Lung cancer remains the primary cause of cancer-related deaths globally, despite significant progress in understanding the aetiology and biology of tumors, as well as the role of enhancing immunologic control and the introduction of advanced treatment modalities (1, 2). Non-small cell lung cancer (NSCLC) is the most common type of lung cancer, accounting for 84% of all lung cancer diagnoses (3, 4). Although chemotherapy remains a common treatment (3, 5), targetable driver gene mutations have revolutionized the therapeutic landscape for patients with advanced NSCLC. The rearranged during transfection (RET) proto-oncogene encodes a transmembrane receptor tyrosine kinase involved in normal embryonic development. RET gene fusions or rearrangements occur in 1%–2% of NSCLC cases (6), particularly in younger (≤ 60 years), nonsmoking patients with adenocarcinoma. These rearrangements may make the cancer more responsive to certain chemotherapy drugs, such as pemetrexed (7). Malignant pleural effusions (MPE) in lung adenocarcinoma frequently harbor RET rearrangements (8). The detection of RET alterations is recommended to identify NSCLC patients who may be eligible for RET inhibitors. Molecular testing techniques available to detect RET rearrangement include next-generation sequencing (NGS), reverse transcription polymerase chain reaction (RT-PCR), fluorescence in situ hybridization (FISH), and immunohistochemistry (9), and the analysis of circulating tumor DNA (ctDNA) (10). Selpercatinib is a novel tyrosine kinase inhibitor that acts as a selective blocker of the activity of the RET protein and its variants. By inhibiting RET, selpercatinib helps to disrupt the signaling pathways that promote cancer growth and survival in cells where RET alterations are present (11). We report a challenging case of a patient treated with selpercatinib in the second line after failure of treatment with chemoimmunotherapy.
Case reportA 59-year-old female patient, a never-smoker, was admitted to our department for acute hypercapnic respiratory failure with right lung consolidation and pleural effusion. Her previous medical history included type II diabetes mellitus, treated with oral hypoglycemics. After thoracentesis, a whole-body CT scan at baseline exhibited a right lower lobe consolidation with enlarged mediastinal lymph nodes, right pleural effusion, and multiple spinal cord lesions. The patient was unable to move from a bed to a standing position, and blood gas analysis showed the presence of respiratory acidosis (pH 7.30), hypercapnia, hypoxemia, and raised lactate (1.8 mmol/L) at rest. Minimal exercise, including a change in position, resulted in severe worsening of hypoxemia. Following mechanical ventilatory support, a chest drain was inserted, and 2 L of brown-colored pleural fluid was removed, with a sample collected for cytological studies. Immunohistochemical analysis of the pleural fluid confirmed the diagnosis of adenocarcinoma and was positive for thyroid transcription factor 1, cytokeratin, low molecular weight cytokeratin, synaptophysin, plasma chromogranin A foci, and cytokeratin 20, and negative for p40 and natural killer CD56. The Ki-67 index was + 30%. Molecular profiling of a cellular sample of pleural effusion resulted in negative findings for epidermal growth factor (EGFR), anaplastic lymphoma kinase (ALK), and proto-oncogene tyrosine-protein kinase 1 (ROS-1); the programmed cell death-ligand 1 (PD-L1) tumor proportion score (TPS) on this sample was between 1% and 49%; RET fusion was not assessed on the tissue sample at this stage. The severe clinical condition of the patient meant that additional invasive procedures to complete diagnosis were contraindicated. A liquid biopsy using the FoundationOne®Liquid CDx assay was performed to analyze ctDNA and obtain genomic information through the NGS method. The panel included over 300 genes, such as RET, EGFR, ROS-1, ALK, mesenchymal-epithelial transition factor (MET), Kirsten rat sarcoma virus (K-RAS), v-raf murine sarcoma viral oncogene homolog B (BRAF), tumor protein p53 (TP53), epidermal growth factor receptor 2 (ERBB2), and fibroblast growth factor receptor 2 (FGFR2). The results revealed RET fusion positivity in the patient’s blood sample. Therefore, the final diagnosis was lung adenocarcinoma, RET fusion-positive, and the clinical stage was IVB (T4N3M1c). Since the Eastern Cooperative Oncology Group performance status (ECOG-PS) at baseline was 4, the patient was only a candidate for best respiratory support care. The chest drain remained in situ for 21 days until the daily pleural drainage volume had sufficiently reduced. Regarding the acute respiratory failure, the patient was initially treated with oxygen therapy via nasal cannula, and due to the worsening of dyspnea, was quickly changed to a high-flow nasal canula (HFNC). The complexity of the case required alternating between HFNC and continuous positive airway pressure (CPAP) cycles. Despite this approach, the patient’s general condition continued to worsen, and blood gas analysis showed acute hypoxemic–hypercapnic respiratory failure with acidosis; therefore, noninvasive ventilation (NIV) with bi-level positive airway pressure (BiPAP) was required. After intensive cardiorespiratory support, a significant improvement in both general and respiratory clinical conditions was achieved (ECOG-PS 2), and the patient was able to start chemotherapy, including cisplatin and pemetrexed, as well as immune check point inhibitors (ICIs) (pembrolizumab). After two cycles of chemoimmunotherapy, the patient experienced severe hematological toxicity [thrombocytopenia and anemia grade 4 according to the Common Terminology Criteria for Adverse Events: CTCAE Ver.5 (12)] and a worsening of the general clinical condition; therefore, we decided to commence second-line therapy with selpercatinib, a tyrosine kinase inhibitor (TKI) selective for RET fusion, at 160 mg bid (ECOG-PS 0-1). However, the treatment plan was agreed upon with the patient. At restaging after 6 months of RET fusion TKI therapy, CT scans documented complete disease response (Figure 1), with no further accumulation of pleural fluid and no evidence of respiratory failure. During treatment with selpercatinib, the patient experienced grade 2 diarrhea and bilateral leg edema, which were managed with dietary changes, antidiarrheals, and diuretics, allowing selpercatinib therapy to continue.
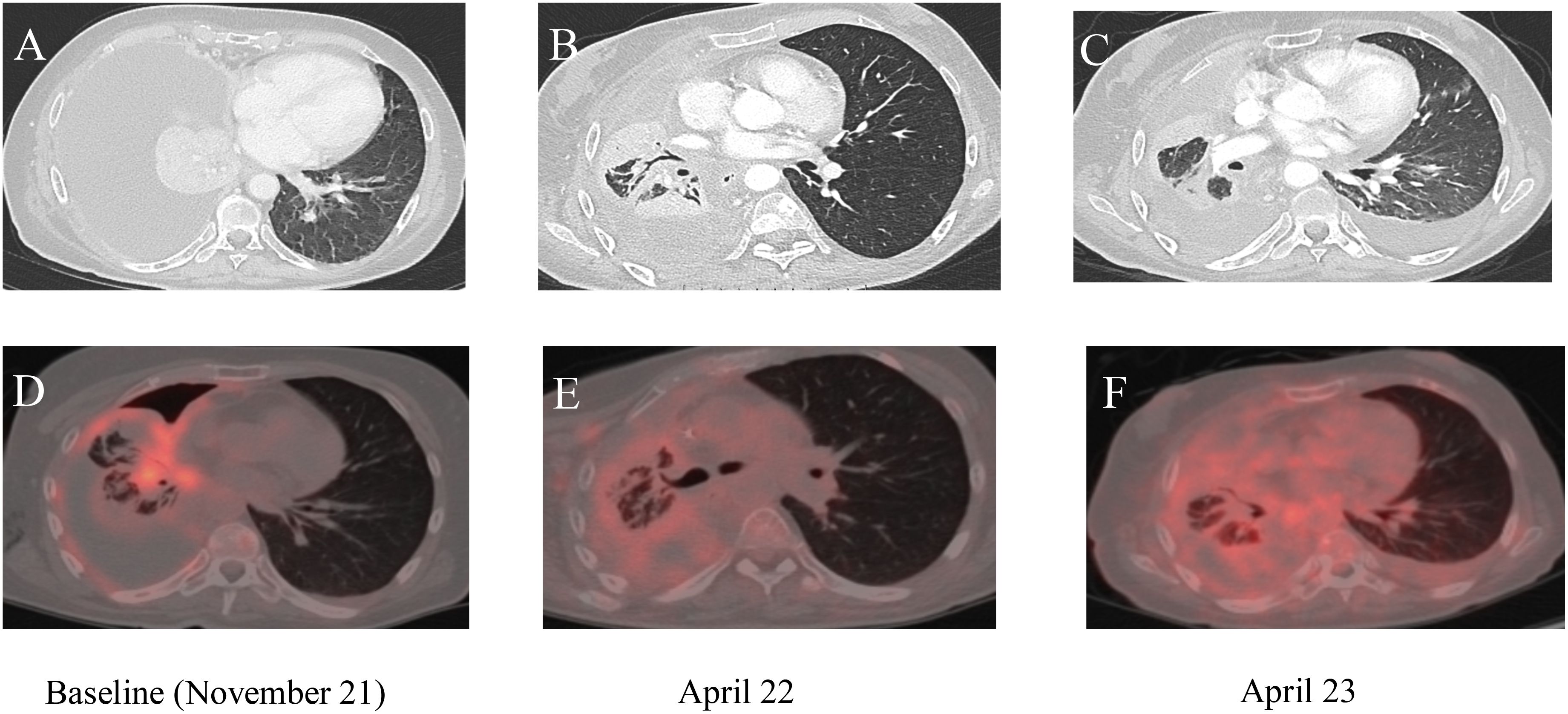
Figure 1. (A–C) Chest computed tomography (CT) images; (D–F) corresponding PET/CT scans. (A, D) Baseline imaging before the start of selpercatinib treatment. (B, E) Imaging results 2 months after the start of treatment. (C, F) Chest CT and PET/CT scans after 14 months of treatment with selpercatinib, highlighting changes in disease progression and response to therapy over time.
After 1 year of treatment with selpercatinib, liver function tests showed an increase in alanine aminotransferase (ALT) and aspartate aminotransferase (AST) (gastrointestinal (GI) toxicity grade 3 according to CTCAE ver.5 (12)), and the CT abdominal scans documented the presence of free fluid in the abdomen. The selpercatinib dose was reduced to 80 mg bid, with transaminase levels dropping to grade 1 within 2 weeks. Therefore, empiric antibiotic therapy was started, and a sample of ascitic fluid was collected for cytological analysis; it showed exclusively fibrin-blood material. After 1 week, the abdominal pain worsened, and abdominal drainage was performed with the removal of approximately 2 L of ascitic fluid. At restaging, the CT confirmed a complete response of the target lesion and an improvement of ascites; therefore, the selpercatinib dose was retitrated to 160 mg bid, and the patient continued selpercatinib medication for about 21 months without major toxicity. The patient died due to metastatic spread of disease to the omentum and peritoneal carcinomatosis, which led to worsening of liver failure. The timeline of treatment is shown in Figure 2.
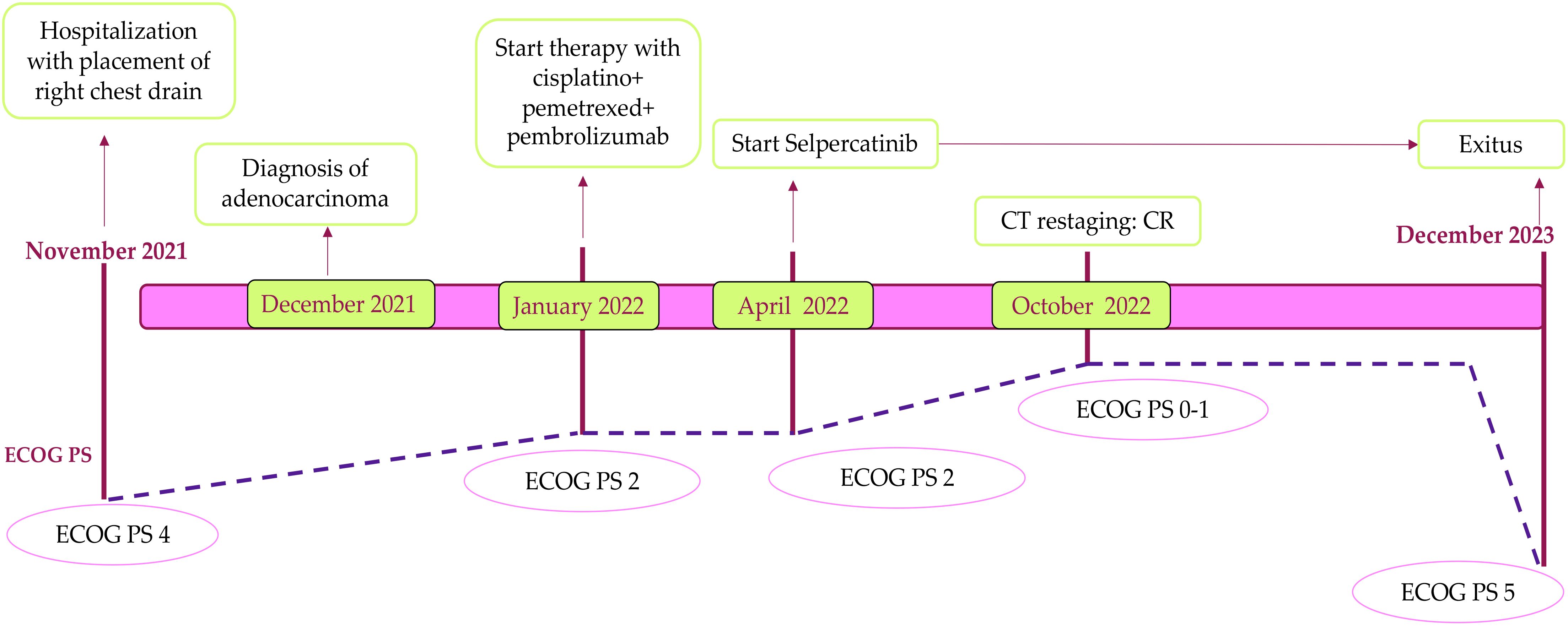
Figure 2. Patient timeline. This figure illustrates the chronological overview of the patient’s treatment and corresponding response, from the baseline in November 2021 through to the time of exit in December 2023. It highlights key points in the patient’s clinical course, including treatment interventions, response assessments, and significant changes in health status. CT, computed tomography; CR, complete response.
DiscussionThis report presents the case of a severely respiratory-compromised patient with grade IV NSCLC harboring a RET fusion mutation, treated with second-line selpercatinib, resulting in a durable response. The patient initially presented with severe clinical conditions (ECOG-PS of 4 at baseline), requiring complex clinical management of respiratory failure and pleural effusion. Significant improvement in clinical status allowed for the initiation of active treatments. During treatment with selpercatinib, grade 3 elevations in transaminase levels and ascites were successfully managed by optimizing the drug dose.
Molecular assessment for RET alterations in NSCLC patients is highly recommended to identify those patients who could potentially benefit from strategies tackling RET inhibition. Various molecular testing methods are available for detecting RET rearrangements. Additionally, the analysis of ctDNA can detect RET rearrangements, offering several benefits such as the ability to test for multiple molecular alterations at once and avoid invasive procedures for patients. Liquid biopsy can be applied to all stages of cancer diagnosis and treatment, allowing noninvasive and real-time monitoring of disease development. To detect these, the NGS method can identify both known and unknown variants exploring genome-wide DNA variations, detecting mutations with a minor allele frequency (MAF) as low as < 1% (13, 14). NGS is increasingly used with targeted panels for highly sensitive detection of specific ctDNA mutations whereas whole genome sequencing (WGS) provides a comprehensive tumor genomic profile, but it is costly and limited when ctDNA concentrations in blood samples are low (15). By enhancing biomarker testing and utilizing targeted therapies, precision medicine can be advanced, although access to these resources may differ between countries. In 2020, the second-generation RET-specific TKIs selpercatinib and pralsetinib were approved by the FDA for RET fusion NSCLC based on the LIBRETTO-001 and ARROW clinical trials (16, 17). In particular, selpercatinib is a first-in-class, highly selective, and potent RET kinase inhibitor with CNS penetration. An updated assessment of the efficacy and safety of selpercatinib in patients with RET fusion-positive NSCLC treated in the phase I/II LI-BRETTO-001 trial showed an overall response rate (ORR) of 84% and 61% in treatment-naive patients and patients with prior platinum-based chemotherapy, respectively. The median progression-free survival (PFS) was 22.0 months for treatment-naive patients and 24.9 months for platinum doublet-pretreated patients (18). Similarly, pralsetinib is a selective and highly potent small molecule inhibitor of wild-type RET and mutated or rearranged RET, with activity against V804 gatekeeper mutations that confer resistance to multikinase inhibitors (19). Regarding safety, the most common high-grade treatment-related adverse events (TRAE) for selpercatinib were high blood pressure (14%), elevated levels of alanine transaminase (ALT) (13%), and aspartate aminotransferase (AST) (10%), while neutropenia (18%), high blood pressure (11%), and anemia (10%) were common for pralsetinib. Most of these side effects did not require drug interruption; however, 30% (selpercatinib) and 38% (pralsetinib) necessitated dose reduction, while a small percentage had to discontinue treatment. Rarely, chylous ascites (CA) may occur as a side effect of RET TKI treatment (20). Given the high efficacy of selective RET inhibitors, it is important to manage side effects to prevent treatment interruption and compromising results. In particular, TKI dose adjustments did not significantly reduce the reaccumulation of effusions (21); however, the interpretation of this result is limited by the small sample size and factors such as home care of indwelling catheters, according to the literature review (22). It will be crucial to assess the effectiveness of different management approaches for chylous effusions in a larger group of patients. In general, for grade ≥ 3 hepatotoxicity, the RET inhibitor should be paused with regular monitoring of ALT/AST until improvement to grade 1, at which point it can be resumed at a reduced dose. In the case of recurrence of hepatoxicity grade ≥ 3, selpercatinib must be discontinued (23). In summary, adverse events of TKIs are usually dose-dependent; the toxicity and effectiveness of TKIs are often linked, meaning that the toxic side effects caused by these drugs can serve as indicators of successful pharmacological inhibition (24). The combined effects of both on- and off-target toxicities can reduce the patient’s quality of life and potentially limit the effective dose of medication (25). Factors such as drug interactions, genetic variations, patient adherence, and the drug’s absorption, distribution, metabolism, and elimination processes must be considered to determine the optimal dosage for each patient (26). Identifying biomarkers that can predict or monitor these toxicities is crucial for optimizing treatment and improving patient outcomes. Some potential biomarkers of toxicity during TKI therapy include circulating cytokines, such as IL-6 and TNF-alpha, which have been associated with inflammatory side effects like skin rashes, gastrointestinal disturbances, and fatigue (27, 28). Monitoring these biomarkers can help identify patients at higher risk for adverse effects, allowing for early intervention and more tailored therapy.
Selpercatinib and pralsetinib are preferred first-line therapy options for patients with RET fusion-positive metastatic NSCLC and are recommended as subsequent therapies if RET inhibitors have not been used in the first-line setting (29). The phase III LIBRETTO-431 trial was designed to define the optimal first-line regimen for patients with RET fusion-positive NSCLC.
ConclusionsIn conclusion, this case report emphasizes the importance of genomic testing across multiple matrices, including liquid biopsy, before excluding any driver gene mutations. It highlights the efficacy and safety of selpercatinib for RET fusion-positive NSCLC. The observed clinical response of our patient further supports the effectiveness of selpercatinib in treating RET fusion-positive NSCLC, even in patients with severe clinical conditions. The long-term durability of response and survival in this challenging case highlights the importance of initiating targeted therapy early for RET fusion-positive patients.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statementWritten informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributionsRP: Writing – original draft. PM: Writing – review & editing. FV: Writing – review & editing. LA: Writing – review & editing. SC: Writing – review & editing. FP: Writing – review & editing. AB: Writing – original draft.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Sung H, Ferlay J, Siegel RL, Laversanne ML, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
PubMed Abstract | Crossref Full Text | Google Scholar
2. Stella GM, Scialò F, Bortolotto C, Agustoni F, Sanci V, Saddi J, et al. Pragmatic expectancy on microbiota and non-small cell lung cancer: A narrative review. Cancer (2022) 14(13). doi: 10.3390/cancers14133131
PubMed Abstract | Crossref Full Text | Google Scholar
3. Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, et al. Cancer statistics for the year 2020: An overview. Int J Cancer. (2021) 149:778–89. doi: 10.1002/ijc.33588
PubMed Abstract | Crossref Full Text | Google Scholar
4. Medusa PM, Gilli M, Notizia L, Pagliaro R, Carro N, Moriello A, et al. Complete response to pembrolizumab as a single agent in a patient with stage III NSCLC with high PD-L1 expression: a case report. Monaldi Arch Chest Dis. (2023) 93:2440. doi: 10.4081/monaldi.2022.2440
PubMed Abstract | Crossref Full Text | Google Scholar
5. Comelia P. Cisplatin/carboplatin + etoposide + vinorelbine in advanced non-small-cell lung cancer: a multicentre randomised trial. Gruppo Oncologico Campano. Br J Cancer. (1996) 74:1805–11. doi: 10.1038/bjc.1996.634
PubMed Abstract | Crossref Full Text | Google Scholar
7. Tsuta K, Kohno T, Yoshida A, Shimada Y, Asamura H, Furuta K, et al. RET-rearranged non-small-cell lung carcinoma: A clinicopathological and molecular analysis. Br J Cancer. (2014) 110:1571–8. doi: 10.1038/bjc.2014.36
PubMed Abstract | Crossref Full Text | Google Scholar
8. Michael F, Newell EW. Parallel identification and profiling of tumour antigen-specific T-cells for biomarker discovery by mass cytometry. Ann Oncol. (2018) 29:ix113. doi: 10.1093/annonc/mdy441
Crossref Full Text | Google Scholar
9. Belli C. ESMO recommendations on the standard methods to detect RET fusions and mutations in daily practice and clinical research. Ann Oncol. (2021) 32:337–50. doi: 10.1016/j.annonc.2020.11.021
PubMed Abstract | Crossref Full Text | Google Scholar
10. Supplee JG, Milan MSD, Lim LP, Potts KT, Sholl LM, Oxnard GR, et al. Sensitivity of next-generation sequencing assays detecting oncogenic fusions in plasma cell-free DNA. Lung Cancer. (2019) 134:96–9. doi: 10.1016/j.lungcan.2019.06.004
PubMed Abstract | Crossref Full Text | Google Scholar
12. Common Terminology Criteria for Adverse Events (CTCAE) Common Terminology Criteria for Adverse Events (CTCAE) v5.0 (2017). Available online at: https://www.meddra.org/ (Accessed November 27, 2017).
14. Gale D, Lawson ARJ, Howarth K, Madi M, Durham B, Smalley S, et al. Development of a highly sensitive liquid biopsy platform to detect clinically-relevant cancer mutations at low allele fractions in cellfree DNA. PloS One. (2018) 13. doi: 10.1371/journal.pone.0194630
PubMed Abstract | Crossref Full Text | Google Scholar
15. Baer C, Kern W, Koch S, Nadarajah N, Schindela S, Meggendorfer M, et al. Ultra-deep sequencing leads to earlier and more sensitive detection of the tyrosine kinase inhibitor resistance mutation T315I in chronic myeloid leukemia. Haematologica. (2016) 101:830–8. doi: 10.3324/haematol.2016.145888
PubMed Abstract | Crossref Full Text | Google Scholar
16. Drilon A, Oxnard GR, Tan DSW, Loong HHF, Johnson M, Gainor J, et al. Efficacy of selpercatinib in RET fusion–positive non–small-cell lung cancer. New Engl J Med. (2020) 383:813–24. doi: 10.1056/nejmoa2005653
PubMed Abstract | Crossref Full Text | Google Scholar
17. Gainor JF, Curigliano G, Kim DW, Lee DH, Besse B, Baik CS, et al. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): a multi-cohort, open-label, phase 1/2 study. Lancet Oncol. (2021) 22:959–69. doi: 10.1016/S1470-2045(21)00247-3
PubMed Abstract | Crossref Full Text | Google Scholar
18. Drilon A, et al. Selpercatinib in patients with RET fusion-positive non-small-cell lung cancer: updated safety and efficacy from the registrational LIBRETTO-001 phase I/II trial. J Clin Oncol. (2022) 41:385–94. doi: 10.1200/JCO.22
PubMed Abstract | Crossref Full Text | Google Scholar
19. Subbiah V, Gainor JF, Rahal R, Brubaker JD, Kim JL, Maynard M, et al. Precision targeted therapy with BLU-667 for RET-driven cancers. Cancer Discovery. (2018) 8:836–49. doi: 10.1158/2159-8290.CD-18-0338
PubMed Abstract | Crossref Full Text | Google Scholar
20. Fricke J, Wang J, Gallego N, Mambetsariev I, Kim P, Babikian R, et al. Selpercatinib and pralsetinib induced chylous ascites in RET-rearranged lung adenocarcinoma: A case series. Clin Lung Cancer. (2023) 24:666–71. doi: 10.1016/j.cllc.2023.08.006
PubMed Abstract | Crossref Full Text | Google Scholar
21. Nardo M, Gouda MA, Nelson BE, Barreto CMN, Slade JH, Poullard A, et al. Strategies for mitigating adverse events related to selective RET inhibitors in patients with RET-altered cancers. Cell Press. (2023). doi: 10.1016/j.xcrm.2023.101332
PubMed Abstract | Crossref Full Text | Google Scholar
22. Kalchiem-Dekel O, Falcon CJ, Bestvina CM, Gainor JF, Drilon A, Lin JJ, et al. Brief report: chylothorax and chylous ascites during RET tyrosine kinase inhibitor therapy. J Thorac Oncol. (2022) 17:1130–6. doi: 10.1016/j.jtho.2022.06.008
PubMed Abstract | Crossref Full Text | Google Scholar
23. Bradford D, Larkins E, Mushti SL, Rodriguez L, Skinner AM, Helms WS, et al. Fda approval summary: Selpercatinib for the treatment of lung and thyroid cancers with ret gene mutations or fusions. Am Assoc Cancer Res Inc. (2021) 2130–5. doi: 10.1158/1078-0432.CCR-20-3558
PubMed Abstract | Crossref Full Text | Google Scholar
24. Moshirfar M, Ronquillo Y, Thomson RJ. Tyrosine Kinase Inhibitors. StatPearls (2024).
26. Terada T, Noda S, Inui KI. Management of dose variability and side effects for individualized cancer pharmacotherapy with tyrosine kinase inhibitors. Pharmacology & Therapeutics (2015) 152:125–34. doi: 10.1016/j.pharmthera.2015.05.009
PubMed Abstract | Crossref Full Text | Google Scholar
27. Yi M, Li T, Niu M, Zhang H, Wu Y, Wu K, et al. Targeting cytokine and chemokine signaling pathways for cancer therapy. Springer nature - Signal Transduction and Targeted Therapy. (2024). doi: 10.1038/s41392-024-01868-3
留言 (0)