Penile cancer is an uncommon genitourinary malignancy that accounts for less than 1% of male cancers in the United States (1). Utilizing the GLOBOCAN database, the estimated incidence rate of penile cancer globally was 0.80 per 100,000 and estimated mortality rate was 0.29 per 100,000 in the year 2020 (2). In other words, there were an estimated 36,068 new cases of penile cancer and 13,211 deaths from penile cancer that year alone. Several factors for the development of penile cancer have been identified including but not limited to smoking, phimosis, obesity, human papilloma virus (HPV), lack of circumcision, and poor personal hygiene (3).
Disparities in incidence based on region of diagnosis have been identified, with higher incidence rates observed in South Asia, Southern Africa, and South America. In fact, the highest incidence rates per 100,000 were estimated to be in the countries of Eswatini (7.0), Uganda (4.6), and Botswana (4.4) (2). Similarly, the highest mortality rates were experienced by Eswatini (3.5 per 100,000) and Uganda (2.4 per 100,000. Indeed, low- and middle-countries appear to shoulder nearly twice the incidence and mortality compared to high-income countries. It is theorized that infectious diseases including HIV and HPV as well as circumcision patterns may explain the differences in incidence of this rare genitourinary malignancy between different regions. Nonetheless, the incidence of penile cancer appears to be increasing in several regions of the world and warrants further attention given its growing burden to healthcare systems globally.
StagingPenile cancer is generally staged using the American Joint Commission on Cancer staging system (8th edition, 2017) incorporating tumor (T), regional lymph nodes (N) and distant metastasis (M) (4, 5). T1 tumors are defined as glans tumors invading the lamina propria, foreskin tumors invading the dermis, lamina propria or dartos fascia, shaft tumors invading connective tissue between the epidermis and corpora, or any site with or without lymphovascular invasion or perineural invasion. T2 tumors are defined as those invading into the corpus spongiosum (either the glands or ventral shaft) with or without the presence of urethral invasion. T3 tumors include those with invasion into the corpora cavernosum including the tunica albuginea with or without the presence of urethral invasion. Finally, T4 tumors invade adjacent pelvic structures such as the prostate, scrotum or pubic bone.
The presence of regional lymph node involvement is critical in the treatment approach of penile cancer. Individuals without palpable or visibly enlarged inguinal lymph nodes are categorized as having cN0 disease, while those with palpable mobile unilateral inguinal lymph nodes are categorized as having cN1 disease. In addition, cN2 disease is defined as the presence of 2 or more palpable mobile unilateral inguinal lymph nodes or bilateral inguinal lymph nodes while cN3 disease is defined as having either unilateral or bilateral fixed inguinal nodes or pelvic lymph nodes.
TreatmentThe treatment approach for penile cancer varies depending on staging, particularly with lymph node involvement or the presence of metastatic disease. In men who have localized T1 disease with low grade tumors may be treated with surgery including local excision, partial penectomy, laser therapy, or radiation therapy. However, men with T2 or greater tumors may be treated with either partial or total penectomy or radiation therapy with or without chemotherapy. For those with low-risk disease, non-palpable inguinal lymph nodes may be monitored with surveillance while in those with T1b or greater disease, non-palpable inguinal lymph nodes may be assessed with bilateral dynamic sentinel node biopsy (DSNB) or treated with bilateral inguinal lymph node dissection (ILND) followed by surveillance. The presence of palpable inguinal or pelvic lymph nodes may warrant percutaneous biopsy and may be treated with either pelvic lymph node dissection (PLND), radiation therapy with or without chemotherapy, or may warrant neoadjuvant chemotherapy such as TIP (paclitaxel, ifosfamide and cisplatin). The detailed management of lymph node positive penile cancer is further described in a recent systemic review by Sachdeva and colleagues (6).
Per the National Comprehensive Cancer Network (NCCN) Guidelines (Version 1.2024), the preferred first-line systemic therapy for metastatic or recurrent penile cancer is TIP. Of note, no randomized controlled trial has been performed in men who had distant metastatic disease as the data for TIP originates from a phase II study including men who had either stage N2 or N3 disease. In the trial, thirty men received four cycles of TIP and had a 50% objective response (7). With a median follow-up of 34 months, the trial found the median time to progression was 8.1 months (95% CI, 5.4 to 50+) while the overall survival was 17.1 months (95% CI, 10.3 to 60+). Among the 30 patients, 20 had died with seventeen of these deaths being attributed to progression of metastatic penile cancer. The other recommended first-line treatment option for metastatic/recurrent penile cancer is 5-fluorouracil (5-FU) in combination cisplatin. The rationale for this combination therapy is based off a pilot study including a total of 8 patients with advanced squamous cell carcinoma of the penis, defined as having either Jackson stages III or IV disease. The study found that 2 (25%) patients had a partial response (8). Given the evidence behind the first-line systemic therapies currently employed in the treatment of metastatic/recurrent penile cancer, we performed a review of the literature to evaluate other systemic therapeutic approaches particularly with the advent of targeted therapies based off genomic aberrations as well as immune checkpoint inhibitors.
Genomic profilingNext generation sequencing has allowed for the rapid identification of potentially actionable genomic alterations in tumor samples. A recent systematic review including 7 studies involving 268 cases of penile squamous cell carcinoma identified TP53, CDKN2A, FAT1, NOTCH-1 and PIK3CA as the most frequently occurring mutations (9). In addition, the study identified alterations in EGFR, which has several corresponding approved therapies in other malignancies such as head and neck cancer as well as non-small cell lung cancer. In one of the largest genomic profile studies to date, 108 samples of penile squamous cell carcinoma were evaluated. The most common mutations identified in the study were as follows in descending order of frequency: TP53 (45.5%), CDKN2A (25.6%), PIK3CA (24.8%), TERT (22.2%), KMT2C (15.9%), NOTCH1 (14.1%), KMT2D (13.6%), FBXW7 (8.8%), NFE2L2 (7.1%), FAT1 (6.9%), NF1 (6.5%), CREBBP (4.4%), and FGFR3 (4.3%) (10). In addition, the study found that 10.7% of tumors had a high tumor mutational burden (TMB) defined as 10 or greater mutations per megabase (Mb), and that 1.1% were microsatellite-high (MSI-H) and as such, may be more responsive to treatment with immune checkpoint inhibitors.
A study of 397 patients with penile squamous cell carcinoma found that 15% of cases had a TMB of 10 mut/Mb or greater, with 4% of tumor specimens harboring a TMB of 20 mut/mb or greater (11). A separate study of 72 cases of penile cancer found PD-L1 expression in 79% of cases, thus further suggestive of the potential responsiveness and rationale for utilizing immune checkpoint inhibitors in treating penile cancer (12). Given genomic profiling suggesting possible clinical benefit, several case series and prospective studies have evaluated the efficacy of targeted therapies and immune checkpoint inhibitors in advanced penile cancer.
A separate retrospective study of patients with penile cancer evaluated the findings of germline testing among 29 patients. The study found that 3 patients (10.3%) harbored pathogenic germline variants including 2 with BRCA2 mutations and one with RAD51C (13). In addition, 16 patients had variants of unknown significance. The study is the first and only known evaluation of the germline mutations in patients with penile cancer, as well as the only known study to identify potentially clinically actionable mutations in those with BRCA2 mutations.
ImmunotherapyIt is estimated that approximately 40% of individuals with cancer in the United States may be eligible for treatment with immune checkpoint inhibitors, which utilize the immune system to kill cancer (14). Given the paucity of data in the treatment of advanced penile cancer, several studies have been undertaken to evaluate the safety and efficacy of immunotherapy in treating this rare malignancy. The first known study to evaluate immune checkpoint inhibitors in advanced penile squamous cell carcinoma involved a patient who was treated with nivolumab after experiencing progression of cancer following chemotherapy and radiation. Treatment with nivolumab led to a partial response with tumor shrinkage, and as such, demonstrated potential efficacy in this patient population (15). A subsequent publication involving two men who had partial penectomy and a combination of chemotherapy and radiation either in the neoadjuvant or adjuvant settings evaluated the efficacy of the immune checkpoint inhibitor pembrolizumab. Both patients’ tumor samples were suggestive of responsiveness to immunotherapy, with one tumor demonstrating a high TMB of 14 mutations/Mb and the second tumor demonstrating positive PD-L1 expression (16). In both instances, patients had a clinical response with the former patient experiencing a complete response for at least 38 months and the latter patient experiencing a partial response for at least 18 months. A separate case series from Hahn and colleagues evaluated three individuals with recurrent, locally advanced or metastatic penile cancer who had experienced progression of cancer on platinum-based chemotherapy. The data from the case series originated from a phase II basket trial for rare malignancies. All three individuals were treated with pembrolizumab, with one patient experiencing a partial response and underwent additional surgery while the two other individuals experiencing progression of their cancer within 3 months of treatment with pembrolizumab (17). Of note, none of the patients experienced grade 3 or worse treatment-related adverse events, thus highlighting the potential safety of this treatment regimen. Finally, the immune checkpoint inhibitor cemiplimab was demonstrated to lead to a complete response in an elderly male with metastatic penile cancer (18). Based off data from these case series and pre-clinical data suggesting potential efficacy for immune checkpoint inhibitors, a limited number of prospective clinical trials have been performed to evaluate the efficacy of immunotherapy in penile cancer.
A multi-center, single-arm phase 2 study evaluated nivolumab plus ipilimumab in patients with advanced rare genitourinary cancers including penile cancer (19). Among the total 55 patients enrolled in the trial, none of the individuals with penile cancer demonstrated a clinical response to treatment with either a partial or complete response. The best response noted among individuals with penile cancer was 2 individuals who experienced stable disease, while 3 experienced progression of their disease.
The first phase II trial evaluating platinum-based chemotherapy plus an immune checkpoint inhibitor was the HERCULES (LACOG 0218) trial (20). In the single arm study conducted at eleven centers in Brazil, a total of 37 patients were enrolled to receive platinum-based chemotherapy plus pembrolizumab for either locally advanced, recurrent or metastatic disease. Of the 33 patients eligible for efficacy analysis, the objective response rate was 39.4% with 1 complete response and 12 partial responses. The response rate was 75% (3 of 4) in patients whose tumors harbored high tumor mutational burden (TMB) status. Moreover, HPV16 positive tumors had a higher response rate (55.6%) in comparison to tumors that were not (35.0%). With a median follow-up of 24.0 months, the median PFS was 5.4 months (95% CI, 2.7-7.2) and median OS was 9.6 months (95% CI, 6.4-13.1). The trial also offered further insight into the most common genomic alterations in the cohort including TP53 (57.1%), CDKN21 (51.4%), and TERT (31.4%).
Despite two positive prospective phase II trials evaluating the use of immunotherapy, the phase II PERICLES trial failed to meet its primary objective of 1-year PFS for the cohort. The study evaluated the efficacy of immune checkpoint inhibitor atezolizumab in 32 patients with stage IVa penile cancer (21). The trial consisted of two cohorts, with cohort A receiving atezolizumab in combination with radiation therapy for those with locoregional disease, and atezolizumab monotherapy in cohort B. The trial found a one-year PFS of 12%, with a median OS of 12 months. Of note, a complete response was noted in two patients with pulmonary metastases. A summary of all clinical trials and case series evaluating the safety and efficacy of immune checkpoint inhibitors is available in Table 1.
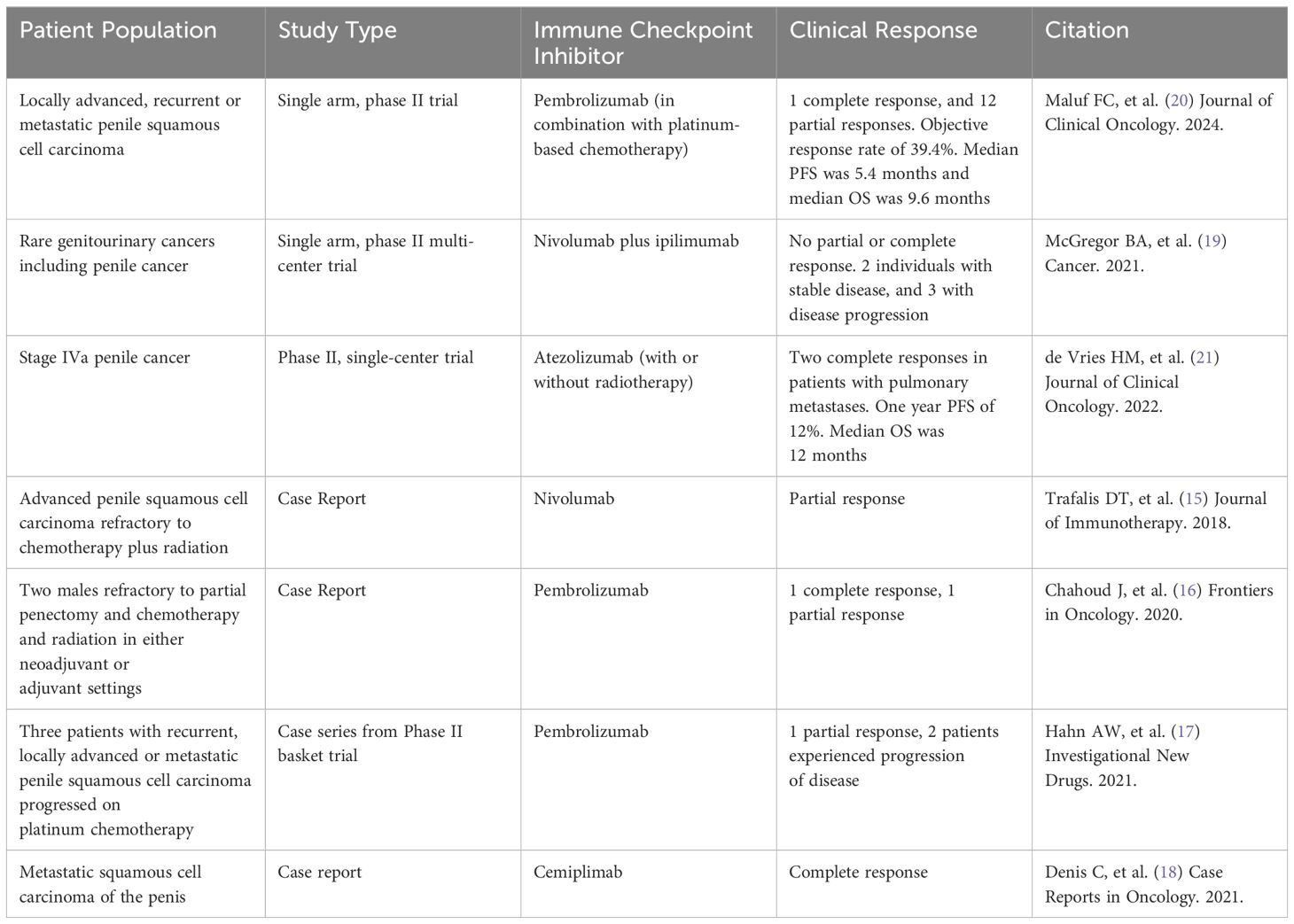
Table 1. Prospective studies and case series utilizing immune checkpoint inhibitors in treating penile cancer.
Targeted therapiesGiven genomic profiling demonstrating the presence of EGFR alterations, EGFR-directed therapies have been utilizing in treatment recurrent or advanced penile cancer. In one of the first case series, 24 patients with advanced penile or scrotal cancer received EGFR-directed therapies including cetuximab, erlotinib and gefitinib (22). Of these patients, 20% (1/5) had a partial response seen on imaging with cetuximab alone, while 25% (3/12) who received cisplatin in combination with cetuximab had partial responses. No individuals treated with gefitinib or erlotinib demonstrated clinical benefit to EGFR-targeted treatment. A subsequent case series evaluated treatment outcomes with EGFR-directed therapies in 3 patients with advanced penile cancer whose cancers were refractory to chemotherapy. Of the three patients, one patient had a complete response to cetuximab and remained disease-free for 42 months while the second patient initially responded to panitumumab before experiencing disease progression (23). The third patient did not experience clinical benefit from EGFR directed therapy, and experienced disease progression.
A case report of metastatic penile cancer in China reported several potentially actionable findings on genomic testing, including a TMB of 13.97 mutations/Mb as well as BRCA2 mutation (24). After experiencing radiographic progression on first-line therapy with chemotherapy and immunotherapy, the patient was treated with poly ADP-ribose polymerase (PARP) inhibitor olaparib in the second line setting and experienced a clinical benefit lasting for 9 months. Of note, the patient was also treated with nivolumab plus ipilimumab in the third line setting but had a PFS of 3 months, thus demonstrating a lack of clinical benefit with dual immune checkpoint inhibition despite genomic profiling revealing TMB high status.
The NTRK inhibitor entrectinib has been studied in several prospective trials in treating locally advanced or metastatic NTRK fusion positive solid tumors. In pooled analysis from three phase 1 and 2 trials, one patient with penile cancer was included in the trial cohort (25). However, tumor response was not specifically reported among tumor types with four or less patients. To our knowledge, no other reports of utilizing targeted therapies based off genomic alterations have been published in the literature thus far.
Ongoing trials and future directionsSeveral prospective trials are evaluating the efficacy of immune checkpoint inhibitors in treating penile cancer including a study combining dostarlimab with PARP inhibitor niraparib (ClinicalTrials.gov ID: NCT05526989). In addition, an ongoing phase 2 trial in men who are unfit or had disease progression on platinum chemotherapy is evaluating the efficacy of immune checkpoint inhibitor avelumab (NCT03391479). Avelumab is also being evaluated as maintenance therapy in patients with locally advanced and metastatic squamous cell penile cancer who had treatment response to chemotherapy (NCT03774901). The EPIC trial is an ongoing phase 2 study in the United Kingdom evaluating the efficacy of cemiplimab as monotherapy or in combination with standard-of-care chemotherapy in locally advanced or metastatic penile cancer (26).
Given overexpression of the vascular growth factor (VEGF) receptor in penile cancer, the tyrosine kinase inhibitor cabozantinib is being further studied in this malignancy (27). CaboPen is a single-arm, single-center, phase 2 trial with patients with either locally advanced penile squamous cell carcinoma or in those with metastatic disease, cabozantinib is being studied until disease progression or unacceptable toxicity (NCT03943602). Cabozantinib is also being studied in combination with nivolumab with or without CTLA-4 inhibitor ipilimumab in patients with metastatic genitourinary tumors (NCT02496208), and in a separate trial combining all three aforementioned therapies in rare genitourinary cancers such as penile cancer (NCT03866382).
As HPV-positive and -negative have distinct molecular features and tumor microenvironments, HPV-directed therapies such as vaccines offer a potential opportunity for prevention or treatment (28–30). However, to our knowledge, no ongoing trials are evaluating HPV-directed therapies for advanced penile cancer.
Antibody drug conjugates (ADCs) such as enfortumab vedotin, which targets nectin-4, and sacituzumab govitecan, which is directed towards Trop-2 expressing cells, are currently approved in the treatment of metastatic urothelial carcinoma. Given the theoretical capability to deliver chemotherapy specifically towards cancer cells expressing these proteins, there has been interest in exploring the effectiveness of these therapies in penile cancer (31). A phase II study of rare genitourinary cancers is currently enrolling and evaluating the use of sacituzumab govitecan with or without the immune checkpoint inhibitor atezolizumab (NCT06161532). In addition, enfortumab vedotin is also being studied in a phase 2 trial in individuals with unresectable or metastatic squamous cell carcinoma of the penis (NCT06104618). These ADCs as well as others in clinical development represent a promising, more targeted approach in delivering cytotoxic payloads to cancer cells (32). A summary of known ongoing and planned prospective clinical trials in advanced penile cancer is also available in Table 2.
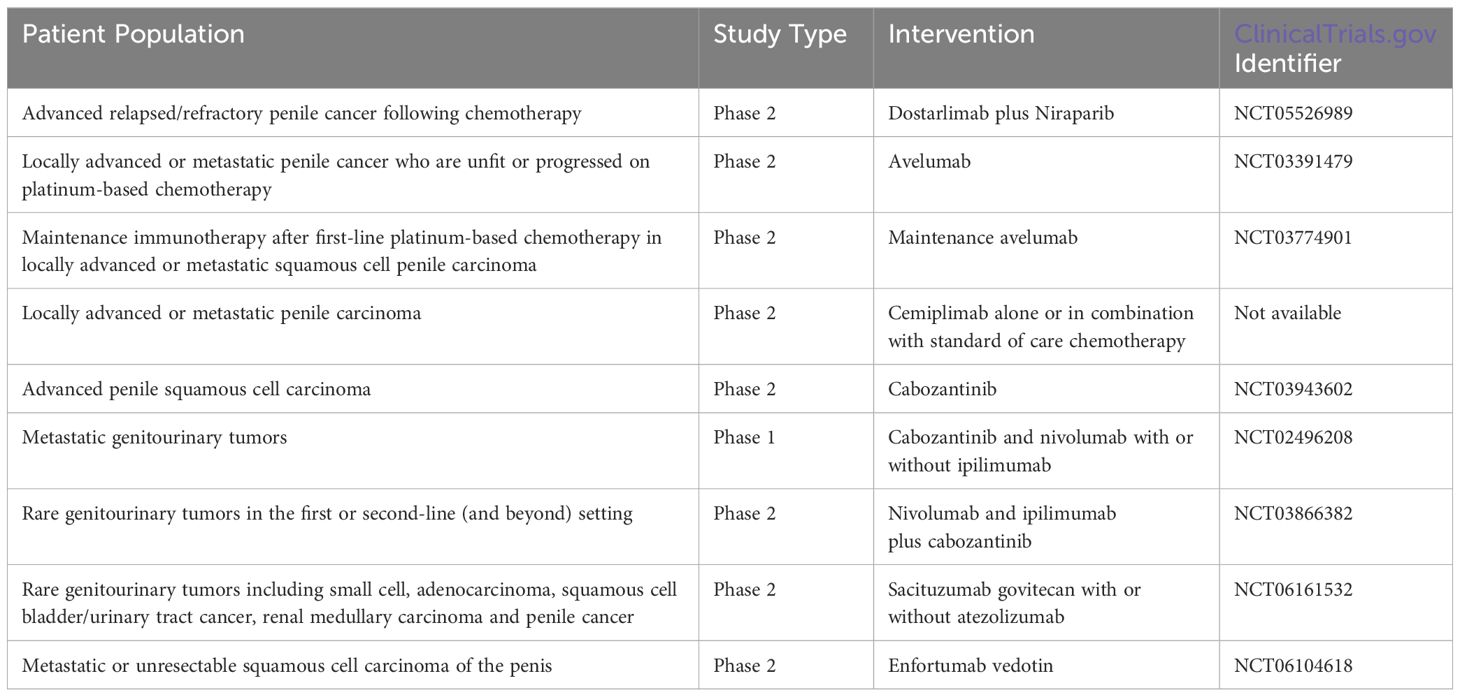
Table 2. Ongoing and planned prospective clinical trials in advanced penile cancer.
ConclusionPenile cancer remains a challenging malignancy to treat in men particularly in advanced disease. Chemotherapy has historically served as the primary treatment modality in recurrent, locally advanced or metastatic penile cancer. Although several case reports have demonstrated potential clinical efficacy in patients whose tumors harbor EGFR or BRCA2 mutations, prospective data lacks in oncogenic driver mutated penile cancer. Several recent phase II trials have demonstrated clinical benefit in a subset of patients who receive treatment with immune checkpoint inhibitors; however, given the genomic profile of penile cancer, it remains unclear if immunotherapy may benefit most patients with penile cancer. Nonetheless, given the paucity of data for currently employed chemotherapy regimens in these aforementioned settings, several ongoing studies aim to evaluate the safety and efficacy of immune checkpoint inhibitors as well as antibody drug conjugates as potential newer generation approaches in treating this uncommon cancer.
Author contributionsDB: Conceptualization, Investigation, Visualization, Writing – original draft, Writing – review & editing. RH: Writing – original draft, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestDB: Consulting/Advisory Role: AIMED BIO, Astellas, AVEO Oncology, Bayer, Eisai, EMD Serono, Exelixis, Janssen, Seagen. Speakers’ Bureau: Merck. Travel and Accommodations: DAVA Oncology, Merck, Seagen. RH is a consultant for Targeted Oncology and has received honoraria from DAVA Oncology and The Dedham Group.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References2. Fu L, Tian T, Yao K, Chen X-F, Luo G, Gao Y, et al. Global pattern and trends in penile cancer incidence: population-based study. JMIR Public Health Surveill. (2022) 8:e34874. doi: 10.2196/34874
PubMed Abstract | Crossref Full Text | Google Scholar
4. Khalil MI, Kamel MH, Dhillon J, Master V, Davis R, Hajiran AJ, et al. What you need to know: updates in penile cancer staging. World J Urol. (2021) 39:1413–9. doi: 10.1007/s00345-020-03302-z
PubMed Abstract | Crossref Full Text | Google Scholar
5. Sanchez DF, Fernandez-Nestosa MJ, Canete-Portillo S, Rodriguez I, Cubilla AL. What is new in the pathologic staging of penile carcinoma in the 8th edition of AJCC TNM model: rationale for changes with practical stage-by-stage category diagnostic considerations. Adv Anat Pathol. (2021) 28:209–27. doi: 10.1097/PAP.0000000000000297
PubMed Abstract | Crossref Full Text | Google Scholar
6. Sachdeva A, McGuinness L, Zapala ŁChecktae, Greco I, Garcia-Perdomo HA, Kailavasan M, et al. Management of lymph node-positive penile cancer: A systematic review. Eur Urol. (2024) 85:257–73. doi: 10.1016/j.eururo.2023.04.018
PubMed Abstract | Crossref Full Text | Google Scholar
7. Pagliaro LC, Williams DL, Daliani D, Williams MB, Osai W, Kincaid M, et al. Neoadjuvant paclitaxel, ifosfamide, and cisplatin chemotherapy for metastatic penile cancer: A phase II study. J Clin Oncol. (2010) 28:3851–7. doi: 10.1200/JCO.2010.29.5477
PubMed Abstract | Crossref Full Text | Google Scholar
9. Ribera-Cortada I, Guerrero-Pineda J, Trias I, Veloza L, Garcia A, Marimon L, et al. Pathogenesis of penile squamous cell carcinoma: molecular update and systematic review. Int J Mol Sci. (2021) 23:251. doi: 10.3390/ijms23010251
PubMed Abstract | Crossref Full Text | Google Scholar
10. Nazha B, Zhuang T, Wu S, Brown JT, Magee D, Carthon BC, et al. Comprehensive genomic profiling of penile squamous cell carcinoma and the impact of human papillomavirus status on immune-checkpoint inhibitor-related biomarkers. Cancer. (2023) 129:3884–93. doi: 10.1002/cncr.34982
PubMed Abstract | Crossref Full Text | Google Scholar
11. Necchi A, Spiess PE, Costa de Padua T, Li R, Grivas P, Huang RSP, et al. Genomic profiles and clinical outcomes of penile squamous cell carcinoma with elevated tumor mutational burden. JAMA Netw Open. (2023) 6:e2348002. doi: 10.1001/jamanetworkopen.2023.48002
PubMed Abstract | Crossref Full Text | Google Scholar
12. Montella M, Sabetta R, Ronchi A, De Sio M, Arcaniolo D, De Vita F, et al. Immunotherapy in penile squamous cell carcinoma: present or future? Multi-target analysis of programmed cell death ligand 1 expression and microsatellite instability. Front Med. (2022) 9:874213. doi: 10.3389/fmed.2022.874213
PubMed Abstract | Crossref Full Text | Google Scholar
13. Sonpavde GP, Nielsen SM, Heald B, Hatchell KE, Esplin ED, Nassar A. Germline genetic testing of patients with penile carcinoma. J Clin Oncol. (2023) 41:10–0. doi: 10.1200/JCO.2023.41.6_suppl.10
Crossref Full Text | Google Scholar
14. Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. (2019) 2:e192535. doi: 10.1001/jamanetworkopen.2019.2535
PubMed Abstract | Crossref Full Text | Google Scholar
15. Trafalis DT, Alifieris CE, Kalantzis A, Verigos KE, Vergadis C, Sauvage S. Evidence for efficacy of treatment with the anti-PD-1 mab nivolumab in radiation and multichemorefractory advanced penile squamous cell carcinoma. J Immunother Hagerstown Md 1997. (2018) 41:300–5. doi: 10.1097/CJI.0000000000000221
PubMed Abstract | Crossref Full Text | Google Scholar
16. Chahoud J, Skelton WP, Spiess PE, Walko C, Dhillon J, Gage KL, et al. Case report: two cases of chemotherapy refractory metastatic penile squamous cell carcinoma with extreme durable response to pembrolizumab. Front Oncol. (2020) 10:615298. doi: 10.3389/fonc.2020.615298
PubMed Abstract | Crossref Full Text | Google Scholar
17. Hahn AW, Chahoud J, Campbell MT, Karp DD, Wang J, Stephen B, et al. Pembrolizumab for advanced penile cancer: a case series from a phase II basket trial. Invest New Drugs. (2021) 39:1405–10. doi: 10.1007/s10637-021-01100-x
PubMed Abstract | Crossref Full Text | Google Scholar
19. McGregor BA, Campbell MT, Xie W, Farah S, Bilen MA, Schmidt AL, et al. Results of a multicenter, phase 2 study of nivolumab and ipilimumab for patients with advanced rare genitourinary Malignancies. Cancer. (2021) 127:840–9. doi: 10.1002/cncr.33328
PubMed Abstract | Crossref Full Text | Google Scholar
20. Maluf FC, Trindade K, Almeida Preto DD, Marques Monteiro FS, Luz M, Beato PM, et al. A phase II trial of pembrolizumab plus platinum-based chemotherapy as first-line systemic therapy in advanced penile cancer: HERCULES (LACOG 0218) trial. J Clin Oncol. (2024) 42:5009–9. doi: 10.1200/JCO.2024.42.16_suppl.5009
Crossref Full Text | Google Scholar
21. de Vries HM, Rafael TS, Gil-Jimenez A, de Feijter JM, Bekers E, van der Laan E, et al. Clinical results of PERICLES: A phase II trial investigating atezolizumab +/- radiotherapy for advanced squamous cell carcinoma of the penis. J Clin Oncol. (2022) 40:3–3. doi: 10.1200/JCO.2022.40.6_suppl.003
Crossref Full Text | Google Scholar
22. Carthon BC, Ng CS, Pettaway CA, Pagliaro LC. Epidermal growth factor receptor–targeted therapy in locally advanced or metastatic squamous cell carcinoma of the penis. BJU Int. (2014) 113:871–7. doi: 10.1111/bju.12450
PubMed Abstract | Crossref Full Text | Google Scholar
23. Brown A, Ma Y, Danenberg K, Schuckman AK, Pinski JK, Pagliaro LC, et al. Epidermal growth factor receptor-targeted therapy in squamous cell carcinoma of the penis: A report of 3 cases. Urology. (2014) 83:159–66. doi: 10.1016/j.urology.2013.08.074
PubMed Abstract | Crossref Full Text | Google Scholar
24. Zhang Q, Li Y, Zhang Y, Deng Z, Ding Y. Case report of penile squamous cell carcinoma continuous treatment with BRCA2 mutation. World J Surg Oncol. (2024) 22:50. doi: 10.1186/s12957-024-03305-9
PubMed Abstract | Crossref Full Text | Google Scholar
25. Demetri GD, De Braud F, Drilon A, Siena S, Patel MR, Chul Cho B, et al. Updated analysis of the efficacy and safety of entrectinib in patients (pts) with locally advanced/metastatic NTRK fusion-positive (NTRK-fp) solid tumors. J Clin Oncol. (2022) 40:3099–9. doi: 10.1200/JCO.2022.40.16_suppl.3099
Crossref Full Text | Google Scholar
26. Bahl A, Foulstone E, Ashurst L, Renninson E, White P, Bravo A, et al. A phase II trial of cemiplimab alone or in combination with standard of care chemotherapy in locally advanced or metastatic penile carcinoma (EPIC trial). J Clin Oncol. (2024) 42:TPS14–4. doi: 10.1200/JCO.2024.42.4_suppl.TPS14
Crossref Full Text | Google Scholar
27. Necchi A, Mariani L, Colecchia M, Giannatempo P, Raggi D, Calareso G, et al. Cabozantinib in patients with advanced penile squamous cell carcinoma (PSCC): the open-label, single-arm, single-center, phase 2, CaboPen trial. Ann Oncol. (2017) 28:v329. doi: 10.1093/annonc/mdx371.081
Crossref Full Text | Google Scholar
28. Xu DM, Chen LX, Zhuang XY, Han H, Mo M. Advances in molecular basis of response to immunotherapy for penile cancer: better screening of responders. Front Oncol. (2024) 14:1394260. doi: 10.3389/fonc.2024.1394260
PubMed Abstract | Crossref Full Text | Google Scholar
29. White J, Mason R, Lawen T, Spooner J, Faria KVM, Rahman F, et al. Therapeutic approaches to penile cancer: standards of care and recent developments. Res Rep Urol. (2023) 15:165–74. doi: 10.2147/RRU.S387228
PubMed Abstract | Crossref Full Text | Google Scholar
32. Dumontet C, Reichert JM, Senter PD, Lambert JM, Beck A. Antibody–drug conjugates come of age in oncology. Nat Rev Drug Discovery. (2023) 22:641–61. doi: 10.1038/s41573-023-00709-2
留言 (0)