The vagus nerve (VN) is the primary component of the parasympathetic nervous system, regulating homeostatic functions throughout the body and brain (Agostoni et al., 1957). A key function of the VN is modulation of the inflammatory reflex, a systemic immune response that peripherally involves the spleen and the activation of choline acetyltransferase (ChAT)-positive (+) cells, i.e., the peripheral cholinergic anti-inflammatory pathway (Pavlov et al., 2003; Pavlov and Tracey, 2012). Additionally, VN activation influences the immune microenvironment of the central nervous system (CNS) through direct release of acetylcholine (ACh) from the nucleus basalis of Meynert (NB), which modulates microglia, the innate immune cells of the CNS (Hays et al., 2013; Nichols et al., 2011). The functional mechanisms of VN immunomodulation have been studied using vagus nerve stimulation (VNS), which uses electrical pulses to modulate VN activity. VNS is also an approved therapy for individuals with refractory epilepsy, treatment-resistant depression, and severe primary headaches (Dawson et al., 2021; Fisher et al., 2020; Silberstein et al., 2016). Given that CNS inflammation (or ‘neuroinflammation’) is associated with neurodevelopmental disorders, VNS may be effective as a therapeutic alternative. In this review we examine the effect of VNS on CNS inflammation, microglial states, and other changes in the microenvironment to explore possible mechanisms and applications of VNS for neurodevelopmental conditions, including schizophrenia, autism spectrum disorder (ASD), and attention-deficit hyperactivity disorder (ADHD).
2 Vagus nerve anatomy and physiologyThe VN, also known as cranial nerve X, is the longest among the twelve paired cranial nerves (Agostoni et al., 1957). These emerge directly from the brain to innervate the head and neck as motor (efferent) nerves, sensory (afferent) nerves, or a combination of both (Agostoni et al., 1957; Goggins et al., 2022). The VN serves as a mixed sensory-motor nerve, comprising approximately 80% afferent and 20% efferent fibers (Foley and DuBois, 1937). Afferent fibers of the VN are primarily composed of small-diameter, unmyelinated C fibers, which conduct afferent visceral information slowly (Ruffoli et al., 2011). A smaller population of larger diameter A and B fibers conduct afferent visceral information, motor input, and parasympathetic input much faster (Ruffoli et al., 2011). These fibers are involved in involuntary reflexes, such as the cough and gag reflex, and the transmission of sensory information (Ruffoli et al., 2011). The efferent VN fibers originate from rootlets exiting from the dorsal motor nucleus of the vagus (DMV) and nucleus ambiguous (NA) in the ventral medulla oblongata (Ruffoli et al., 2011; Wiles et al., 2007). These are responsible for stimulation of branchial arch striated muscles and control of parasympathetic functions (Ruffoli et al., 2011).
Understanding the basic anatomy and physiology of the VN in both the brain and body is essential to grasping the full breadth of its many functions. The Latin word “vagus,” meaning “wandering,” is aptly applied to the VN due to its extensive and complex path of innervation throughout the body (Ruffoli et al., 2011). Upon exiting the base of the skull, the VN subsequently innervates structures of the head and neck such as the larynx and pharynx, and additionally sends fibers that make up the auricular branch of the VN (ABVN) to innervate the outer ear (Howland, 2014). The two sides of the VN asymmetrically innervate the heart, with the right VN specifically innervating the sinoatrial node, which is the heart’s pacemaker, while the left innervates the atrioventricular node (Ruffoli et al., 2011). In the thorax, the VN additionally innervates the lungs and esophagus that it follows down into the abdomen (Ruffoli et al., 2011). The VN extensively innervates the stomach, which is its largest source of sensory information, as well as numerous abdominal organs, with its furthest reaching fibers innervating the distal third of the transverse colon (Ruffoli et al., 2011).
The VN collects peripheral information from its visceral branches and sends it through afferent projections to the tractus solitarius, which utilizes glutaminergic neurotransmission to synapse to the nucleus tractus solitarius (NTS) (Wang et al., 2021; Snell, 2010; Andresen and Yang, 1990). The NTS is a major sensory processing center in the brain that sends projections to numerous brain regions for further signaling (Figure 1), including major structures like the thalamus, hippocampus, rostral ventrolateral medulla, amygdala, and cerebral cortex (Breit et al., 2018). One important NTS projection connects to the NB, a region of the basal forebrain responsible for producing the neurotransmitter ACh for the prefrontal cortex, hippocampus, and amygdala (Hulsey et al., 2016). ACh is an important neurotransmitter that has been implicated in cognitive aspects like memory, attention, and motivation, as well as in regulating inflammation (Gombkoto et al., 2021). Stimulation of NB cells can modulate the release of ACh in its interconnected brain regions to enact a cholinergic response in the brain (Hays et al., 2013; Nichols et al., 2011; Ananth et al., 2023). The NTS can also modulate the NE concentrations through a di-synaptic pathway that connects the NTS to the locus coeruleus (LC), a region responsible for the production of norepinephrine (NE) (Manta et al., 2009a). NE activates α -adrenergic receptors ( α -ARs) and β -ARs in the brain, initiating increased arousal, attention, and the formation and retrieval of memories (Hays et al., 2013). The LC is part of the circuit that connects the VN to the serotonergic dorsal raphe nucleus (DRN), which enables the VN-mediated modulation of serotonin (5-HT) (Manta et al., 2009a). Chronic application of VNS in rats has demonstrated that prolonged production of NE can indirectly control the release of 5-HT in the DRN and ultimately increase 5-HT transmission in the hippocampus (Manta et al., 2009a). The LC is also known to send one-way connections to the NB that can excite cholinergic neurons to stimulate ACh release, establishing a direct link between the adrenergic and cholinergic systems in the CNS (Taylor et al., 2022). Projections from the NTS directly connect to the paraventricular nucleus (PVN), which is responsible for modulating corticotrophin-releasing hormone (CRH) as part of the hypothalamus-pituitary–adrenal (HPA) axis (Pavlov et al., 2003). The downstream effect of HPA axis stimulation is stress mediation through increased production of cortisol, a potent inflammatory inhibitor (Bonaz et al., 2017). This link between the NTS and PVN provides the VN a pathway to modulate the neuro-hormonal anti-inflammatory responses in the body (Pavlov et al., 2003). Additionally, the NTS harbors synaptic connections to the rostral ventrolateral medulla (RVM), which plays a role in cardiovascular homeostasis (Pavlov et al., 2003). Finally, the VN can mediate hippocampal functions and the production of brain-derived neurotrophic factor (BDNF) through connections arising from the NTS, LC, and DRN (Pavlov et al., 2003).
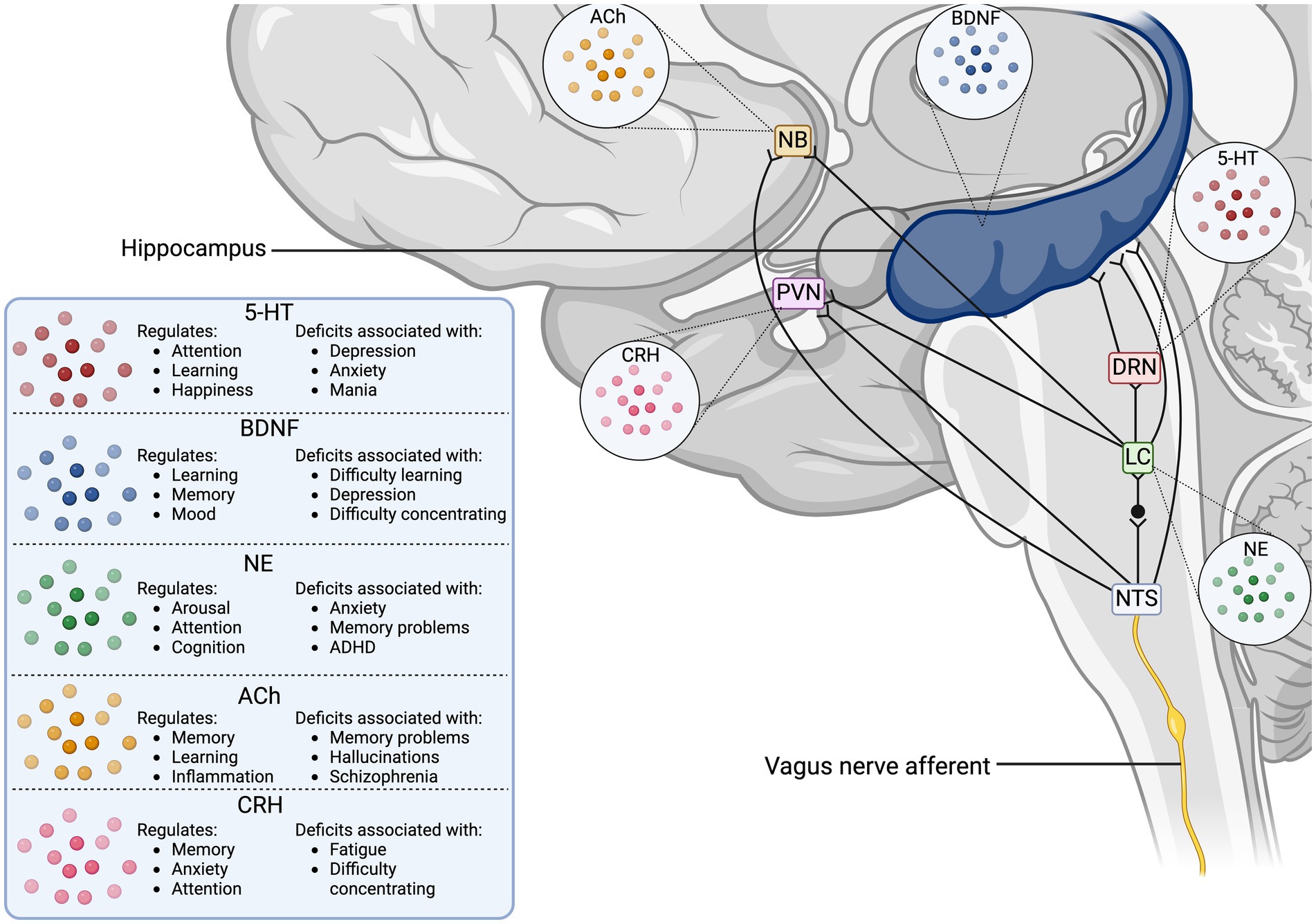
Figure 1. Vagus nerve neurotransmitter and synaptic pathways in the human brain. The illustration displays the direct synaptic connections from the incoming vagus nerve afferent signals to brain regions associated with neurotransmitter release. The table outlines the cognitive functions the stimulated neurotransmitters modulate, as well as the disorders associated with deficits in the neurotransmitter concentrations. 5-HT, serotonin; ACh, acetyl choline; BDNF, brain-derived neurotrophic factor; CRH, corticotropin releasing hormone; DRN, dorsal raphe nucleus; LC, locus coeruleus; NB, nucleus basalis of Meynert; NE, norepinephrine; NTS, nucleus tractus solitarius; PVN, paraventricular nucleus.
Vagal afferents also establish direct connections to the area postrema (AP) and DMV, which, along with the NTS, form the dorsal vagal complex (Pavlov et al., 2003). The DMV, serving as the motor component of the VN, receives processed NTS signals via gamma-aminobutyric acid (GABA) to regulate visceral functions through efferent signaling (Davis et al., 2004). Vagovagal reflexes, those mediating digestive functions, are controlled by inhibitory connections sent from the NTS to the DMV after afferent processing (Davis et al., 2004; Li and Owyang, 2003). In addition, the highly vascularized AP acts as a circumventricular organ, enabling the brain to have access to toxins, cytokines, and circulating hormones in the blood without crossing the blood–brain barrier (BBB), thus allowing for direct humoral immune-to-brain communication (Price et al., 2008). The AP possesses receptors for interleukin (IL)-1R1, which can induce c-Fos signals in the NTS and PVN, while IL-1 β -induced activation of the HPA axis also depends on the AP (Price et al., 2008).
3 The vagus nerve and the systemic immune systemThe body’s innate immune system is a major component of immune responses, responsible for the defense against infection and injury (Pavlov and Tracey, 2004). Innate immune cells, such as granulocytes, macrophages, and dendritic cells, are activated by pathogen-associated and danger-associated molecular patterns received by pattern recognition receptors on the cell surface, including Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain-like receptors (NLRs) (Pavlov et al., 2003; Pavlov and Tracey, 2012). As a result of downstream signaling cascades, there is an increased production and release of pro-inflammatory mediators like tumor necrosis factor (TNF), IL-6, and IL-1 β , which play critical roles in homeostatic immune response, such as extracellular pathogen clearance, neutrophil recruitment, and vasodilation (Pavlov and Tracey, 2004). Localized increases in TNF lead to common signs of inflammation, such as heat, swelling, pain, and redness in the skin (Pavlov and Tracey, 2004). The immune response is normally localized to the site of injury or infection, and is regulated by the release of anti-inflammatory mediators such as TGF β , IL-4 and IL-10 and through ACh signaling (Pavlov and Tracey, 2012). However, disturbances in the regulation and activity of the innate immune system can lead to chronic inflammation, caused by the continuous release of pro-inflammatory cytokines and decreased VN activity (Pavlov and Tracey, 2012). Chronic inflammation can then lead to the development of several diseases, including cardiovascular disease, diabetes mellitus, and neurodegenerative disorders (Furman et al., 2019). Additionally, inflammation during critical developmental periods, such as during pregnancy, infancy, and early childhood, can result in increased risk for neurodevelopmental disorders such as ASD, schizophrenia, and epilepsy (Jiang et al., 2018).
Immunomodulation via the VN is a crucial homeostatic function that relies on bidirectional communication between the brain and body. Afferent VN fibers detect immune imbalances and synapse the information to the NTS and its connected brain regions for processing, while efferent signals are generated within the CNS and sent to the DMV to conduct an immune response (Davis et al., 2004). Vagal motor neurons and efferent fibers originating from the DMV and NA then provide parasympathetic regulation to the body through the principle neurotransmitter ACh (Pavlov et al., 2003). Synthesis of ACh begins with a reaction between choline and acetyl coenzyme A, catalyzed by ChAT (Grando et al., 2003). The action of ACh is terminated by its hydrolysis by acetylcholinesterase or butyrylcholinesterase (Leuzinger et al., 1968). ACh acts on ionotropic nicotinic (nAChRs) and metabotropic muscarinic receptors (Gombkoto et al., 2021). Nicotinic receptors are ligand-gated ion channels with α - and β -subunits that form 12 subtypes ( α2–10 and β2–4), while muscarinic ACh receptors are G-protein-coupled receptors with five subtypes, divided into excitatory (M1, M3, and M5) and inhibitory (M2 and M4) receptors (Eickhoff et al., 2022). ACh plays an important role in immunomodulation, being released by immune cells, such as T cells, natural killer cells, and lymphocytes, as a response to infection (Suarez et al., 2018). ACh mediates concentration-dependent decreases in the pro-inflammatory mediator TNF and other pro-inflammatory mediators, such as IL-1 β , IL-6, and IL-18, through post-transcriptional mechanisms (Borovikova et al., 2000; Cox et al., 2020).
3.1 The humoral immune pathwayThe sympathetic nervous system is complexly connected to the VN, and its parasympathetic functions and regulation of the autonomic nervous system can lead to functional and structural changes in the CNS. Environmental factors, such as psychological stress, diet, infection, and pollution, can influence the function of the VN in neurodevelopment and cognitive processes. Stress, for example, is a major modulator of VN function and excessive stress can lead to epigenetic alterations in synaptic structure and function (Murphy and Heller, 2022). In response to stress, the body activates the autonomic nervous system – comprised of the sympathetic and parasympathetic branches (Murphy and Heller, 2022). The HPA axis is a primary component of the sympathetic nervous system – the “fight or flight” response – and stimulates the secretion of glucocorticoids and catecholamines (Murphy and Heller, 2022). These hormones influence changes in emotional and arousal states, increase heart rate and blood pressure, decrease gut motility and secretion, and decrease bronchi diameter (Murphy and Heller, 2022). Once stress has been mitigated, the body returns to its homeostatic functions, as mediated by the parasympathetic nervous system – the “rest and digest” condition – which is primarily controlled by the VN (Murphy and Heller, 2022). However, in rodent models exposed to chronic stress, the autonomic nervous system can become dysregulated, leading to elevated glutamate levels and dendritic shrinkage in the CA1, CA3 and dentate gyrus regions of the hippocampus, as well as in the medial amygdala and prefrontal cortex (McEwen, 2017; Chaouloff et al., 2007). To prevent excessive activation and maintain homeostasis, the autonomic nervous system meets at nerve junctions (plexuses) to bridge communication between the sympathetic and parasympathetic systems (Howland, 2014). The VN can also directly communicate with components of the sympathetic nervous system in the CNS, such as the HPA axis, and indirectly connect to the sympathetic preganglionic neurons in the upper spinal cord (Pavlov et al., 2003). These CNS communication lines are crucial for the sympathetic and parasympathetic systems to work synergistically in humoral and VN-mediated parasympathetic immune responses throughout the body (Pavlov et al., 2003).
The humoral immune pathway for immune-to-brain communication relies on the HPA axis as the major component to initiate immune response. This pathway involves circulating cytokines, including TNF and IL-1 β , that cross the BBB and act on surface receptors on the brain capillary endothelium to enhance the release of prostaglandins, whose diffusion into the parenchyma mediates fever response and triggers HPA axis activation (Pavlov et al., 2003). Furthermore, the AP is a circumventricular organ that acts as a transduction site, allowing direct systemic signaling to the NTS and RVM, which further synapse to the HPA axis and sympathetic nervous system (Pavlov et al., 2003). The VN-mediated activation of PVN cells causes CRH to be synthesized and enter the pituitary portal system, where it stimulates adrenocorticotrophin hormone synthesis in the anterior pituitary, which in turn stimulates the release of cortisol from the adrenal cortex (Pavlov et al., 2003). The HPA axis is regulated through multiple negative feedback loops, such as the inhibition of CRH by adrenocorticotrophin hormone, and modulation by neural ACh, GABA, and 5-HT (Pavlov et al., 2003). Cortisol influences the inflammatory response by binding intracellular receptors and suppressing nuclear factor κB activity (NF- κB) expression, which is linked to pro-inflammatory cytokine synthesis (Pavlov et al., 2003). The sympathetic nervous system plays a role in both pro-inflammatory and anti-inflammatory processes through the LC and RVM, which connect to the sympathetic preganglionic cholinergic neurons in the spinal cord (Pavlov et al., 2003; Elenkov et al., 2000). These neurons then synapse with the postganglionic neurons, using NE as their primary neurotransmitter (Pavlov et al., 2003; Elenkov et al., 2000). During early inflammatory stages, the sympathetic nervous system can activate the inflammatory response at a local level through stimulation of α2-ARs (Pavlov et al., 2003; Elenkov et al., 2000). Activation of β -ARs on lymphocytes and macrophages by NE inhibits pro-inflammatory cytokine production via the β2-AR-cAMP-protein kinase A pathway and elevate anti-inflammatory cytokine levels (Pavlov et al., 2003).
3.2 The inflammatory reflexThe inflammatory reflex is a VN-mediated response to immune challenge comprised of two arms: afferent and efferent (Pavlov et al., 2003; Pavlov and Tracey, 2012). Peripheral pro-inflammatory molecules are received by afferent VN fibers, and signals are sent to the NTS, where they synapse to interconnected brain regions, such as the hypothalamic nuclei, amygdala, and insular cortex, to coordinate autonomic and endocrine responses (Pavlov et al., 2003; Pavlov and Tracey, 2012). One study demonstrated that intraportal administration of IL-1 β results in a dose-dependent increase in afferent activity in the hepatic branch of the VN in rats, which was not observed following hepatic vagotomy, therefore suggesting the presence of IL-1 β receptors on VN afferents (Niijima, 1996). Furthermore, the administration of IL-1 β resulted in a reflex activation of the sympathetic splenic nerve, which was similarly lacking in vagotomised rats (Niijima, 1996). Further studies implicated the participation of IL-1 β receptors in VN afferents and chemosensory cells in the paraganglia surrounding the afferent endings of the VN (Goehler et al., 1999; Ek et al., 1998). However, studies using the inflammogen lipopolysaccharides (LPS) via intraperitoneal injection or using intraperitoneal injection of IL-1 β in vagotomised rodents found that high levels of circulating IL-1 β could produce fever and sickness behaviors by bypassing neuronal circuits and acting directly on the brain through circumventricular organs like the AP, or other humoral mechanisms. Therefore, VN-mediated responses work in a dose-dependent fashion and appear especially important at early stages of infection, when circulating IL-1 β levels are low (Goehler et al., 2000; Hansen et al., 2001). Additionally, endocrine processes can be slower in comparison to neural regulation, emphasizing the crucial role of the VN and sympathetic nervous system in eliciting a rapid initial immunoregulatory response (Pavlov and Tracey, 2004). Signal integration in the NTS and associated brain regions, such as the PVN, RVM, and LC, creates the substrate for the HPA axis and sympathetic nervous systems to interact with the VN playing a central immunomodulatory role (Pavlov et al., 2003).
The efferent arm of the inflammatory reflex is constituted by the cholinergic anti-inflammatory pathway. When activated through the NTS-DMV synapse, the efferent signals travel to the celiac superior mesenteric ganglion complex, where they connect to the splenic nerve (Pavlov and Tracey, 2012). Stimulation of the splenic nerve by the efferent VN fibers leads to the release of NE in the spleen (Pavlov and Tracey, 2012). Splenic NE binds to β2-ARs found on the surface of memory CD4+ T cells expressing ChAT (Pavlov and Tracey, 2012). This subsequently triggers the synthesis and release of ACh from the T cells (Pavlov and Tracey, 2012). It was originally thought that ACh is directly released from the nerves, however studies found that the inflammatory reflex failed to inhibit TNF release in T cell-deficient nude mice, indicating that T cells play a role in the process (Rosas-Ballina et al., 2011). When T cells were repopulated in the T-cell deficient nude mice, the response was restored, suggesting direct signaling via NE due to the proximity of splenic lymphocytes to the adrenergic nerve endings (Rosas-Ballina et al., 2011). Together, these findings supported the role of ChAT T-cells in the ACh release required for the inflammatory reflex. Peripheral immune cells, such as macrophages, dendritic cells, and monocytes, are a major source of TNF, and the expression of α7AChR in bone marrow-derived cells is essential for ACh regulation of TNF release (Pavlov and Tracey, 2012). The ChAT T-cell derived ACh binds α7AChR to affect downstream signaling pathways, inhibiting NF- κB nuclear translocation and activating the Janus kinase 2-signal transducer and activator of transcription 3 mediated signaling cascade (Pavlov and Tracey, 2012; de Jonge et al., 2005). This results in the inhibition of TNF transcription along with other pro-inflammatory mediators. A study using human macrophage cultures exposed to LPS demonstrated that ACh can inhibit pro-inflammatory cytokines without affecting the release of anti-inflammatory cytokines (Pavlov and Tracey, 2012; Borovikova et al., 2000). Electrical stimulation of the VN also effectively decreases serum TNF levels in wild-type mice, but is ineffective in mice lacking nicotinic receptors, further validating this important pathway (Pavlov et al., 2003).
4 Microglia, central nervous system inflammation, and the vagus nerveThe VN plays a complex role in influencing the systemic immune response and has an impact on the immune microenvironment within the CNS. In the CNS, homeostatic balance and modulation of neuroinflammation is mediated by microglia, a type of glial cell that acts as the resident innate immune cells (Tremblay, 2020). Microglia originate from yolk sac primitive macrophages, entering the brain during early embryonic development, around day 9.5 in mice or gestational week 4.5 in humans, which is equivalent to the first trimester (Monier et al., 2007; Ginhoux et al., 2010). Microglia express a variety of morphologies that are closely associated with function. In the healthy brain, surveying microglia are the predominant morphology, using numerous, highly branched, and dynamic thin processes to constantly survey the parenchyma for homeostatic changes (Shigemoto-Mogami et al., 2014; Ueno et al., 2013; Miyamoto et al., 2016; Tremblay, 2021). Surveying microglia have various functions, including modulation of neurons and glial cells, facilitating the formation and pruning of synaptic elements, and maintenance of myelination, and are an integral component of the neurovascular unit (Shigemoto-Mogami et al., 2014; Ueno et al., 2013; Miyamoto et al., 2016; Tremblay, 2021).
Microglia host a large variety of well-established surface receptors (Figure 2) as part of their sensome, including those for DA, adenosine, opioids, cannabinoids, and CRH (Watters and Pocock, 2014; Liu et al., 2016). The innate immune response in the CNS is initiated by microglial TLRs, NLRs, and triggering receptors expressed on myeloid cells (Rodríguez-Gómez et al., 2020). Stimulation of TLRs leads to NF- κB and mitogen-activated protein kinase cascades activation, subsequently leading to pro-inflammatory mediator transcription and phagocytosis of nearby damaged neuronal cells (Watters and Pocock, 2014; Rodríguez-Gómez et al., 2020). Microglia also express ARs, including β1-AR, β2-AR, α1A-AR, and α2A-AR, for NE, as well as α3, α5, α6, α7, and β4 nicotinic receptors for ACh, and GABAA and GABAB receptors, all of which promote a neuroprotective, anti-inflammatory microglial phenotype (Watters and Pocock, 2014; Liu et al., 2016). Activation of microglial α7nAChRs results in transcriptional alterations such as increased antioxidant genes and decreased phosphorylation of NF- κB, thereby reducing pro-inflammatory cytokine release (Han et al., 2014). Additionally, one study analyzed the role of ACh in LPS-elicited microglial inflammatory response using rat neuron-microglial co-cultures and found that higher levels of ACh reduced the concentration of TNF α and inhibited hippocampal neuronal apoptosis (Li et al., 2019). Inhibition of TNF α by α7nAChR is mediated by reduced extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase signaling (Li et al., 2019). Additionally, microglia express the ionotropic glutamate receptors α -amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type GluR1-GluR4 and kainate, and all three metabotropic glutamate receptors (Watters and Pocock, 2014; Liu et al., 2016). The AMPA receptors have been shown to both inhibit and stimulate the release of TNF α , whereas metabotropic glutamate and kainate receptors increase TNFα (Watters and Pocock, 2014; Liu et al., 2016). There is support for the presence of N-methyl-D-aspartate (NMDA) receptors on microglia, which enhance the release of TNF α , IL-1, and nitric oxide (Watters and Pocock, 2014; Liu et al., 2016).
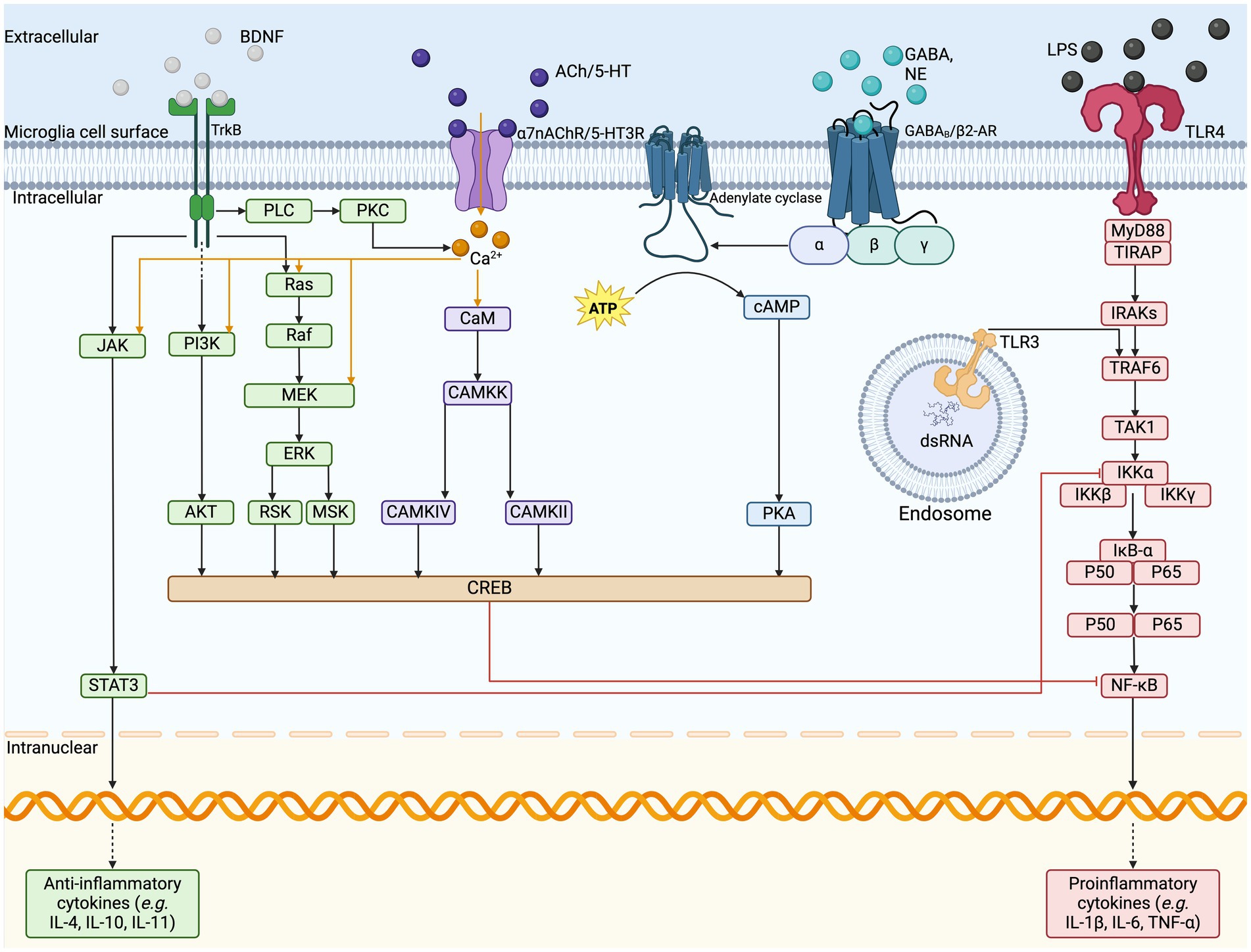
Figure 2. Microglia cell surface sensome and anti-inflammatory pathways. The illustration depicts the cell surface receptors found on microglia and the respective cell signaling cascades. VNS stimulates the release of various neurotransmitters, such as BDNF, ACh, NE, GABA, and glutamate, which act on their respective cell surface receptors on microglia. In response to pro-inflammatory signaling on TLRs, the signal cascades can inhibit the transcription of pro-inflammatory cytokine release and upregulate the release of anti-inflammatory cytokines to resolve inflammation. ACh, acetylcholine; AKT, protein kinase B; ATP, adenosine triphosphate; BDNF, brain-derived neurotrophic factor; CAMKK, calcium/calmodulin-dependent protein kinase; cAMP, cyclic adenosine monophosphate; NE, norepinephrine; dsRNA, double stranded ribonucleic acid; ERK, extracellular signal regulated kinases; GABA, gamma-aminobutyric acid; GPCR, G-protein coupled receptor; IL, interleukin; IRAKs, interleukin-1 receptor associated kinase; JAK, Janus kinase; LCIG, ligand-gated ion channels; LPS, lipopolysaccharide; MEK, mitogen activated protein kinase-kinase; MSK, mitogen and stress activated kinase; MyD88, myeloid differentiation primary response; NF-kB, nuclear factor kappa B; PI3K, phosphoinositide 3-kinase; PKA, protein kinase A; PKC, protein kinase C; PLC, phospholipase C; RSK, ribosomal S6 kinase; RTK, receptor tyrosine kinase; STAT3, signal transducer and activator of transcription 3; TAK1, mitogen-activated protein kinase kinase kinase; TIRAP, TIR domain containing adaptor protein; TLR, Toll-like receptor; TNF, tumor necrosis factor; TRAF6, TNF receptor associated factor 6; VNS, vagus nerve stimulation.
A neurotransmitter modulator associated with microglial function is BDNF. The neurotrophic factor BDNF plays a critical role in neuronal plasticity and is mainly produced by neurons, where it can be utilized by other neurons or microglia (Andero et al., 2014). Studies have shown that microglia also produce BDNF, although the impact of microglial BDNF is currently debated (Honey et al., 2022; Onodera et al., 2021). Some studies have found expression levels of microglial BDNF to be either absent or too low to noticeably modulate neuronal function (Honey et al., 2022; Onodera et al., 2021). However, others have highlighted the importance of microglia-derived BDNF in learning-induced synapse formation in mice, and its expression on pro-inflammatory microglia following adverse early life experiences (Komori et al., 2024; Parkhurst et al., 2013). Dimerized BDNF binds tropomyosin-related kinase B (TrkB) receptors and p75 neurotrophin receptor in neurons (Wang et al., 2015; Zhang et al., 2003; Frisén et al., 1993). However, the expression of TrkB on microglia also remains a topic of debate due to the heterogeneity of microglia across species, ages, and brain regions (Wang et al., 2015; Zhang et al., 2003; Frisén et al., 1993). One mouse study observed that neuronal BDNF prevented microglia from engulfing mossy fiber synapses in the hippocampus and increased microglial motility and synapse engulfment when BDNF was blocked (Onodera et al., 2021). Another mouse study found that microglial exposure to BDNF reversed LPS-induced inflammatory responses (Charlton et al., 2023). These studies both indicate that BDNF may have some impacts on microglial functions in the hippocampus.
Microglia have high levels of cellular plasticity, resulting in many diverse structural states that allow them to shift between functions of surveillance, neuroprotection and neurotoxicity (Hickman et al., 2013). One phenotype, surveillant microglia, present long processes, capable of crosstalk with neurons and monitoring homeostatic changes in the CNS microenvironment (Savage et al., 2020). Upon homeostatic challenge and across aging, microglia may express states including dystrophic and senescent states (Savage et al., 2020; Bisht et al., 2016). These states often display a more ameboid-like morphology, with shorter, thicker, and less branched processes (Savage et al., 2020; Bisht et al., 2016). Dark microglial states, present in early development and in pathology, display makers of cellular stress (Savage et al., 2020; Bisht et al., 2016). After the detection of an immune insult, microglia can change their morphology, proliferative state, phagocytic activity, and antigen presentation capacity to contribute to an immune response (Bachiller et al., 2018). As part of the pro-inflammatory response and when regulating neuronal activity and homeostasis, microglia can produce pro-inflammatory cytokines and chemokines in the brain, such as IL-6, IL-8, and TNFα (Bachiller et al., 2018). Microglial phenotypes are diverse, making them difficult to universally define, as different stimuli and CNS conditions lead to differential responses and states other than the previously defined status “activated” and “resting,” or “M1” and “M2,” being considered limited and not reflecting the current understanding (Watters and Pocock, 2014; Rodríguez-Gómez et al., 2020; Friedman et al., 2018). Indeed, microglia are always active, in health and disease, and they can co-express M1 and M2 markers in their different states (Paolicelli et al., 2022). Microglial release of pro-inflammatory mediators is necessary for physiological processes, acting as a defence and repair mechanism that is held under tight regulation by anti-inflammatory mediators (Rodríguez-Gómez et al., 2020). Previous studies have suggested that microglial involvement in neurodevelopmental disorders, like ASD and schizophrenia, is linked to elevated levels of microglial neuregulin and irregular pro-inflammatory cytokine production associated with altered physiological activities in patients (Ikawa et al., 2017; Rodriguez and Kern, 2011). Some maternal factors, such as immune activation, can also influence prenatal microglial immune reactivity, as shown by a mouse study which demonstrated that microglia could become blunted, presenting a long-lived decrease in immune reactivity, after maternal immune activation (Hayes et al., 2022). In vitro this was seen as a reduction of IL-6 and TNF α release from primary microglial cells from the maternal immune activation group after LPS stimulation compared to the control (Hayes et al., 2022). Additionally, in vivo results indicated decreased CD68+ lysosomes in microglia, smaller microglia size, and an imbalance in the microglial mitochondrial pathways in the immune activation group compared to the control (Hayes et al., 2022).
5 Vagus nerve stimulationVNS is a neuromodulation technology approved for patients with severe neurological disorders, including drug-refractory epilepsy, stroke neurological sequelae, cluster headaches, and migraines, alongside neuropsychiatric disorders like major depression (Dawson et al., 2021; Fisher et al., 2020; Silberstein et al., 2016). The most common form of VNS is invasive VNS (iVNS), which involves surgical implantation of a programmable pulse generator device for electrical stimulation of the left cervical VN (Howland, 2014). The procedure is typically performed on an outpatient basis under general anesthesia, involving subcutaneous implantation of the generator and attaching the electrode wire to the mid-cervical VN (Howland, 2014). A programmable wand placed outside the skin controls stimulation features, such as the current charge, pulse width, pulse frequency, on/off duty cycle, and more (Howland, 2014). Approved parameters range from 0.25–3 mA for the current intensity, 300–500 μs for the pulse width, and a frequency of 20–50 Hz, alongside timing parameters (Badran and Austelle, 2022). Since the right VN directly innervates the sinoatrial valve in the heart, stimulation could cause cardiac effects, including bradycardia and asystole (Howland, 2014). Therefore, left VN stimulation has been preferentially approved as the primary treatment modality (Howland, 2014). Adverse effects, such as wound infection and hoarseness, are mostly associated with the surgical procedure and only occur in about 1% of patients (Howland, 2014). Stimulation-related effects are limited to the short period during stimulation and can be mediated by decreasing stimulation parameters, but can include voice alteration, cough, dyspnea, and changes in breathing patterns during sleep, resulting in apneas (Howland, 2014). Due to the invasiveness, the treatment is limited to individuals resistant to conventional therapies, and its high, unsubsidized cost further limits its use (Yap et al., 2020). Regardless, iVNS devices were approved by the United States FDA for refractory epilepsy in 1997 and for chronic treatment-resistant depression in 2005 (Howland, 2014). As of 2021, more than 125,000 patients have received iVNS devices for treatment (Fetzer et al., 2021).
Early studies of VNS in animals found that iVNS had a potent anti-convulsive effect (Zabara, 1992; Lockard et al., 1990). Further studies confirmed the viability of the treatment for epilepsy, as thoroughly outlined in another review (Milby et al., 2009). The iVNS therapy for epilepsy was approved for use in 1997 for adults in the USA and Canada and demonstrates a 50–60% response rate in patients with over 50% seizure reduction (Dolezalova et al., 2022). It also improved the mood of epilepsy patients, leading to further studies into its use in treatment-resistant depression, which was later approved for use in 2005 (Howland, 2014). Other conditions iVNS is approved for include ischemic stroke, tinnitus, traumatic brain injury, and spinal cord injury (Hulsey et al., 2016). Non-invasive VNS devices are being investigated for a wide variety of disorders, such as cluster headaches and migraines, tinnitus, schizophrenia, and ASD (Hasan et al., 2015; Hyvärinen et al., 2015; Nesbitt et al., 2015). Furthermore, the discovery of the inflammatory reflex encouraged new studies to investigate VNS as a possible treatment modality for inflammatory conditions, such as inflammatory bowel disease, rheumatoid arthritis, and Crohn’s disease (Bonaz et al., 2017; Koopman et al., 2016). Clinical studies focusing on iVNS showed efficacy in rheumatoid arthritis and Crohn’s disease in small cohorts, while transcutaneous methods have shown efficacy in alleviating CNS inflammation by altering microglial response to a neuroprotective phenotype in mouse models in vitro (Bonaz et al., 2017; Koopman et al., 2016; Zhao et al., 2019). Additionally, both invasive and transcutaneous devices have shown some success in treating inflammatory conditions, like rheumatoid arthritis, by reducing mouse and human serum levels of TNF, IL-6, and IL-1β (Hong et al., 2019; Addorisio et al., 2019).
Alternative methods of VNS include transcutaneous VNS (tVNS), which is non-invasive and targets either the ABVN (auricular VNS) or the cervical branch of the VN (cervical VNS) (Howland, 2014). Auricular VNS targets the cymba conchae, which is the only known location with 100% VN innervation (Wang et al., 2021). Stimulation parameters have a much wider range, with current intensity from 0.13–50 mA, pulse width from 20–500 μs, and frequency between 1–30 Hz (Badran and Austelle, 2022). These parameters can be higher than in iVNS due to the insulative properties of the skin (Badran and Austelle, 2022). Functional magnetic resonance imaging studies have confirmed that auricular VNS stimulates the same brain regions as iVNS, such as the brain stem, hippocampus, amygdala, prefrontal cortex, and thalamus (Badran and Austelle, 2022). Further, the higher stimulation parameters increase brain response without hanging the regional specificity (Badran and Austelle, 2022). One barrier with this method is that the afferent pathway of the ABVN remains poorly understood. FDA-approved devices for auricular VNS include the NEMOS and NET-2000 (Wang et al., 2021). These devices were authorized for the treatment of epilepsy and depression in 2010, and pain management in 2012 (Howland, 2014). Cervical VNS uses gammaCore devices on the anterolateral surface of the neck to target the VN in the carotid sheath (Wang et al., 2021). The electrodes are placed on the sternocleidomastoid muscle, where the VN is close to the surface of the neck and provides a convenient marker for placement (Wang et al., 2023). Stimulation parameters are commonly adjusted up to a maximum of 60 mA, a pulse width of 1,000 μs, and a frequency of 25 Hz (Miyatsu et al., 2024). This device was approved for the treatment of cluster headaches in 2017, migraine in 2018, and hemicrania continua in 2021 (Howland, 2014). One limitation of cervical VNS concerns the position of the VN. While the stimulation device is placed on the same VN location as iVNS electrodes, the electric pulse must navigate through around 2 mm of skin, 3–6 mm of superficial fascia, and 5–6 mm of the sternocleidomastoid muscle, making selective stimulation difficult and increasing the likelihood of stimulating both afferent and efferent fibers (Yap et al., 2020). However, magnetic resonance imaging (MRI) has shown promise in tailoring stimulations based on individual characteristics, such as skin conductivity and tissue thickness (Kaczmarczyk et al., 2018). It has also been suggested that lower frequency stimulations could favor efferent over afferent fiber activation (Meneses et al., 2016). Both tVNS mechanisms stimulate various brain regions, such as the NTS, parabrachial area, hypothalamus, amygdala, nucleus accumbens, and LC, as shown by MRI (Yakunina et al., 2017). For the purposes of this review, both types of tVNS will be referred to interchangeably in further discussion unless otherwise stated. The value of tVNS as an alternative treatment modality lies in its limited side effects. The common side effects include some pain or itching at the stimulation site, and, in less common cases (<1%), patients may experience nausea or vomiting, headache, heart palpitations, facial drooping, dizziness, and vocal hoarseness (Yap et al., 2020). Despite the possibility of side effects, they are generally less severe and less frequent than those associated with iVNS. Furthermore, tVNS avoids the need for costly surgery, making it a viable option for further research.
6 Neuronal mechanisms of vagus nerve stimulation 6.1 Microglia and inflammationOne possible action of VNS in the brain is through the modulation of inflammation. Studies testing VNS for rheumatoid arthritis have shown that VNS inhibits joint inflammation and the release of inflammatory cytokines (Zhang et al., 2008). Clinical studies have also indicated that VNS might be beneficial against rheumatoid arthritis owing to a reduction of TNF- α release ex vivo and might lead to improvements in disease severity (Koopman et al., 2016; Genovese et al., 2020). In addition, the inflammation that underlies the pathogenesis of CNS diseases, such as multiple sclerosis, Alzheimer’s disease, epilepsy, and schizophrenia, is of great interest for VNS research (Kelly et al., 2022). Experimental studies in animal models have revealed that VNS plays a significant role in altering inflammatory responses (Howland, 2014). Additionally, a human trial in patients with refractory epilepsy found a decrease in IL-8 during long-term VNS (6 months), with no significant changes in the expression of IL-1 β , TNF- α , IL-6 or IL-10 (De Herdt et al., 2009). In a study of cerebral ischemia researchers also observed that VNS downregulated IL-1 β and IL-18 in brain tissues from the cortex, but the neuroprotective effects offered by VNS were reversed by the administration of an α7nAChR antagonist (Tang et al., 2022). These results support a role of α7nAChR in the anti-inflammatory action of VNS. Given the anatomical connections of the VN to the NB, the direct modulation of ACh levels may explain the link of this mechanism. A study on rats focused on depression and stress moreover revealed decreased IL-1 β , TNF- α , and IL-6 levels in hippocampal tissues after VNS, as well as morphological changes in hippocampal microglia, notably from amoeboid to surveillant states, with an increased expression of α7nAChR (Namgung et al., 2022). In traumatic brain injury, VNS reduced TNF- α levels in the serum and brain tissue in a rabbit model (Zhou et al., 2014). Elsewhere, a study showed that VNS significantly reduced the release of pro-inflammatory cytokines such as TNF- α , IL-1 β , and IL-6 in the ischemic penumbra cortex 24-h after ischemia through α7nAChR activation in microglia from mice subjected to vascular occlusion (Jiang et al., 2014).
Microglia hold a central place in VNS-induced cholinergic anti-inflammatory mechanisms. Numerous CNS conditions have been examined to determine the role of microglia in the actions of VNS, including epilepsy, stress, ischemic stroke and spinal cord injury (Namgung et al., 2022; Chen et al., 2022; Zhang et al., 2021). TLR4 is an important mediator in neuroinflammatory-related disease that is primarily expressed by microglia and has been demonstrated to have a role in traumatic brain injury and ischemic stroke (Yao et al., 2017; Tian et al., 2019). One study on rats found that VNS inhibited the TLR4/myeloid differentiation primary response 88/NF- κB pathway in microglia, thereby reducing the release of the pro-inflammatory cytokines IL-1 β and IL-6 (Zhang et al., 2021). Activation of α7nAChR stimulates adenylyl cyclase 6, promoting the degradation of TLR4, which is consistent with their findings of reduced TLR4 expression after VNS in rats (Zhang et al., 2021; Kim et al., 2014; Zhu et al., 2021). Additionally, VNS can increase ACh levels in the rat brain, thereby activating α7nAChR in microglia after ischemic stroke, which inhibits the peripheral inflammatory response in part through the TLR4/NF- κB pathway (Zhang et al., 2021; Kim et al., 2014). A study in mice subjected to transient middle cerebral artery occlusion also found that VNS treatment, given for 60 min before, during, and after the occlusion, preserved microglial α7nAChR expression in the penumbra regions and inhibited NLRP3 inflammasome activation (Xia et al., 2024). Proposed mechanisms for the central reduction of inflammation involve the VNS-induced release of NE from the LC, which has been shown to be essential for the anti-convulsive effects of VNS, by activating β2-ARs on microglia, astrocytes, and neurons to reduce inflammatory responses via the NF- κB pathway (Krahl et al., 1998; Laureys et al., 2014). Additionally, activation of the NB triggers the release of ACh to target α7nAChRs, which induce an anti-inflammatory response (Kaczmarczyk et al., 2018). Studies on spinal cord injury models and stress models in rats found similar results, with VNS downregulating pro-inflammatory cytokine release and promoting microglia to release anti-inflammatory mediators via α7nAChR upregulation (Namgung et al., 2022; Chen et al., 2022).
Studies have shown evidence of anti-inflammatory effects in human rheumatoid arthritis upon treatment with VNS, as well in reducing the central inflammatory response in murine autoimmune encephalomyelitis (Koopman et al., 2016; Hao et al., 2011). One study, utilizing LPS in mice to induce increased pro-inflammatory cytokine levels found that the whole brain pro-inflammatory cytokine levels were significantly reduced after VNS, alongside a significant reduction in the percent of microglia (CD11b+/CD45low) as seen by flow cytometry of whole brains (Meneses et al., 2016). Additionally, they found a decreased expression of the microglia/macrophage marker Iba1 in the hippocampus of VNS-treated mice, suggesting the treatment normalized microglial reactivity which could be linked to reduced CNS inflammation (Meneses et al., 2016). A study also tested VNS in a rat model of LPS-induced demyelination and found a reduced microglial response to inflammation and improved remyelination around the lesion border, indicated by a 57.4% reduction in demyelination compared to the sham (Bachmann et al., 2024). However, there was no preventative effect of VNS on demyelination (Bachmann et al., 2024). They suggest that VNS may enhance microglial clearance of debris to favor remyelination (Bachmann et al., 2024). Additionally, this study found a reduced Iba1 expression and cell count, without a reduction in surveillant microglia, indicating that VNS may reduce microglial reactivity and promote neuroprotective microglial populations (Bachmann et al., 2024). This is supported by a study that used a mouse model of maternal immune activation to study the effect of auricular VNS in the treatment of ASD-phenotypes in offspring (Zhang et al., 2024). Using Iba1 as a microglial marker and CD15, a marker found in immune cells and used as a marker for reactive microglia, they found 7 days of auricular VNS in adult maternal immune activation exposed mice decreased microglial proliferation and decreased the number of reactive microglia in the medial prefrontal cortex (Zhang et al., 2024). They additionally used an anti-IL17a model to compare against VNS treated mice and suggest the mechanism may be mediated through the IL-17a inflammatory pathway (Zhang et al., 2024).
Other hypotheses implicate that the central role of α7nAChR involves the expression of peroxisome proliferator-activated receptor γ (PPAR γ ), which is upregulated by α7nAChR activation (Jiang et al., 2015). PPAR γ is a ligand-activated nuclear receptor that plays a role in adipocyte differentiation, lipid metabolism, and insulin resistance, as well as inhibits the synthesis and secretion of pro-inflammatory cytokines in the CNS (Jiang et al., 2015; Kapadia et al., 2008). Hypotheses stipulate that VNS may upregulate the expression of PPAR γ for participation during VNS-induced neuroprotection, while its activation can exert anti-inflammatory effects in both the periphery and CNS (Jiang et al., 2015). In line with this, studies using a rat model of right middle cerebral ischemia have demonstrated that VNS decreased pro-inflammatory cytokine expression and upregulated PPAR γ gene expression via activation of α7nAChR (Jiang et al., 2015). Additionally, the authors found decreased neuronal damage, and improved neurofunctional recovery after ischemic stroke (Jiang et al., 2015).
The mechanisms underlying the anti-inflammatory effects of VNS occur through various pathways. Another possible pathway is the cholinergic anti-inflammatory pathway, where α7nAChR in splenic macrophages inhibits the release of pro-inflammatory cytokines (Chen et al., 2018). The connection between the microbiota, gut, and brain, notably through the microbiota-gut-brain axis, can play an important role in modulating inflammatory responses via the VN and is associated with disorders, such as neurodevelopmental, neuropsychiatric and neurodegenerative conditions (Wang et al., 2021). The cells in the gut called enteroendocrine cells detect chemical signals and transmit them to the VN through the enteric neurons, triggering an anti-inflammatory response (Wang et al., 2021). Permeability in the BBB can allow for peripheral immune signaling that triggers an inflammatory response, which can be regulated by the VN. For example, neurodegenerative diseases and ischemic stroke are characterized by functional and structural changes in the BBB (Jin et al., 2023). When glial cells in the brain parenchyma bind to damage-associated molecular patterns, they undergo phenotypic changes leading to an increased release of pro-inflammatory mediators that affect BBB permeability (Jin et al., 2023). Besides, studies have shown that epilepsy is associated with structural and functional changes in the BBB (Kaya et al., 2008; Kaya et al., 2013). VNS treatments were shown to strengthen BBB integrity, likely through the modulation of α7nAChR’s, notably improving the barrier’s structural and functional components (Kaya et al., 2008; Kaya et al., 2013). Specifically, VNS-mediated α7nAChR-induced upregulation in splenic macrophages was shown to inhibit the release of pro-inflammatory factors, thereby protecting the initial degradation of the BBB (Chen et al., 2018). Additionally, VNS reversed BBB permeability and decreased pro-inflammatory microglial responses and TNF- α levels in mice with cerebral microinfarction and colitis (Chen et al., 2018). Under this model, the actions of the cholinergic anti-inflammatory pathway provide a possible route to influence CNS inflammation.
6.2 Modulation of plasticity: ACh and BDNFACh and BDNF are both central neurotransmitters in neuronal plasticity and have been implicated in the mechanism of VNS-mediated plasticity modulation. Activation of the NB was found to be essential to the plasticity-enhancing effects of VNS in the motor cortex (Hulsey et al., 2016). For example, increased ACh release has been associated with increased visual cue detection in the prefrontal cortex and cognitive performance in learning and spatial memory tasks in the hippocampus in rats (Gombkoto et al., 2021). Nicotinic and muscarinic receptors can also modulate the release of other neurotransmitters, such as glutamate, DA, 5-HT, and NE (McGehee et al., 1995; Picciotto et al., 2012). The interaction of the cholinergic system with the dopaminergic pathway has led to suggestions that nAChRs and M1-muscarinic ACh receptors could be involved in dopaminergic dysregulation in patients with schizophrenia (Eickhoff et al., 2022). As previously discussed, ACh also ties into microglial modulation, which could also play important roles in increasing plasticity.
BDNF is another neurotransmitter that plays a crucial role in hippocampal neuronal plasticity. Activation of the TrkB receptor leads to activation of downstream pathways to recruit molecules like phosphoinositide 3-kinase, whose downstream pathways promote cell growth and proliferation (Yang et al., 2020). Studies in rat models have supported these connections through findings indicating that VNS influenced the expression of BDNF, 5-HT, NE, and inflammatory mediators involved in hippocampal neurogenesis (Manta et al., 2009a; Biggio et al., 2009; Follesa et al., 2007). One study observed reduced BDNF mRNA expression in the hippocampal CA1, CA3, and dentate gyrus of vagotomised rats and mice, as well as decreased adult hippocampal cell proliferation and decreased survival of newly born neurons in these animals (Andero et al., 2014; O’Leary et al., 2018). The gut has also been implicated in VN-mediated hippocampal plasticity. The VN facilitates bidirectional communication between the gut and the brain through complexly branching afferent fibers that extensively cover the stomach and receive substantial sensory data (Breit et al., 2018; O’Leary et al., 2018). Evidence from mouse studies support the role of the gut microbiota in influencing hippocampal neuronal plasticity, reducing depression-like behaviors, and altering protein expression in the hippocampus (O’Leary et al., 2018; Bravo et al., 2011). Additionally, the VN processes gut signals, such as mechanical distension and nu
留言 (0)