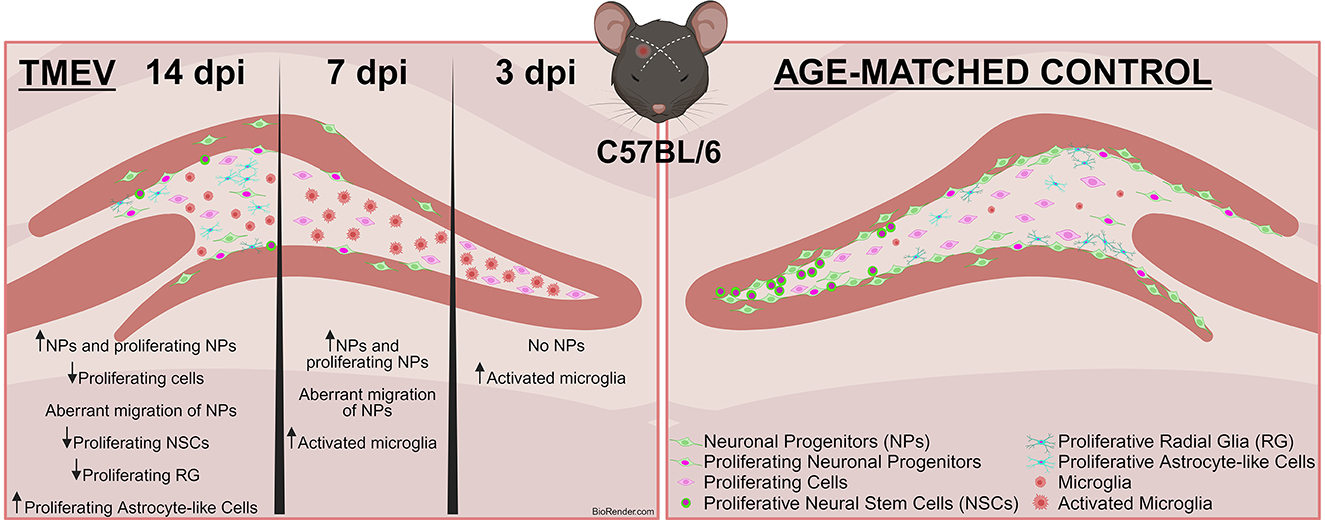
Graphical Abstract. Infection-Induced Seizures Alter Neurogenesis in the TMEV Model. This study employed the Theiler's Murine Encephalomyelitis Virus (TMEV) model to investigate the effects of virus-induced seizures on neurogenesis at 3, 7, and 14 days post-infection (dpi) in adult C57BL/6J mice. Immunohistochemical analysis revealed a reduction in proliferating cells post-infection. Notably, neuronal progenitors (DCX+) were nearly absent at 3 dpi, resumed proliferation by 14 dpi, but did not reach control (CTR) levels and displayed abnormal migration. Additionally, the number of proliferating neural stem cells (NSCs) decreased significantly in TMEV mice at 14 dpi, while a higher population of proliferating astrocytes was observed. Most changes were similar between seizing and non-seizing infected mice.
1 IntroductionEpilepsy is a profoundly debilitating neurological disorder characterized by recurrent unprovoked seizures, afflicting over 70 million people worldwide. Currently available antiseizure drugs offer symptomatic seizure control but are often accompanied by adverse effects and regrettably demonstrate inefficacy in nearly one-third of the patients (Löscher et al., 2020), underscoring the imperative for innovative therapeutic approaches. One such pioneering intervention is regenerative medicine which could offer more than just symptomatically balancing inhibition and excitation in the epileptic brain: Replacing lost inhibitory neurons via transplantation of gamma-aminobutyric acid (GABA)-ergic interneurons derived from human stem cells has shown promising results in experimental epilepsy models (Bershteyn et al., 2023; Cunningham et al., 2014; Upadhya et al., 2019; Waloschková et al., 2021). However, critical concerns regarding tumorigenicity and invasive delivery into the patient's brain must be addressed (Yasuhara et al., 2017). Alternatively, targeting endogenous repair mechanisms, such as the birth of new neurons (adult neurogenesis), to counteract progressive neuron loss and aberrant network connectivity, presents a promising therapeutic strategy. In fact, recent investigations (Jessberger and Parent, 2015) have highlighted the complex and important relationship between epileptic disease progression and adult neurogenesis, which introduces a largely unexplored delicate equilibrium between the brain regenerative processes and pathological outcomes.
Neurogenesis, the process of generating and integrating new neurons into specific brain regions, has undergone a paradigm shift from being largely attributed to early brain development to being acknowledged as an ongoing phenomenon in specific brain neurogenic niches during adulthood (Gross, 2000; Hussain et al., 2024; Wang et al., 2022). These niches, namely the dentate gyrus (DG) in the hippocampus and the subventricular zone (SVZ) of the lateral ventricles, have the capacity to generate new neurons (Abbott and Nigussie, 2020; Kempermann et al., 2018; Schlessinger et al., 1975). In fact, despite the inherent constraints on neuronal regeneration within the mature brain, a notable 6% of cellular constituents within the DG are identified as adult-born neurons (Cameron and McKay, 2001), and are known to persist long term, as substantiated by rodent studies conducted by Dayer et al. (2003). Key to the dynamics of adult neurogenesis are the NSCs within the DG, characterized by their radial morphology. NSCs mature into neurons, migrate to their appropriate locations within existing neural circuits, and establish functional connections. Adult-born neurons must correctly and precisely integrate into the already existing neural networks to be able to contribute to the physiological brain function such as cognitive abilities, including learning and memory (Bao and Song, 2018; Christian et al., 2014; Deng et al., 2010; Sahay and Hen, 2007). Emerging evidence unveiled the unique role of immature adult-born neurons in modulating neural networks. These immature neurons exhibit heightened excitability and plasticity, attributes that enable them to differentially influence mature neuron firing, synchronization, and network oscillations, thus contributing to cognitive processes (Bao and Song, 2018; Song et al., 2012). Moreover, their connections within the hippocampal circuitry and broader connectivity to other brain regions underscore their significance in regulating vast neural dynamics (Bao and Song, 2018; Barbosa et al., 2015; Lugert et al., 2010; Pilz et al., 2018).
Given its importance, the process of adult neurogenesis is under stringent regulation by a diverse array of intrinsic and extrinsic factors: Environmental enrichment, physical activity, learning experiences, and exposure to novel stimuli enhance neurogenesis, whereas stress, aging, and specific neurological pathologies like epilepsy can alter the process. Aberrations in adult neurogenesis have been implicated in a range of cerebral disorders such as major depression, neurodegenerative diseases, and epilepsy (Christian et al., 2014; Kang et al., 2016; Sahay and Hen, 2007; Winner et al., 2011). In fact, extended seizures trigger adult neurogenesis in several animal models, presumably as a compensatory mechanism against neuronal loss (Bengzon et al., 1997; Parent et al., 1997). However, neurons born during and after seizures exhibit structural and functional abnormalities, leading to enduring changes in hippocampal morphology and heightened neuronal hyperexcitability (Jessberger et al., 2007).
Our understanding of (aberrant) adult neurogenesis in epilepsy is primarily based on traditional epilepsy models where seizures are induced chemically or electrically. However, the etiology of epilepsy encompasses an array of central nervous system (CNS) insults, including genetic causes, traumatic brain injuries, brain tumors, and infections (Fisher et al., 2017). Notably, brain inflammation due to cerebral infections (encephalitis) emerges as a pivotal risk factor for acute seizures and subsequent epilepsy onset. Numerous viruses, including herpes simplex virus type-1 (HSV-1), non-polio picornaviruses, Zika virus (ZIKV), West Nile virus (WNV), Japanese encephalitis virus, cytomegalovirus, human herpes virus-6, and SARS-CoV-2, can trigger encephalitis in humans (Bartolini et al., 2019; DePaula-Silva et al., 2021; Ellul et al., 2020; Solomon et al., 2000; Suzuki et al., 2008). Importantly, the repercussions of viral encephalitis extend beyond the acute phase. After virus infection, an initiated tangled response characterized by acute inflammation induces modification of the brain circuitry thereby possibly contributing to the development of epilepsy months to years later (Gerhauser et al., 2019; Libbey et al., 2008; Stewart et al., 2010). This transient provocation of acute seizures first—and the likely occurrence of chronic epilepsy later, propel viral encephalitis into the forefront of scientific inquiry and therapeutic investigation. However, the intersection of infection, neurogenesis, and possible consequent seizure occurrence remains incompletely elucidated. We aim to shed light onto the complex mechanisms governing this relationship and possibly finding new targets for developing innovative therapies.
To investigate the impact of viral infections on adult neurogenesis and its potential implications for epilepsy onset, our study utilized a model of viral encephalitis-induced seizures, the Theiler's Murine Encephalomyelitis Virus (TMEV) model. TMEV's experimental deployment as an epilepsy model is predominantly focused on the assessment of antiseizure drugs (Batot et al., 2022; Metcalf et al., 2022). Recently, it has emerged as a promising model for unveiling various mechanisms underlying virus-induced epilepsy due to its remarkable parallels with human temporal lobe epilepsy (Bröer et al., 2017, 2016; Cusick et al., 2017; DePaula-Silva et al., 2021; Hanak et al., 2019; Howe et al., 2022; Kirkman et al., 2010; Loewen et al., 2016; Sanchez et al., 2019; Waltl et al., 2018a,b). The presented research seeks to start unraveling the temporal dynamics and specific cell types arising from adult-born cells during acute encephalitis, which can be accompanied by seizures, and ends with viral clearance and resolving of symptoms. Thus, in the TMEV model, the acute phase lasts up to 14 days post-infection (dpi).
2 Materials and methods 2.1 Animal modelProcedures involving animals and their care were conducted in conformity with the institutional guidelines and in compliance with national and international laws and policies [EEC Council Directive 2019/1010, June 5, 2019, EU Directive 2010/63/EU for animal experiments, and the ARRIVE guidelines (Percie du Sert et al., 2020)], as well as authorized by the Institutional Animal Care and Use Committee at the University of Utah (protocol number: 21-11009, Dept. of Pharmacology and Toxicology) or the local government [Berlin State Office for Health and Social Affairs (LAGeSo) Berlin, Germany, permission number G0015/21]. All experiments were designed and planned to minimize the number of animals used and their suffering. Animals were housed at constant temperature (23 ± 1°C) and relative humidity (60 ± 5%) with ad libitum access to food and water and maintained on a fixed 12 h light/dark cycle. Each cage was enriched with environmental stimuli, including shelters, nesting materials, and plastic and cardboard rolls. TMEV-infected and perfused brain samples were obtained from male and female adult C57BL/6J mice, kindly provided by Prof. Dr. Karen Wilcox from the College of Pharmacy, University of Utah, USA. Additionally, age-matched, in-house-bred naïve male and female C57BL/6J mice were utilized as control (CTR) animals, alongside Dulbecco's modified eagle Medium (DMEM)-injected animals as a vehicle control (TMEV vehicle group). Statistical analyses confirmed no significant differences between naïve CTR and DMEM vehicle controls, allowing the use of CTR animals as representative of baseline physiological conditions (Supplementary Figures 1A–D). Additionally, given that no statistical differences were observed between male and female mice, data from both sexes were combined when appropriate (filled circles indicate male mice, while hollow circles denote females). Finally, required group sizes were determined a priori by power analyses (Faul et al., 2009).
2.2 TMEV infection and seizure monitoringMice were infected using the Daniel's strain (DA) of TMEV in the laboratory of Prof. Dr. Karen Wilcox as detailed in a prior publication (Batot et al., 2022; Figure 1A). Briefly, 7–8 weeks old mice were anesthetized by inhalation of isoflurane and, using a free-hand method of infection that obviated the need for a stereotaxic frame, 20 μL of virus suspension containing 4 × 104 plaque-forming units (PFU) of DA-TMEV, was injected at a depth of 1.5 mm in the right temporal lobe region above the hippocampal formation. Injection coordinates were selected according to stereotaxic injection in relation to bregma [−2.0 (AP); +3.0 (ML); −1.5 (DV)] Paxinos and Franklin (2004). Mice were randomly assigned to three distinct experimental cohorts: 3 (seizure onset), 7 (seizure peak), and 14 dpi (virus clearance and several days after seizures end). Evaluation of the development, intensity, and number of generalized acute seizures was assessed in the laboratory of Prof. Dr. Karen Wilcox and involved monitoring handling-induced behavioral seizures for 1 h twice daily (between 9 AM and 12 PM and between 1 PM and 4 PM), with a minimum 3 h interval between sessions, as outlined by Batot et al. (2022). Generalized motor seizure severity was scored using a modified Racine scale (Racine, 1972; Table 1).
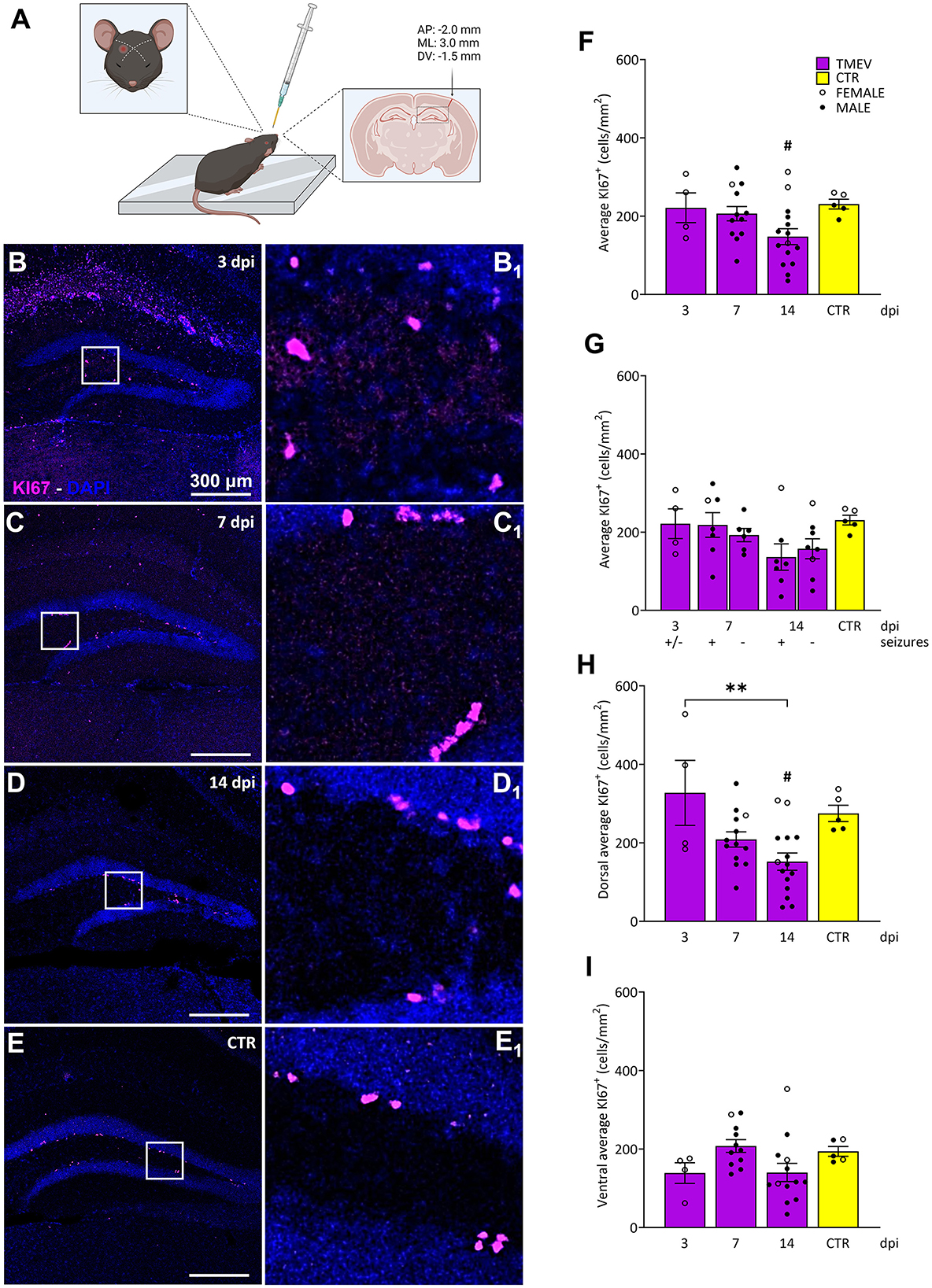
Figure 1. Impact of TMEV CNS infection on DG cell proliferation. (A) Schematic illustration depicting the procedure for TMEV injection. Commencing from the left: For administering an injection into the right parietal cortex, the needle is introduced slightly lateral to an imaginary line drawn between the eye and the ear on the contralateral side. The depth control collar is distinctly outlined in yellow to ensure accurate insertion depth. The trajectory of the injection is discernible in the coronal cross-section of the brain, delineated by the arrow. The specified injection site corresponds to the provided coordinates positioned above the arrow. This visual depiction was generated utilizing biorender.com and adapted from Batot et al. (2022). (B–E) Representative images showing IHC of KI-67 in C57BL/6J-mouse hippocampal slices at different time points post infection [3 dpi (n = 4) (B), 7 dpi (n = 13) (C), 14 dpi (n = 16) (D), and CTR (n = 5) (E)]. (B1–E1) Insets show magnified views of the corresponding ROIs in (B–E). (F) Average KI-67+ cell density was analyzed across all the TMEV-infected mice, divided by time point and CTR. A significant reduction in cell proliferation was observed at 14 dpi compared to CTR (14 dpi vs. CTR: #p = 0.0270). (G) Analyses of seizing (+) and non-seizing (–) mice revealed no significant differences in cell proliferation at 7 and 14 dpi. Mice at 3 dpi were defined as (+/–) due to potential seizure occurrence outside the single observational period before their sacrifice. (H) When analyzing the dorsal hippocampi separately, a significant reduction in cell proliferation was detectable at 14 dpi compared to 3 dpi and to CTR (3 dpi vs. 14 dpi: **p = 0.0046, 14 dpi vs. CTR: #p = 0.0411). (I) No significant differences in cell proliferation were detected in the ventral DG. The scale bar in (B) also applies to (C–E). Filled circles indicate male mice, while hollow circles denote females. Data are mean ± SEM, normality was tested using the Shapiro-Wilk test, statistical analyses were performed using Ordinary one-way ANOVA followed by Bonferroni's multiple comparison test. Statistical significance was set at the adjusted P-value ≤ 0.05.
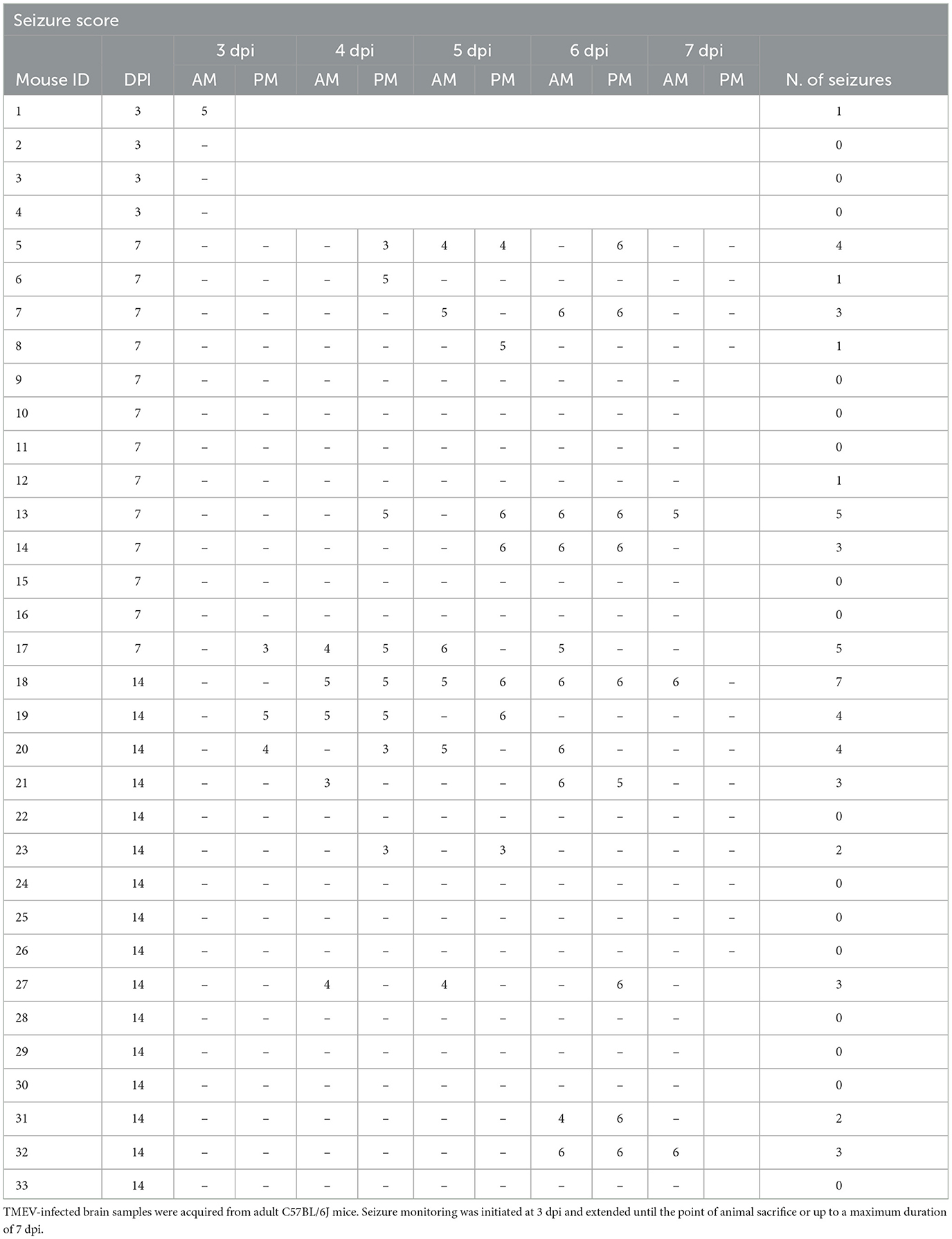
Table 1. List of infected mice, seizure scores, and number of seizures (N. of seizures).
2.3 Sample collectionMice were transcardially perfused at various time points (3, 7, and 14 dpi), thus the maximum age difference between the groups was 11 days. Briefly, mice were deeply anesthetized via intraperitoneal pentobarbital injection or sacrificed through isoflurane inhalation. After cessation of breathing, transcardial perfusion was carried out using 1x phosphate-buffered saline (PBS; Thermo-Fischer), followed by a 4% paraformaldehyde [Sigma-Aldrich (PFA)] solution. Brains were then resected and stored overnight (ON) in 4% PFA at 4°C. On the subsequent day, brains were placed in vials containing 30% cold sucrose (Sigma-Aldrich) and stored at 4°C until complete dehydration. Hippocampi of both TMEV-infected and CTR mice were sectioned into 40 μm thick coronal slices using a freezing microtome (HM 400, MICROM GmbH, Germany). Slices were cut approximately from Bregma −1.22 to −3.80 mm (Paxinos and Franklin, 2004). These slices were divided into 8 series to represent the hippocampal dorso-ventral extent (a section every 280 μm per series). Every vial was pre-filled with cryoprotective solution [30% glycerol, 30% ethylene glycol, and 40% PBS (Roth, Germany)] and, after the procedure, contained the same number of slices. The vials were then stored at −20°C upon use. In this study a total of 33 TMEV injected mice divided across different dpi groups and 9 CTR mice were used.
2.4 Immunohistochemistry (IHC)Theiler's Murine Encephalomyelitis Virus infected and CTR hippocampal slices were washed five times for 15 min with 1x PBS to remove the cryoprotective solution and residual PFA. After permeabilization and blocking (1 h, RT, constantly mixed) with 10% normal goat serum [Merck, Germany, Cat. #: S26-100ml, Lot #: 4064259 (NGS)] in 0.2% Triton X-100 [Merk, Germany, Cat. #: T8787-100ml, Lot #: SLCJ6163 (PBST)], samples were incubated ON at room temperature in 1x PBST containing 4% NGS and primary antibodies while being constantly mixed. Depending on the experimental design, different primary antibodies were used per one of the eight series of hippocampal sections: guinea pig anti-Doublecortin [Merck, Germany, Cat. #: AB2253, Lot #: 3850474, (DCX), neuronal progenitor cells, 1:1500], mouse anti-Nestin [Merck, Germany, Cat. #: MAB353, Lot #: 4040753, (NES), NSC 1:200] (Merck, Germany), rabbit anti-KI-67 (Abcam, Germany, Cat. #: Ab15580, Lot #: GR3397465-1, proliferative cells, 1:1000), and rabbit anti-Ionized Calcium-Binding Adapter Molecule 1 [Abcam, Germany, Cat. #: Ab178847, Lot #: GR3229566-16 (IBA1), microglia, 1:1000]. The following day, slices were washed three times for 15 min with 1x PBST and incubated for 2 h at RT in 1x PBS with 4% NGS and secondary antibodies conjugated with Alexa Fluor-488 (Invitrogen, Germany, Cat. #: A11008, Lot #: 2557379) and Alexa Fluor-568 (Invitrogen, Germany; Cat. #: A11011, Lot #: 2500544) dilution 1:1000 each, constantly mixed. Samples were then washed again three times for 15 min in 1x PBST and two times for 15 min in 1x PBS. Slices were mounted on slides and the cell nuclei were labeled using Fluoromount G® containing DAPI (Invitrogen, Germany, Cat. #: 4959, Lot #: 142202). Finally, samples were covered with coverslips and stored at 4°C before the imaging process. For Glial Fibrillary Acidic Protein (GFAP, astrocytic marker) labeling, a Mix-n-Stain™ CF™647 kit (Sigma-Aldrich, Germany, Cat. #: MX647S20) was used. This disposable kit allowed for mixing the primary antibody (Mouse anti-GFAP, chosen dilution 1:2000) with the secondary (conjugated with Alexa Fluor-647) before use, thus avoiding the need for a second day of incubation. For each round of experiments, one slice was used as a negative control to evaluate the quality of the staining.
2.5 ImagingHippocampal slices processed as described above were used for imaging acquisition. Single-plane images of one series per animal (8 slices in total per animal) labeled for DCX, KI-67 and IBA1 were acquired by using a fluorescent microscope (Leica DMi8®, Leica, Germany). The hippocampal DG was visualized at 20x magnification with the focus corrected for all the channels. One image of all three channels was acquired [1,024 × 1,024 pixels (px), 1.3 μm/px]. Differently, the colocalization of GFAP+/NES−, NES+/GFAP−, and GFAP+/NES+ cells with KI-67 was acquired using a confocal laser scanning microscope (Leica SP8®, Leica, Germany). This approach was necessary as the signal from single-plane images acquired with the fluorescent microscope was insufficient to evaluate real colocalization. For acquisition, the laser's power was set to 1–2% and the photomultiplier tubes were adjusted to avoid saturation. The pinhole was set to 0.1 Airy Unit and the line average to 3. Then, a Z-stack of the entire DG (1,024 × 1,024 px, 0.75 μm/px, objective magnification: 20x, zoom factor: 0.75, distance in Z-direction: 2 μm) was acquired with a 20x objective. For this set of experiments, one dorsal and one ventral both ipsi- and contra-lateral hippocampal slice per animal per experimental group at the same coordinates were acquired as representative of the whole dorsal or ventral area. Regardless of the microscope used, all settings were kept constant throughout the acquisitions.
2.6 Imaging analysisAll acquired images were analyzed in a randomized order using ImageJ by an experimenter blinded to the animal ID and group allocation. Each sample acquisition was split into different channels with DAPI labeling used to identify the DG region. For single-plan acquisitions, a region of interest (ROI) was defined by tracing the external boundaries of the DG stratum granulare (SG) in each analyzed image and the corresponding area was calculated. For Z-stack acquisitions, the DG area was measured in the central imaged plane. Cell counts within the DG ROI were performed manually on single-plane acquisitions (DCX, KI-67, DCX/KI-67) and on different stacks (GFAP/KI-67, NES/KI-67, and GFAP/NES/KI-67), or on single-plane acquisitions (IBA1) using the “Analyse Particle” function. Additionally, the “Analyse Particle” was also used to calculate the area of each detected IBA1 cell. To this extent, the same ROI defined for calculating the DG area was used to identify the mean fluorescence intensity of the IBA1 labeling separately for each sample in the same experimental group. Then, the IBA1 channel was selected, filtered with the “Gamma filter” set to 1.5 and the “Gaussian Blur” set to 1 to improve image quality. Subsequently, twice the detected mean fluorescent intensity of the entire IBA1 dataset per group was used to threshold the images. To analyze both the number and area of IBA1 labeling, the “Analyse Particles” function was used, with the particle size, expressed as “pixel units,” set to 8 to remove background non-specific signals. This cutoff was chosen based on resolution limitation of the fluorescent microscope (Supplementary Figures 1E–G). Independent of the counting method, DCX, KI-67, and IBA1 cell density was calculated as a ratio between the number of positive cells per image and the ROI defined around the DG area of the same analyzed images. The result was then multiplied by a factor of 1,000,000 and the average per animal, expressed as cells/mm2, was calculated and used for statistical analysis. Differently, the density of colocalizing GFAP+/NES−, NES+/GFAP−, and GFAP+/NES+ cells with KI-67 was calculated within the Z-stack DG volume and approximated to mm3 by multiplying the resulting cell density of each analyzed image by a factor of 1,000,000,000. The approximated density was then averaged per animal, expressed as cells/mm3 and used for statistics.
To analyze the percentage of KI-67+ (proliferating) DCX+, GFAP+/NES−, NES+/GFAP−, and GFAP+/NES+ cells, a ratio of the total number of detected and manually counted double-positive cells for each specific marker to the total number of detected and manually counted KI-67+ cells in the DG for each imaged brain slice per animal was calculated. This ratio was then multiplied by 100 to obtain a percentage per imaged DG and subsequently averaged for each animal across the different groups. The final percentage per animal was used for statistics.
To evaluate possible aberrant neuronal progenitor cell migration, three reference lines were manually defined across the DG in each acquired image: one at the top, one at the center, and one at the bottom of the SG. Two additional lines were drawn between these, dividing the SG into 4 distinct layers (Figures 3A, B). Cells were assigned an arbitrary migration score (1 = normal migration, 4 = completely aberrant migration) based on their position within the defined layers. A score of 1 was assigned to cells located at the interface between the SG and the DG hilus. A score of 2 was given to cells situated between the first and the third lines, without touching the first line but crossing or touching the third. Cells between the third and the fifth lines received a score of 3, while a score of 4 was assigned to cells outside the SG, either in the molecular layer of the DG or within the hilus. Finally, the total number of scored cells per layer and hemisphere (ipsilateral or contralateral to the TMEV injection) was expressed as cell density. This was calculated as the number of manually counted cells per score per image (8 slices per animal across consistent series for all experimental groups), normalized to the DG area in the corresponding image. To standardize the data, the resulting value was multiplied by a factor of 1,000,000 to yield cell density in cells/mm2. For each animal, we determined the average density for each score, and group means were subsequently calculated. To express the results as percentages, the total number of cells across all four scores within each experimental group (3, 7, 14 dpi, and CTR), separated by hemisphere, was defined as 100%. The proportion of cells assigned to each score was then expressed as a percentage of this total, which was used for descriptive analysis.
2.7 StatisticsData were unblinded and all statistical analyses were performed using GraphPad Prism Version 9. Normality distribution of the data was tested using the Shapiro-Wilk test. Multiple comparisons between normally or non-normally distributed data were further analyzed using either One-Way ANOVA followed by Bonferroni's multiple comparisons test or Kruskal-Wallis ANOVA followed by Dunn's multiple comparisons test. For comparisons between two groups, either an Unpaired t-test or Mann–Whitney test was used. Correlation analyses were conducted utilizing either the Pearson correlation coefficient or the Spearman correlation method, contingent upon the characteristics of the data distribution. Statistical significance was set at the adjusted (multiple comparisons) or exact P-value ≤ 0.05.
3 Results 3.1 Seizure occurrence after infectionThe TMEV model was employed due to its translational relevance as a model of viral encephalitis-induced seizures. In this paradigm, intracerebral TMEV infection in C57BL/6J mice leads to the initiation of acute behavioral seizures in 50–70% of mice within a defined window spanning 3 to 8 dpi, followed by a seizure-free latency period. Around 14 dpi, the virus is cleared presumably due to activation of the adaptive immune response (DePaula-Silva et al., 2017). Acute seizures were recorded and are detailed in Table 1. To align with the study's core objective of examining the effects of virus infection and/or the occurrence of seizures on adult neurogenesis, each cohort included mice with and without seizures. Since seizure observation began at 3 dpi, animals from this group were monitored for seizures once before sacrifice and denoted as “+/–.”
3.2 Impact of TMEV infection on hilar cell proliferationTo comprehensively investigate the effect of TMEV infection on cell proliferation dynamics during the acute infection period (3–14 dpi), we conducted an analysis of KI-67+ cells in hippocampal slices from TMEV-infected mice at distinct time points: 3 dpi (n = 4), 7 dpi (n = 13), 14 dpi (n = 16; Table 1 and Figure 1). We included hippocampal slices from age-matched CTR mice (n = 5) to establish a baseline comparison for cellular proliferation. KI-67, a nuclear protein expressed throughout active phases of the cell cycle (G1, S, G2, and mitosis), remains undetectable in quiescent cells (G0; Bruno and Darzynkiewicz, 1992; Bullwinkel et al., 2006; Cuylen et al., 2016; Darzynkiewicz et al., 2015; Scholzen and Gerdes, 2000; Sobecki et al., 2016), making it a reliable marker for assessing cell proliferation. Cell density analyses were performed on the DG of all processed hippocampal samples (Figures 1F–I), stratified based on total average (Figure 1F), seizures detection (Figure 1G), dorsal/ventral (Figures 1H, I), and left/right hippocampus (data not shown). Notably, TMEV infection itself, rather than the occurrence of seizures, induced alterations in cell proliferation during the early post-virus infection stages, specifically at 14 dpi, when compared to the CTR group (Figures 1F, G). During the acute infection phase, cell proliferation in the dorsal DG was significantly reduced at 14 dpi in comparison to 3 dpi and to CTR (Figure 1H). No statistically significant differences in cell proliferation were observed between dorsal and ventral slices at any time point (data not shown). Additionally, no discernible variations in KI-67 expression were detected between the dorsal and ventral regions among both seizing and non-seizing mice (data not shown). An exploration into potential correlations between DG KI-67 cell density in seizing mice (including total, dorsal, or ventral) and seizure-related parameters such as number of seizures, seizure onset, and cumulative seizure score did not yield statistically significant findings (Supplementary Figures 2A, C, E).
3.3 TMEV infection immediately impairs DG neuronal progenitor densityNext, we sought to elucidate the impact of TMEV infection on DG neuronal progenitors (DCX+; Figure 2). DCX is a microtubule-associated protein expressed by actively dividing neuronal precursor cells and immature neurons in both embryonic and adult brains, maintaining stable expression as the cells differentiate into neurons (Brown et al., 2003). Quantitative cell density analyses unveiled a striking and immediate depletion of DCX+ neuronal progenitor cells in the DG during the acute phase of TMEV infection compared to the CTR group (Figures 2E–H). At 3 dpi, the depletion was nearly complete, indicating a rapid and drastic effect on the neuronal progenitor population. However, by 7 and 14 dpi, a partial recovery was observed, with DCX+ cell density at 14 dpi being significantly higher than at 3 dpi across the total DG, as well as within the dorsal and ventral hippocampal regions (Figures 2E, G, H). Despite this recovery, the density of DCX+ cells at 14 dpi remained significantly lower than that in the CTR group, except in the ventral hippocampal area (Figures 2E, G, H). To further investigate the potential influence of seizure activity on neuronal progenitor cells in TMEV-infected brains, we compared mice that developed seizures with infected non-seizing mice (Figure 2F): Interestingly, mice experiencing seizures exhibited a more pronounced reduction in neuronal progenitor cell density compared to the CTR group. No significant differences were observed between the dorsal and ventral regions in seizing and non-seizing mice (data not shown). Consistent with our findings using KI-67 as a marker, correlation analyses between DG DCX cell density in seizing mice (total, dorsal, or ventral) and seizure parameters did not yield statistically significant results (Supplementary Figures 2B, D, F).

Figure 2. Theiler's Murine Encephalomyelitis Virus infection severely impacts neuronal progenitor proliferation. (A–D) Representative images showing IHC of DCX in C57BL/6J-mouse hippocampal slices at different time points post infection [3 dpi (n = 4) (A), 7 dpi (n = 13) (B), 14 dpi (n = 16) (C), and CTR (n = 5) (D)]. (E) Average DCX+ cell density in TMEV-infected mice at different dpis. (A1–D1) Insets show magnified views of the corresponding ROIs in (A–D). Immature neurons were significantly reduced over the acute phase of infection compared to CTR (3 dpi vs. CTR: ####p < 0.0001, 7 dpi vs. CTR: ##p = 0.0047, 14 dpi vs. CTR: #p = 0.0259). DCX cell population slowly increased over time (3 dpi vs. 14 dpi: *p = 0.0376). (F) Differences in DCX expression were mainly due to the occurrence of seizures [3 dpi (+/–) vs. CTR: ####p < 0.0001, 7 dpi (+) vs. CTR: ##p = 0.0027, 14 dpi (+) vs. CTR: #p = 0.0223]. (G, H) Neuronal progenitor cell density analysis in both dorsal (G) and ventral (H) hippocampi. A partial recovery was observed over the different time points in both dorsal and ventral hippocampi [(G) 3 dpi vs. 14 dpi: *p = 0.0404; (H) 3 dpi vs. 14 dpi: *p = 0.0374]. The impact on neuronal progenitors seemed to be more severe in the dorsal hippocampal areas than in the ventral ones [(G) 3 dpi vs. CTR: ###p = 0.0001, 7 dpi vs. CTR: ##p = 0.0033, 14 dpi vs. CTR: #p = 0.0307; (H) 3 dpi vs. CTR: ###p = 0.0001, 7 dpi vs. CTR: #p = 0.0125]. The scale bar in (A) also applies to (B–D). Filled circles indicate male mice, while hollow circles denote females. Data are mean ± SEM, normality was tested using the Shapiro-Wilk test, statistical analyses were performed using Kruskal-Wallis test followed by Dunn's multiple comparison test. Statistical significance was set at the adjusted P-value ≤ 0.05.
3.4 Neuronal progenitor cell migration is affected by TMEV infectionTo evaluate the repercussions of TMEV infection on neuronal progenitor cell migration, we scrutinized the migration pattern of DCX+ cells on both the contralateral (left hemisphere) and ipsilateral (right hemisphere) sides relative to the TMEV injection (3 dpi (n = 4), 7 dpi (n = 13), 14 dpi (n = 16), as well as in CTR mice (n = 5) as shown in Figures 3A, B and expressed as percentage. Our analysis unveiled that CTR mice exhibited a parallel stratification of migrating neuronal progenitor cells in both analyzed hemispheres (Figures 3C, D). Indeed, over 90% of cells resided within regions categorized as score 1 or 2, characteristic of typical DCX+ cell locations, such as the subgranular zone of the DG and the ventral part of granule cell layer itself. In contrast, the migration pattern in infected animals was changed (Figures 3E–J), especially on the ipsilateral hemisphere to the infection: a migration score 4, representing neuronal progenitor migration outside of the typical areas within or below the granule cell layer (e.g., toward the hilus or stratum moleculare) was already visible at early time points (Figures 3F, H, J). At 3 dpi, despite very few DCX+ cells remaining after infection, a discernible aberration in the migratory behavior of DCX+ cells was evident (Figure 3F): 50% of the detectable DCX+ cells exhibited a migration score 4 in TMEV-infected mice. Neuronal progenitor cell migration on the contralateral side exhibited a smoother progression across various scores during the acute infection phase (Figures 3E, G, I). Specifically, migration score 4 was observed in the contralateral side starting at 14 dpi (Figure 3I). By this time point, the distribution of cells across different migration scores was almost equivalent in both sides (Figures 3I, J), with a notable surge in score 4 cells relative to prior time points. Analysis performed between seizing and non-seizing mice did not show any significance (data not shown).
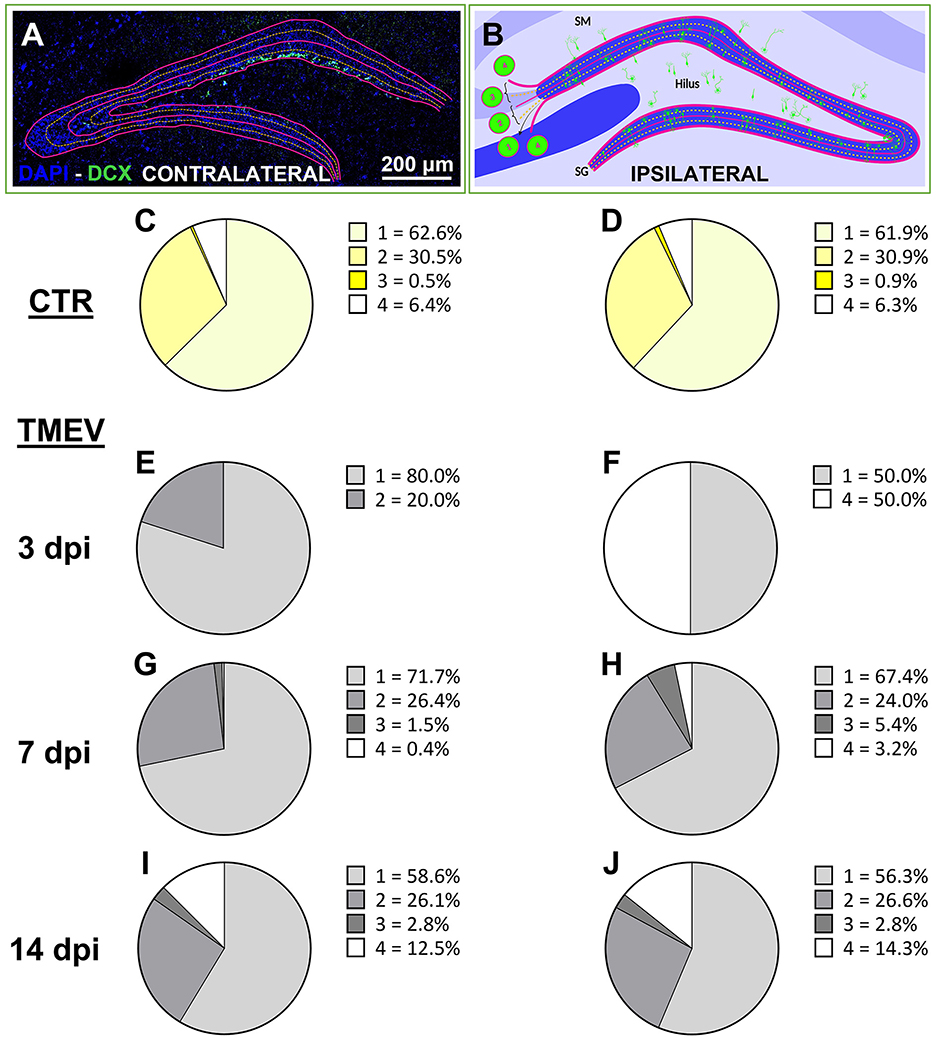
Figure 3. Neuronal progenitor cells aberrantly migrate over the acute phase after TMEV infection. (A, B) Aberrant migration was evaluated by analyzing ipsilateral and contralateral DG labeled for DCX at different time points [3 dpi (n = 4), 7 dpi (n = 13), 14 dpi (n = 16) compared to CTR (n = 5)]. (C, D) CTR showed identical score in both hemispheres. (E, G, I) In the acute phase of infection (from 3 to 14 dpi), the contralateral side showed a smoother transition of aberrant neuronal progenitor cell migration over the analyzed time points if compared to the ipsilateral side (F, H, J) which showed a completely aberrant migration already at 3 dpi (F). (I, J) At 14 dpi, the contralateral and the ipsilateral side of infection displayed identical migration scores.
3.5 TMEV infection impacts on the proliferative state of different classes of cellsAfter having found that immature neurons were depleted, we wanted to investigate whether they were replenished by cell proliferation. To investigate the impact of TMEV infection on neuronal progenitor proliferation, we conducted a focused analysis on the percentage of double-stained DCX+/KI-67+ cells. For this investigation, we utilized TMEV-infected mice from various time points (3 dpi (n = 4), 7 dpi (n = 13), 14 dpi (n = 16), compared to CTR (n = 5; Figures 4A–D). Quantitative analysis of the double-stained cells (Figure 4E) revealed that at 3 and 7 dpi, DCX+ neuronal progenitor cells demonstrated significantly reduced proliferation compared to CTR. This was accompanied by a progressive increase in proliferating neuronal precursors across the different time points, consistent with the upregulation of DCX expression (Figure 2). To further elucidate this phenomenon, we conducted region-specific analyses on the dorsal, ventral, left, and right DG regions (Supplementary Figure 4). The significant reduction in the percentage of proliferating neuronal progenitors observed at 3 and 7 dpi, compared to CTR, was consistent across all regions (Supplementary Figures 4A–D). Overall, only a small percentage of proliferative cells represented neuronal progenitors, even in the CTR group (6.4% of KI-67+ cells). This prompted us to assess the identity of the other proliferating cells, such as NSC (NES+/GFAP−), radial glia (GFAP+/NES+) and astrocyte-like cells (GFAP+/NES−), in order to determine the specific proliferating cell types. The rationale for selecting these cell types stemmed from literature suggesting that the stem cell niche is giving rise to either neuronal progenitor cells (in a physiological environment) or astrocytes (in a pathological environment; Hattiangady and Shetty, 2010). To gain deeper insights, we focused our analyses on 14 dpi for multiple reasons: (1) 14 dpi marks the endpoint for virus clearance in this model and represents the conclusion of the acute infection phase, (2) 14 dpi exhibited the highest expression of DCX+ cells during the acute phase, and (3) the 14-dpi cohort comprised both seizing and non-seizing mice. To conduct this experiment, we randomly selected 3–7 mice from each group (seizing and non-seizing), performed IHC on NES, GFAP, and KI-67 (Figures 4F–I), and analyzed the GFAP+/NES+/KI-67+, and GFAP+/NES−/KI-67+ cell populations, similar to our previous analysis of DCX+/KI-67+. Subsequent cell count analysis revealed that TMEV infection significantly reduced the percentage of proliferating NSC cell populations compared to CTR (Figure 4J) in all analyzed regions (Supplementary Figures 4E–H). Analysis of GFAP and NES co-labeled cells showed a significant reduction compared to CTR (Figure 4K), predominantly observed in the left hemisphere (Supplementary Figure 4K). Additionally, the percentage of proliferating astrocytes (Figure 4L) was significantly higher than in CTR, validating the presence of an inflammatory substrate, as indicated by our analysis of IBA1+ cell density and morphology (Figure 5). However, this increase in proliferating astrocytes was only statistically significant vs. CTR in the dorsal and ventral areas (Supplementary Figures 4M, N), while there was no difference between the infected right and left hemispheres (Supplementary Figures 4O, P). Despite conducting an analysis of the percentage of proliferating neuronal precursor cells, proliferating NSCs or proliferating astrocytes in relation to seizure-related parameters, such as number of seizures, seizure onset and cumulative seizure score, no statistically significant results were obtained (data not shown). To present a comprehensive overview of proliferating cells at the end of the acute phase following TMEV infection compared to CTR, we normalized the dataset for each individual cell marker used to detect proliferating cells to the KI-67+ total percentage (Figure 4M). This analysis yielded intriguing differences between CTR and TMEV-infected mice at 14 dpi. Specifically, not only did the percentage of proliferating early progenitors (NES+/KI-67+ and NES+/GFAP+/KI-67+) significantly decrease in TMEV-infected mice compared to CTR, but also the percentages of proliferating neuronal progenitor cells (DCX+/KI-67+) were notably lower. Conversely, the percentage of proliferating astrocytes (NES−/GFAP+/KI-67+) exhibited a striking increase in TMEV-infected mice compared to CTR.
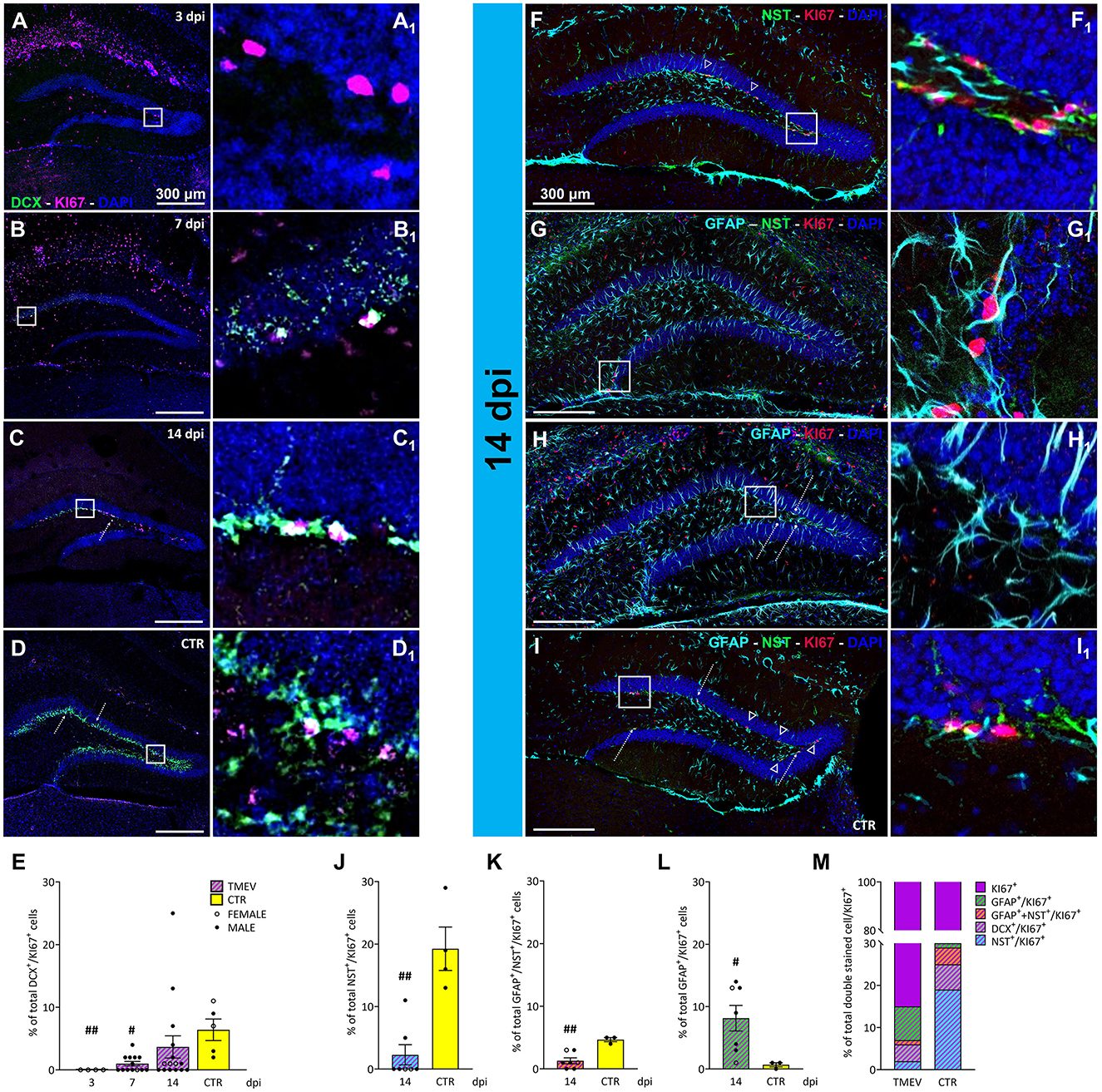
Figure 4. Proliferation state of different classes of cells during TMEV infection. (A–D) Representative images showing IHC of DCX and KI-67 in C57BL/6J-mouse hippocampal slices at different time points post infection [3 dpi (n = 4) (A), 7 dpi (n = 13) (B), 14 dpi (n = 16) (C), and CTR (n = 5) (D)]. (A1–D1) Insets show magnified views of the corresponding ROIs in (A–D). (E) Percentage of DCX+/KI-67+ analyzed in all the TMEV-infected mice divided per time points. Neuronal progenitor cells demonstrated significantly reduced proliferation at 3 and 7 dpi compared to CTR (3 dpi vs. CTR: ##p = 0.0037, 7 dpi vs. CTR: #p = 0.0201). (F–I) Identification of proliferative NSC cells and mature/immature astrocytes by IHC of NES+/KI-67+ (arrowheads; F, I), GFAP+/NES+/KI-67+ (G), and GFAP+/KI-67+ (bullet arrows; H, I), in a sub-cohort (n = 7) of 14 dpi C57BL/6J-mouse hippocampal slices. (F1–I1) Insets show magnified views of the corresponding ROIs in (F–I). (J) The percentage of proliferating NSC populations was significantly reduced in TMEV-infected mice compared to CTR (14 dpi vs. CTR: ##p = 0.0030) as well as (K) the percentage of proliferating GFAP and NES co-labeled forms (14 dpi vs. CTR: ##p = 0.0083). (L) The percentage of proliferating mature astrocytes was significantly higher compared to CTR (14 dpi vs. CTR: #p = 0.0333). (M) Normalized overview of the analyzed proliferating cell populations at the end of the acute phase following TMEV infection compared to CTR. The percentage of proliferating NSC (NES+/KI-67+), immature astrocytes (GFAP+/NES+/KI-67+), and immature neurons (DCX+/KI-67+) is significantly lower in TMEV-infected mice compared to CTR, conversely, the percentage of proliferating mature astrocytes (GFAP+/KI-67+) exhibited a striking increase in TMEV-infected mice compared to CTR. The scale bar in (A) also applies to (B–D) whilst the scale bar in (F) also applies to (G–I). Filled circles indicate male mice, while hollow circles denote females. Data are mean ± SEM, normality was tested using the Shapiro-Wilk test, statistical analyses were performed either using Kruskal-Wallis test followed by Dunn's multiple comparison test (E) or Mann-Whitney test (J–L). Statistical significance was set at the adjusted (multiple comparisons) or exact P-value ≤ 0.05.
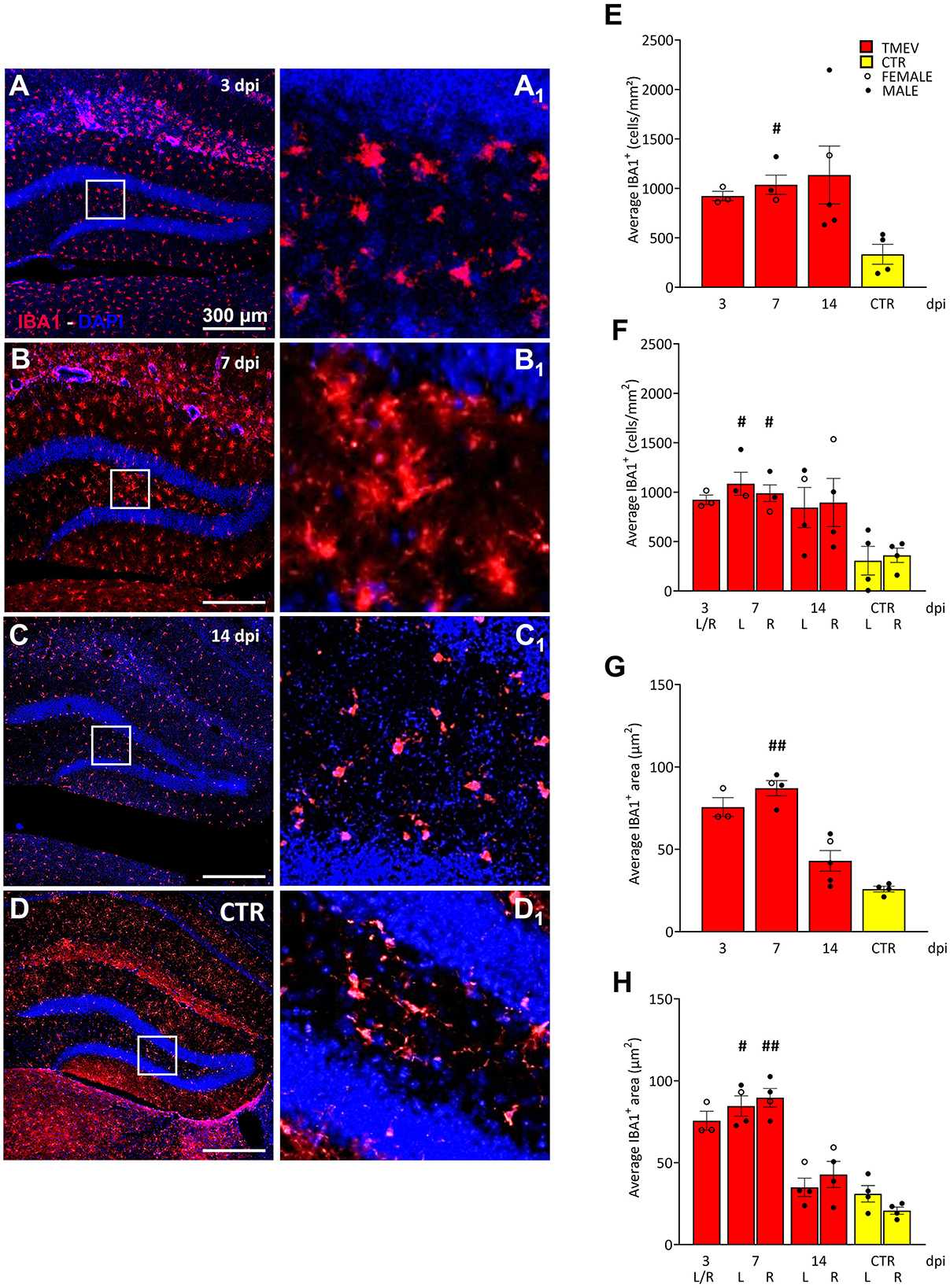
Figure 5. Theiler's Murine Encephalomyelitis Virus infection activates microglia response. (A–D) Representative images showing IHC of IBA1 in hippocampal slices of a subcohort of both seizing and non-seizing C57BL/6J-mice at different time points post infection [3 dpi (n = 3) (A), 7 dpi (n = 4) (B), 14 dpi (n = 5) (C), and CTR (n = 3) D]. (A1–D1) Insets show magnified views of the corresponding ROIs in (A–D). (E) DG IBA1+ cell density and (G) morphology showed an increase at 7 dpi relative to CTR [(E) 7 dpi vs. CTR: #p = 0.0451; (G) 7 dpi vs. CTR: ##p = 0.0032]. (F, H) This phenomenon occurred in both hemispheres (F) 7 dpi (L) vs. CTR (L) #p = 0.0372, 7 dpi (R) vs. CTR (R) #p = 0.0482; [(H) 7 dpi (L) vs. CTR (L) #p = 0.0445, 7 dpi (R) vs. CTR (R) ##p = 0.0017]. No significant differences between left (L) and right (R) as well as between cohorts were detected at any analyzed time point (E–H). The scale bar in (A) also applies to (B–D). Filled circles indicate male mice, while hollow circles denote females. Data are mean ± SEM, normality was tested using the Shapiro-Wilk test, statistical analyses were performed using Kruskal-Wallis test followed by Dunn's multiple comparison test. Statistical significance was set at the adjusted P-value ≤ 0.05.
3.6 Microglia activation in the mouse DG in the acute phase of virus infectionSince seizures did not seem to have a major effect on cell proliferation, contrarily to what was previously published in etiologically different models of seizures and epilepsy (Bengzon et al., 1997; Gray and Sundstrom, 1998; Parent et al., 2006; Parent and Murphy, 2008; Parent et al., 2002, 1997; Scott et al., 1998), we compared the rate of inflammation after virus infection to identify a potential influence on cell proliferation, and/or seizure occurrence. To achieve this, we examined a randomized sub-cohort of mice, consisting of both seizing and non-seizing animals (n = 3–5), over the course of infection from 3 to 14 dpi. CTR mice (n = 4) were included as a reference group for comparative analyses. As microglia play a crucial role in neuroinflammation (Bröer and Pauletti, 2024), we employed IBA1 as a well-established marker to assess reactive microglia (Figure 5). IBA1 is known to be upregulated during macrophage/microglia activation in various brain diseases (Hoogland et al., 2015; Ito et al., 1998; Lier et al., 2021), including TMEV infection (DePaula-Silva et al., 2021, 2019; Jafari et al., 2012; Loewen et al., 2016). In order to assess the extent of the inflammatory response occurring within the hippocampus during the acute stage of the infection (3–14 dpi), our investigation centered upon the quantification of IBA1 cell density and the characterization of alterations in their morphology (Supplementary Figures 1E–G). This approach was adopted with the dual purpose of identifying any potential augmentation in the presence of infiltrated microglial cells within the DG, while concurrently estimating their level of activation. This rationale is grounded in established literature, which stipulates that microglial activation engenders discernible shifts in cellular dimensions (as evidenced by an increase in cell size). By analyzing both the cell density and the area of each positive cell, we confirmed earlier work (Bröer et al., 2017, 2016; Cusick et al., 2017; DePaula-Silva et al., 2021; Hanak et al., 2019; Howe et al., 2022; Kirkman et al., 2010; Loewen et al., 2016; Sanchez et al., 2019; Waltl et al., 2018a,b) that a significant increase in recruited and activated immune cells was evident when comparing TMEV-infected mice with CTR at 7 dpi (Figures 5E–H). This increase in IBA1+ cell density and morphology was observed not only in the ipsilateral hemisphere, but also in the contralateral hemisphere to the site of virus injection (Figures 5F, H), indicating a bilateral microglial response during TMEV infection. We further sought to elucidate whether the microglial activation differed across distinct time points during the acute phase of infection: No statistical differences in IBA1 cell density and morphology were observed between 3, 7, and 14 dpi (Figures 5E–H), suggesting that microglia activation reaches a relatively stable state during this early phase of infection. Furthermore, we explored potential correlations between DG IBA1 cell density in seizing mice (total, dorsal, or ventral) and seizure-related parameters, such as number of seizures, seizure onset, and cumulative seizure score. However, no significant correlations were found in our analyses (data not shown). Moreover, the correlation analyses conducted between total DCX cell density and total IBA1 cell density (Supplementary Figures 3A, B) or IBA1 morphology (Supplementary Figures 3C, D) at 7 and 14 dpi did not attain statistical significance notwithstanding an apparent negative correlation trend evident in all depicted data.
4 DiscussionThe primary objective of this investigation was to conduct an initial assessment of the impact of viral encephalitis-induced neuroinflammation and seizures on adult neurogenesis during the acute phase of infection. To achieve this objective, we employed a viral encephalitis model using male and female C57BL/6J mice which were subjected to intracortical injection of the neurovirulent DA strain of TMEV. This strain is known for its infection of CA1 and CA2 pyramidal neurons, resulting in substantial neuronal loss within the hippocampal region (Bröer et al., 2017, 2016; Gerhauser et al., 2019) and acute polioencephalitis (Dal Canto and Lipton, 1982; Jafari et al., 2012; Lipton et al., 2005). This viral spread coincides with significant microglial activation, macrophage infiltration, and the release of pro-inflammatory cytokines, which play a crucial role in exacerbating neuroinflammation and modulating neuronal excitatory patterns, thus contributing to the onset of acute seizures (Bröer et al., 2017, 2016; Cusick et al., 2017; DePaula-Silva et al., 2021, 2018; Di Nunzio et al., 2021; Hanak et al., 2019; Howe et al., 2022; Kirkman et al., 2010; Loewen et al., 2016; Sanchez et al., 2019; Waltl et al., 2018a,b). Following infection, ~50%−70% of C57BL/6J mice exhibit transient early (acute) afebrile seizures along with impaired motor function and coordination within 3–7 dpi. This is followed by a seizure-free period, viral clearance by the immune system within 2–3 weeks, and a subsequent reduction in seizure thresholds months after infection, as well as chronic epilepsy in about 25%−40% of infected mice (Ba
留言 (0)