Nematode infestations significantly threaten global agriculture, causing substantial economic losses of over USD 80 billion annually (Nicol et al., 2011; Abd-Elgawad, 2024). Plant-parasitic nematodes (PPNs) are highly diverse and include various species, such as root-knot nematodes (Meloidogyne spp.), cyst nematodes (Heterodera and Globodera spp.), lesion nematodes (Pratylenchus spp.), and reniform nematodes (Rotylenchulus reniformis). These nematodes exhibit unique parasitic mechanisms. Hence, their management in agricultural systems is challenging.
Root-knot nematodes invade root tissues and induce the formation of specialized feeding structures called giant cells, diverting host resources and stunting plant growth. Bacillus subtilis produces nematicidal enzymes, such as proteases, which degrade nematode cuticles, reducing mobility and infectivity. Secondary metabolites, such as fengycin and surfactin, exhibit potent activity by disrupting nematode cell membranes, causing cell lysis and death (Jiang et al., 2021). Moreover, these metabolites inhibit egg hatching and juvenile development, effectively suppressing the nematode life cycle. On the other hand, cyst nematodes form syncytia in root tissues, resulting in long-term nutrient extraction. Lesion nematodes produce migratory lesions that compromise root integrity and increase susceptibility to secondary infections (Gupta et al., 2023). These adaptations result in yield losses, with root-knot nematodes alone accounting for an estimated loss of over 5% globally. Their adaptability and multiple life cycles in warm climates exacerbate this damage (Subbotin et al., 2021). Similarly, cyst nematodes survive under unfavorable conditions by forming resilient cysts containing eggs, enabling extended dormancy in the soil (Moens et al., 2018). B. amyloliquefaciens plays a crucial role in managing cyst nematodes by inducing systemic resistance in plants, thereby suppressing the formation of syncytia within root tissues. This bacterium also produces chitinases to degrade cyst shells, preventing hatching and subsequent infestations (Ngalimat et al., 2021). Given these functions of Bacillus spp. and their role in improving plant vigor, they are effective against cyst nematodes in diverse agricultural systems.
The survival strategies of nematodes demand tailored management approaches that account for the distinct biological traits of each group. For instance, root-knot nematodes secrete effector proteins that suppress key host plant defense pathways, such as those mediated by jasmonic acid (JA) and salicylic acid (SA), while cyst nematodes release effector proteins that alter root architecture to facilitate syncytium formation (Ahmad et al., 2021). Moreover, lesion nematodes disrupt cell walls enzymatically, contributing to extensive root decay. Understanding these intricate molecular interactions is crucial for devising effective and sustainable management strategies.
Traditional control methods, such as crop rotation, the use of resistant cultivars, and the use of chemical nematicides, are limited by the biological versatility of nematodes and the environmental concerns associated with chemical usage. The ability of root-knot nematodes to overcome resistant cultivars further complicates breeding efforts (Pradhan et al., 2023). Moreover, although chemical nematicides are initially effective, they pose risks to nontarget organisms and contribute to environmental degradation (Kumar et al., 2017). These limitations underscore the need for safer, eco-friendly alternatives.
Recent advances in biocontrol have demonstrated the potential of Bacillus spp. in combating specific PPNs. Bacillus spp. employ various mechanisms, such as the production of nematicidal metabolites (e.g., lipopeptides and proteases), the induction of systemic resistance in plants, and competition with nematodes for resources (Patil et al., 2019; Jiang et al., 2021). For instance, B. subtilis produces fengycin and surfactin lipopeptides, which disrupt root-knot nematode cuticles, while B. amyloliquefaciens induces systemic resistance in plants, enhancing defenses against cyst nematodes (Lin et al., 2020). Understanding the mechanisms underlying these distinct interactions is crucial for optimizing their applications in nematode management programs and ensuring that they also contribute positively to soil health. This review emphasizes the targeted use of Bacillus spp. against root-knot and cyst nematodes, detailing their distinct survival strategies and biocontrol mechanisms.
Given the diversity of PPNs and the limitations of conventional management strategies, this review focuses on Bacillus spp. as biocontrol agents, discussing their mechanisms, efficacy, and potential for integration into sustainable nematode management programs. The discussion covers multiple PPNs, focusing on crop nematodes, especially root-knot, cyst, lesion, and reniform nematodes. The literature is sourced from reputable databases, including Elsevier, Springer, and MDPI, ensuring the inclusion of high-quality and relevant studies.
Major phytopathogenic nematodes in global agriculturePhytopathogenic nematodes pose a significant threat to global agriculture. They impact a wide range of crops by feeding on plant roots, disrupting nutrient uptake, and serving as vectors for other pathogens. The most harmful genera include Meloidogyne, Heterodera, Globodera, Pratylenchus, Radopholus, Rotylenchulus, Ditylenchus, and Bursaphelenchus, each exhibiting unique life cycles, modes of action, and seasonal habitats that contribute to pathogenicity (Mesa-Valle et al., 2020; Palomares-Rius et al., 2020).
Root-knot nematodes (Meloidogyne spp.), including M. incognita, M. javanica, and M. arenaria, are particularly damaging. Their life cycles progress from eggs to infective juveniles and adults, with juveniles primarily causing damage by penetrating plant roots. These nematodes thrive in warm climates and cause peak damage during spring and summer, contributing to significant yield losses in various crops, such as tomatoes, soybeans, and cotton in Brazil, China, and other regions (Blouin et al., 1998; Subbotin et al., 2021). Cyst nematodes (Heterodera and Globodera spp.) pose unique challenges because of their ability to form cysts containing eggs. Consequently, they can survive for long durations under adverse conditions. The soybean cyst nematode H. glycines and the golden potato cyst nematode G. rostochiensis cause substantial crop losses, particularly in temperate regions. Their dormant cysts hatch under favorable environmental conditions, typically in spring, aligning with the planting season (He et al., 2022). Lesion nematodes (Pratylenchus spp.) are migratory endoparasites that create lesions in root tissues as they feed, significantly impairing plant health. These nematodes are active throughout the year in warm, moist environments, such as those in tropical agricultural regions, causing severe yield losses in various crops, such as banana, coffee, and soybean (Saikai and MacGuidwin, 2022; Riascos-Ortiz et al., 2022). Similarly, burrowing nematodes (Radopholus similis) and stem nematodes (Ditylenchus dipsaci) exhibit seasonal activity, with the former thriving in wet tropical climates and the latter affecting bulbous plants in cooler climates (Mathew and Opperman, 2019; Sturhan and Brzeski, 2020). The global burden of these nematodes is substantial. Hence, there is an urgent need for sustainable, effective management strategies to mitigate the impact of these nematodes on global food security.
Traditional methods of nematode control and their limitationsTraditional nematode management approaches, including cultural practices, biocontrol methods, and chemical treatments, have been widely implemented to mitigate the detrimental effects of nematodes and maintain crop health and productivity (Elango et al., 2020). Cultural methods, such as crop rotation, soil solarization, and sanitation, aim to interrupt the life cycle of nematodes, thereby diminishing their populations in the soil (Oka, 2010). Biocontrol methods leverage natural predators and antagonistic plants to maintain the ecological balance of nematode populations (El-Saadony et al., 2021). Chemical treatments, which involve the application of nematicides, can directly target nematodes and rapidly reduce their populations.
Despite their extensive use, these conventional methods have several limitations that undermine their long-term efficacy and sustainability (Sikora and Roberts, 2018). Although cultural practices, such as crop rotation, are theoretically effective, they require extensive knowledge and labor and can yield inconsistent results because of environmental variations (Grubišić et al., 2018). Biocontrol methods, including the use of antagonistic plants, such as marigold (Tagetes spp.) and neem (Azadirachta indica), offer environmentally friendly alternatives; however, they often fail to exhibit adequate suppressive effects and may require considerable time to be effective (Waller and Thamsborg, 2004). Moreover, the efficacy of biocontrol methods can significantly vary depending on the species involved and the environmental conditions.
Although chemical treatments provide rapid and effective nematode control, they pose significant risks to human health, nontarget organisms, and the environment. The persistent use of nematicides has led to the emergence of resistant nematode strains, thereby diminishing their long-term effectiveness (Timper, 2014). The regulatory restrictions posed on many effective nematicides because of their adverse environmental impacts have further limited their availability and use (Grubišić et al., 2018).
These inherent limitations of traditional nematode control methods highlight the need for innovative and sustainable approaches. Integrated pest management (IPM) strategies that combine traditional practices with modern technological advancements present a promising solution. These strategies aim to enhance the effectiveness of nematode control while minimizing the associated environmental and health risks.
Biocontrol strategies for nematodes with a focus on Bacillus sppBiocontrol strategies are being recognized as sustainable and environmentally friendly alternatives to chemical nematicides for managing nematode infestations. Various microbial agents and botanical extracts have shown potential for reducing nematode populations. For instance, fungal strains, such as Auxarthron reticulatum DY-2, Verticillium saksenae A-1, Lecanicillium psalliotae A-1, and L. antillanum B-3, have been explored for their effectiveness in parasitizing and reducing nematode populations (Oh et al., 2014a,b; Nguyen et al., 2014). Additionally, extracts of Cinnamomum cassia bark and C. aromaticum have demonstrated enzyme-inhibitory and nematicidal properties, thereby serving as potential agents for botanical interventions (Nguyen et al., 2009, 2012; Nguyen and Jung, 2014). Nguyen et al. (2011) demonstrated that treatment with C. cassia crude extracts significantly reduced gall formation and nematode growth in a dose-dependent manner in root-knot nematode-infested cucumber plants. This treatment also enhanced the activities of antioxidative enzymes, such as SOD, CAT, and APX, in cucumber leaves, indicating a strengthened defense response against the nematode. Furthermore, bark extracts of Terminalia nigrovenulosa and related compounds have been found to disrupt nematode life cycles (Seo et al., 2013).
In addition to fungi and botanical extracts, entomopathogenic nematodes (EPNs), such as Steinernema and Heterorhabditis spp., are known for their ability to release symbiotic bacteria (e.g., Xenorhabdus and Photorhabdus spp.) that produce toxins lethal to nematodes (El Aimani et al., 2022). Furthermore, predatory fungi, such as Paecilomyces and Arthrobotrys spp., can trap and digest nematodes, while endophytic fungi, such as Trichoderma spp., can colonize plant roots and produce enzymes and metabolites that can inhibit nematode activity and enhance plant resistance (Singh et al., 2019). The incorporation of organic amendments, such as compost and green manure, into the soil can also boost the populations of beneficial microbes that compete with or directly antagonize nematodes. These biocontrol strategies can not only reduce the reliance on chemical nematicides but also promote sustainable agricultural practices by enhancing soil health and biodiversity. The schematic representation of comparison of chemical pesticide-based nematode management with Bacillus-based biocontrol approaches, showcasing differences in mode of action, scalability, production costs, environmental impacts, non-target species effects, soil health, economic value, and sustainability was displayed (Figure 1).
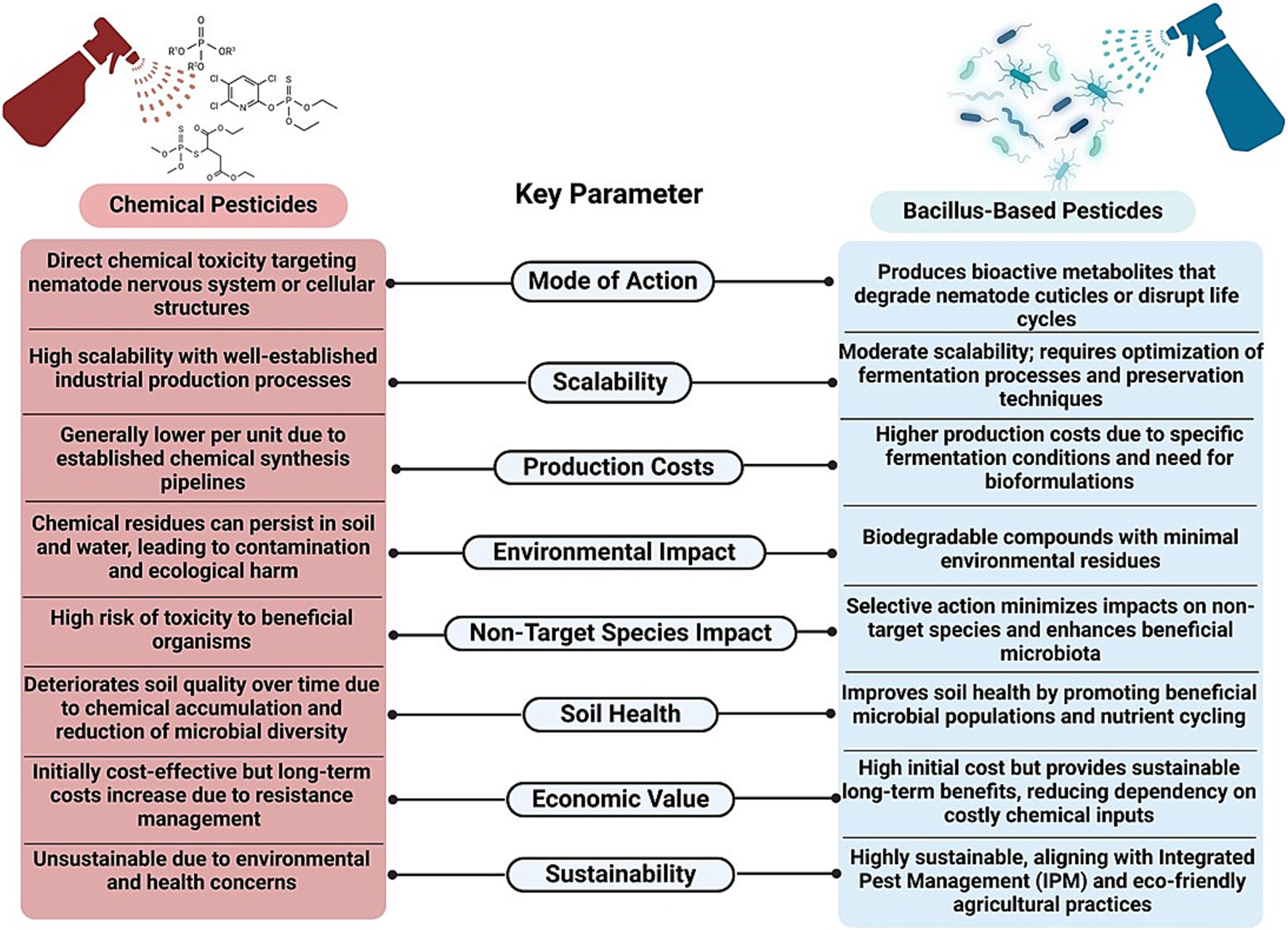
Figure 1. Schematic representation of comparison of conventional and Bacillus-based nematode management strategies.
Bacterial antagonists are among the most promising biocontrol agents. They suppress PPNs through multiple mechanisms, including the production of nematicidal lipopeptides, such as surfactin and fengycin, which disrupt nematode cuticles and membranes. Bacillus spp. produce various enzymes, such as chitinases and proteases, which degrade nematode eggshells and cuticles, effectively reducing juvenile development and reproduction (Yang et al., 2013). In particular, B. subtilis triggers systemic resistance in plants by activating JA and SA signaling pathways, thereby enhancing the natural defenses of plants against nematode attacks (Chowdhury et al., 2015). The antagonistic effects of Paenibacillus elgii HOA73 and P. illinoisensis KJA-424 were evaluated through in vitro nematicidal assays and greenhouse experiments. Key methodologies included assessing nematode motility and mortality using bacterial supernatants and evaluating the activity of enzymes, such as chitinases and proteases. Greenhouse trials confirmed reductions in nematode gall formation and reproduction in infested tomato plants (Jung et al., 2002; Nguyen et al., 2013). Bacillus spp., in particular, are a diverse group of gram-positive, rod-shaped, endospore-forming bacteria commonly found in soil and plant environments. They can produce various bioactive compounds, including enzymes, antibiotics, and toxins, which enhance their effectiveness in controlling plant pathogens and promoting plant health (El Aimani et al., 2022). Some Bacillus spp. are notably effective against nematodes and other plant pathogens, making them valuable for sustainable agricultural practices.
Bacillus spp. produce various nematicidal compounds, including lipopeptides, proteases, and chitinases, which target nematodes at various life stages (Tran et al., 2019). These soil-dwelling bacteria produce spores that can endure extreme environmental conditions, making them ideal candidates for sustainable nematode management (Singh et al., 2019). They can directly antagonize nematodes by producing toxins, enzymes, and other bioactive compounds that impact nematode mobility, development, and reproduction (Migunova and Sasanelli, 2021). Bacillus spp., such as B. thuringiensis (Bt) and B. firmus, have been extensively studied for their nematicidal activities (Zuckerman et al., 1993). For instance, Bt produces crystal (Cry) proteins that are toxic to a broad range of nematodes and can cause cell lysis and death upon ingestion (Forghani and Hajihassani, 2020). Similarly, B. firmus produces enzymes and secondary metabolites that degrade the nematode cuticle and interfere with physiological processes. The use of Bacillus spp. not only reduces the reliance on chemical nematicides, thereby mitigating environmental impacts, but also promotes soil health by maintaining beneficial microbial populations (Tran et al., 2019).
Bacillus spp. can effectively manage PPN infestations through various biocontrol strategies (Tian et al., 2007; Gamalero and Glick, 2020; Diyapoglu et al., 2022). The nematicidal activity of B. subtilis was assessed through in vitro bioassays focusing on lipopeptides, such as surfactin and fengycin, which can cause significant disruption of nematode cell membranes, resulting in mortality (El Aimani et al., 2022). Similarly, studies on B. amyloliquefaciens have revealed its efficacy in IPM programs. By producing antifungal and antibacterial metabolites, the bacterium could exhibit dual efficacy against PPNs and secondary infections in plants under controlled and field conditions (Cetintas et al., 2018). These strategies highlight the versatility of Bacillus spp. as biocontrol agents through multiple mechanisms, including direct toxicity, the inhibition of nematode development, and the enhancement of plant resistance. These bacteria also induce systemic resistance in plants, enhancing their defensive capabilities against nematode attacks (Yang et al., 2022). They produce chitinase and other enzymes that can degrade nematode eggshells, thereby reducing hatching rates and subsequent infection levels. Field trials have also revealed that formulations containing Bacillus spp. can significantly reduce root galling and improve plant health, demonstrating their practical applicability in agricultural settings (Forghani and Hajihassani, 2020).
In summary, Bacillus spp. employ various proteins and secondary metabolites to exhibit nematicidal effects. The key proteins include Cry proteins from Bt, which act by forming pores in the gut cells of nematodes, causing cell lysis and death (Forghani and Hajihassani, 2020; Diyapoglu et al., 2022). B. firmus produces chitinase, an enzyme that breaks down chitin in nematode eggshells, thereby preventing hatching and reducing nematode populations (Tran et al., 2019). Additionally, B. subtilis produces lipopeptides, such as surfactin and fengycin, which disrupt nematode cell membranes, causing the loss of cell integrity and cell death (El Aimani et al., 2022). B. amyloliquefaciens produces proteases, which degrade nematode cuticles and interfere with their physiological processes, resulting in reduced viability and reproduction (Cetintas et al., 2018). The primary modes of action through which Bacillus spp. target nematodes include direct toxicity by producing toxins and enzymes, the inhibition of egg hatching and juvenile development, the induction of systemic resistance in plants, and the disruption of physiological processes by degrading structural components (e.g., cuticles) and interfering with metabolic pathways essential for nematode survival (Shafi et al., 2017). The detailed mechanisms of action underlying the efficacy of Bacillus spp. against PPNs are presented in Figure 2.
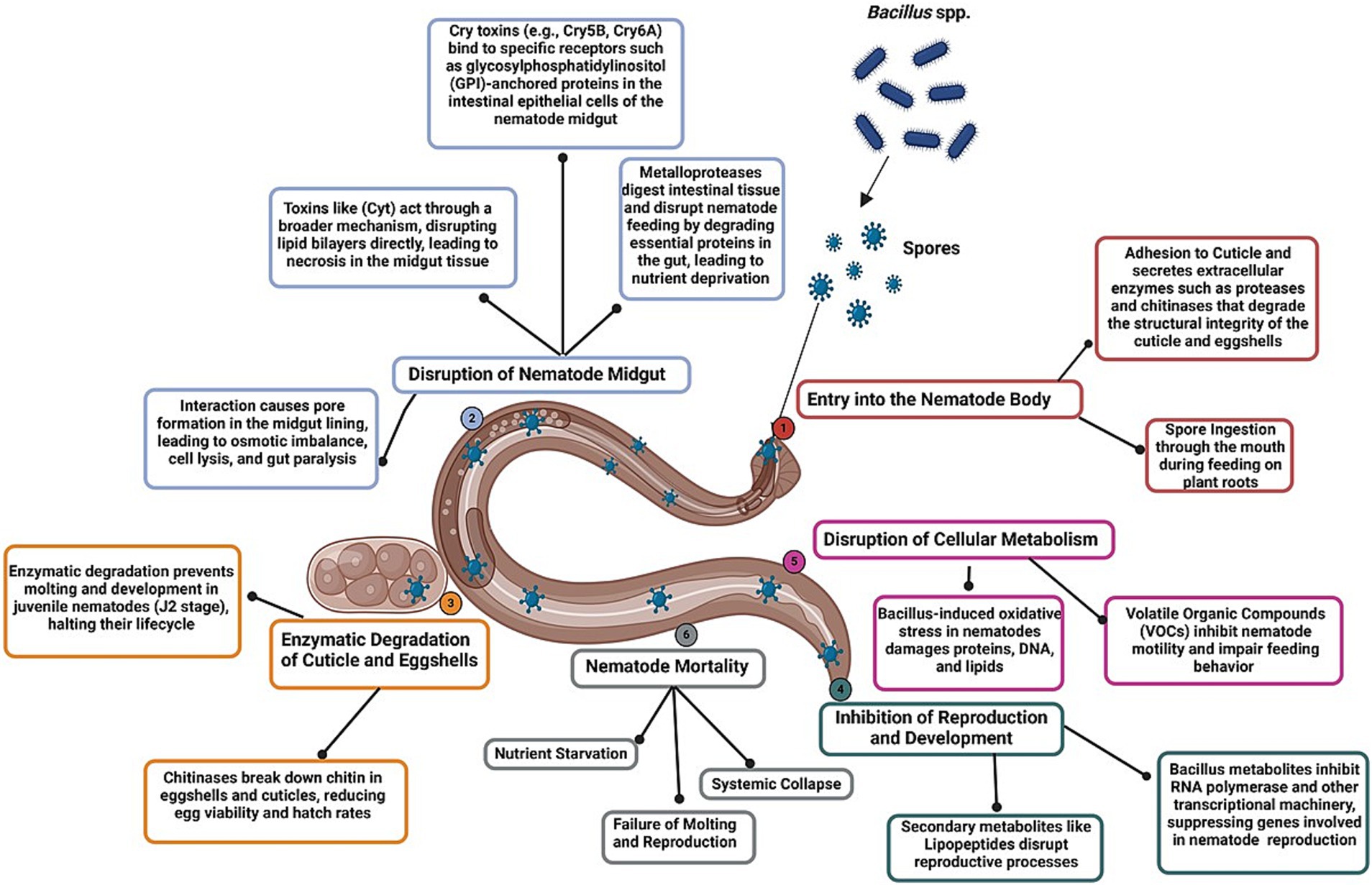
Figure 2. Mode of action of Bacillus spp. against plant-parasitic nematodes. The figure illustrates the sequential mechanisms of Bacillus species, including the entry of spores into the nematode body via ingestion or adhesion to the cuticle, enzymatic degradation of structural components (such as cuticles and eggshells), and disruption of intestinal cells through Cry and Cyt toxin-induced pore formation. The figure also highlights the inhibition of nematode reproduction, the disruption of cellular metabolism, and systemic physiological collapse, ultimately resulting in nematode mortality.
Historical perspective on the use of Bacillus spp. as biocontrol agentsThe historical development of Bacillus spp. as biocontrol agents against plant pathogens, particularly nematodes, highlights significant advancements in scientific understanding and practical applications. Bacillus spp. were first identified by Ferdinand Cohn in the late 19th century. Early research highlighted their roles in improving soil health and promoting plant growth through the production of nematicidal compounds, such as enzymes and secondary metabolites (Brzezinska et al., 2020).
The mid-20th century marked a pivotal advancement with the discovery of Bt and its insecticidal Cry proteins, forming the foundation for experimental biocontrol applications (Sanahuja et al., 2011). A timeline highlighting significant milestones in the development of Bacillus species as biocontrol agents, from their initial discovery to advancements in genetic engineering and sustainable agricultural practices, emphasizing their expanding role in integrated pest management, is presented (Figure 3). Initial studies on nematode management focused on nematicidal compounds, such as chitinases and proteases, (Bacon et al., 2006). By the 1970s and 1980s, researchers identified specific toxins and enzymes produced by Bacillus spp., revealing their targeted actions against nematodes (Van Frankenhuyzen, 2009, 2013). Field trials in the 1990s evaluated the efficacy of Bacillus-based biocontrol agents under various environmental and agronomic conditions. These studies highlighted the importance of application methods, soil properties, and microbial interactions in achieving consistent nematode suppression (Etesami et al., 2023; Serrão et al., 2024). With advancements in genomic technologies, researchers unraveled genes and regulatory pathways responsible for the biocontrol properties of Bacillus spp. in the early 21st century. This enabled the development of genetically enhanced strains with improved efficacy and environmental resilience (Carmona-Hernandez et al., 2019). Given the commercial success of Bacillus-based products, these biocontrol agents were further integrated into IPM systems, offering sustainable alternatives to chemical nematicides (Castillo et al., 2013). Current research underscores the role of Bacillus spp. in promoting soil biodiversity and enhancing plant microbiomes, which contribute to long-term nematode suppression (Calvo et al., 2010). Biotechnological advances, including CRISPR and synthetic biology, have further expanded the potential of Bacillus spp., enhancing their stability, specificity, and ability to produce nematicidal compounds (Baptista et al., 2022). Key Bacillus spp., including Bt, B. subtilis, and B. cereus, are crucial because they produce diverse nematicidal compounds, such as Cry proteins, chitinases, and lipopeptides, which exhibit broad-spectrum activity against nematodes (Jouzani et al., 2017; Saxena et al., 2020; Ahmad et al., 2021). Comparative studies have demonstrated the unique strengths of Bacillus spp., providing insights into their compatibility with specific crops and soil environments. For example, B. subtilis induces systemic resistance in plants, Bt acts through direct toxin-mediated gut disruption, and B. cereus enhances soil health through microbial synergism (Diyapoglu et al., 2022; Tran et al., 2019). This historical trajectory highlights the evolution of Bacillus spp. from their initial discovery to becoming cornerstones of sustainable agriculture. The roles of Bacillus spp. in nematode biocontrol highlight their potential as integral components of IPM strategies, addressing key challenges in plant health management (Sanahuja et al., 2011; Raymond and Federici, 2017).
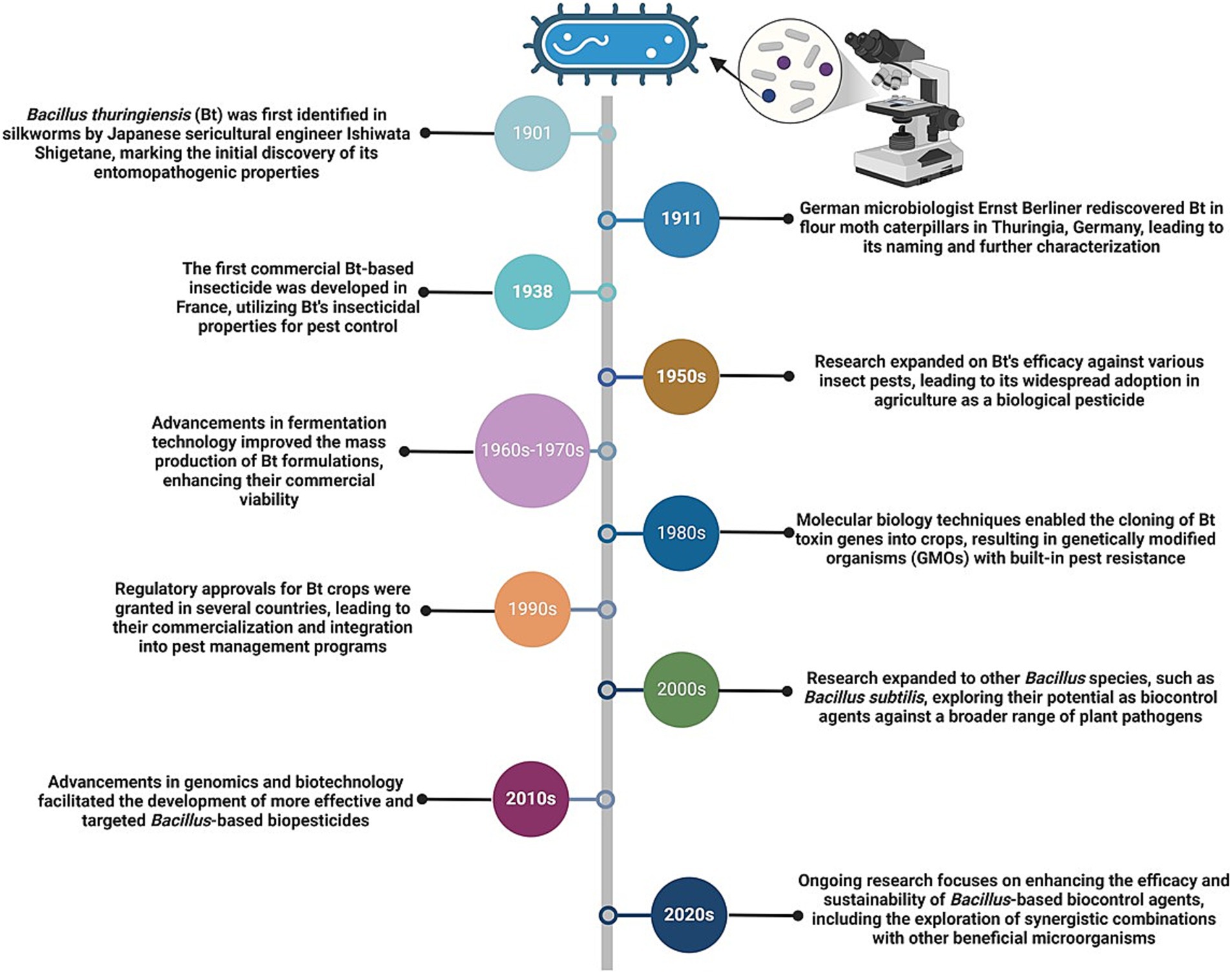
Figure 3. Timeline of Bacillus species development as biocontrol agents. This timeline highlights significant milestones in the development of Bacillus species as biocontrol agents, from their initial discovery to advancements in genetic engineering and sustainable agricultural practices.
Key Bacillus spp. and their efficacy against nematodes BtBt is widely recognized for its potent nematicidal activity, primarily mediated by the production of insecticidal Cry and cytolytic (Cyt) proteins. These proteins, synthesized as protoxins during sporulation, exhibit significant efficacy against various PPNs, including Meloidogyne and Heterodera spp. (Verduzco-Rosas et al., 2021; Kahn et al., 2021). Experimental studies on the efficacy of Bt toxins generally utilize nematode bioassays, in which second-stage juveniles (J2) of Meloidogyne spp. are exposed to varying concentrations of Cry and Cyt proteins under controlled environmental conditions. Mortality, hatching inhibition, and mobility reduction are the commonly measured endpoints in such studies. Upon ingestion, the alkaline gut environment of nematodes solubilizes these protoxins, which are then activated by specific gut proteases. The activated Cry proteins bind to gut epithelial receptors, such as cadherin-like proteins, aminopeptidases, and alkaline phosphatases, inducing structural changes that facilitate membrane insertion and pore formation (Griffitts et al., 2005; Schnepf et al., 1998). This pore formation disrupts osmotic balance, causing cell lysis, gut paralysis, and eventual nematode death due to starvation or secondary infections (Bravo et al., 2007). Cyt proteins complement Cry proteins by targeting the lipid components of nematode cell membranes, thereby inducing cell lysis through distinct pore-forming mechanisms (Gill et al., 1992; Wei et al., 2003). In laboratory assays, Cry5B has been found to interact with glycosylphosphatidylinositol-anchored proteins in the gut cells of M. incognita, causing cell swelling and epithelial rupture. Cry6A specifically targets aspartyl protease and alkaline phosphatase receptors, initiating apoptosis and disrupting gut integrity (Barros dos Santos et al., 2022; Shi et al., 2020). These experiments typically involve histological analysis of nematode midgut tissues and the use of advanced imaging techniques to confirm receptor interactions and cellular damage. The specificity and effectiveness of Bt toxins vary among nematode species because of differences in gut receptor structures and proteolytic activation. Nematodes can use innate defenses, such as enzyme detoxification and pH modulation, to mitigate Bt toxicity, highlighting the complexity of host–pathogen interactions (Zhang et al., 2012). These interactions underscore the versatility and adaptability of Bt in managing diverse nematode infestations. Advances in molecular biology have facilitated the engineering of transgenic crops expressing Cry proteins, conferring continuous protection against nematodes. For example, in field trials, transgenic rice expressing Cry6A exhibited significant resistance to M. graminicola, with the nematode populations decreasing by 80% and yield improving by 30% (Lilley et al., 2011; Berlitz et al., 2014). Such experiments typically involve randomized field plots, and the efficacy of treatments is compared with those of chemical nematicides and untreated controls. Nematode population dynamics and yield data are analyzed to assess efficacy. The integration of Bt formulations with organic amendments, such as chitin or neem extracts, can further enhance their efficacy through synergistic effects (Chen et al., 2000; Radwan, 2007). Field applications of Bt-based biopesticides can be evaluated using standardized protocols. For instance, Cry55A-containing formulations have shown notable efficacy in reducing M. incognita populations under greenhouse and field conditions, with Cry55A-treated soil exhibiting 70% lower nematode gall formation than untreated controls. These findings highlight the potential of Cry55A as a soil inoculant (Manivannan et al., 2019; Ramalakshmi et al., 2020). Innovative delivery systems, such as seed treatments and soil inoculants, ensure early and sustained activity throughout the growing season (Etesami et al., 2023). These advancements align with sustainable agricultural practices, offering an eco-friendly alternative to chemical nematicides (Hui et al., 2012; Chen et al., 2024). Given its robust mechanisms of action, adaptability to various nematode species, and compatibility with sustainable practices, Bt plays a crucial role in modern nematode management frameworks. Comparative insights across species and delivery systems underscore its effectiveness as a cornerstone of nematode biocontrol strategies.
B. subtilisB. subtilis, a versatile PGPR, exhibits remarkable efficacy against PPNs through diverse mechanisms. This bacterium produces lipopeptides, such as surfactins, fengycins, and iturins, which disrupt nematode cell membranes, causing cell lysis and death (Heerklotz and Seelig, 2007; Henry et al., 2011). In vitro studies can confirm these effects by exposing Meloidogyne juveniles to purified lipopeptides and assessing mortality through microscopic observations and viability staining. Additionally, B. subtilis secretes hydrolytic enzymes, such as chitinases and proteases, which degrade nematode eggshells and cuticles, thereby inhibiting juvenile emergence and reproduction (Hu et al., 2007; Huang et al., 2008). Enzymatic activity is often assessed using substrate degradation assays, in which enzymatic activity is correlated with nematode population decline. B. subtilis also induces systemic resistance in plants by activating JA and SA pathways, thereby enhancing the production of phenolics and defense proteins that limit nematode penetration (Adiwena et al., 2023). In greenhouse studies, RT-qPCR and phenolic quantification assays can be used to validate these responses. Volatile organic compounds (VOCs), such as 2,3-butanediol and acetoin, further suppress nematode motility and reproduction while promoting rhizosphere health (Henry et al., 2011). These VOCs can be identified through GC–MS analysis, and their inhibitory effects can be confirmed by performing bioassays. The applications of B. subtilis include seed treatments, soil drenching, and foliar sprays. Seed treatments ensure early root colonization, while soil drenching targets root zones for sustained nematode suppression. Foliar sprays activate induced systemic resistance (ISR) pathways, indirectly reducing nematode infestations (Barnawal et al., 2017; Basiouny and Abo-Zaid, 2018). In field trials, these methods can be assessed through randomized designs to monitor nematode levels and yield improvements. When integrated into IPM frameworks, B. subtilis performs synergistically with organic amendments and other biocontrol agents, enhancing efficacy and promoting soil health (Cavalcanti et al., 2024). These combined strategies can maximize nematicidal potential and support sustainable agriculture. The multifaceted actions of B. subtilis highlight its pivotal role in reducing nematode infestations and promoting eco-friendly pest management practices.
B. cereusB. cereus exhibits robust nematicidal activity against PPNs through diverse mechanisms. It secretes metalloproteinases, such as neutral protease (Npr) and bacillolysin (BlyA), which degrade nematode cuticle proteins, thereby causing structural collapse and death (Yin et al., 2021a,b; Kulkova et al., 2023). Enzyme assays have confirmed the degradation of nematode cuticles, correlating enzymatic activity with nematode mortality. Lipopeptides, such as surfactin and fengycin, disrupt nematode cell membranes via pore formation, causing cell leakage and lysis (Tong-Jian et al., 2013; Hu et al., 2020). Fluorescent dyes have been used to validate membrane disruption.
B. cereus also produces siderophores, such as bacillibactin, which can chelate iron, thereby depriving nematodes of essential nutrients (Köhl et al., 2019). Furthermore, they produce bacteriocins, such as cerein, which can act as antibiotics and target nematode cellular processes. Bioassays have confirmed nutrient depletion and reduced viability in treated nematodes. Nano-bioformulations have further improved the stability and bioavailability of these bioactive compounds, ensuring prolonged nematode suppression in diverse soils (Kumar et al., 2021). Field trials have highlighted their extended activity and reduced application frequencies. Optimized delivery methods include soil drenching, seed treatments, and foliar sprays. Soil drenching ensures uniform root-zone colonization, while seed treatments enable early protection during crucial growth stages (Ahmed et al., 2019). Randomized trials have revealed significant reductions in M. incognita populations and improvements in yield. When combined with mycorrhizal fungi, B. cereus exhibits synergistic effects, enhancing soil microbial diversity and plant resilience (Hu et al., 2017). Genetic engineering approaches, including CRISPR, are being used to enhance the production of bioactive compounds and target-specific nematicidal properties (Mohamed et al., 2021). Through its diverse mechanisms of action, including enzyme secretion, nutrient competition, and direct nematode disruption, B. cereus offers a sustainable biocontrol option for PPN management. Its integration into IPM strategies and compatibility with sustainable agriculture highlight its crucial role in reducing chemical nematicide usage while improving crop health and productivity.
B. megateriumB. megaterium is a robust biocontrol agent that has been proven to be effective against PPNs by producing various bioactive compounds and enzymes. It secretes proteases, such as neutral and serine proteases, which degrade structural proteins in nematode cuticles, causing severe damage and death (Padgham and Sikora, 2007). Lipopeptides, such as surfactin and iturin, disrupt nematode cell membranes through pore formation, causing cell leakage and lysis (Pueyo et al., 2009). Additionally, B. megaterium synthesizes siderophores, such as bacillibactin, which can chelate iron in the rhizosphere, thereby depriving nematodes of vital nutrients and suppressing their populations while promoting a balanced microbial community. These processes have been validated through enzyme assays, correlating siderophore activity with nematode suppression (Huang et al., 2010). Nano-bioformulations have further enhanced the stability and bioavailability of B. megaterium metabolites, ensuring prolonged nematode suppression and reduced application frequency (Kumar et al., 2021). Various application techniques, including soil drenching and seed treatments, have been optimized for efficient delivery. Soil drenching ensures deep root penetration, while seed treatments facilitate early root colonization, offering sustained protection during crucial growth stages (Padgham and Sikora, 2007; Raza et al., 2024). These strategies have been effective against root-knot nematodes, such as M. incognita, significantly improving plant health and yields in field trials (Mostafa et al., 2018). Genetic engineering approaches, such as the overexpression of genes responsible for lipopeptide synthesis and VOC production, have been employed to enhance nematicidal efficacy. These efforts have shown promise in increasing activity against nematodes while maintaining environmental safety (Grage et al., 2017; Hartz et al., 2021). Through its multifaceted nematicidal mechanisms, B. megaterium serves as an eco-friendly alternative to chemical nematicides. Its adaptability and integration into IPM strategies make it a cornerstone of sustainable pest management. It can support agricultural productivity while minimizing environmental impacts.
B. pumilusB. pumilus employs diverse nematicidal mechanisms, making it a powerful biocontrol agent against PPNs. It acts by secreting proteolytic enzymes, such as subtilisin, which can degrade nematode cuticle proteins, causing osmotic imbalance and eventual death (Ramezani Moghaddam et al., 2014). Lipopeptides, such as pumilacidin and bacilysin, disrupt nematode cell membranes and induce pore formation, ion leakage, and cytoplasmic efflux, thereby causing rapid cell lysis (Dobrzyński et al., 2023). B. pumilus also synthesizes siderophores, such as bacillibactin, which can chelate iron and other essential nutrients, depriving nematodes of crucial resources and fostering beneficial microbial competition in the rhizosphere (Lee et al., 2016). Additionally, B. pumilus produces antimicrobial compounds, including bacteriocins, which disrupt nematode metabolic pathways. A guanidine compound from B. pumilus strain LYMC-3 exhibited potent activity against Bursaphelenchus xylophilus; the LC50 values were 113.5 and 62.5 mg/L after 24 and 48 h, respectively, highlighting its targeted efficacy (Li et al., 2018). Nano-bioformulations have improved the stability and bioavailability of B. pumilus metabolites, ensuring consistent nematode suppression in different agricultural conditions (Mahmoud et al., 2016). B. pumilus differentiates itself by integrating siderophore-mediated nutrient deprivation with enzymatic and antimicrobial strategies, unlike Bt (which relies on Cry proteins) or B. cereus (which relies on lipopeptides). Its compatibility with agronomic practices, such as seed treatments and soil drenching, facilitates early root colonization and uniform metabolite distribution, enhancing field performance. Furthermore, its synergy with mycorrhizal fungi and other beneficial microbes enhances nutrient cycling and plant resilience, creating a holistic defense against nematodes (Carriel and Soto, 2022). Through its multifaceted actions and adaptability, B. pumilus exhibits significant potential for integration into IPM strategies. Further research on genetic optimization, delivery systems, and formulations is warranted to sustainably maximize its agricultural impact.
B. licheniformisB. licheniformis employs diverse mechanisms, including enzymatic degradation, antimicrobial activity, and soil microbiome modulation, to manage PPNs. Its nematicidal activity is attributed to the secretion of hydrolytic enzymes, such as proteases and chitinases, which target the cuticles and eggshells of nematodes, impairing their mobility, reproduction, and viability (Park et al., 2015). For example, strain MH48 effectively degrades nematode structures, particularly in B. xylophilus (Jeong et al., 2015). Additionally, B. licheniformis produces lipopeptides, such as bacillomycin and fengycin, which disrupt nematode and fungal cell membranes, causing ion leakage and cytoplasmic loss. Thus, it exhibits dual functionality as a biocontrol agent (Stoica et al., 2019). B. licheniformis strains, such as strain XF32, have exhibited enhanced production of fengycin through genetic modifications, highlighting their potential for agricultural and industrial applications (Zhaojian et al., 2021). Furthermore, strain JF-22 was found to reduce M. incognita populations and enrich beneficial microbial communities in tomato rhizospheres, promoting soil health and plant resilience (Du et al., 2022). Unlike Bt, which relies on Cry proteins, or B. pumilus, which relies on nutrient deprivation, B. licheniformis integrates enzymatic lysis with microbiome enhancement to suppress nematodes. It also supports plant defenses indirectly. Studies have indicated its ability to bolster the resistance of C. elegans to bacterial infections through hormonal signaling pathways, such as those involving serotonin, suggesting its potential for inducing systemic resistance in plants (Yun et al., 2014). Advances in genetic engineering, such as promoter and ribosome binding site engineering, have increased the capacity of B. licheniformis to produce antimicrobial compounds and enzymes, enhancing its biocontrol potential (Xiao et al., 2024). Field trials have highlighted its dual role in managing nematodes and promoting plant growth. For instance, strain MH48 was found to reduce fungal infections and improve nutrient availability in pine seedlings (Won et al., 2018). The synergy of B. licheniformis with other biocontrol agents further enhances its effectiveness in IPM strategies.
B. firmusB. firmus exhibits remarkable versatility in suppressing nematode populations and enhancing plant growth. As an alkaliphilic, endospore-forming bacterium, it thrives in various soil environments, making it suitable for diverse agricultural systems (Settu et al., 2024). It is distinguished from other Bacillus spp. by its ability to colonize plant roots and induce systemic resistance, exhibiting both direct nematicidal effects and indirect plant-protective effects (Huang et al., 2021). A primary mode of action of B. firmus involves the production of lytic enzymes, such as chitinases and proteases. These enzymes target the structural integrity of nematode eggshells and cuticles, resulting in the degradation and reduced viability of eggs and juveniles. Genomic studies on B. firmus strains, such as strain TNAU1, have identified genes like chiA and chiB, which are involved in the synthesis of chitinase, an enzyme crucial for breaking down the chitinous components of nematode structures (Settu et al., 2024). This enzymatic degradation not only disrupts nematode development but also facilitates nutrient recycling in the rhizosphere, indirectly benefiting plant health. Moreover, B. firmus produces antimicrobial peptides, including surfactin and fengycin, which disrupt nematode cell membranes. These lipopeptides interact with membrane lipids, forming pores that cause ion imbalance, cytoplasmic leakage, and eventual nematode death (Daulagala, 2021). For example, strain YBf-10 can significantly reduce M. incognita populations by producing these bioactive compounds, effectively suppressing nematode-induced damage, such as gall formation and egg mass production (Xiong et al., 2015). Among Bacillus spp., B. firmus is distinguished by its efficacy in reducing nematode reproductive potential. Strain I-1582, widely studied for its nematicidal efficacy, can suppress egg hatching and juvenile viability by producing proteases and secondary metabolites. These metabolites interfere with nematode signaling pathways essential for reproduction and development, offering a comprehensive mechanism for population control (Huang et al., 2021). Furthermore, B. firmus promotes plant growth by enhancing nutrient uptake and root colonization, thereby effectively mitigating the damage caused by nematode infestations. Comparative analyses have revealed that B. firmus differentiates itself from other Bacillus spp. through its robust adaptability to diverse soil pH levels and its ability to induce systemic resistance. Unlike Bt, which relies on Cry proteins for specific gut receptor targeting, or B. subtilis, which is known for its VOC-mediated effects, B. firmus integrates multiple mechanisms, including enzymatic degradation, lipopeptide production, and systemic resistance induction, to combat nematodes and support plant health. The dual role of B. firmus in nematode suppression and plant growth promotion highlights its suitability for sustainable agricultural practices. Recent advancements in genomic studies have further elucidated the biocontrol potential of B. firmus. For instance, strain TNAU1 harbors genes encoding nematode-virulent proteases and other antimicrobial compounds, which can enhance its specificity and efficacy against PPNs. Additionally, B. firmus YBf-10 can modulate microbial communities in the rhizosphere, enriching beneficial microbes and suppressing harmful pathogens. Thus, it can play a role in IPM strategies (Marin-Bruzos et al., 2021). Field applications of B. firmus include soil drenching and seed treatments, which ensure effective delivery of bioactive compounds to nematode hotspots. Pot experiments using soil-drenched YBf-10 revealed substantial reductions in nematode populations and an increase in overall plant growth, showcasing its practical applicability in real-world agricultural systems (Xiong et al., 2015). B. firmus employs a multifaceted approach involving enzymatic lysis, antimicrobial activity, and systemic resistance induction for managing nematodes. Its ability to thrive in diverse soil environments, its biocontrol efficacy, and its plant growth-promoting properties underscore its potential as a key agent in sustainable nematode management and IPM strategies.
B. nematocidaB. nematocida is a spore-forming bacterium with distinct nematicidal properties. Thus, it is a pivotal agent for managing PPNs. This bacterium is predominantly found in soil and plant rhizospheres. It utilizes a multifaceted approach involving enzymatic, biochemical, and molecular strategies, which collectively contribute to its efficacy (Huang et al., 2005). Its nematicidal action is attributed to its ability to secrete lytic enzymes, such as chitinases and proteases, which are encoded by genes like chiA, chiB, aprE, and nprB. These enzymes target and damage the structural integrity of nematode eggshells and cuticles, directly impairing nematode survival and reproduction. The breakdown of these protective structures not only suppresses nematode populations but also releases essential nutrients, thereby enhancing soil fertility (Sun et al., 2024). Moreover, B. nematocida produces antimicrobial lipopeptides, such as fengycin, surfactin, and bacillomycin. These bioactive metabolites disrupt nematode cell membranes by interfering with lipid bilayers, resulting in pore formation, ion leakage, and eventual mortality (Niu et al., 2006; Niu et al., 2011; Niu et al., 2016; Bo et al., 2022). This biochemical disruption demonstrates the potent antagonistic effects of the bacterium on nematode physiology. A unique aspect of the mode of action of B. nematocida is the synthesis of 2-heptanone, a volatile compound that acts as a nematode attractant. These chemical lures nematodes toward the bacterium, enhancing its ability to target and infect nematodes with high precision. This mechanism exemplifies an evolutionary adaptation for host–pathogen interactions, as highlighted by Zhu et al. (2019). Such attractant-based pathogenicity differentiates B. nematocida from other Bacillus spp., adding a layer of specificity to its biocontrol efficacy. Recent studies have identified adaptive molecular responses in B. nematocida under stress conditions. For example, Sun et al. (2018) reported that protein acetylation modulates the enzymatic activity of the bacterium, enhancing its nematicidal efficacy. This adaptive regulation reflects a dynamic interaction between B. nematocida and its nematode targets, showcasing the ability of the bacterium to respond to environmental stimuli. Comparative analyses have revealed that B. nematocida utilizes a highly specialized approach compared with other Bacillus spp. Unlike B. subtilis, which primarily induces systemic resistance in plants and produces VOCs, or Bt, which relies on Cry proteins for gut-specific toxicity, B. nematocida integrates enzymatic degradation, membrane disruption, and chemical attraction to exhibit nematicidal effects. This multipronged strategy underscores its effectiveness in managing PPNs while minimizing collateral effects on nontarget organisms. The practical application of B. nematocida has shown promising results in field trials, with its soil drench formulations and seed treatments effectively reducing nematode populations and enhancing plant growth. The specificity of B. nematocida for nematodes reduces the ecological risks often associated with broad-spectrum chemical nematicides. Furthermore, its potential for integration into IPM strategies highlights its role in promoting sustainable agriculture. B. nematocida is an advanced biocontrol agent characterized by enzymatic degradation, biochemical toxicity, and adaptive molecular interactions. Its unique mechanisms of action and its specificity for nematodes make it a promising alternative to chemical nematicides, contributing to environmentally sustainable agricultural practices.
B. amyloliquefaciensB. amyloliquefaciens exhibits robust nematicidal activity. It is distinct from other Bacillus spp. because of the production of diverse enzymes and bioactive secondary metabolites. Its efficacy is mainly attributed to its ability to synthesize lipopeptides, such as fengycin and iturin, which disrupt nematode cell membranes. These lipopeptides interact with lipid bilayers and cause pore formation and subsequent cell lysis, resulting in nematode mortality (Ngalimat et al., 2021). Moreover, B. amyloliquefaciens secretes hydrolytic enzymes, such as chitinases and proteases, which enzymatically degrade nematode cuticles and eggshells, thereby inhibiting juvenile development and reducing nematode reproduction rates (Migunova and Sasanelli, 2021). Genomic studies have highlighted the roles of various genes, such as fenA and ituD, in the biosynthesis of these lipopeptides, underscoring the genetic adaptability of the bacterium for biocontrol applications (Luo et al., 2022). In addition to exhibiting direct nematicidal effects, B. amyloliquefaciens significantly contributes to soil health and plant growth. It stimulates plant development by producing phytohormones and promotes nutrient availability by altering the soil microbiome. For instance, VOCs produced by B. amyloliquefaciens not only suppress pathogens but also enhance root growth and nutrient uptake, reinforcing its dual role as a biocontrol agent and a growth promoter (Chowdhury et al., 2015). Strain FZB42 exhibits these attributes by inducing systemic resistance in plants. ISR is achieved through the activation of JA and ethylene (ET) signaling pathways, resulting in the increased production of defense-related enzymes and antimicrobial compounds that protect plants from nematodes and other pathogens (Chowdhury et al., 2015). The genetic manipulation of B. amyloliquefaciens has further enhanced its efficacy. For example, the fusion of B. amyloliquefaciens SA5 with Lysinibacillus sphaericus created a hybrid strain (Bas8) with elevated chitinase production. This strain exhibited significant nematicidal effects against M. incognita in controlled trials (Abdel-Salam et al., 2018). Similarly, Liu et al. (2013) demonstrated that the deletion of the gene RBAM_007470, responsible for the synthesis of plantazolicin, reduced the nematicidal efficacy of strain FZB42, highlighting the importance of specific metabolites in biocontrol strategies. Field and greenhouse trials have substantiated the biocontrol potential of B. amyloliquefaciens. For example, applications of this bacterium at varying concentrations (50–200%) effectively suppressed M. javanica in common beans by inhibiting juvenile hatching and reducing motility. These effects were observed both in vitro and in vivo, showcasing its adaptability across different environmental conditions (Messa et al., 2019). Furthermore, the spiral nematode Helicotylenchus dihystera was effectively controlled in soybean fields treated with B. amyloliquefaciens-based formulations, with the nematicidal effects being comparable to those of chemical nematicides, such as abamectin. Improvements were also noted in soybean yield and soil health (Camatti et al., 2023). Compared with other Bacillus spp., B. amyloliquefaciens uniquely combines potent direct nematicidal mechanisms with plant growth-promoting traits. While Bt primarily relies on Cry proteins for nematode control and B. subtilis relies on systemic resistance induction, B. amyloliquefaciens integrates membrane disruption, enzymatic degradation, and systemic resistance induction, making it a versatile and holistic agent for nematode management. Its ability to modulate the soil microbiome and enhance nutrient cycling further distinguishes it as an indispensable component of sustainable agricultural practices. Overall, B. amyloliquefaciens employs a synergistic blend of biochemical, enzymatic, and ecological strategies to control PPNs and enhance plant health. Continued research on its genetic pathways, interaction mechanisms, and field applications can further enhance its role in IPM and sustainable agriculture (Table 1).
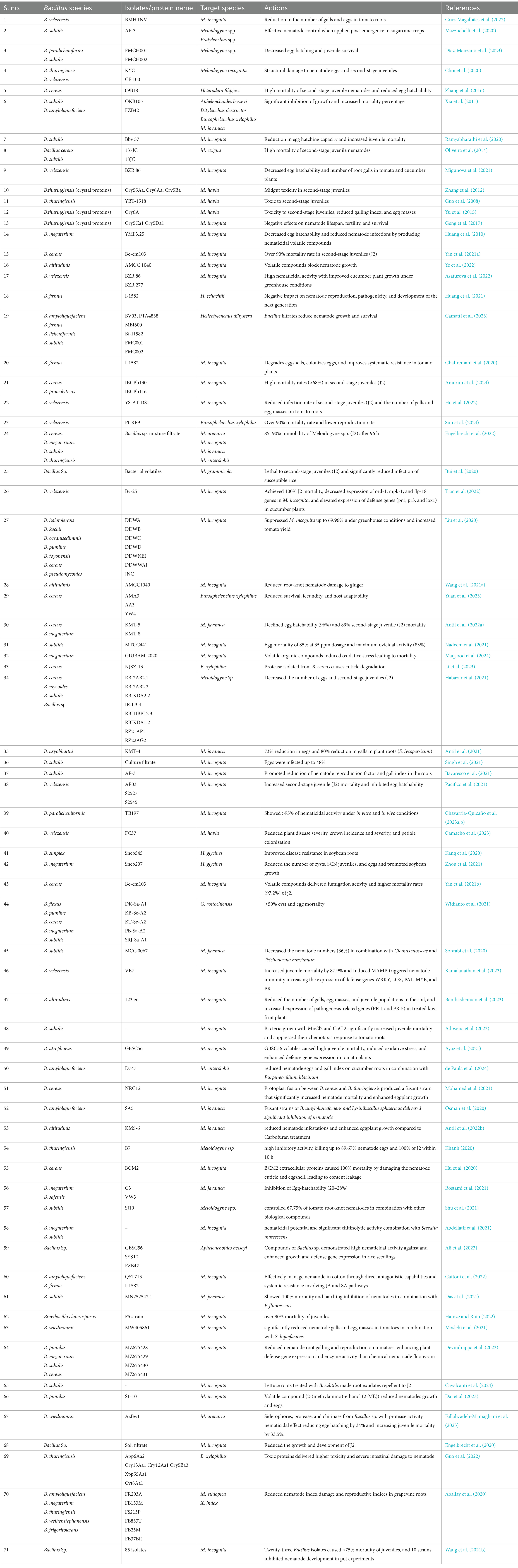
Table 1. Mode of actions of different isolates or proteins from Bacillus species against major pytopathogenic nematodes.
B. velezensisB. velezensis, a species closely related to B. amyloliquefaciens, exhibits substantial nematicidal activity by producing diverse bioactive compounds, making it a key player in sustainable agriculture. Its effects are mainly attributed to the production of lipopeptides (surfactin, fengycin, and iturin), polyketides, and siderophores, which collectively target PPNs and other phytopathogens (Rabbee et al., 2019, 2023). These compounds act by disrupting cell membranes, interfering with metabolic pathways, and creating a hostile environment for pathogens. Moreover, B. velezensis contributes to soil health by promoting beneficial microbial communities and enhancing nutrient cycling, making it a multifunctional agent in IPM systems. The nematicidal efficacy of B. velezensis has been well documented in controlled environments (Wu et al., 2023). For instance, strain YS-AT-DS1 was found to significantly reduce M. incognita infection rates in tomato plants by affecting water and solute transport mediated by TIP genes, without activating the JA or SA pathway (Hu et al., 2022). This finding highlights the unique mode of action of the strain compared with other Bacillus spp., which often rely heavily on ISR through JA/SA pathway activation. Another prominent strain, GB03, has been extensively studied for its ability to enhance plant growth and immunity by producing VOCs that prime plant defenses by inducing systemic resistance (Jang et al., 2023). Strain GB03 is recognized for its practical applications. It has also been validated by the U.S. EPA as
留言 (0)