A thorough understanding of infant motor development is fundamental to the practice of pediatric physiotherapists. Infant motor development encompasses the development of postural control, movement patterns, and coordination, which are essential for the acquisition of motor skills. The complex and dynamic process of infant motor development is affected by several factors, including genetic, physical, neurological, social, and environmental factors (1). As infants grow, their movement repertoires expand and adjust in response to the challenges and learning opportunities presented by changing environments and task-specific contexts (2). These factors contribute to shaping the trajectory and speed of infant motor development (3).
Assessment tools offer comprehensive insights into infant motor development and are frequently used by pediatric physiotherapists alongside clinical observations (4, 5). Reliable, validated tools are recommended for consistent administration and scoring (6, 7). These tools help identify atypical or delayed motor development early, ensuring only infants in need receive interventions, thus preventing unnecessary treatment for typically developing infants (8, 9).
It is important to consider possible differences in pace of motor trajectories when using an assessment tool outside its original context (10–12). Hence, part of cross-cultural validity may entail an adaptation of the scores to the cultural context, and development of standards. This approach helps prevent insufficient or excessive follow-up due to invalid norms (13), thereby striking a balance that promotes accurate monitoring and appropriate intervention for each infant's unique developmental pathway.
The Alberta Infant Motor Scale (AIMS) is a widely used standardized tool for identifying infants with atypical or delayed motor function, also in Norway (5, 9, 13). A systematic review found the AIMS to have limited cross-cultural validity in terms of different trajectories for motor development across cultures, with several studies suggesting that the Canadian norms were excessively strict (13). Alternative AIMS reference values from Brazilian, Dutch, Polish, and Thai infants have been introduced to provide more culturally appropriate standards (14–17). These studies showing that the AIMS has low cross-cultural validity underline the need to examine whether the Canadian norms are valid for Norwegian infants.
Investigating the validity of the AIMS within the Norwegian infant population is important as the AIMS, with Canadian norms, is currently the most used assessment tool by Norwegian pediatric physiotherapists in primary- and specialist healthcare (5). Findings suggest that Norwegian children start walking independently significantly later than the Canadian AIMS norm reference (18). Additionally, Norwegian infants are found to achieve gross motor milestones later than other populations (3) all of which supports the need to investigate validity.
In this article, the cross-cultural validity of the AIMS will be investigated by comparing the scoring distributions. The primary aim of this cross-sectional study was to investigate the cross-cultural validity of the AIMS Canadian norm reference for Norwegian infants aged 6–9 months. The secondary aim was to compare the Norwegian sample's AIMS scores with those of the Dutch norms for infants in the same age range. The choice of Dutch norms for comparison stems from the geographical proximity and cultural similarities between the Netherlands and Norway.
The 6–9-month age interval range was targeted in this study because the most compelling evidence for identifying delayed motor function with the AIMS typically appears after eight months of corrected age (9). In this age range, infants are likely to be assessed with more items than in the younger and older age groups. Moreover, other studies on the cross-cultural validity of the AIMS have included this age range (14–17, 19–25), which allows for comparison. This phase is also crucial for infants as they refine their muscle coordination, heavily influenced by explorative activities that are essential for motor development (26). Additionally, the largest number of infants in the available sample was in the age range 6–9 months.
This investigation serves as a preliminary investigation, and the findings of this study will indicate whether there is a need for a comprehensive cross-cultural validation of the AIMS across all age groups in Norway.
Materials and methods Design, participants and recruitmentThe data of Norwegian infants used in this cross-sectional study was extracted from a previous study on infant motor assessment (27). The inclusion criteria were infants aged 3–18 months, corrected for prematurity. The exclusion criteria were infants with severe medical conditions that precluded assessment, and those whose parents did not speak and understand Norwegian or English. Data from all infants aged 6–9 months were extracted for this study.
The sample was recruited from four municipalities in western and southeastern Norway; Porsgrunn, Bamble, Tønsberg, and Bergen, between October 2015 and June2020. Public health nurses assisted in recruiting all eligible parents or legal guardians of infants during regular checkups in well baby clinics. Participants were also recruited through word of mouth from former participants.
Detailed informed consent was provided to establish predictability and ensure that parents were fully aware of the study's aim, their role, and the handling of their data (27). The consent letter assured parents that they and the public health nurse would be notified of any concerns regarding the infant's motor function identified during the assessment. Parents were also informed, verbally and in writing, of their right to withdraw from the project at any time without affecting the follow-up service provided by the child healthcare centers. No participants withdrew from the study.
Method of data collectionScoring based on the Canadian AIMS norm reference was conducted in first half of 2023 using video recordings of assessments performed between October 2015 and June 2020 (27, 28). The third author (KMT), a specialist in pediatric physical therapy, conducted all assessments. Each infant was assessed once with minimal physical handling, ensuring they were in an alert, non-crying state. Various settings were chosen for conducting the assessments, including well baby clinics, the infant's home, the Western Norway University of Applied Sciences, and the Children's Physiotherapy Center in Bergen. Demographic characteristics of the sample and general population of Norwegian infants were obtained from the Medical Birth Registry of Norway (27, 29).
MeasuresThe main variables of interest in this study were the AIMS scores from the Canadian, Dutch, and Norwegian samples (9, 17). The AIMS assesses infants from term (40 weeks gestation) to 18 months post-term, based on observation of qualitative and functional aspects of spontaneous movement (9). Assessment is conducted using 58 observational items in the prone, supine, sitting, and standing positions. Each item is scored as 1 (observed) or 0 (not observed), with a total possible score of 58 points. Age-specific norms, adjusting for corrected age for preterm birth (before 37 weeks gestation), are used for identifying potential motor delays by applying cut-off percentiles. The cut-off for infants up to 8 months is the 10th percentile, and the 5th percentile is used from 8 months onwards (9). The AIMS is considered a cost and time-effective tool with robust psychometric properties (4, 9).
A previous study on the cross-cultural validation of the AIMS norms established a threshold for clinically significant differences as a variation of two points in the raw score (20). This threshold was also applied in this study.
Statistical analysisBackground characteristics of the Norwegian sample were analyzed using descriptive statistics. To determine the sample's representativeness of the Norwegian infant population, we compared it with open-access data from the Medical Birth Registry of Norway. The chi-square test was applied to assess differences in the categorical variables, while an independent t-test was utilized for the continuous variables. The sample distribution on the Canadian and Dutch AIMS percentiles was analyzed using descriptive statistics to calculate frequencies.
The total raw AIMS scores from the Canadian norms were compared to those of the Norwegian sample using an independent t-test for each age group, with a statistically significant level set at p < 0.05.
The same comparison with the Dutch norms was not possible because the total raw AIMS scores for the Dutch sample were not available (17). However, the Dutch material did provide a table of scores corresponding to each percentile in the norms, a format also available in the Canadian material (9). To facilitate comparison, we created a similar table for the Norwegian sample. This was done by sorting the infants by month and ranking them according to their AIMS scores, we determined the number of points required for the 5th–90th percentiles in the Norwegian sample.
Statistical analysis was performed using IBM SPSS Statistics (Version 29) and Microsoft Excel. Additionally, a medical statistician was consulted to ensure the robustness and validity of the statistical process employed.
Ethical considerationsThis study utilizes previously collected data, where ethical approval was obtained from the Regional Committee for Medical and Health Research Ethics (2016/566 REK vest) and by the Norwegian Social Science Data Service (project no. 45014/3/MSS) (27). Informed consent was obtained from all parents or legal guardians involved.
Results Sample characteristicsDetailed characteristics of the sample, including information about infants and their mothers, are presented in Table 1. The sample comprises 189 infants, distributed across each age interval with a range of 44–51 infants per group. In the group of 9-month-old infants, there was a larger proportion of males, preterm infants, young mothers and variation of birth weight. None of the preterm infants were extremely preterm or had a very low birthweight (Table 1). They were thus considered low-risk infants.
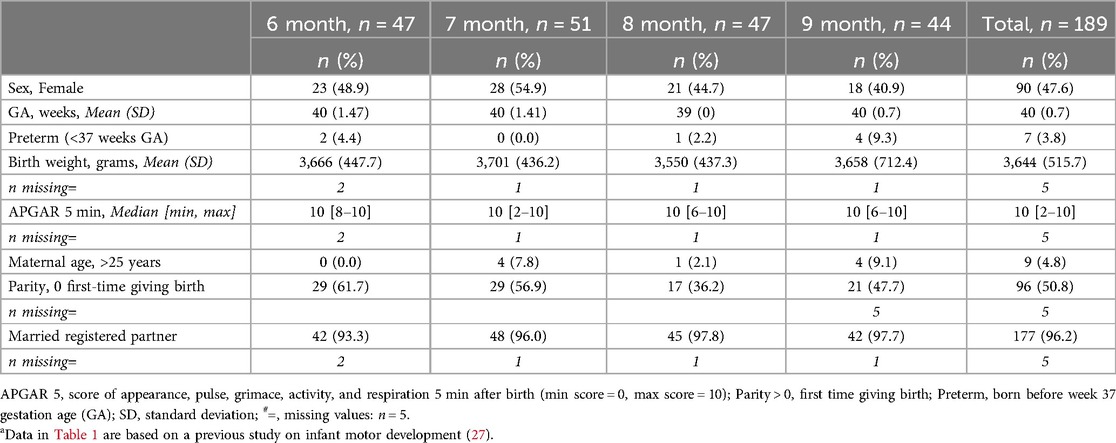
Table 1. Background characteristics of the Norwegian sample, infants aged 6–9 monthsa.
No significant differences were found regarding infant sex, preterm birth status, or maternal marital status when comparing the sample characteristics to those of the Norwegian infant population. Significant differences in maternal age and parity were observed; our sample included a smaller percentage of mothers under the age of 25 and a higher percentage of first-time mothers. Additionally, the sample showed a significantly higher average birth weight than the general population. Overall, the sample was considered a low-risk group with demographics representative of the Norwegian infant population.
Comparison of percentiles in the Canadian, Dutch, and Norwegian sampleFigure 1 presents a tentative Norwegian percentile rank based on the scores of the sample. The Canadian norms consistently show the highest percentile ranks, followed by the Norwegian sample, and then the Dutch norms. The observed differences are considered clinically significant between Canadian and Norwegian norms, and Dutch and Norwegian norms. The Canadian norms are at least two points higher than the Norwegian ranks, with exceptions at the 5th percentile for ages 6, 8, and 9 months, and the 10th percentile for 7-month-old infants. Conversely, the Dutch norms are generally more than two points lower than the Norwegian ranks, except at the 90th percentile for infants aged 8 and 9 months.
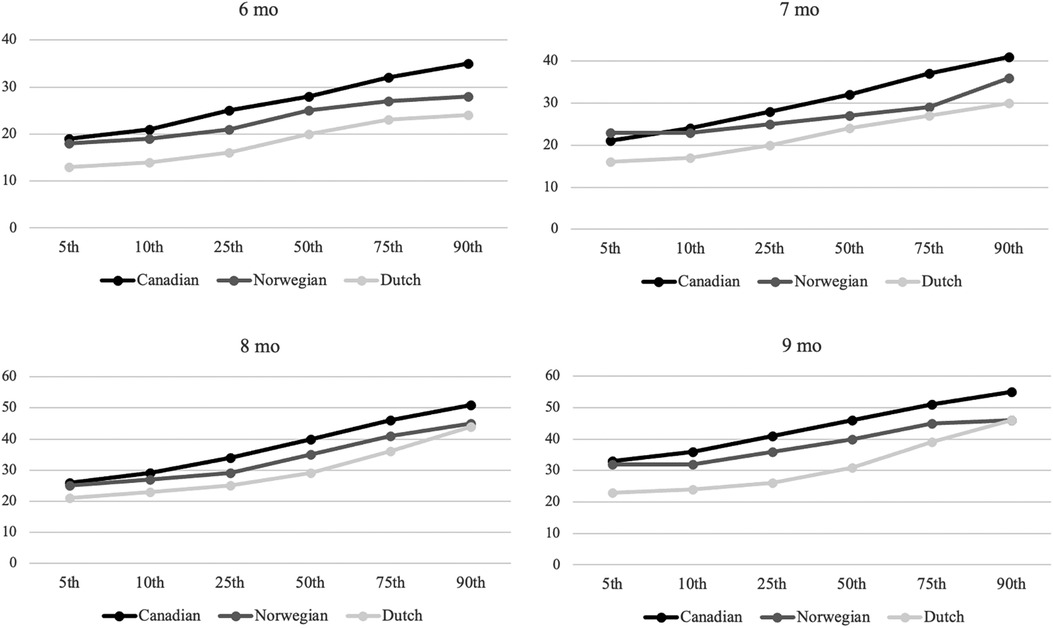
Figure 1. Percentile ranks for Canadian, Norwegian, and Dutch samples aged 6–9 months. Y-axis: AIMS total score, X-axis percentiles.
Distribution of Canadian and Dutch AIMS percentiles in the Norwegian sampleUsing Canadian norms, the median percentile rank for the Norwegian sample is the 50th, and the mean percentile value is 29.8. The Norwegian sample displays a left-skewed distribution with a higher prevalence of lower AIMS scores, as seen in Figure 2. Overall, 81% of the Norwegian sample scored at or below the 50th percentile, with 18% falling at or below the cut-off, indicating a possible motor delay. A substantial proportion of infants across all age groups scored at or below the 50th percentile: 94% at 6 months, 84% at 7 months, 70% at 8 months, and 77% at 9 months. Regarding cut-off scores, 30% at 6 months and 18% at 7 months scored below the 10th percentile. Additionally, 9% at 8 months and 16% at 9 months scored at or below the 5th percentile threshold.
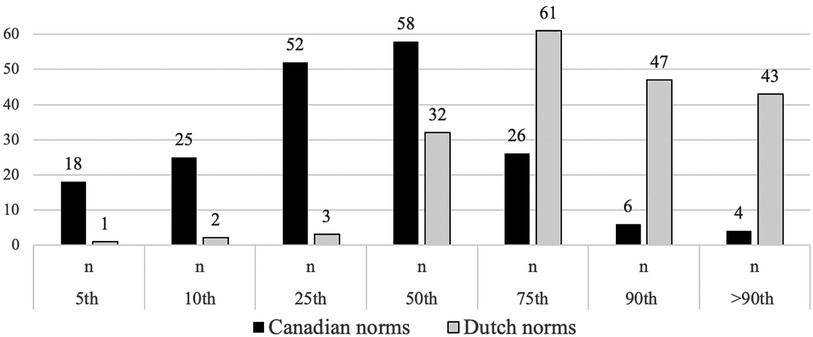
Figure 2. Number of infants aged 6–9 months from the Norwegian sample falling within the Canadian and Dutch percentiles of the Alberta AIMS. Y-axis: Number of children, X-axis: percentiles.
Using Dutch norms, the median percentile rank is the 75th. This indicates a right-skewed distribution with a higher prevalence of higher AIMS scores (Figure 2). Overall, 20% of the Norwegian sample scored at or below the 50th percentile, with only 1% falling at or below the cut-off. Within each age group, a smaller proportion scored at or below the 50th percentile: 23% at 6 months, 22% at 7 months, 28% at 8 months, and 5% at 9 months. Regarding cut-off scores, none of the infants at 6 or 7 months scored below the 10th percentile. At 8 months, 2% fell below the 5th percentile, and at 9 months, no infants scored below this cut-off.
Comparison of the mean total raw score in the Canadian and Norwegian sampleFigure 3 displays the comparison of the mean total raw AIMS scores between the Canadian normative sample and the Norwegian sample. There is a statistically significant difference across all age intervals with p-values below 0.001. The variation in mean total raw scores ranges from 4.1 to 5.6 points.
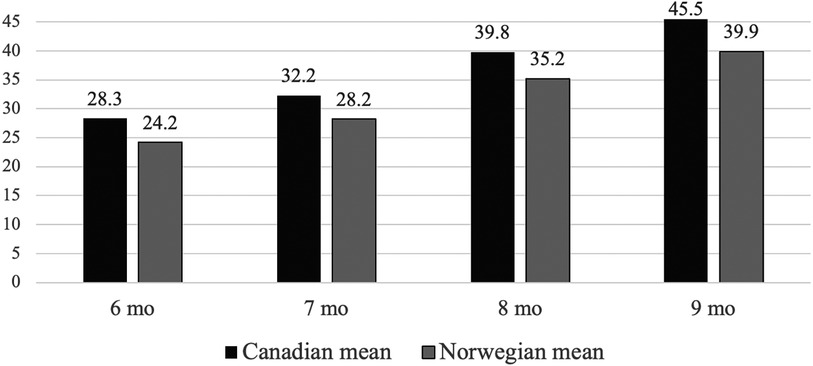
Figure 3. The mean total AIMS score of Norwegian infants and Canadian normative sample aged 6–9 months categorized in monthly intervals.
DiscussionThe primary finding suggests that the Canadian AIMS norm reference is not valid for the sample of Norwegian infants aged 6–9 months. The sample had significantly lower mean total scores than their Canadian peers across all corresponding age groups. This is clinically relevant since the observed differences, ranging from 4.1 to 5.6 points, exceed the two-point threshold for clinically significant differences (20). Additionally, the Canadian AIMS percentile rank was also higher than the Norwegian sample's, further indicating a clinically significant difference.
The secondary finding indicates that the Dutch percentile ranks were lower than those of the Norwegian sample, a difference considered clinically significant due to a general trend of exceeding the two-point threshold.
The sample in the current study mirrors the demographic characteristics of the general population of Norwegian infants, which enhances the generalizability of the results (11). Our findings also align with research suggesting that Norwegian infants' motor skill development pace differs from that of peers in other cultures (3, 18), further lending credibility to our observations.
Our findings support previous research indicating that the AIMS norms are overly strict and have limited cross-cultural validity (14–17, 19–22, 24, 30). A comprehensive cross-cultural validation of the AIMS is warranted to address the observed discrepancies among Norwegian infants.
The findings of this study align with those of a systematic review which indicates that several standardized assessment tools developed in North America may not have universal validity, particularly for assessing motor development in children aged 0–2 years (13). Motor development is known to be influenced by a variety of factors, including biological aspects such as genetics, prenatal health, prematurity, birth complications, physical health, and nutrition, as well as environmental influences like socioeconomic status, environmental stimulation, and psychosocial factors (3, 13, 17).
Cultural caregiving practices are a prominent environmental influence that contribute to differences in motor development (3, 31). Comparing cultural caregiving practices across Canada, the Netherlands, and Norway is challenging due to the subjective nature of cultural norms and their impact on motor development. However, research suggests that cultural norms in North America often promote early sitting and active training (13, 32). In contrast, Norwegian and Dutch cultural norms tend to support a more natural progression, allowing infants to develop at their own pace (3, 17, 18, 31).
Cultural caregiving practices provide diverse experiences, such as positioning and handling routines that encourage movements against gravity (26). These opportunities for motor exploration afforded by the environment and trial-and-error experiences contribute to the acquirement of motor skills (2, 10). North American caregiving practices may potentially lead to an overall faster trajectory of motor development as infants are exposed to positions that require postural control.
On the other hand, the more lenient approach by Norwegian and Dutch caregivers may result in the later attainment of postural control and overall motor development. For example, infants with limited prone position experience often exhibit temporary motor delays, as this position is crucial for developing the upper body strength and motor control needed for movements against gravity (33, 34). These skills are fundamental elements of later motor skills, thereby underlining the influence of early experiences on future motor outcomes (2).
Strengths and limitations of the studyThe sample size in our study was deemed adequate for a preliminary investigation into cross-cultural differences of the AIMS. It is estimated that a minimum of 20 infants per age group can provide 80% power for assessing the cross-cultural validity of the AIMS (35). Additionally, our sample size aligns with those of other studies that have studied the cross-cultural validity of the AIMS (20, 21, 36–39). The sample size was deemed too small to establish reliable percentile ranks (9, 40). Therefore, it is imperative to note that the Norwegian percentile values presented here are provisional and should be interpreted with caution when compared to Canadian and Dutch percentiles. The constrained sample size could potentially introduce bias into the comparison.
The sample was considered a low-risk group with demographics representative of the Norwegian infant population; however, additional examination is necessary. The study's scope was limited to two geographical areas and a few demographic variables. Hence, it is important to acknowledge that these may not encompass all the potential discrepancies between the sample and the broader population. Notably, influential factors such as socioeconomic background and health literacy were not accounted for in the analysis. The potential of self-selection bias ought to be considered, as participants in similar research in Norway tend to have higher socioeconomic background and enhanced health literacy (41–43).
Statistical considerations include the use of an independent samples t-test to compare AIMS mean total scores, despite the non-normal distribution of the Norwegian sample. The absence of necessary data for non-parametric tests within the AIMS material (9), necessitated the use of a parametric test for group comparison, which may be considered a study limitation. However, a Mann–Whitney U test was also conducted to compare the groups under the assumption that the Canadian values followed a perfectly normal distribution. This assumption introduces yet another potential limitation. Despite these methodological challenges, both tests indicated significant differences between the Canadian and Norwegian samples. After consulting with a medical statistician, we selected the independent samples t-test for its alignment with the methods used in other studies that have undertaken cross-cultural validation of the AIMS (14–16, 19, 20, 22, 35, 37, 44, 45). We consider this consistency in methodology as a strength of our research.
Clinical implicationsThe stringent Canadian norms, currently used in Norway (5), may lead to the misclassification of normal motor development variations as delays. This could result in unnecessary referrals to pediatric physiotherapy and early intervention services, wasting resources and causing undue stress for families of healthy infants. Conversely, the more lenient Dutch norms might fail to identify infants with genuine delayed motor development among Norwegian infants. This could delay crucial early interventions, necessary service referrals, and the provision of adequate support to families truly in need.
These findings highlight the critical need for validated, culturally appropriate reference values for Norwegian infants to accurately evaluate motor development and prevent misclassification risks. Norwegian reference values should be generated from a large sample that accurately represents the proportion of preterm infants, socioeconomic background, and cultural diversity of the population (46). These considerations are key as these factors are known to influence infant motor development (4, 8, 13, 47–49).
In addition to creating a normative reference for the Norwegian population, it is important to recognize that language and cultural context significantly influence the validity of assessment tools (12). Cultural adaptations must extend beyond mere direct translation and should encompass a thorough translation process of the test manual conducted by a group of experts (50). Such a method ensure that cross-cultural adaptations are tailored to the specific context and are systematically validated, thereby contributing to more accurate assessments that can be reliably used in clinical practice (40).
In the absence of a Norwegian-adapted version of the AIMS, pediatric physiotherapists should cautiously interpret AIMS results, acknowledging that Norwegian infants may exhibit slower motor development compared to Canadian peers. It is important to recognize the limitations of standardized assessments, which might not capture an infant's complete motor skills, potentially leading to discrepancies in observed behaviors (4, 8). The AIMS should be considered one component of a comprehensive evaluation that includes clinical assessments, clinical reasoning, and critical evaluations (5).
ConclusionThe Canadian and Dutch AIMS norm references are indicated to have limited applicability for Norwegian infants aged 6–9 months in this study, with Canadian norms being too strict and Dutch norms too lenient. A thorough cross-cultural validation to establish Norwegian-specific AIMS norms is recommended.
Data availability statementThe datasets presented in this article are not readily available because Ethical approval presupposes that the data will only be used for the project and the research questions for which approval has been sought. Requests to access the datasets should be directed to Datasets are not available on request due to ethical restrictions.
Ethics statementThe studies involving humans were approved by Regional Committees for Medical and Health Research Ethics, Norway. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributionsAG: Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. KR: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing. KT: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. The work was supported by the Norwegian Fund for Post-Graduate Training in Physiotherapy. The funding source had no involvement in study design, data collection, or interpretation of results.
AcknowledgmentsWe thank all infants and parents who participated in the study. Artificial intelligence has been used for writing assistance (51).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1511965/full#supplementary-material
References1. Adolph KE, Franchak JM. The development of motor behavior. Wiley Interdiscip Rev Cogn Sci. (2017) 8(1–2):e1430. doi: 10.1002/wcs.1430
Crossref Full Text | Google Scholar
3. Organization WH. Assessment of sex differences and heterogeneity in motor milestone attainment among populations in the WHO multicentre growth reference study. Acta Paediatr. (2006) 95:66–75. doi: 10.1111/j.1651-2227.2006.tb02377.x
Crossref Full Text | Google Scholar
4. Spittle AJ, Doyle LW, Boyd RN. A systematic review of the clinimetric properties of neuromotor assessments for preterm infants during the first year of life. Dev Med Child Neurol. (2008) 50(4):254–66. doi: 10.1111/j.1469-8749.2008.02025.x
PubMed Abstract | Crossref Full Text | Google Scholar
5. Melfald Tveten K, Olsen SA, Riiser K. Å teste eller ikke teste?—en studie av norske fysioterapeuters praksis for kartlegging av motorisk funksjon. Fysioterapeuten. (2024) 91(5):22–7.
6. Noritz GH, Murphy NA, Panel NSE, Murphy NA, Hagan JF Jr, Lipkin PH, et al. Motor delays: early identification and evaluation. Pediatrics. (2013) 131(6):e2016–e27. doi: 10.1542/peds.2013-1056
PubMed Abstract | Crossref Full Text | Google Scholar
8. Palisano RJ, Orlin MN, Schreiber J. Physical Therapy for Children. St. Louis, MI: Elsevier (2014).
9. Piper M, Darrah J. Motor Assessment of the Developing Infant. 2nd ed. St. Louis, MI: Elsevier (2022).
10. Cintas HL. Cross-cultural similarities and differences in development and the impact of parental expectations on motor behavior. Pediatr Phys Ther. (1995) 7(3):103–11. doi: 10.1097/00001577-199500730-00004
Crossref Full Text | Google Scholar
11. de Vet HCW, Terwee CB, Mokkink LB, Knol DL. Measurement in Medicine: A Practical Guide. Cambridge: Cambridge University Press (2011).
12. Gjersing L, Caplehorn JR, Clausen T. Cross-cultural adaptation of research instruments: language, setting, time and statistical considerations. BMC Med Res Methodol. (2010) 10:1–10. doi: 10.1186/1471-2288-10-13
PubMed Abstract | Crossref Full Text | Google Scholar
13. Mendonça B, Sargent B, Fetters L. Cross-cultural validity of standardized motor development screening and assessment tools: a systematic review. Dev Med Child Neurol. (2016) 58(12):1213–22. doi: 10.1111/dmcn.13263
PubMed Abstract | Crossref Full Text | Google Scholar
14. Eliks M, Anna S, Barbara S, Gajewska E. The standardization of the polish version of the Alberta Infant Motor Scale. BMC Pediatr. (2023) 23(1):236. doi: 10.1186/s12887-023-04055-5
PubMed Abstract | Crossref Full Text | Google Scholar
15. Saccani R, Valentini NC, Pereira KR. New Brazilian developmental curves and reference values for the Alberta Infant Motor Scale. Infant Behav Dev. (2016) 45:38–46. doi: 10.1016/j.infbeh.2016.09.002
PubMed Abstract | Crossref Full Text | Google Scholar
16. Tupsila R, Bennett S, Mato L, Keeratisiroj O, Siritaratiwat W. Gross motor development of Thai healthy full-term infants aged from birth to 14 months using the Alberta Infant Motor Scale: inter individual variability. Early Hum Dev. (2020) 151:105169. doi: 10.1016/j.earlhumdev.2020.105169
PubMed Abstract | Crossref Full Text | Google Scholar
17. van Iersel PA, la Bastide-van Gemert S, Wu Y-C, Hadders-Algra M. Alberta Infant Motor Scale: cross-cultural analysis of gross motor development in Dutch and Canadian infants and introduction of Dutch norms. Early Hum Dev. (2020) 151:105239. doi: 10.1016/j.earlhumdev.2020.105239
PubMed Abstract | Crossref Full Text | Google Scholar
19. De Kegel A, Peersman W, Onderbeke K, Baetens T, Dhooge I, Van Waelvelde H. New reference values must be established for the Alberta Infant Motor Scales for accurate identification of infants at risk for motor developmental delay in flanders. Child Care Health Dev. (2013) 39(2):260–7. doi: 10.1111/j.1365-2214.2012.01384.x
PubMed Abstract | Crossref Full Text | Google Scholar
20. Fleuren K, Smit L, Stijnen T, Hartman A. New reference values for the Alberta Infant Motor Scale need to be established. Acta Paediatr. (2007) 96(3):424–7. doi: 10.1111/j.1651-2227.2007.00111.x
PubMed Abstract | Crossref Full Text | Google Scholar
21. Lopes VB, de Lima CD, Tudella E. Motor acquisition rate in Brazilian infants. Infant Child Dev. (2009) 18(2):122–32. doi: 10.1002/icd.595
Crossref Full Text | Google Scholar
22. Saccani R, Valentini NC. Reference curves for the Brazilian Alberta Infant Motor Scale: percentiles for clinical description and follow-up over time. J Pediatr (Rio J). (2012) 88:40–7. doi: 10.2223/JPED.2142
PubMed Abstract | Crossref Full Text | Google Scholar
23. Saccani R, Valentini NC. Cross-cultural analysis of the motor development of Brazilian, Greek and Canadian infants assessed with the Alberta Infant Motor Scale. Rev Paul Pediatr. (2013) 31:350–8. doi: 10.1590/S0103-05822013000300012
PubMed Abstract | Crossref Full Text | Google Scholar
24. Suir I, Boonzaaijer M, Nijmolen P, Westers P, Nuysink J. Cross-cultural validity: canadian norm values of the Alberta Infant Motor Scale evaluated for Dutch infants. Pediatr Phys Ther. (2019) 31(4):354–8. doi: 10.1097/PEP.0000000000000637
PubMed Abstract | Crossref Full Text | Google Scholar
27. Melfald Tveten K. Motor function in infants attending primary care: assessment and associations with maternal folic acid and multivitamin intake during pregnancy [Philosophiae doctor (Ph.D)]. Høgskulen på Vestlandet (2022).
28. Hadders-Algra M. Heineman KR. The Infant Motor Profile: Routledge (2021).
30. Aimsamrarn P, Janyachareon T, Rattanathanthong K, Emasithi A, Siritaratiwat W. Cultural translation and adaptation of the Alberta Infant Motor Scale Thai version. Early Hum Dev. (2019) 130:65–70. doi: 10.1016/j.earlhumdev.2019.01.018
PubMed Abstract | Crossref Full Text | Google Scholar
31. van Schaik SD, Oudgenoeg-Paz O, Atun-Einy O. Cross-cultural differences in parental beliefs about infant motor development: a quantitative and qualitative report of middle-class Israeli and Dutch parents. Dev Psychol. (2018) 54(6):999. doi: 10.1037/dev0000494
PubMed Abstract | Crossref Full Text | Google Scholar
32. Kretch KS, Willett SL, Hsu L-Y, Sargent BA, Harbourne RT, Dusing SC. “Learn the signs. Act early.”: updates and implications for physical therapists. Pediatr Phys Ther. (2022) 34(4):440–8. doi: 10.1097/PEP.0000000000000937
PubMed Abstract | Crossref Full Text | Google Scholar
33. Pin T, Eldridge B, Galea MP. A review of the effects of sleep position, play position, and equipment use on motor development in infants. Dev Med Child Neurol. (2007) 49(11):858–67. doi: 10.1111/j.1469-8749.2007.00858.x
PubMed Abstract | Crossref Full Text | Google Scholar
34. Salls JS, Silverman LN, Gatty CM. The relationship of infant sleep and play positioning to motor milestone achievement. Am J Occup Ther. (2002) 56(5):577–80. doi: 10.5014/ajot.56.5.577
PubMed Abstract | Crossref Full Text | Google Scholar
35. Kepenek-Varol B, Hoşbay Z, Varol S, Torun E. Assessment of motor development using the Alberta Infant Motor Scale in full-term infants. Turk J Pediatr. (2020) 62(62):94–102. doi: 10.24953/turkjped.2020.01.013
PubMed Abstract | Crossref Full Text | Google Scholar
36. Jeng S-F, Yau K-IT, Chen L-C, Hsiao S-F. Alberta Infant Motor Scale: reliability and validity when used on preterm infants in Taiwan. Phys Ther. (2000) 80(2):168–78. doi: 10.1093/ptj/80.2.168
PubMed Abstract | Crossref Full Text | Google Scholar
37. Manuel A, Burger M, Louw Q. Validation of the Canadian norms for the Alberta Infant Motor Scale for infants in a South African region aged four to twelve months; a pilot study. S Afr J Physiother. (2012) 68(2):23–8. doi: 10.4102/sajp.v68i2.12
Crossref Full Text | Google Scholar
38. Morales-Monforte E, Bagur-Calafat C, Suc-Lerin N, Fornaguera-Martí M, Cazorla-Sánchez E, Girabent-Farrés M. The Spanish version of the Alberta Infant Motor Scale: validity and reliability analysis. Dev Neurorehabil. (2017) 20(2):76–82. doi: 10.3109/17518423.2015.1066461
PubMed Abstract | Crossref Full Text | Google Scholar
39. Wang H, Li H, Wang J, Jin H. Reliability and concurrent validity of a Chinese version of the Alberta Infant Motor Scale administered to high-risk infants in China. Biomed Res Int. (2018) 2018:2197163. doi: 10.1155/2018/2197163
PubMed Abstract | Crossref Full Text | Google Scholar
40. Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, et al. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol. (2010) 63(7):737–45. doi: 10.1016/j.jclinepi.2010.02.006
PubMed Abstract | Crossref Full Text | Google Scholar
41. Magnus P, Birke C, Vejrup K, Haugan A, Alsaker E, Daltveit AK, et al. Cohort profile update: the Norwegian mother and child cohort study (MoBa). Int J Epidemiol. (2016) 45(2):382–8. doi: 10.1093/ije/dyw029
留言 (0)