrovascular disease associated with high mortality and disability rates. ICH constituted 27.9% of stroke occurrences in 2019 (Collaborators, 2021), and there were 10–30 cases of cerebral hemorrhage per 100,000 individuals globally (Qureshi et al., 2009). In low-income areas, the age-standardized stroke-related mortality rate is 3.6 times higher than that in high-income areas (Collaborators, 2021). Furthermore, from 1990 to 2019, there was a significant increase in the incidence of ICH (Collaborators, 2021); thus, ICH presents a considerable challenge as a serious global public health risk, especially in economically disadvantaged nations. In the past decade, an increasing number of intervention measures have been used to target early acute hematoma in ICH (Al-Kawaz et al., 2020). However, existing treatment for ICH is insufficient. Therefore, recent studies have focused on identifying the precise therapeutic targets and developing more effective alternative therapies for ICH.
HPL is a biomaterial rich in bioactive factors (Schallmoser et al., 2020). In recent years, the beneficial effects of HPL in the treatment of neurological diseases have become increasingly evident (Zhang et al., 2015; Brambilla et al., 2023). Furthermore, owing to the simplicity of HPL production and the ability of HPL to reduce unreasonable platelet waste, it holds great promise as a therapeutic, especially in low-income countries. Research on the use of HPL for the treatment of ICH is currently in the preclinical stage. However, the significant amount of experimental data on HPL in neurological diseases suggests that the transition to clinical trials is iminent (Nebie et al., 2022). This review investigated the relationship between platelet count and ICH pathogenesis. We reviewed the current state of research on the clinical application of ICH, highlighting their limitations, and describe the preclinical evidence supporting the use of HPL in the treatment of ICH. Moreover, we described the history of research on HPL and details of its production process. In addition, we describe the synergistic effects of bioactive molecules in HPL on ICH treatment. Finally, we illustrate the issues that need to be addressed to develop ICH as a therapeutic agent.
2 Search strategyA literature search using the PubMed and Web of Science databases was conducted, with the search scope ending in December 2024. The following keywords were used for both database searches: human platelet lysates, intracerebral hemorrhage, pathophysiology, brain, neuroinflammation, neuronal death, blood–brain barrier, quality, and safety. No filter conditions were applied during the search process.
A search for the keyword “human platelet lysate” in PubMed revealed 1,926 related papers as of November 2024, with the earliest document dating back to 1959. The number of studies on HPL began to significantly increase approximately by 2013, and numerous studies exist on the potential value of HPL in research on various diseases. Searches using the keywords “human platelet lysate” and “brain” revealed 62 relevant papers. The earliest preclinical study exploring the therapeutic potential of HPL in neurological diseases was published by Hayon et al. (2013). Since then, several preclinical studies have been published on the role of the HPL in various brain diseases, including Parkinson’s disease, traumatic brain injury, cerebral infarction, and spinal cord injury (SCI). However, to date, no randomized controlled clinical studies have been conducted. Additionally, when searching across all platforms using the keywords “platelet lysate” and “intracerebral hemorrhage,” we found only one recently published article that described the potential of HPL to treat secondary injuries in ICH (Qiu et al., 2024), indicating that more in-depth research is needed in this area.
3 Relationship between platelets and ICH pathophysiology 3.1 New perspectives on plateletsPlatelets are non-nucleated cells found in the blood and are essential for maintaining effective blood clotting within blood vessels. These cells contain various particles (Iyer and Dayal, 2020), such as alpha, dense, and lysosomal granules, which contain various bioactive molecules, including growth factors, cytokines, and enzymes (Nebie et al., 2022). The platelet surface is equipped with multiple receptors, including the glycoprotein Ib-IX-V and IIb-IIIa complexes. These receptors are essential for facilitating the attachment of platelets to blood vessel walls and promoting platelet clustering. Upon activation, platelets undergo a morphological transformation from a disk shape to an irregular form, accompanied by the release of granular contents, which promotes blood coagulation (Golebiewska and Poole, 2015; Holinstat, 2017). After injury to the blood vessels, platelets quickly attach to the wall of the damaged vessel, creating a plug made of platelets. Subsequently, the coagulation cascade is triggered by the release of various factors by platelets, ultimately creating stable fibrin clots.
Furthermore, platelets are vital for managing anticoagulant and fibrinolytic systems, ensuring proper blood fluidity and preventing excessive clotting (Golebiewska and Poole, 2015). However, recent studies have suggested that platelets could be classified as a novel type of immune cell. Platelets interact with immune cells through various mechanisms and participate in inflammatory responses. Additionally, platelets can release cytokines such as platelet factor 4 (PF4), which regulates immune cell activity (Golebiewska and Poole, 2015; Nicolai et al., 2024). Platelets are activated during inflammation to release bioactive factors that exert pro-inflammatory and anti-inflammatory effects. This bidirectional regulatory capacity allows platelets to assume complex roles in the inflammatory response. Consequently, platelets significantly contribute to many systemic diseases, particularly central nervous system disorders, such as ICH. They are not only involved in maintaining the integrity of the blood–brain barrier (BBB) but may also interact with other immune cells, influencing both inflammatory responses and neuroregeneration processes (Ebermeyer et al., 2021; Mao et al., 2022). It’s worth noting that a clinical study also demonstrated the predictive ability of platelets in the prognosis of ICH (Xu et al., 2024).
3.2 ICH pathophysiologyPrimary brain injury (PBI) occurs when mechanical damage to blood vessels causes blood to flow into the brain parenchyma, forming a hematoma that compresses the surrounding brain tissue. Mechanical compression leads to ischemia of blood vessels surrounding the hematoma, resulting in swelling of adjacent brain tissue and increased intracranial pressure (ICP) (Peng et al., 2019). This can be fatal if the hematoma compresses the brainstem (Wilkinson et al., 2018). The occurrence of hematoma is a dynamic process, with approximately 30% of patients experiencing hematoma growth after onset (Davis et al., 2006); therefore, preventing hematoma growth is crucial for preventing ICH (Veltkamp and Purrucker, 2017).
Secondary brain injury (SBI) occurs after primary injury, with microglial activation occurring minutes after ICH. Neutrophils are observed in the hematoma region at 4 h post-ICH and can peak within 3 days. These cells release pro-inflammatory factors, chemokines, and oxidative stress mediators that contribute to neuroinflammation, oxidative stress, and damage to the BBB (Mracsko and Veltkamp, 2014; Chen Y. et al., 2021; Magid-Bernstein et al., 2022). The products of red blood lysis can induce oxidative stress, thereby exacerbating SBI. These products include hemoglobin and Fe2+ (Figure 1) (Keep et al., 2014). Administering lysed red blood cells into the cerebral ventricles of rats results in quicker and more pronounced brain edema compared with the effects of administering concentrated red blood cells (Xi et al., 1998). Furthermore, administration of iron chelators can mitigate brain damage in animal models (Gu et al., 2009; Okauchi et al., 2010). Thrombin plays a crucial role in the development of SBI. While high concentrations of thrombin can lead to nerve damage in laboratory settings (Donovan et al., 1997), low concentrations possess neuroprotective properties (Vaughan et al., 1995). In addition, thrombin has been found to have the potential to promote neurogenesis (Yang et al., 2008).
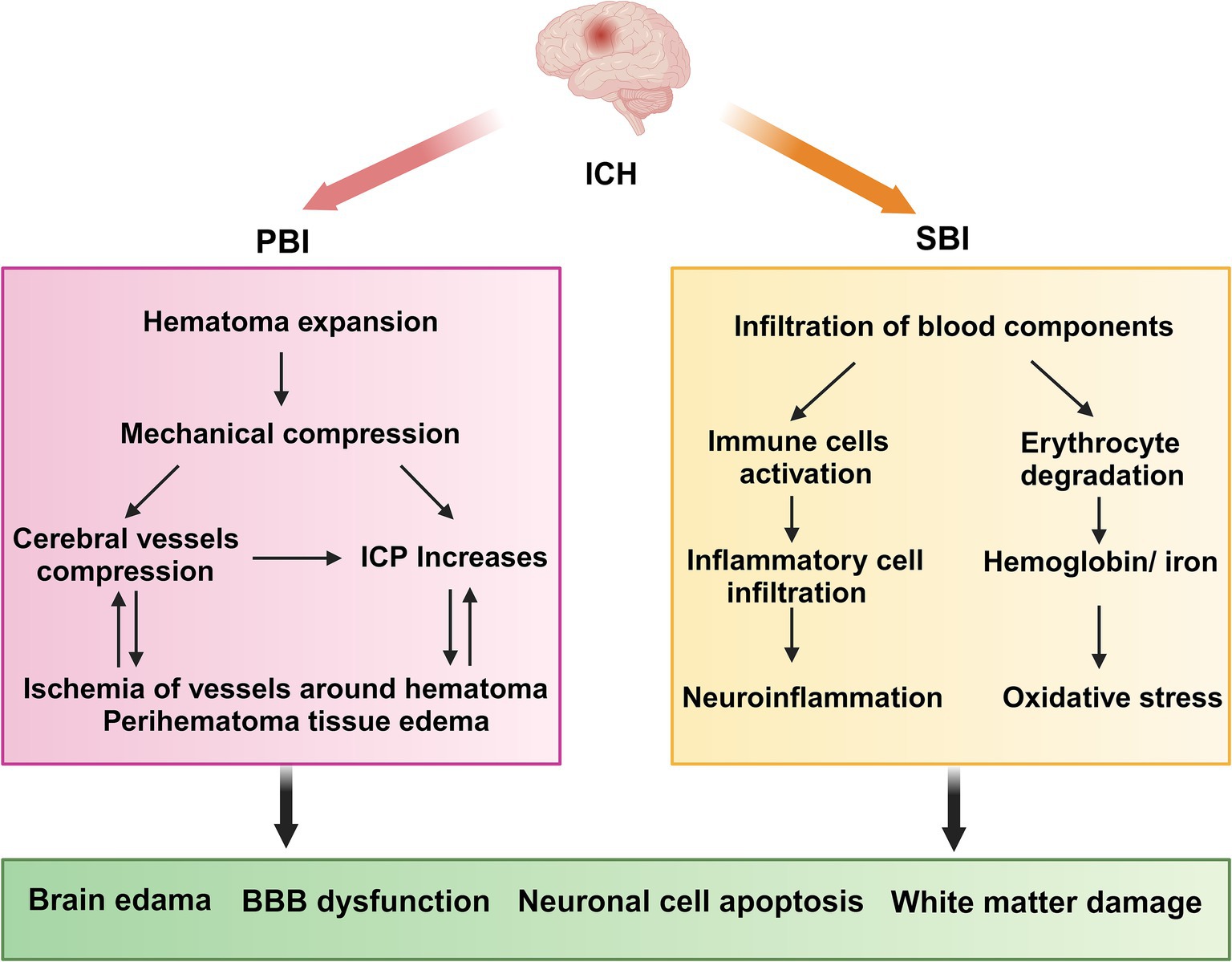
Figure 1. ICH pathophysiology. ICH, Intracerebral hemorrhage; PBI, primary brain injury; SBI, Secondary brain injury; ICP, intracranial pressure; BBB, blood–brain barrier. PBI: The growth of a hematoma causes compression of nearby blood vessels, which leads to ischemia and swelling in the adjacent brain tissue, along with a rise in intracranial pressure. SBI: neutrophils move into the hematoma region while resident immune cells, including microglia, activate and generate pro-inflammatory cytokines. Concurrently, erythrocytes from the hematoma are broken down, releasing hemoglobin and Fe2+. Collectively, these factors contribute to neuroinflammation and oxidative stress, which ultimately result in damage to the blood–brain barrier, exacerbating cerebral hematoma and inducing adjacent edema.
3.3 Role of platelets in ICH pathophysiologyPrevious reports have described the structural similarities between platelets and neuronal cells (Canobbio, 2019). The alpha granules in platelets, which store bioactive molecules, are analogous to the large dense-core vesicles found in neurons (Burnouf and Walker, 2022); the process through which platelets transmit signals bears a subtle resemblance to the transmission of signals at neuronal terminal synapses. In both cases, exocytosis is triggered by Ca2+ (Reed et al., 2000; Burnouf and Walker, 2022). Dense platelet granules are rich in neurotransmitters such as epinephrine, dopamine, glutamate, and gamma-aminobutyric acid (GABA) (Kaneez and Saeed, 2009; Burnouf and Walker, 2022).
Platelets play a crucial role in neuroimmune responses. They bind to the corresponding ligands on neutrophils via receptors on their surfaces, which promote the activation and aggregation of neutrophils, ultimately contributing to BBB damage and exacerbating neurological injury, as shown in detail in Figure 2 (Chou et al., 2023). A previous review highlighted an innovative function of platelets in immune responses, emphasizing their role in the activation, differentiation, and suppression of T cells such as Th1, Th17, and Treg cells. Thus, studying the relationship between platelets and T cells could serve as a paradigm for understanding the interaction between the immune and nervous systems (Ponomarev, 2018). Although the immune response serves as a defense mechanism, it can also damage the body. This protective effect is associated with bioactive factors that are stored in the alpha granules of platelets and are closely linked to the regeneration and repair processes. Recently, these bioactive factors have demonstrated positive effects in various animal disease models, including those of diseases involving the nervous system (Griffith et al., 2021; Nebie et al., 2021b; Nebie et al., 2022; Alhawari et al., 2023; Grzelak et al., 2024). These factors can enhance the neurotrophic properties of adipose stem cells to a greater extent than differentiated Schwann cells (Brambilla et al., 2023), improve wound healing, promote neuronal differentiation in vitro, and promote angiogenesis and neurogenesis, while exerting neuroprotective effects in rats following stroke (Hayon et al., 2013; Nebie et al., 2021a). Although several of the above-mentioned studies have revealed positive effects of the bioactive factors on neuroimmunity, the potential role of the HPL in ICH pathogenesis has not yet been elucidated. Further research in this area is required to address this gap.
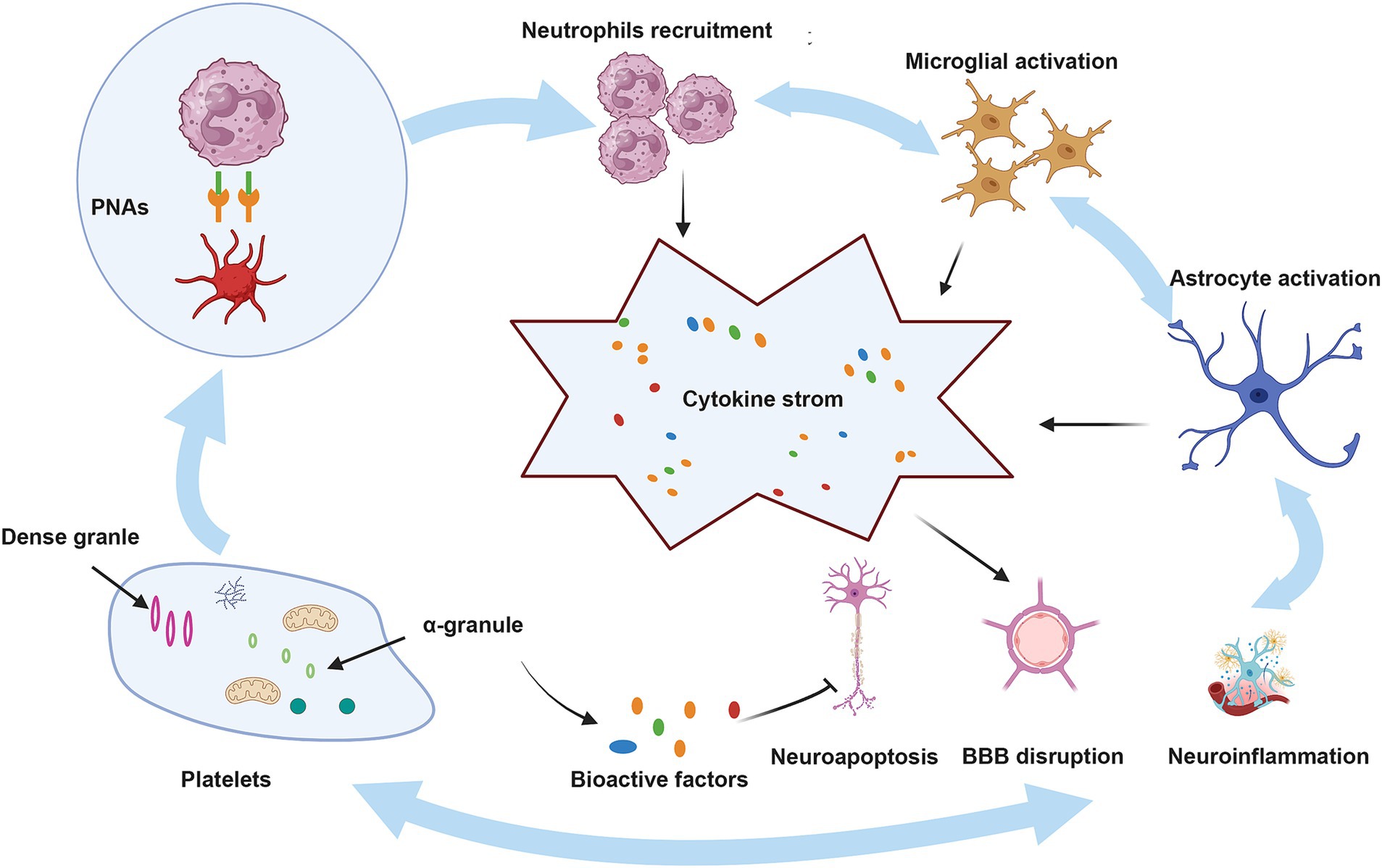
Figure 2. The role of platelets in ICH. BBB, blood–brain barrier; PNAs, platelet–neutrophil aggregates. Upon injury, platelets that have been activated attach to neutrophils through PSGL-1 and CD40, utilizing the surface receptors CD62P and CD40L, which leads to the development of PNAs. These aggregates enhance neutrophil function by releasing inflammatory mediators and cytokines. Moreover, neutrophils that are recruited, in conjunction with activated microglia and astrocytes, produce a range of pro-inflammatory substances, resulting in a cytokine storm that intensifies ICH. Conversely, bioactive factors present in the alpha granules of platelets may mitigate these pathological processes by reducing neuroinflammation, neuroapoptosis, and damage to the BBB.
4 Progress in ICH treatment research and its limitations 4.1 SurgeryIndividuals experiencing severe ICH generally receive urgent surgical intervention shortly following the occurrence of the injury. Surgical procedures may involve external ventricular drainage (EVD), craniotomy to remove hematomas, and less invasive surgical methods. Notably, over 40% of those with ICH are likely to experience intraventricular hemorrhage (IVH) (Hallevi et al., 2008). However, Infections caused by EVD are frequently fatal in neurologically critical patients, and the high incidence of this complication warrants significant attention (Zhou et al., 2023). Increased intracranial pressure resulting from the obstruction of cerebrospinal fluid flow due to IVH necessitates urgent EVD. In certain cases, combined thrombolysis may also be required. While craniotomy for hematoma extraction is the most thoroughly researched surgical technique at present, it continues to be a subject of debate. The earliest experimental findings indicate that craniotomy offers no substantial advantage regarding mortality rates (McKissock et al., 1961). Although prompt surgical intervention can critically preserve patients’ lives, existing studies do not show a considerable advantage of early surgery concerning long-term mortality and disability. This is mainly attributed to the risk of surgical complications, such as bleeding and infections (Mendelow et al., 2013). As a result, in craniotomy procedures requiring the extraction of substantial bone flaps and the exposure of broad sections of brain tissue, different surgical approaches have been established, such as minimally invasive techniques. In contrast to open surgery, minimally invasive methods can significantly lower the risks linked to hematoma extraction and surgical trauma. In 1989, Dr. Auer carried out the initial controlled study on minimally invasive surgery, showing that this method is beneficial for mortality rates as well as overall patient results (Auer et al., 1989), however, these findings were restricted to patients with subcortical hemorrhage. Recent studies have indicated that minimally invasive surgery does not provide advantages in long-term functional outcomes when compared to conservative treatments (de Oliveira Manoel, 2020).
4.2 Blood pressure control and reversal antiplatelet drugsBlood pressure is acknowledged as a crucial element in the worsening of acute ICH as the hematoma enlarges. An examination of findings from two extensive randomized controlled trials suggests that higher early variability in blood pressure correlates with less favorable functional outcomes (Chung et al., 2018; Divani et al., 2019; Moullaali et al., 2019). However, results from the large randomized controlled trial of the INTERACT2 study indicated that in patients with ICH, intensive blood pressure lowering did not significantly reduce the primary outcomes of mortality or severe disability (Anderson et al., 2013). The reversal of antiplatelet therapy is an essential element in cases of treatment for ICH. Currently, ICH related to antiplatelets represents a considerable challenge in medical treatment. A frequently utilized approach for reversing antiplatelet therapy is platelet transfusion; nonetheless, this procedure may worsen thrombotic events. As of now, there are no dedicated medications specifically designed to counteract the impacts of antiplatelet therapy. While not commonly used in medical practice, idarucizumab has demonstrated efficacy in reversing anticoagulant actions in a major study involving individuals with gastrointestinal bleeding (Pollack et al., 2017). Multiple studies have shown that andexanet-α exhibits significant hemostatic effects (Connolly et al., 2016; Connolly et al., 2019). However, approximately 10% of all trial subjects experienced thrombotic events (Siegal et al., 2015). A significant randomized study involving the antifibrinolytic medication tranexamic acid (TXA) showed a decrease in early mortality rates among individuals with ICH treated with TXA. Nevertheless, it did not indicate a meaningful effect on functional outcomes at 90 days or on overall mortality rates (Sprigg et al., 2018).
4.3 Targeting neuroinflammationInvestigating critical components of the inflammatory pathway is presently regarded as an encouraging therapeutic approach to suppress neuroinflammation after ICH. The activation of microglia and macrophages is vital in numerous inflammatory processes, playing a key part in the SBI and acting as a major source of chemokines and inflammatory factors (Gang et al., 2018), Modulating microglia and macrophages to decrease the ratio of M1 /M2 polarized cells is currently a prominent focus in the research and treatment of ICH. In mice with ICH, it has been demonstrated that Irisin reduces M1 macrophage levels while promoting an increase in M2 macrophages, thus exhibiting an anti-neuroinflammatory effect. Furthermore, a different preclinical investigation revealed that a Dectin-1 inhibitor can facilitate the conversion of M1 macrophages to M2 macrophages (Fu et al., 2021; Wang et al., 2022). While many research efforts have illustrated the capability of these agents to suppress neuroinflammation, most have not advanced to the stage of clinical trials. Earlier studies indicated that antagonists of TNF-α can decrease PHE and improve neurological results (King et al., 2013). Nevertheless, there is a significant lack of clinical studies examining the effectiveness of TNF-α antagonists for addressing ICH.
4.4 Targeting oxidative stressCurrently, there are no specific therapies targeting oxidative stress that are widely implemented in the clinical treatment of ICH. After ICH occurs, the infiltration of blood and the breakdown of red blood cells are major contributors to oxidative stress injury and are intricately linked to ferroptosis. An earlier investigation demonstrates that iron chelators can alleviate the detrimental impacts of hemoglobin and Fe2+ in relation to ICH (Wu et al., 2012). Ceruloplasmin (CP) has been shown to reduce brain injury by promoting the transformation of Fe2+ into Fe3+ (Chen et al., 2022). GPX has emerged as a central inhibitory factor of ferroptosis in recent years, capable of reducing oxidative stress by inhibiting ferroptosis. Therefore, GPX mimics is a promising strategy for mitigating oxidative stress. Ebselen, a well-known GPX mimic, has demonstrated its antioxidant potential in several clinical trials (Yamaguchi et al., 1998; Ogawa et al., 1999). Employing Nrf2 activators is also seen as a method to boost antioxidant capacity and reduce damage caused by oxidative stress after ICH. Recombinant C1q/TNF-related protein 9 (rCTRP9) has the ability to stimulate the AdipoR1/APPL1/AMPK/Nrf2 signaling pathway, which helps decrease neurological impairment in a mouse model of ICH (Zhao et al., 2021). The research shows that albumin reduces oxidative stress injury in a rat model of cerebral hemorrhage by activating the ERK/Nrf2/HO-1 signaling pathway (Deng et al., 2021).
4.5 Targeted peripheral hematoma edemaAt present, no formal guidelines exist for the management and treatment of peripheral hematoma edema (PHE). Several factors limit research on treatment approaches, such as the expansion of hematomas and the different techniques employed to assess PHE (Al-Kawaz et al., 2020). Hypertonic treatments, including mannitol and hypertonic saline, are extensively employed in clinical settings. Although the short-term effects of these therapies have been confirmed, their impact on long-term neurological results is still unclear (Wang et al., 2015). Studies have shown that sulfonylureas can reduce PHE after ICH (Irvine et al., 2019). Additionally, there is a study investigating the role of glyburide in the treatment of PHE in ICH (GATE-ICH, NCT03741530).
The intricacies of ICH pathophysiology are multifaceted, and we still do not fully comprehend certain underlying mechanisms. This leads to considerable difficulties in the treatment of ICH. Surgical methods have not demonstrated improvements in long-term results, and pharmacological therapies lack specificity. Consequently, the available treatment options for ICH are restricted.
In comparison to traditional therapies, (1) HPL could enhance nerve regeneration. Research indicates that HPL has the ability to stimulate the growth of endogenous neural stem cells, aiding in nerve regeneration and repair (Nebie et al., 2019). Conversely, conventional treatments typically prioritize symptom management rather than actively facilitating nerve regeneration. (2) Enhance cognitive abilities. HPL might positively influence cognitive function, representing a significant benefit during recovery following intracerebral hemorrhage (Nebie et al., 2021b). (3) Being a biological agent, HPL could present a reduced likelihood of adverse effects. Traditional therapies, particularly surgical approaches, often involve a higher potential for complications, including infection or postoperative hemorrhage. (4) HPL is easy to utilize. The process of preparing and administering HPL is generally straightforward and applicable in various contexts, whereas traditional treatments frequently necessitate more sophisticated medical infrastructure and expertise. (5) Reduced economic burden. While there may be some costs associated with the preparation and administration of HPL, it might offer a more economical alternative when compared to high-risk surgical procedures and prolonged medication regimens (Nebie et al., 2022). Considering the constraints of conventional treatment approaches for ICH, an increasing number of investigations are exploring innovative methods for its management, leading to significant advancements. Dr. Xu and his team developed a novel hydrogel substance to address ICH, reporting a strategy that integrates neuroprotection with the stimulation of intrinsic nerve regeneration, showcasing considerable therapeutic promise (Wang Y. et al., 2024; Zhang et al., 2024; Xu et al., 2025). Notably, HPL has also indirectly shown neuroprotective and neuroregenerative properties across various studies.
5 Historical highlights and preparation of HPL 5.1 History of HPL in biomedicineHPL is currently defined internationally as a biological material rich in protein and growth factors and free of cells (Schallmoser et al., 2020). The development of HPL is strongly connected to research on platelet-rich plasma (PRP), especially because of its significant cytokines content. In the 1980s and 1990s, researchers identified multiple growth factors derived from platelets and explored their possible functions in healing wounds and regenerating tissue (Lynch et al., 1987; Pierce et al., 1989; Pierce et al., 1991; Hosgood, 1993). In 1983, Cowan employed the freeze–thaw method to prepare PL to investigate its effects on tumors (Cowan et al., 1983). In 2005, Doucet was the first to utilize HPL as a substitute for fetal bovine serum (FBS) to promote mesenchymal stem cell growth, revealing its significant potential for stem cell cultivation (Doucet et al., 2005). This discovery is of great significance, as it indicates that cell therapy applied to humans can eliminate the need for animal-derived biological materials in culture. This advancement reduces the risk of transmission of zoonotic infections and minimizes immunogenicity. Nowadays, HPL is employed as a substitute for FBS in the media used for cell cultures across different cell types, reducing the likelihood of immune reactions and infections linked to biological materials derived from bovine sources (Burnouf et al., 2016; Schallmoser et al., 2020; Oeller et al., 2021). Investigations into utilizing HPL as an alternative to FBS in the cultivation of different stem cells for treating clinical diseases are consistently on the rise. A systematic review by Palombella demonstrated that, compared to FBS, HPL can enhance the proliferation of adipose stem cells (ASCs) and bone marrow stem cells (BMSCs) by increasing the doubling rate and reducing the doubling time (Palombella et al., 2022). The study by Ballesteros further investigated the effects of varying concentrations of HPL on the proliferation and phenotypic marker expression of ASCs. The findings indicated that higher concentrations of HPL can enhance adipogenesis and osteogenesis while concurrently reducing the expression of the chemotactic factor receptors CXCR2 and CXCR3 (Ballesteros et al., 2020). This shows that by adjusting the HPL concentration, stem cells’ biological characteristics can be regulated to a certain extent, providing the possibility of directional differentiation of stem cells. Based on the application of HPL in cell therapy, neuroscientists have increasingly focused on neurological diseases. Recent studies have demonstrated that Schwann cells differentiated from adipose stem cells (SC-ASCs) can produce abundant neurotrophic factors, indicating their potential utility in the treatment of peripheral nerve injury (PNI). However, challenges remain in the clinical translation of this cell therapy. Brambilla found that ASCs cultured with HPL produce a greater quantity of neurotrophic factors than SC-ASCs cultured under the same conditions. This solves the problems in the clinical translation process of SC-ASCs cell therapy (Brambilla et al., 2023). Other similar studies have demonstrated the significant potential of HPL in innovative cell therapies for the treatment of neurological diseases (Tan et al., 2016; Palombella et al., 2020). The aforementioned studies illustrate the capacity of HPL to address neurological diseases through cell therapy indirectly.
5.2 Preparation of HPLHPL is mainly extracted from platelet concentrate (PC). The platelet content of PC is four to five times that of circulating blood (Schallmoser et al., 2020). Although PC is not difficult to obtain, the preproduction of raw materials for HPL must meet strict standards. (1) The collection, testing, storage, and transportation of raw plasma should comply with regulations, and the quality and legality of the source of raw plasma must be ensured. Raw plasma may contain pathogens associated with blood-borne diseases, such as HIV, HBV, and HCV (Burnouf et al., 2019). (2) Production facilities must obtain appropriate licensing permits (Schallmoser et al., 2020). (3) The production process must be strictly controlled, particularly the removal and/or inactivation of viruses (Bieback et al., 2019). (4) A tracking system must be established from the source of blood collection to the end of production to enable possible problems to be traced (Strunk et al., 2018b).
Moreover, as the starting material for the production of HPL, PC should have the following qualities (Schallmoser et al., 2020): (1) test negative for infectious diseases, viruses, bacteria, and fungi; (2) pH > 6.4 at the end of the shelf life; (3) at least 0.6 × 1011 platelets per 40 mL; and (4) a number of platelets per unit >2 × 1011 and a number of white blood cells <1 × 106.
Different methods of obtaining PCs may affect the variability of HPL fractions, and several previous studies have reported a reduction in the variability of bioactive factors in PCs from different sources by pooling PCs (Horn et al., 2010; Lohmann et al., 2012). Currently, there is no consensus on the standard size of PCs produced by the Good Manufacturing Practice (GMP) HPL production method, which has been adopted worldwide, with reported sizes ranging from 4 to 125 PC units (Strunk et al., 2018a). Some publications use 10–15 PC units, which is equivalent to the amount of plasma donated by 40–50 people (Schallmoser et al., 2007; Bieback et al., 2009). Thus, 10–15 PC units is the best quantity to reduce variability. However, according to the recommendations of the European Pharmacopeia, the number of PCs should be limited to reduce the risk of infection (Schallmoser et al., 2020).
HPL production typically involves physical and biochemical methods. The most prevalent technique is the freeze–thaw method (74%), followed by the use of biochemical agents to activate platelets (13%), ultrasound (8%), and solvent detergent (2%) (Schallmoser et al., 2020). The freeze–thaw method involves placing platelet-rich plasma at −80°C overnight, then incubating it at 37°C, repeating the process three times, and obtaining a protein-rich solution through repeated centrifugation and filtration (Astori et al., 2016). Ultrasound at a frequency of 20 kHz can also be used to prepare HPL. After 30 min of ultrasonic lysis, 74% of PDGF-AB is released from the platelet granules (Bernardi et al., 2013). Table 1 summarizes the details of the various preparation methods for HPL. The preparation process for HPL is simple, and the primary raw materials required are easy to obtain. This can also prevent PC waste owing to its short shelf life. As a low-cost blood product, HPL holds great medical value. However, strict and unified standards for acquiring and producing raw materials must be established.
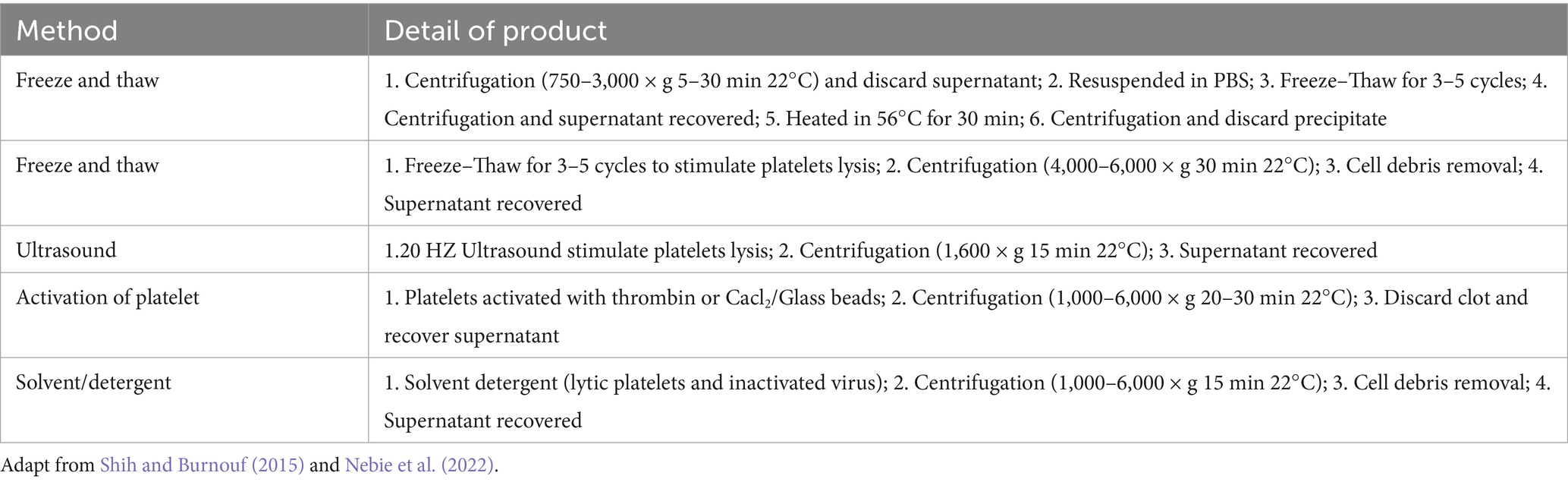
Table 1. Details of different production methods of HPL.
5.3 Therapeutic potential of bioactive factors in HPLDetected by Elisa and multiplex assays, HPL is abundant in bioactive factors, such as growth factors like brain-derived neurotrophic factor (BDNF), platelet-derived growth factor (PDGF), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), transforming growth factor-β (TGF-β), insulin-like growth factor (IGF), insulin-like growth factor-1 (IGF-1), fibroblast growth factor (FGF), angiopoietin-1, and IGF-binding protein 3 (IGF-BP3) (Astori et al., 2016; Viau et al., 2019; Oeller et al., 2021). Additionally, HPL also contains various chemokines, interleukins, and antioxidants (Nebie et al., 2022). Such as platelet factor 4 (PF4), CC-chemokine ligand 3 (CCL3), macrophage inflammatory protein (MIP-1), macrophage migration inhibitory factor (MIF), interleukin-4 (IL-4), interleukin-10 (IL-10), catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPX), these bioactive factors not only aid in wound repair and vascular regeneration but also contribute to combating neuroinflammation, oxidative stress, and reducing cell apoptosis (Hassamal, 2023). Table 2 summarizes the bioactive factors present in HPL and their active roles in ICH based on previously reported platelet components (Viau et al., 2019).
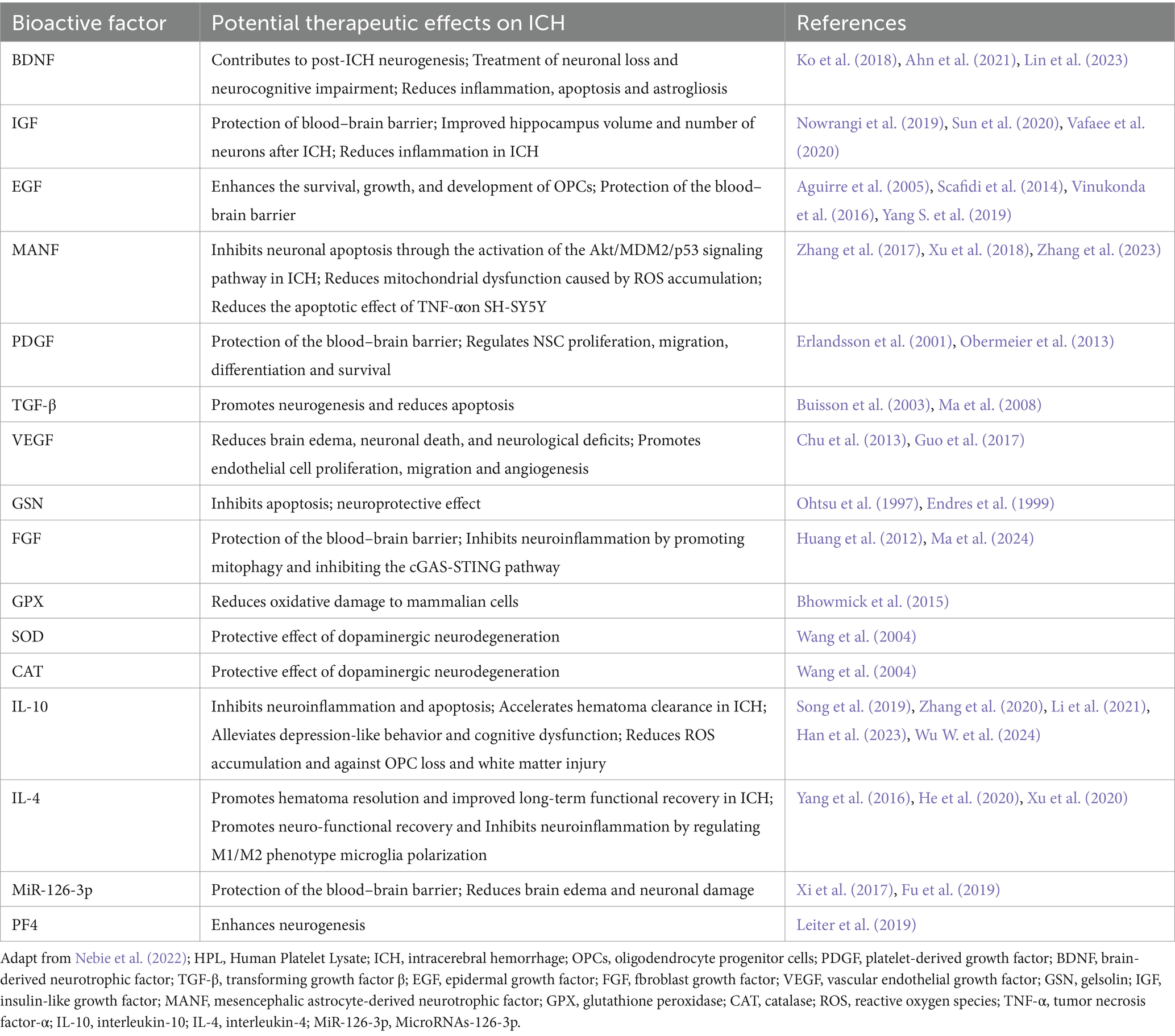
Table 2. Bioactive factors contained in HPL and their potential therapeutic effects in ICH.
Several key factors among these bioactive components, such as BDNF, TGF-β, and IL-10, have been the subject of extensive investigation concerning their involvement in ICH. Notably, BDNF is widely acknowledged as a neurotrophic factor. Earlier studies have shown that BDNF is capable of stimulating the downstream PI3K/Akt signaling pathway via TrkB receptor activation, which in turn boosts the anti-apoptotic potential of neurons and diminishes secondary neuronal damage after cerebral hemorrhage (Hasegawa et al., 2020). Insufficient levels of BDNF result in decreased interactions among endothelial cells, potentially leading to ICH. An adequate supply of BDNF helps sustain endothelial cell stability, fosters cell-to-cell interactions, preserves the blood–brain barrier’s integrity, and consequently lowers the likelihood of bleeding (Donovan et al., 2000). BDNF is thought to be involved in the modulation of neuronal energy metabolism, which in turn boosts cellular resilience during ischemic or hemorrhagic occurrences. By augmenting the energy status within cells, BDNF allows neurons to more effectively endure different stress scenarios (Schäbitz et al., 2007). The external administration of BDNF could potentially enhance neurological recovery following a cerebral hemorrhage. Studies have demonstrated that BDNF administration results in notable neuroprotective effects and functional enhancements (Han et al., 2011; Lin et al., 2023).
Previous publications reported that the expression of TGF-β is significantly increased in the pathological state of ICH (Yang H. et al., 2019). According to Zhang et al., an increase in TGF-β following cerebral hemorrhage might play a role in the repair and regeneration processes of neurons and glial cells. TGF-β aids in the development of scar tissue and restricts the spread of the damaged region by enhancing glial cell proliferation and facilitating matrix remodeling (Zhang and Yang, 2020). Moreover, TGF-β is significant in modulating microglial activation and inflammatory responses, which are vital for managing neuroinflammation under pathological circumstances (Li Y. et al., 2017). TGF-β signaling can suppress heightened inflammatory responses, thereby safeguarding neurons. Additionally, TGF-β participates in the modulation of angiogenesis within the brain, which is crucial for the repair of injured tissue and enhancement of blood flow. It facilitates the development of new blood vessels by influencing the activities of endothelial cells (Zhang and Yang, 2020).
The possible involvement of IL-10 in neurological disorders is evidenced by its ability to inhibit neuroinflammation. During the recovery phase following intracerebral hemorrhage, the levels of IL-10 may further rise. This prolonged expression aids in both the resolution of inflammation and the restoration of injured tissue. Research indicates that the delivery of recombinant IL-10 enhances the long-term outcomes in mice deficient in IL-10, highlighting the crucial function of IL-10 in the recovery process (Han et al., 2023). Mice deficient in IL-10 demonstrate greater pathological injury following intracerebral hemorrhage, characterized by heightened brain edema and damage to white matter. In these mice, the phagocytic functions of macrophages and microglia were diminished, leading to a slower removal of hematoma, which in turn exacerbated tissue impairment (Li et al., 2021). Additionally, variations in IL-10 expression are significantly linked to other cytokines. For instance, the elevated levels of IL-17A following a stroke are associated with reduced IL-10, and the suppression of IL-17A may enhance the outcomes in mice lacking IL-10 (Piepke et al., 2021). This indicates that IL-10 not only contributes to its own anti-inflammatory properties but also interacts with other pro-inflammatory agents to collectively modulate the inflammatory response following cerebral hemorrhage.
5.4 Preclinical evidence of HPL therapy for ICHFurthermore, additional research has provided evidence that HPL directly influences areas related to neurological diseases for therapeutic purposes (Figure 3). An investigation into the intracerebroventricular delivery of HPL using a rat model characterized by middle cerebral artery occlusion marks the initial report validating the neuroprotective benefits of HPL in neurological disorders. Findings revealed that HPL was effectively administered to the lateral ventricle, facilitating angiogenesis, neurogenesis, and neuroprotection and leading to a decrease in behavioral impairments after cerebral ischemia (Hayon et al., 2013). Figure 4 shows the efficacy of HPL in mitigating ischemia–reperfusion injury in adult rats (Zhang et al., 2015). This research confirmed the neuroprotective properties of HPL, demonstrating its capability to decrease infarct volume and neurological impairments in experimental ischemic stroke. This effect may stem from the anti-apoptotic and anti-inflammatory properties of the growth factors present in HPL, as well as its potential to enhance vascular development and nerve regeneration. The benefits of HPL include: in contrast to individual exogenous neurotrophic or growth factors, the growth factors found in HPL are present in their natural forms and might exhibit synergistic effects. Furthermore, HPL possesses autologous qualities, minimizes the risk of infection or immune reactions, and is more cost-effective.
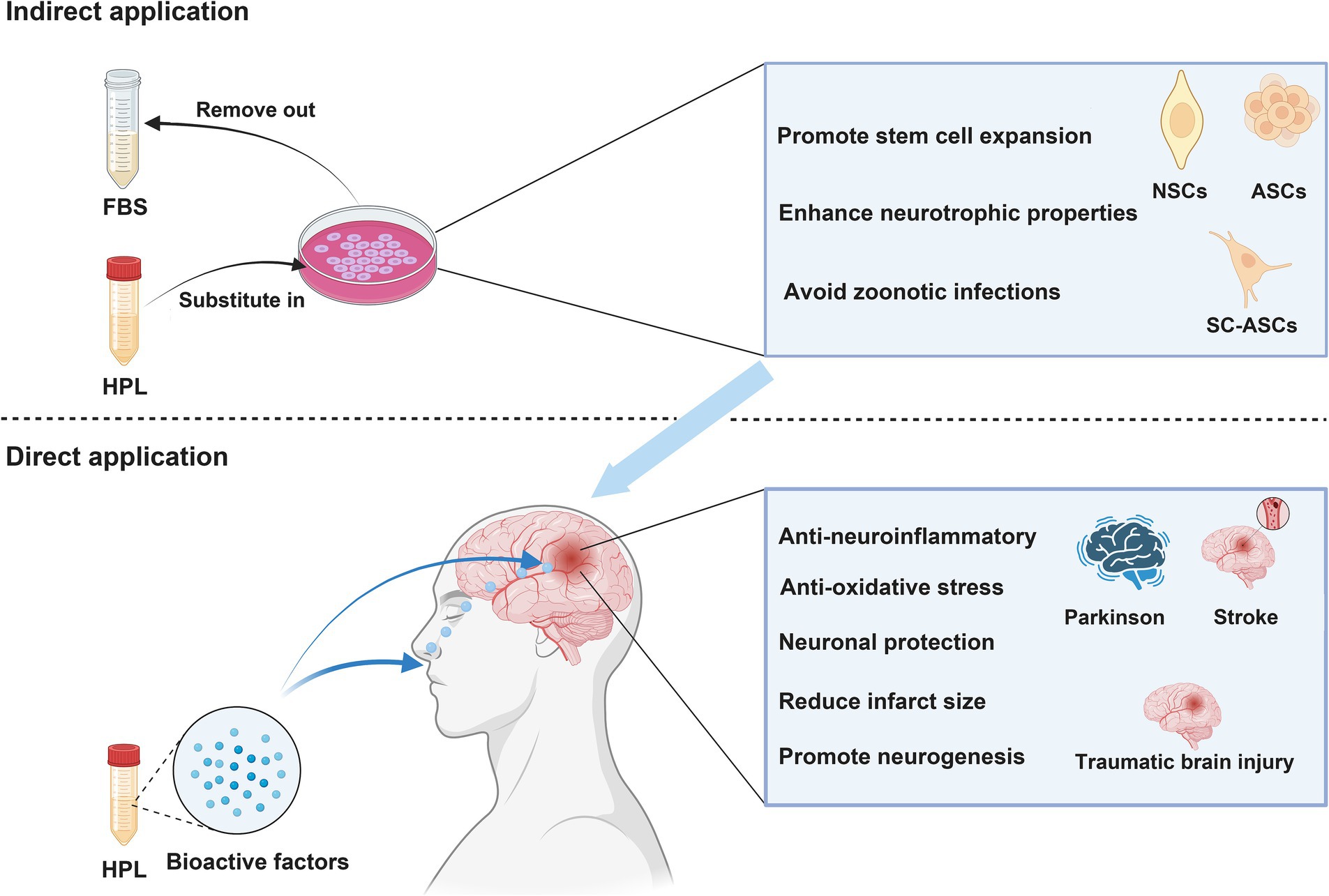
Figure 3. NSCs, neural stem cells; ASCs, adipose-derived stem cells; BMSCs, Bone marrow mesenchymal stromal cells. Indirect application: HPL increases the efficiency of cell therapy in treating neurological disease by culturing various types of stem cells. Direct application: HPL allows bioactive factors to directly reach the injured site and exert therapeutic effects through intranasal or targeted administration.
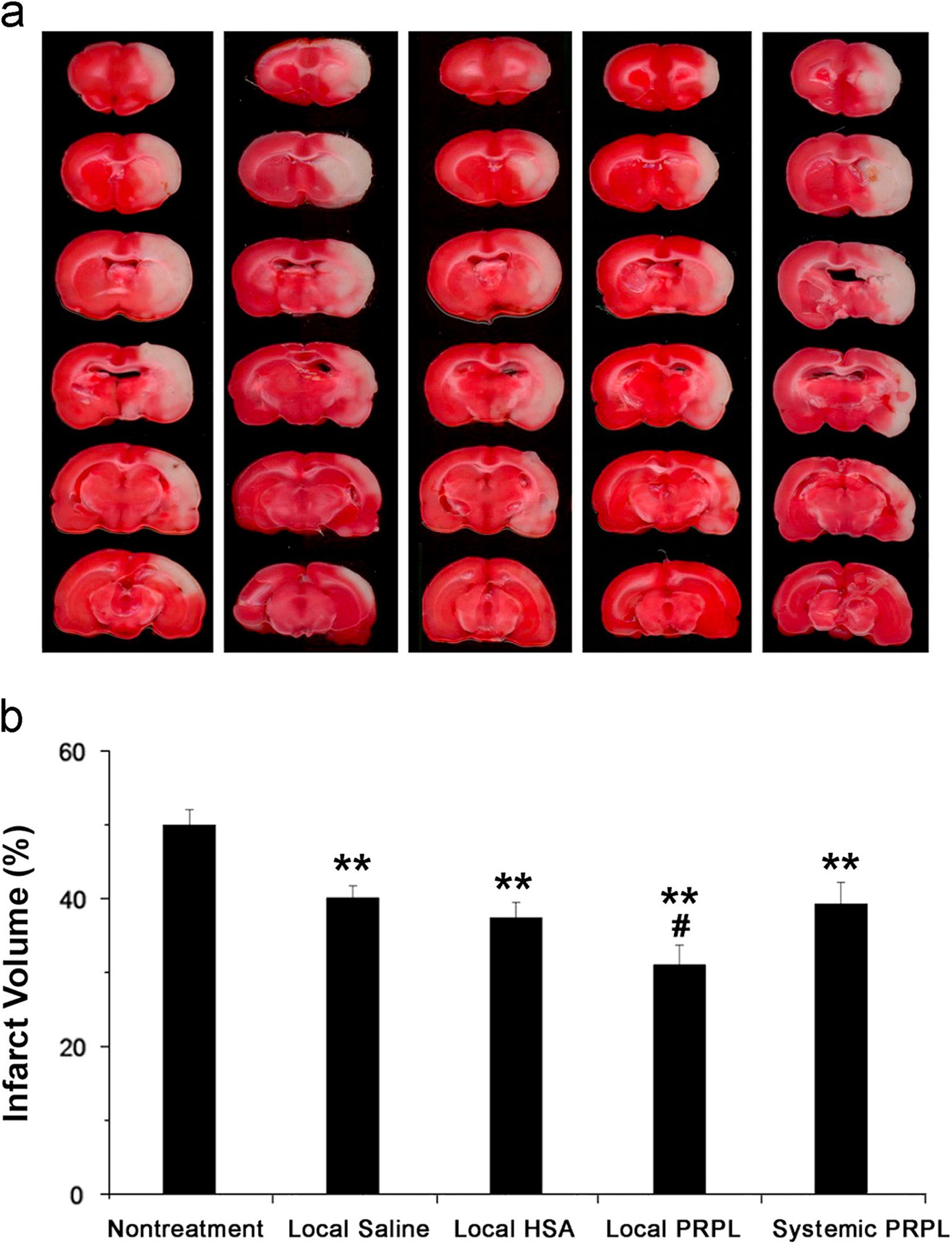
Figure 4. (A) Brain slices obtained from representatives stained with TTC 24 h post-reperfusion in cases of stroke, both with and without intervention. (B) Assessment of infarct volumes quantitatively across various groups shows that the infusion of local human PRP lysate markedly decreases infarct volume in comparison to the group with no treatment for stroke (**p < 0.01). Additionally, a further decrease is observed when this group is compared to the other treatment groups (#p < 0.05). The data is cited from a previous research (Zhang et al., 2015), this citation is permitted by the journal (License Number 5886510366832).
Nebie et al. employed HPL to deliver therapy to mice with traumatic brain injury (TBI) through intranasal administration. The group receiving treatment demonstrated enhancements in cognitive abilities and motor functions relative to the control group, alongside decreased levels of cortical neuroinflammation and oxidative stress (Nebie et al., 2021b). This research presents compelling support for HPL as a possible intervention for TBI. The investigators not only confirmed the functional efficacy of HPL in an in vitro setting, but they also assessed its therapeutic impact in two distinct in vivo systems, which enhanced the credibility of the findings. Additionally, the study indicated that HPL may operate through various mechanisms, such as enhancing neurotrophic factors, reducing inflammation, providing antioxidant protection, and facilitating neural repair. However, the dosages and methods of administration of HPL utilized in the experiments were derived from mouse studies, and these might not be directly relevant to human conditions. Furthermore, the investigation was solely reliant on animal studies and in vitro analyzes, lacking direct data from human clinical trials. Clinical trials involving humans are essential to confirm the safety and effectiveness of HPL. These aspects require further exploration during the translational medicine process.
In vitro studies have demonstrated that HPL provides a robust protective effect on neuronal cells subjected to ferroptosis inducers (Nebie et al., 2019). Additionally, in an Amyotrophic Lateral Sclerosis (ALS) mouse model, the administration of HPL through intracerebroventricular delivery and intranasal routes has been shown to extend the lifespan of the mice (Gouel et al., 2022). A 10-fold concentration of HPL, combined with heat treatment at 56°C, resulted in the diffusion of neurotrophic factors in the striatum following intranasal administration to mice with Parkinson’s disease, demonstrating neuroprotective effects (Chou et al., 2017). Anitua et al. evaluated the therapeutic effect of a specific human platelet lysate (HPL) enriched in growth factor plasma (PRGF-Endoret) in a mouse model of Alzheimer’s disease, The intranasal application of PRGF-Endoret in APP/PS1 mice showed a notable decrease in the accumulation of Aβ in the brain and a reduction in tau phosphorylation. Moreover, APP/PS1 mice treated with PRGF-Endoret demonstrated less astrocyte reactivity and a safeguarding effect against synaptic protein loss. In vitro experiments suggest that treatment with PRGF-Endoret influences astrocyte activation diminishes inflammatory responses, and enhances Aβ degradation. Furthermore, PRGF-Endoret administration resulted in significant improvements in behaviors related to anxiety, learning, and memory (Anitua et al., 2014). In the rat model of cerebral infarction, HPL has demonstrated significant therapeutic potential. Systemic infusion of HPL can reduce the size of cerebral infarction and enhance neurological scores in mice, particularly in the surrounding area of the lesion. Notably, local arterial administration exhibits a more pronounced therapeutic effect on cerebral infarction (Zhang et al., 2015). Additionally, HPL is utilized as a culture supplement for SH-SY5Y and BV-2 microglia cultures to assess cytotoxicity, evaluate inflammatory responses, and determine the capacity to stimulate wound healing, HPL modulates microglial activity while promoting both wound healing and neuronal differentiation (Nebie et al., 2021a). Another study evaluated the toxicity and neuroprotective activity of HPL on LUHMES cells and primary cortical/hippocampal neurons. The results indicated that HPL enhanced the expression of tyrosine hydroxylase and neuron-specific enolase in LUHMES cells, while it did not significantly affect synaptic protein expression in primary neurons. Furthermore, the tested virus-inactivated platelet lysates demonstrated potent neuroprotective effects on LUHMES and primary neurons exposed to erastin, a known inducer of ferroptotic cell death (Nebie et al., 2019). HPL demonstrates notable neuroprotective properties in cell-based models of Parkinson’s disease and amyotrophic lateral sclerosis, specifically in LUHMES and NSC-34 cell lines, respectively. It exhibits resistance to certain cell death pathways, including apoptosis and ferroptosis, as well as to specific oxidative stress inducers such as 1-methyl-4-phenylpyridine (MPP+) and menadione. The protective effects of HPL may be associated with the activation of the AKT pathway and the involvement of the MKE pathway (Gouel et al., 2017). A recent investigation thoroughly showcased the therapeutic capabilities of HPL in ICH, highlighting its role in decreasing neuroinflammation, mitigating oxidative stress, and offering neuronal protection (Qiu et al., 2024). Nevertheless, this research does not provide insights into the underlying mechanisms responsible for these effects. Consequently, future research avenues in clinical translation will concentrate on understanding the mechanisms by which these therapeutic effects manifest. More information is detailed in Table 3.
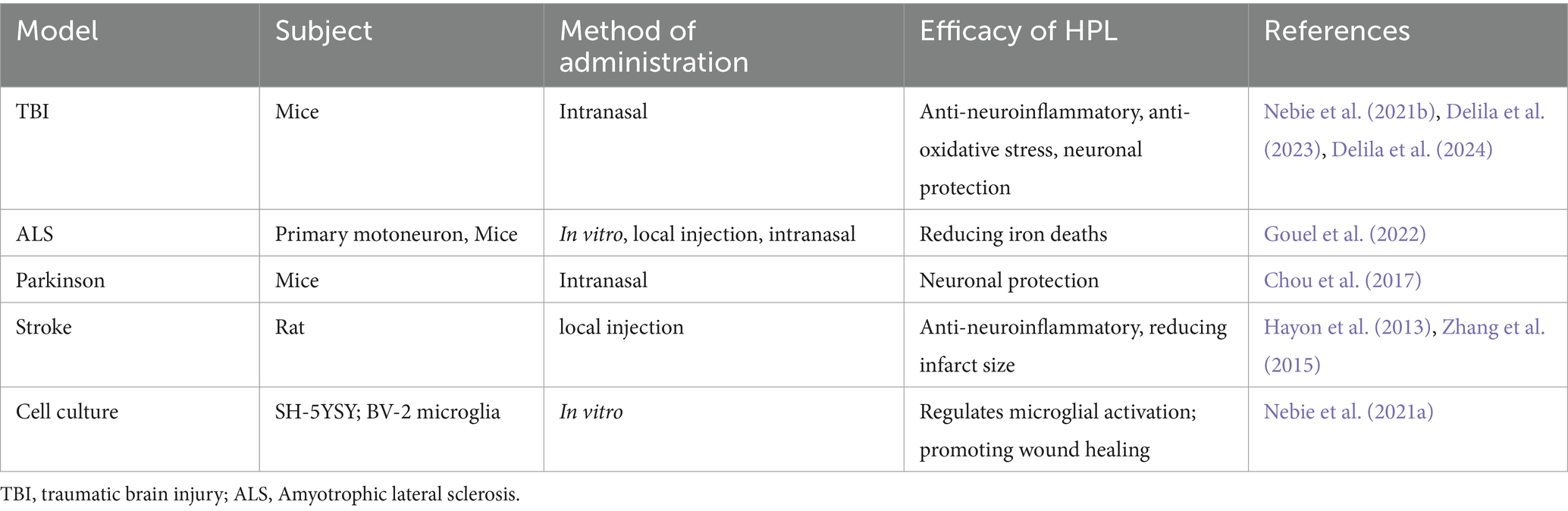
Table 3. HPL directly targets lesions to treat neurological diseases.
6 Synergistic effect of bioactive molecules in HPL in the treatment of ICH 6.1 NeuroinflammationNeuroinflammation is frequently associated with both local and systemic damage. As inflammation propagates, it can initiate a positive feedback damage cascade. HPL contains numerous anti-inflammatory bioactive factors, including TIMP-1, IL-4, and miR-126-3p. Research has demonstrated that these factors exert synergistic effects on anti-neuroinflammation through various mechanisms (Wu et al., 2023). In recent years, the bioactive molecule tissue inhibitor of metalloproteinase TIMP-1 has been widely recognized for its inhibitory effect on inflammation. Furthermore, the imbalance between matrix metalloproteinases (MMPs) and TIMP-1 is thought to be associated with various neuroinflammatory diseases (Gardner and Ghorpade, 2003). Previous reports have indicated that neuroinflammatory diseases are associated with a reduction in TIMP-1 expression and an increase in MMP levels (Gardner et al., 2006). Current research suggests that TIMP-1 can inhibit MMP and exert anti-neuroinflammatory effects by binding to cell surface receptors (Thevenard et al., 2014; Knight et al., 2019; Nicaise et al., 2019). However, there are currently no studies reporting that HPL can increase the level of TIMP-1 in the body after ICH in some way. This is still a direction that we need to delve into in preclinical research. In addition, the bioactive molecule IL-4 in HPL is also an essential bioactive factor in reducing neuroinflammation (Nebie et al., 2022). A study examining the effects of IL-4 treatment on surgery-induced neuroinflammation in rats found that IL-4 significantly reduced microglial activation and the release of pro-inflammatory factors (Li Z. et al., 2017). In addition, the therapeutic effect of IL-4 on neuroinflammation was demonstrated in mice using exosomes containing IL-4 (Casella et al., 2018). IL-4 ameliorates neuroinflammatory diseases by interacting with IL-4 receptors in the nervous system (Vogelaar et al., 2018). The study conducted by Lima et al. also demonstrated the anti-neuroinflammatory effects of IL-4 in the context of spinal cord injury (Nebie et al., 2022). These studies demonstrate the broad therapeutic potential of IL-4 against neuroinflammation under different disease perspectives and different administration methods. There are also studies showing the anti-inflammatory effect of miR-126-3p in platelet exosomes and inhibiting the autophagy and death of cortical neurons by targeting the phosphoinositide-3-kinase regulatory subunit 2 (PIK3R2) in neonatal rats treated with hypoxia-reoxygenation injury (HI) (Ge et al., 2023).
6.2 Oxidative stressOxidative stress is primarily attributed to an increase in oxygen free radicals resulting from mitochondrial dysfunction (Ma et al., 2014) Other factors contributing to the production of reactive oxygen species (ROS) include the degradation of hemoglobin, which generates a substantial amount of ROS (Yang et al., 2017), and the infiltration of neutrophils, which also produces ROS (Zhao et al., 2017; Wu X. et al., 2024). Furthermore, studies indicate that inhibiting microglial activity can significantly reduce ROS levels (Wang and Tsirka, 2005; Yang et al., 2017; Zhao et al., 2017). Currently, there are no anti-oxidative stress drugs available for clinical use (Zheng et al., 2022), which is currently the biggest challenge to oxidative stress damage. Helmut Sies believes that oxidative stress is caused by the imbalance between oxygen free radicals and the body’s antioxidant capacity (Forman and Zhang, 2021). This indicates that HPL possesses the capability to reduce oxidative stress, as it contains a variety of antioxidant factors, including catalase (CAT), ceruloplasmin (CP), GPX, SOD, and GCLM (Nebie et al., 2021b). Research involving CP-deficient mouse models indicates that CP may mitigate oxidative stress by oxidizing non-iron substrates, including catecholamines and aromatic amines (Hineno et al., 2011). Folle discovered that GCLM can safeguard red blood cells by alleviating oxidative stress, which, in turn, diminishes the oxidative stress intensified by the degradation of red blood cells (Föller et al., 2013). Bhowmick et al. developed highly effective mimetics of GPx and Prx, which showed the ability to safeguard human cells against oxidative harm by displaying outstanding antioxidant properties in the presence of cellular thiols (Bhowmick et al., 2015). Studies have shown that administering exogenous superoxide dismutase (SOD) can lessen brain tissue injury caused by excitotoxic effects from sodium glutamate while also offering protection to mitochondria (Liao et al., 2020). Moreover, Research on Huntington’s disease has also indicated the antioxidant properties of GPX (Mason et al., 2013). The studies above provide compelling evidence that HPL effectively treats ICH by mitigating oxidative stress. Although these findings are primarily derived from preclinical research, the current absence of adequate clinical treatments for ICH underscores the necessity for further investigation into the potential of HPL as a therapeutic option. Moreover, several studies have shown that HPL is capable of activating the PI3K/AKT signaling pathway (Ranzato et al., 2009; Chadid et al., 2018). Interestingly, earlier research has proved that the activation of this pathway could reduce oxidative stress-related damage (Zheng et al., 2023; Wang J. B. et al., 2024).
6.3 NeuroapoptosisRecent studies have highlighted the significant roles of Gelsolin, MANF, VEGF, and PDGF, bioactive factors that are abundant in HPL, in the protection of neuronal cells. Over the past few years, researchers have recognized at least 10 different mechanisms through which nerve cell death occurs. The pathways that result in the death of nerve cells are intricate and involve multiple factors. As a result, simply blocking a particular mechanism of nerve cell death might not be enough to avert cell loss (Fricker et al., 2018). Caspase, a protein specific to cysteinyl aspartate, is essential for the process of apoptosis and has an important function in neuronal cell death (Eskandari and Eaves, 2022). TNF-αattaches to death receptors on the cell membrane, resulting in the recruitment of Fas-associated death domain protein (FADD). This protein can interact with pro-caspase-8 to subsequently activate caspase-8, leading to the initiation of downstream signaling pathways that activate caspase-3 and ultimately induce apoptosis in the cell (Fricker et al., 2018). Studies suggest that a lack of neuron-s
留言 (0)