In newborns, subcutaneous fat necrosis (SCFN) is an uncommon and self-limiting non-infectious panniculitis (1). It typically occurs in full-term newborns in the first weeks of life, following perinatal asphyxia, hypothermia, or obstetrical complications during labor or delivery (2). Hypercalcemia may develop and has been implicated as the cause of severe complications, including nephrocalcinosis, hypertension, vomiting, growth failure, irritability, and even seizures.
Herein, we report two cases of newborns with hypercalcemia and subcutaneous fat necrosis after therapeutic hypothermia (TH) associated with nephrocalcinosis; we discuss these findings with their similarities and differences and review the recent literature.
2 MethodsIn order to review literature, a PubMed (MEDLINE) database search from 1995 onwards was performed using the medical subject headings “subcutaneous fat necrosis” AND “nephrocalcinosis” AND “newborn”; this search revealed few case reports of subcutaneous fat necrosis and nephrocalcinosis in newborns. The search was limited to English for language; the date last searched was March 1st, 2024. Only papers reporting newborns with SCFN and hypercalcemia after therapeutic hypothermia, followed by nephrocalcinosis, were identified in the search.
3 Case presentation 3.1 Patient 1A female term child, born at 39 weeks gestational age to a primigravida mother with gestational diabetes, was delivered by emergency cesarean section because of fetal distress in a level II hospital. The birth weight was 3,850 g (large for gestational age according to the Italian Neonatal Anthropometric Charts (3). Apgar scores were 3, 5, and 7 at 1/5/10 min, respectively. The infant was intubated within 2 min of life and placed on mechanical ventilation for respiratory failure. The cord arterial pH was 6.97, and the base excess was −16.8. The infant was moved to the III-level Neonatal intensive care unit (NICU) of Fondazione Policlinico “A. Gemelli” (Rome, Italy) because of moderate encephalopathy on Sarnat staging to start therapeutic hypothermia within the 6-hour window.
On admission to our NICU, she was cooled to 33.5°C for 72 h according to the national neuroprotective cooling protocol, using whole-body cooling. Blood cultures were drawn, and prophylactic antibiotic therapy was started. She was parenterally nourished during the procedure, and no sequelae were observed. After 72 h of therapeutic hypothermia, gradual rewarming to 37°C was performed; mechanical ventilation was gradually withdrawn. Blood exams were always within normal reference ranges during TH and after rewarming, with no remarks on physical examination. On day 5 of life, some red, painful, non-tender subcutaneous lesions appeared on her back in a wide area up to her bottom. The pain was controlled with acetaminophen.
A clinical diagnosis of SCFN was made. Biopsy of the skin and subcutaneous fat was not done, and SCFN was defined by the presence of characteristic clinical features documented by the examining physician.
Blood cultures and colliquated area swabs remained negative. A metabolic screening revealed a serum calcium of 18.6 mg/dl (normal range: 8.6–10.2 mg/dl), with a normal creatinine level (Figure 1). This observation led to further tests being performed: serum parathormone by radioimmunoassay was suppressed, and 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels were within the normal ranges (Table 1).
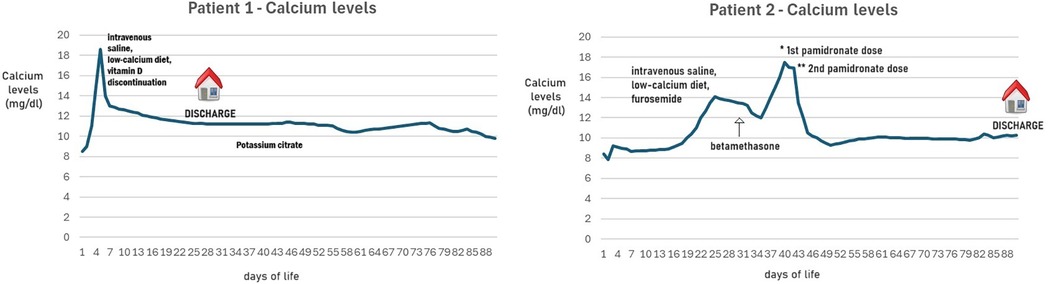
Figure 1. Calcium levels nobserved in patients 1 and 2, treated with TH.
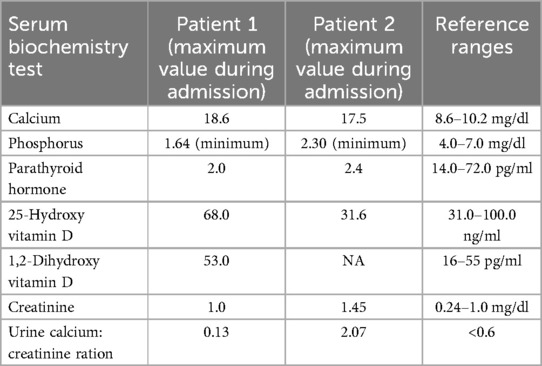
Table 1. Laboratory data of our patients.
Considering asymptomatic hypercalcemia, the child was treated with intravenous saline, a low-calcium diet, and vitamin D discontinuation. Neither corticosteroids nor bisphosphonates were used to normalize hypercalcemia.
Magnetic resonance imaging performed on day 5 of life displayed focal brain ischemia in the left basal ganglia, associated with bilateral white matter ischemic spots and cerebral swelling.
The renal ultrasonography showed, at 20 days of age, a bilateral increased echogenicity of medullary pyramids, suggesting a grade 2 nephrocalcinosis secondary to SCFN and hypercalcemia, although renal function was normal.
The child was discharged after twenty-eight days, with a serum calcium level of 11.2 mg/dl. A urine calcium to creatinine ratio of 0.13 (normal: <0.6) excluded hypercalciuria; no evidence of proteinuria was observed.
During follow-up, her serum calcium remained always normal, but considering the persistence of a grade 2 nephrocalcinosis, the child was treated with a potassium citrate supplementation. At 9 months of age, the skin lesions had almost disappeared; however, the renal ultrasonography assessed the persistence of bilateral nephrocalcinosis despite the potassium citrate treatment.
Follow-up visits up to 18 months of age showed normal growth and a mild neurodevelopmental delay, according to brain injury.
3.2 Patient 2A male child, born at 39 weeks gestational age to a tertigravida mother, was delivered by emergency cesarean section because of fetal bradycardia in a level I hospital. The birth weight was 3,220 g (adequate for gestational age according to Italian Neonatal Anthropometric Charts (3). Apgar scores were 1, 1, and 4 at 1/5/10 min, respectively. The infant needed advanced cardiopulmonary support, and he was intubated within 1 min of life and placed on mechanical ventilation for respiratory failure. The cord arterial pH was 6.89, and the base excess was −24. Neurological examination showed a severe encephalopathy on Sarnat staging. The infant was moved to the III-level NICU of Bambino Gesù Children Hospital (Rome, Italy) to start therapeutic hypothermia within the 6-h window.
On admission to our NICU, he was cooled to 33.5°C for 72 h, using whole-body cooling, according to the national neuroprotective cooling protocol. He was in critical condition and required mechanical ventilation and inotropic treatment to maintain hemodynamic stability. Blood cultures were drawn, and empiric antibiotic therapy was started. He was parenterally nourished during the procedure, and no sequelae were observed. At 12 h of life he presented seizures on the amplified electroencephalography (aEEG) and phenobarbital therapy was started.
After 72 h of therapeutic hypothermia, gradual rewarming to 37°C was performed; mechanical ventilation and inotropic therapy were gradually withdrawn and then suspended at 10 days of life. Blood exams were always within normal reference ranges during TH and after rewarming, with no remarks on physical examination.
Magnetic resonance imaging performed on day 7 of life displayed diffuse signal alteration, with hyperintensity in T2-weighted sequences and restriction of diffusivity of the subcortical and deep white matter in the supratentorial hemispheric area bilaterally, of the corpus callosum, of the posterior limb of the internal capsule and of the external capsule bilaterally, of the basal nuclei and of the thalamic region. Similar lesions were evident in the brainstem and the cerebellar cortico-subcortical area bilaterally.
On day 25 of life, some red, painful, non-tender subcutaneous lesions appeared on his back in a wide area up to the bottom and on the back of his arms bilaterally (Figure 2). The pain was controlled with acetaminophen.
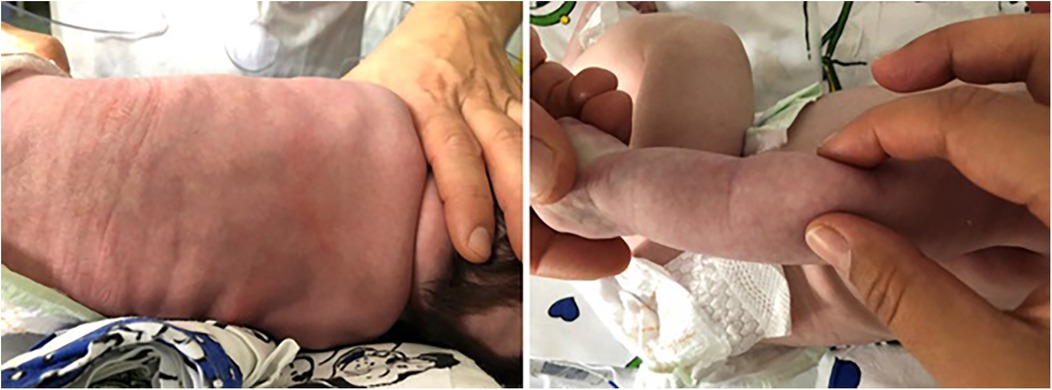
Figure 2. Red, painful, non-tender subcutaneous lesions appeared in patient 2.
A clinical diagnosis of SCFN was made. Biopsy of the skin and subcutaneous fat was not done, and SCFN was defined by the presence of characteristic clinical features documented by the examining physician.
A metabolic screening revealed a serum calcium of 14.1 mg/dl (normal range: 8.6–10.2 mg/dl), with a normal creatinine level (Figure 1). This observation led to further tests being performed: serum parathormone by radioimmunoassay was suppressed, and 25-hydroxyvitamin D level was within the normal ranges (Table 1).
The renal ultrasonography, normal at birth, showed a bilaterally increased echogenicity of medullary pyramids, suggesting a grade 2 nephrocalcinosis secondary to SCFN and hypercalcemia, although the renal function was normal.
Considering asymptomatic hypercalcemia, the child was initially treated with hyperhydration of intravenous saline, a low-calcium diet, and furosemide. After a few days, considering the persistence of hypercalcemia and the pain related to the lesions, a course of betamethasone was started with rapid benefit on the skin lesions. Since calcium levels continued to increase (maximum 17.5) at 40 days of life, pamidronate was used in two doses, the first at 0.25 mg/kg and the second at 0.5 mg/kg after 48 h. Calcium levels came back to normal ranges 72 h after the second dose.
The child was discharged at three months of life with a serum level of calcium of 10.3 mg/dl. The skin lesions had disappeared. A urine calcium to creatinine ratio of 0.17 (normal: <0.6) excluded hypercalciuria; no evidence of proteinuria was observed. Considering the presence of grade 2 nephrocalcinosis, the child was treated with a potassium citrate supplementation.
During follow-up, his serum calcium always remained normal, but the last renal ultrasonography, at 12 months of age, assessed the persistence of bilateral nephrocalcinosis despite the potassium citrate treatment.
Follow-up visits up to 24 months of age showed a severe neurodevelopmental delay with bilateral neurosensorial hypoacusis, according to brain injury.
4 ResultsSixteen cases were identified (3–11), some of them with incomplete information. In Table 2, we systemically collected and summarized information on patients' characteristics, diagnostic features, laboratory values, duration of symptoms, and evolution under treatment and compared them to our case. Different studies described SCFN and hypercalcemia case reports (10, 12), while we reviewed only the cases of newborns with SCFN, hypercalcemia, and associated nephrocalcinosis after TH.
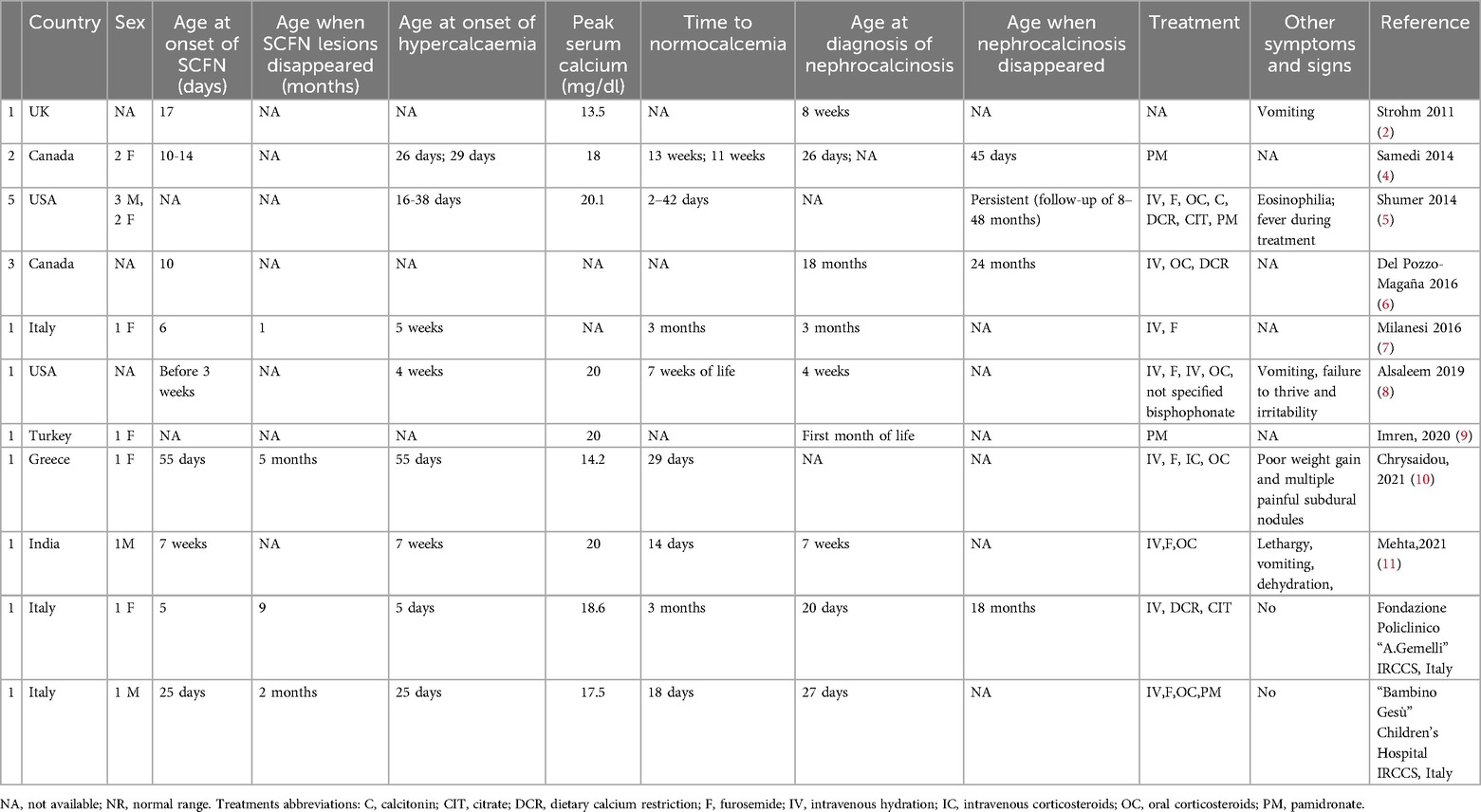
Table 2. Case reports of subcutaneous Fat necrosis and nephrocalcinosis in newborns managed with therapeutic hypothermia.
5 DiscussionThe newborn's subcutaneous fat necrosis (SCFN) is an uncommon transient disorder. Usually, it develops within the first weeks of life in full-term newborns who experience perinatal distress, including hypoxic-ischemic encephalopathy (HIE). SCFN is a noninfectious panniculitis characterized by firm subcutaneous nodules, a few millimeters to several centimeters in size, typically found over the face, back, gluteus, arms, and thighs. Resolution of nodules usually takes place over months. These children could present a potentially life-threatening hypercalcemia–hypercalciuria, whose pathogenesis is still unknown. Hypercalcemia, which presents with irritability, lethargy, hypotonia, poor weight gain, and feeding difficulties, must be treated in time to avoid serious complications, such as nephrocalcinosis, nephrolithiasis, and renal failure. Nephrocalcinosis due to SCFN appears after several weeks or months (12).
To the best of our knowledge, the case of patient 1 was interesting because, beyond the rarity of this association, our patient developed SCFN and hypercalcemia early and almost simultaneously. Hypercalcemia has been previously reported to appear only after resolution of skin lesions (4–10). The age of onset of nephrocalcinosis was also early, at 20 days of life, similar to another Canadian clinical report (4). At the time of writing, nephrocalcinosis was still present in our patient at 18 months of life.
Conversely, the case of patient 2 was interesting because the onset of hypercalcemia and SCFN was delayed after the first week of life, but it was prolonged and resistant to the first line of treatment with hyperhydration and diuretic therapy. The calcium levels start to diminish significantly only after pamidronate treatment. In the second case, nephrocalcinosis was evident at the time of diagnosis of SCFN and was still present at the last follow-up at 12 months of age.
Since the introduction of TH for HIE, a few case reports have been published reporting SCFN and nephrocalcinosis. In the United Kingdom, the Total Body Hypothermia (TOBY trial) cooling registry reported incidence of SCFN in 12 of 1,239 (about 1%) newborns who underwent whole-body cooling treatment for HIE; renal ultrasound scan revealed nephrocalcinosis in one of these twelve (about 0.1%) (2). However, the incidence of SCFN and nephrocalcinosis could be underestimated. In our combined multicenter experience, during a six-year period (2018–2023), we have observed 10 cases of SCFN in a multicenter cohort of 315 HIE newborns (about 3.2%), of whom only 2/10 cases with associated nephrocalcinosis (about 0.6%).
Many authors tried to explain the still unknown pathogenesis of SCFN in newborns with perinatal asphyxia. Impaired tissue perfusion and hypoxemia seem to lead to the crystallization of free fatty acids, with subcutaneous adipose tissue storage of them; as a result, tissue necrosis occurs (13). Some authors suggested that the development of SCFN is related to the biochemical characteristics of neonatal adipose tissue: a wrong ratio of unsaturated fatty acid to saturated fatty acids (such as stearic and palmitic acids) permits a setting in which higher melting and solidification points produce fat crystals and tissue necrosis (14). In cold stress conditions such as hypothermia, neonatal adipose tissue is more susceptible to injury, resulting in necrosis of the skin and granulomatous infiltration (13). The differential diagnoses of SCFN include sclerema neonatorum, erythema nodosum, and bacterial cellulitis (4). Bao et al. summarized in a systematic review that patients with SCFN rarely required surgical management: surgery was indicated when the lesions were hemorrhagic, necrotic, or digressed from an ordinary presentation (resembling an infection or a malignancy) (15).
Common complications associated with SCFN include hypoglycemia, anemia, thrombocytopenia, hypertriglyceridemia, and hypercalcemia. In most SCFN cases reported in the literature, hypercalcemia was reported when skin lesions began to resolve. However, there is little data regarding its clinical course: hypercalcemia was mild and follow-up short (16).
Shumer et al. chose to study infants with severe hypercalcemia, probably the ones at the highest risk of complications, in the largest cohort described to date (5). However, few cases reported the association of SCFN with hypocalcemia (17), but the pathogenesis is unknown; perinatal asphyxia may lead to transient pseudohypoparathyroidism and, ultimately, hypocalcemia (18).
Hypercalcemia is the most serious complication of SCFN and usually occurs between one to six months after the fat necrosis is resolved (16, 19). Severe hypercalcemia is rare, but it should be treated immediately and aggressively to avoid serious complications; in particular, nephrocalcinosis is a common complication. In Shumer's cohort, nephrocalcinosis was present in 5/7 patients (83%) with SCFN and hypercalcemia (5).
Khedr et al. reported a case of occult massive visceral fat necrosis following therapeutic hypothermia in a term infant who expired on the 25th day of life following a neonatal course complicated by severe encephalopathy, pulmonary artery hypertension, persistent thrombocytopenia, hypoglycemia, and severe basal ganglia-thalamic abnormalities on magnetic resonance imaging. The postmortem examination indicated extensive necrosis of fat adjacent to ribs, thymus, kidneys, and pancreas, characterized by widespread adipocyte necrosis, granulomatous inflammation, and scattered microcalcifications. Subcutaneous (white) fat was spared. This case highlights the need for close monitoring of encephalopathic newborns for fat necrosis complications, such as hypercalcemia and nephrocalcinosis, as less severe cases may go unnoticed in hypoxic-ischemic newborns, particularly those treated with hypothermia (20).
Concerning the therapeutic approach, the classic treatment of hypercalcemia includes intravenous hyperhydration and restriction of vitamin D and calcium. Indeed, vitamin D supplementation should be avoided (21). However, this first-line therapy could be insufficient and does not change the natural course of the disease and its complications. So, calcium-wasting diuretics (furosemide) and corticosteroids (prednisolone) are used; however, it was described that they increase renal calcium excretion, raising the risk of nephrocalcinosis (22).
Despite the increasing use of bisphosphonates in children for the treatment of primary and secondary forms of osteoporosis and hypercalcemic disorders, inhibiting activity of osteoclasts and bone resorption, few cases of SCFN and hypercalcemia treated with bisphosphonates are reported (23–25). We suggest discussing the possibility of using bisphosphonates when the first-line therapy fails. Pamidronate has been reported as the first-line treatment for severe hypercalcemia, with hypercalciuria complicating SCFN to prevent and decrease the risk of nephrocalcinosis (21). Because it reduces the renal calcium load, it does not increase the risk of nephrocalcinosis as instead corticosteroids and furosemide do (26, 27).
Militello et al. (28) reported a case of a full-term newborn, admitted at 3 weeks of life for failure to thrive and poor feeding, who had at birth a mild perinatal asphyxia that needed no TH (therefore not included in Table 2). Her serum calcium level was 16.6 mg/dl: a renal ultrasound detected bilateral medullary nephrocalcinosis and only later developed SCFN. After intravenous hydration and treatment with furosemide and then intravenous methylprednisolone, they administered a single low dose of zoledronic acid (0.025 mg/kg), and subsequently, the serum calcium rapidly decreased, subcutaneous nodules progressively decreased, and renal findings improved over time.
The use of bisphosphonates in pediatric patients has been proven safe; however, the risk of possible side effects should be kept in mind: flu-like syndrome, hypocalcemia, atypical femur fractures, osteonecrosis of the jaws, orbital inflammation, and growth impairment (25). Their use in newborns should be carefully weighed because there is still insufficient safety data. In our patient, pamidronate was given after unsuccessfully using the classic treatment regimens. Since patient 2 already had nephrocalcinosis, we preferred to avoid long-term diuretic or steroid medication that would have aggravated the renal status, and we observed no side effects after pamidronate administration.
We are aware that the small sample size, with only two cases of SCFN with associated nephrocalcinosis among a multicenter cohort of 315 HIE infants, limits the possibility of generalizing these findings; furthermore, the retrospective nature of this case series introduces potential biases in data collection. Moreover, although HT appeared to have a protective role towards the kidney, as systematically summarized by van Wincoop et al. (29), the follow-up duration, particularly for nephrocalcinosis, may not be sufficient to assess long-term renal outcomes. Indeed, Frank et al. reported that nephrocalcinosis could also be found years later among infants with SCFN (however, including children with SCFN from different causes) (30).
From the review of the literature and the analysis of our case, we suggest strictly monitoring HIE infants who underwent TH with a clinical and ultrasound follow-up, screening them for SCFN and mainly for hypercalcemia already at the onset of skin lesions and also for the possible development of nephrocalcinosis (12). After discharge, parents of infants treated with TH should be, therefore, instructed about all complications and to consult a physician if any symptom of hypercalcemia develops. Even if initially negative, it could be useful to schedule a renal ultrasound again, for example, 3–6 months later, in case of SCFN and/or hypercalcemia during hospitalization.
6 ConclusionAlthough the association among SCFN, hypercalcemia, and nephrocalcinosis is uncommon, it could be potentially harmful to infants who underwent TH. A multi-disciplinary approach (neonatologist, pediatric endocrinologist, and nephrologist) is required to tailor therapeutic interventions (31). Further studies are needed to evaluate the real incidence of SCFN, hypercalcemia, and nephrocalcinosis in these newborns and standardize the treatment of hypercalcemia to prevent complications.
We suggest that all these infants should be screened for these findings to be treated promptly: our study may help contribute towards a higher level of awareness of this uncommon association.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statementEthical approval was not required for the study involving human samples in accordance with the local legislation and institutional requirements because this preliminary study reported only a retrospective analysis of data available through the Institutional Databases. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributionsDD: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. CM: Conceptualization, Data curation, Investigation, Writing – original draft. GS: Data curation, Investigation, Writing – review & editing. FC: Data curation, Investigation, Writing – review & editing. AG: Data curation, Investigation, Writing – review & editing. LM: Data curation, Investigation, Writing – review & editing. IS: Data curation, Investigation, Writing – review & editing. IB: Data curation, Investigation, Writing – review & editing. GU: Data curation, Investigation, Writing – review & editing. FS: Data curation, Investigation, Writing – review & editing. SC: Data curation, Investigation, Writing – review & editing. AB: Data curation, Investigation, Writing – review & editing. FG: Data curation, Investigation, Writing – review & editing. GV: Data curation, Investigation, Methodology, Supervision, Writing – review & editing. AD: Conceptualization, Data curation, Investigation, Methodology, Supervision, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Italian Ministry of Health with the Current Research funds.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Ladoyanni E, Moss C, Brown RM, Ogboli M. Subcutaneous fat necrosis in a newborn associated with asymptomatic and uncomplicated hypercalcaemia. Pediatr Dermatol. (2009) 26(2):217–9. doi: 10.1111/j.1525-1470.2009.00884.x
PubMed Abstract | Crossref Full Text | Google Scholar
2. Strohm B, Hobson A, Brocklehurst P, Edwards AD, Azzopardi D, UK TOBY Cooling Register. Subcutaneous fat necrosis after moderate therapeutic hypothermia in neonates. Pediatrics. (2011) 128(2): e450–2. doi: 10.1542/peds.2010-3508
PubMed Abstract | Crossref Full Text | Google Scholar
3. Bertino E, Spada E, Occhi L, Coscia A, Giuliani F, Gagliardi L, et al. Neonatal anthropometric charts: the Italian neonatal study compared with other European studies. J Pediatr Gastroenterol Nutr. (2010) 51(3):353–61. doi: 10.1097/MPG.0b013e3181da213e
PubMed Abstract | Crossref Full Text | Google Scholar
4. Samedi VM, Yusuf K, Yee W, Obaid H, Al Awad EH. Neonatal hypercalcaemia secondary to subcutaneous fat necrosis successfully treated with pamidronate: a case series and literature review. Am J Perinatol Rep. (2014) 4:e93–6. doi: 10.1055/s-0034-1395987
Crossref Full Text | Google Scholar
5. Shumer DE, Thaker V, Taylor GA, Wassner AJ. Severe hypercalcaemia due to subcutaneous fat necrosis: presentation, managementand complications. Arch Dis Child Fetal Neonatal Ed. (2014) 99:F419–21. doi: 10.1136/archdischild-2014-306069
PubMed Abstract | Crossref Full Text | Google Scholar
7. Milanesi E, Loda C, Drera B, Poggiani C. Hypercalcaemia and nephrocalcinosis complicating subcutaneous fat necrosis in a newborn after therapeutic hypothermia. Minerva Pediatr. (2016) 68(4):316–7.27277205
PubMed Abstract | Google Scholar
8. Alsaleem M, Saadeh L, Elberson V, Kumar VHS. Subcutaneous fat necrosis, a rare but serious side effect of hypoxic-ischemic encephalopathy and whole-body hypothermia. J Perinat Med. (2019) 47(9):986–90. doi: 10.1515/jpm-2019-0172
PubMed Abstract | Crossref Full Text | Google Scholar
9. İmren IG, Demirkan N, Duygulu Ş. Subcutaneous fat necrosis and severe hypercalcemia as a complication of therapeutic hypothermia in a newborn with asphyxia. Dermatol Ther. (2020) 33(6):e13952. doi: 10.1111/dth.13952
PubMed Abstract | Crossref Full Text | Google Scholar
10. Chrysaidou K, Sargiotis G, Karava V, Liasis D, Gourvas V, Moutsanas V, et al. Subcutaneous fat necrosis and hypercalcemia with nephrocalcinosis in infancy: case report and review of the literature. Children. (2021) 8:374. doi: 10.3390/children8050374
PubMed Abstract | Crossref Full Text | Google Scholar
11. Mehta S, Gupta NP, Batra A, Sharma R. Subcutaneous fat necrosis in an infant with hypoxic ischaemic encephalopathy stage 3: an uncommon association. BMJ Case Rep. (2021) 14(7):e237933. doi: 10.1136/bcr-2020-237933
PubMed Abstract | Crossref Full Text | Google Scholar
12. Stefanko NS, Drolet BA. Subcutaneous fat necrosis of the newborn and associated hypercalcaemia: a systematic review of the literature. Pediatr Dermatol. (2019) 36(1):24–30. doi: 10.1111/pde.13640
PubMed Abstract | Crossref Full Text | Google Scholar
13. Cote NL, Patterson JE. Panniculitis. In: Fitzpatrick JE, Morelli JG, editors. Dermatology Secrets Plus. 3rd ed. Philadelphia: Mosby (2007). p. 156–65.
14. Chuang SD, Chiu HC, Chang CC. Subcutaneous fat necrosis of the newborn complicating hypothermic cardiac surgery. Br J Dermatol. (1995) 132:805–10. doi: 10.1111/j.1365-2133.1995.tb00731.x
PubMed Abstract | Crossref Full Text | Google Scholar
15. Bao E, Villavisanis DF, Ibelli TJ, Levy L, Taub PJ. Subcutaneous fat necrosis of the newborn: a systematic review of surgical management and outcomes. J Plast Reconstr Aesthet Surg. (2024) 91:293–301. doi: 10.1016/j.bjps.2024.02.027
PubMed Abstract | Crossref Full Text | Google Scholar
16. Mahé E, Girszyn N, Hadj-Rabia S, Bodemer C, Hamel-Teillac D, De Prost Y. Subcutaneous fat necrosis of the newborn: a systematic evaluation of risk factors, clinical manifestations, complications and outcome of 16 children. Br J Dermatol. (2007) 156:709–15. doi: 10.1111/j.1365-2133.2007.07782.x
PubMed Abstract | Crossref Full Text | Google Scholar
17. Onyiriuka AN, Utomi TE. Hypocalcemia associated with subcutaneous fat necrosis of the newborn: case report and literature review. Oman Med J. (2017) 32(6):518–21. doi: 10.5001/omj.2017.99
PubMed Abstract | Crossref Full Text | Google Scholar
18. De Rose DU, Perri A, Gallini F, Priolo F, Tiberi E, Vento G, et al. Neonatal transient pseudohypoparathyroidism (ntPHP): could it be included among inactivating PTH/PTH-related protein signalling disorders (iPPSD)? Ann Pediatr Endocrinol Metab. (2019) 24(2):129–32. doi: 10.6065/apem.2019.24.2.129
PubMed Abstract | Crossref Full Text | Google Scholar
19. Borgia F, De Pasquale L, Cacace C, Meo P, Guarneri C, Cannavo SP. Subcutaneous fat necrosis of the newborn: be aware of hypercalcaemia. J Pediatr Child Health. (2006) 42:316–8. doi: 10.1111/j.1440-1754.2006.00862.x
PubMed Abstract | Crossref Full Text | Google Scholar
20. Khedr S, Piskorski A, Bingham AR, Goldstein J, Laptook AR, De Paepe ME. Occult massive visceral fat necrosis following therapeutic hypothermia for neonatal encephalopathy. Pediatr Dev Pathol. (2018) 21(5):502–6. doi: 10.1177/1093526617737881
PubMed Abstract | Crossref Full Text | Google Scholar
21. Improda N, Capalbo D, Poloniato A, Garbetta G, Dituri F, Penta L, et al. Perinatal asphyxia and hypothermic treatment from the endocrine perspective. Front Endocrinol. (2023) 14:1249700. doi: 10.3389/fendo.2023.1249700
PubMed Abstract | Crossref Full Text | Google Scholar
22. Canpolat N, Özdil M, Kuruğoğlu S, Çalışkan S, Sever L. Nephrocalcinosis as a complication of subcutaneous fat necrosis of the newborn. Turk J Pediatr. (2012) 54(6):667–70.23692798
PubMed Abstract | Google Scholar
23. Alos N, Eugène D, Fillion M, Powell J, Kokta V, Chabot G. Pamidronate: treatment for severe hypercalcaemia in neonatal subcutaneous fat necrosis. Horm Res. (2006) 65(6):289–94. doi: 10.1159/000092602
PubMed Abstract | Crossref Full Text | Google Scholar
24. Khan N, Licata A, Rogers D. Intravenous bisphosphonate for hypercalcaemia accompanying subcutaneous fat necrosis: a novel treatment approach. Clin Pediatr (Phila). (2001) 40:217–9. doi: 10.1177/000992280104000407
PubMed Abstract | Crossref Full Text | Google Scholar
25. Lombardi G, Cabano R, Bollani L, Del Forno C, Stronati M. Effectiveness of pamidronate in severe neonatal hypercalcaemia caused by subcutaneous fat necrosis: a case report. Eur J Pediatr. (2009) 168(5):625–7. doi: 10.1007/s00431-008-0797-8
PubMed Abstract | Crossref Full Text | Google Scholar
26. Cranefield DJ, Odd DE, Harding JE, Teele RL. High incidence of nephrocalcinosis in extremely preterm infants treated with dexamethasone. Pediatr Radiol. (2004) 34:138–42. doi: 10.1007/s00247-003-1090-7
PubMed Abstract | Crossref Full Text | Google Scholar
27. Alon US, Scagliotti D, Garola RE. Nephrocalcinosis and nephrolithiasis in infants with congestive heart failure treated with furosemide. J Pediatr. (1994) 125:149–51. doi: 10.1016/S0022-3476(94)70143-1
PubMed Abstract | Crossref Full Text | Google Scholar
28. Militello MA, Re MP, Vitaliti G, Finazzo F, Manzoni P, Vitaliti SM. Use of zoledronic acid in a neonate with subcutaneous fat necrosis complicated with severe, refractory hypercalcemia. Am J Perinatol. (2019) 36(S 02):S134–8. doi: 10.1055/s-0039-1691777
PubMed Abstract | Crossref Full Text | Google Scholar
29. van Wincoop M, de Bijl-Marcus K, Lilien M, van den Hoogen A, Groenendaal F. Effect of therapeutic hypothermia on renal and myocardial function in asphyxiated (near) term neonates: a systematic review and meta-analysis. PLoS One. (2021) 16(2):e0247403. doi: 10.1371/journal.pone.0247403
PubMed Abstract | Crossref Full Text | Google Scholar
30. Frank L, Brandt S, Wabitsch M. Subcutaneous fat necrosis in newborns: a systematic literature review of case reports and model of pathophysiology. Mol Cell Pediatr. (2022) 9(1):18. doi: 10.1186/s40348-022-00151-1
PubMed Abstract | Crossref Full Text | Google Scholar
31. Rodd C, Schwieger-Briel A, Hagmann C, Newborn Brain Society Guidelines and Publications Committee. Subcutaneous fat necrosis associated with hypercalcemia in neonates with neonatal encephalopathy treated with therapeutic hypothermia. Semin Fetal Neonatal Med. (2021) 26(4):101269. doi: 10.1016/j.siny.2021.101269
留言 (0)