Angelica sinensis is the dry root of the umbelliferae plant Angelica sinensis (Oliv.) Diels, which is recorded in Chinese Pharmacopoeia (2020) (Figures 1A, B). The object exhibits a light brown to brown coloration, characterized by longitudinal wrinkles and transverse lens-like perforations. Its fried slices are typically round, oval, or irregular in shape, displaying a yellow-white to light brown hue with a flat surface that may feature cracks. A light brown cambium ring is present at the center, accompanied by several brown oil spots (Figures 1C, D). Angelica sinensis is mainly produced in southeastern Gansu, followed by Yunnan, Sichuan, Shaanxi, Hubei and other provinces. Angelica sinensis was first described in Shennong Ben Cao Jing and is known for its ability to tonify blood. It is a traditional medicinal and edible plant that has long been used for invigorating the blood, promoting circulation, lubricating the intestines, regulating menstruation as well as relieving pain, treating female irregular menstruation and amenorrhea (Erlin et al., 2019).

Figure 1. (A, B) Angelica sinensis herbs (Plant Photo Bank of China, PPBC). (C) Angelica sinensis original medicinal materialsand (Baidu Gallery). (D) Angelica sinensis slices (Herbal medicine room, Affiliated Hospital of Gansu University of Chinese Medicine).
ASP are natural macromolecular compounds and the main active substances of Angelica sinensis. They offer a wide range of pharmacological functions, for example, promoting hematopoiesis and improving immunity, antioxidation, and antitumour effects (Jin et al., 2012). It has been widely used owing to its low toxicity, residue-free nature, and nontolerant properties (Jin et al., 2012). As a result, the utilization of ASP is gaining more attention from scholars worldwide. With the deepening of research, the medical potential of ASP is gradually explored. This paper provides an overview of the current research on the extraction, isolation, and pharmacological effects of ASP. Additionally, it analyzes and summarizes the potential value of ASP, thus, serving as a reference for further research on ASP and its application in the fields of food, health products, and pharmaceuticals.
2 MethodologyThis review article has been retrieved in the form of a database search. The search terms are in the form of subject words combined with free words. We systematically searched Baidu Literature, China National Knowledge Infrastructure, VIP Datas, PubMed, Google-Scholar and Web of Science. Using“Angelica sinensis”or “Angelica sinensis polysaccharides”, and “pharmacological effects”, “extraction” and “structure”, as the search terms from scientific ethnobotany and ethnomedicine databases (up to September 2024), total of 663 articles were retrieved, and 484 were duplicated by software and manual removal. We carefully reviewed and classified the title, abstract and full text of the literature, and finally obtained 21 articles on ASP extraction, separation and structural analysis, 90 pharmacological research studies. The research methods we included include clinical studies, clinical trials, cell experiments, animal experiments, literature reviews, network pharmacology, etc. We extracted study details, including the relevant information on the pharmacological action and chemistry attributes of ASP, as well as the study status. Finally, through literature review and summary, it was found that ASP have ten pharmacological effects, as shown in the following Figure 2.
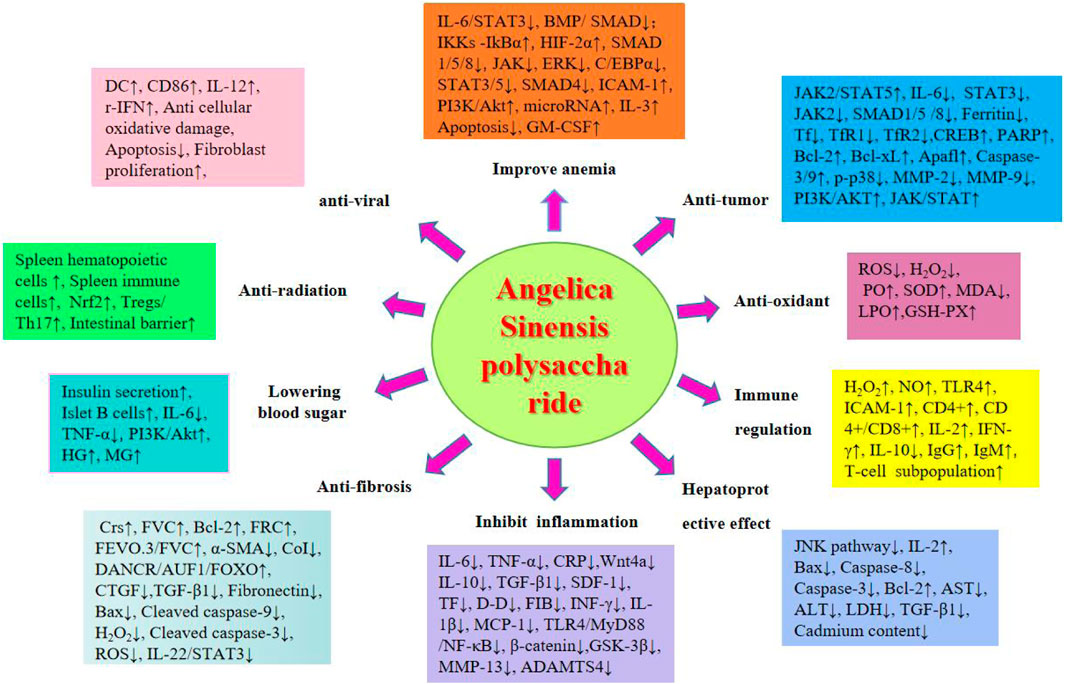
Figure 2. Pharmacological effects of ASP.
2.1 Extraction of ASPASP is a typical traditional Chinese medicinal polysaccharide, and its extraction method is similar to that of other traditional Chinese medicinal polysaccharides. Common extraction methods include water extraction and alcohol precipitation, ultrasound extraction, enzyme extraction, and microwave-assisted extraction.
2.1.1 Water extraction and alcohol precipitation methodsAt present, the extraction process of polysaccharides is mostly water extraction and alcohol precipitation, which means that the medicinal material of Angelica sinensis is first defatted with organic solvents, then extracted with hot water, and then subjected to alcohol precipitation with a certain concentration of ethanol. The water extraction and alcohol precipitation method has the advantages of simplicity, convenience, low cost, no pollution, and suitability for industrial production. However, a high temperature is required during the extraction process, resulting in a low extraction rate, significant loss of polysaccharide activity, and difficult purification (Huang, 2010). Yu et al. (2013) used response surface methodology and reported that the extraction time and solid‒liquid ratio are the two most important factors affecting the yield of ASP in hot water extraction. Zhang et al. (2016b) soaked Angelica sinensis slices in 95% ethanol by volume for 24 h and then extracted them with hot water, followed by alkali and acid precipitation and alcohol precipitation to obtain water-soluble ASP. Wu et al. (2015) adopted an orthogonal design to improve the hot water extraction method of ASP and obtained the optimal extraction process by using 10 cycles of water and 60 min of continuous extraction three times. The obtained aqueous extract was concentrated to 1:8, and 95% ethanol was added to a concentrated solution of 65% to obtain 0.265 mg/g ASP. Zhang et al. (2016a) extracted crude polysaccharides from Angelica sinensis using three methods and compared the yield of polysaccharides (fresh Angelica sinensis after natural air drying, boiled directly in water; Angelica sinensis was processed into small pieces, soaked in 75% ethanol for 1 week, with a solid‒liquid ratio of 1:10, and the residue after alcohol extraction was boiled in water; Angelica sinensis was processed into small pieces, soaked in 78% ethanol for 1 week, with a solid‒liquid ratio of 1:10, and then infiltrated with 76% ethanol until the exudate was colorless. The infiltrated Angelica sinensis was boiled in water at a solid‒liquid ratio of 1:30 during decoction and boiled for 30 min. The extraction solutions obtained from the three processing methods were all precipitated with ethanol at a final concentration of 80%, subjected to precipitation and decolorization treatment, and then freeze-dried to obtain crude polysaccharides from Angelica sinensis. The results showed that treatment with ethanol could change the yield of crude polysaccharides from Angelica sinensis, and the ethanol infiltration method had the highest yield of crude polysaccharides from Angelica sinensis.
2.1.2 Ultrasonic extraction methodThe ultrasonic extraction method has the advantages of short extraction time, simplicity and speed, and good separation. Zhao et al. (2016) screened the optimal parameters for the ultrasonic extraction of ASP using response surface methodology (a material–liquid ratio of 7, an extraction time of 45 min, an extraction temperature of 90°C, and an ultrasonic power of 180 W). Several scholars (Nai et al., 2021; Tian et al., 2017) have investigated three factors, namely, the material-liquid ratio, sonication time and sonication power, for the extraction of ASP by ultrasound through single-factor tests and orthogonal tests and optimized the extraction solutions by the response surface method. The optimal conditions were a material-liquid ratio of 1:43, an ultrasonication time of 28 min, and a power of 396 W using multiple regression analysis, resulting in a polysaccharide yield of 21%.
2.1.3 Enzyme extraction methodThe principle of enzyme extraction is to use enzymes to hydrolyze the cell wall to fully dissolve the substances contained in the cells in water to achieve efficient extraction. Zhang et al. (2012a) used enzyme-assisted extraction technology to extract ASP and optimized the extraction conditions. The results showed that the enzyme extraction method improved the extraction rate of polysaccharides, and the operation was simple, low cost and had little chemical pollution. Zhang and Lv (2013) compared four methods, namely, hot water extraction, ultrasonic extraction, cellulase extraction and pectinase extraction, of ASP, with the yield of ASP as the index. The results showed that the enzymatic method was the better choice for the extraction of ASP. The optimum extraction conditions for the cellulase method were as follows: enzyme dosage, 1.0%; extraction temperature, 60°C; extraction time, 60 min; and pH, 6.0. The optimum extraction conditions for the pectinase method were as follows: enzyme dosage, 1.0%; extraction temperature, 50°C; extraction time, 90 min; and pH, 5.5. Therefore, enzymatic hydrolysis has considerable development prospects.
2.1.4 Microwave-assisted extractionMicrowave-assisted extraction of polysaccharides is a potential new technology that has the advantages of speed, low solvent consumption, high extraction rate and low cost (Zhang, 2006). Jin et al. (2007) studied the extraction of ASP via orthogonal experiments. The results showed that compared with ultrasonic extraction and direct heat extraction, microwave extraction has the advantages of time savings, high efficiency and energy savings. Li et al. (2012) used a single factor grouping test and orthogonal optimization test to investigate the process conditions of microwave-assisted extraction of polysaccharides from Angelica sinensis. The results showed that the polysaccharide extraction rate was 7.82% at a power of 500 W, an extraction time of 20 min, and a solid‒liquid ratio of 1:15.
2.2 Separation purification of ASPASP extracts often contain impurities, such as proteins and pigments, which need to be further separated and purified. Protein impurities are usually removed by the Sevag method (Wang et al., 2019) or the repeated freeze‒thaw method (Yang et al., 2008b). The separation and purification methods used are the common alcohol precipitation method (Wang et al., 2019) and the column chromatography method (Yang et al., 2008b). Among them, the column chromatography methods can be divided into anion exchange chromatography and gel filtration chromatography. Cao et al. (2008b) separated the crude polysaccharide obtained by water extraction and alcohol precipitation and repeated freezing and thawing to remove protein by a DEAE-Sephadex A-25 column and obtained three polysaccharide components. APS-2 was further separated on a Sephacryl S-400 column to obtain two polysaccharide components (APS-2a and APS-2b). Cao et al. (2006) treated ASP with ethanol to remove pigments, extracted it with hot water, and precipitated it with ethanol to obtain crude polysaccharides. The proteins were removed by the freeze‒thaw method, filtered through a 0.65 μm membrane filter, and loaded onto a DEAE Sephadex A-25 column for elution. The eluent was collected, lyophilized, and further passed through a Sephacryl S-400 column to obtain three components (APS-1a, APS-1b, and APS-1c). After concentration, dialysis, and lyophilization, APS-1c was further purified on a SephadexG-100 column to obtain two purified components (APS-1cI and APS-1cII). Zhang et al. (2016b) obtained crude polysaccharides by hot water extraction and ethanoprecipitation. The protein was removed by the freeze‒thaw method, and then, the filtrate was dialyzed and loaded on a Sephadex G-50 column, eluted with distilled water, collected and concentrated, and lyophilized to obtain ASP. With the continuous improvement and development of scientific and effective methods for the preparation of Chinese medicines, approaches for the separation and purification of ASP have been explored.
2.3 Structural studies on the ASPASP consists of at least 10 identical or different monosaccharides linked by α- or β-glycosidic bonds and is widely found in nature (Chen and Huang, 2018). Based on their structural characteristics, ASP can be divided into two types: homopolysaccharides, which are composed of one type of monosaccharide, and heteropolysaccharides, which are composed of two or more monosaccharides, such as glucose, galactose, arabinose, and rhamnose, but their components are mainly glucose (Tian et al., 2024). The polysaccharide APS-bII is the first polysaccharide isolated from Angelica sinensis in the form of a white powder (Nai et al., 2021). Currently, the polysaccharides extracted from Angelica sinensis are mainly heteropolysaccharides (Figure 3), with the main structural backbone being α (1,4)-Glc and a relative molecular weight distribution of 5.1 to 2,300 kDa. The active polysaccharides are mainly water soluble. The physicochemical properties and biological activity of polysaccharides are closely associated with structural parameters such as the monosaccharide backbone, monosaccharide composition, relative molecular mass, conformation, position of glycosidic bonds, functional groups, branching degree, and advanced conformation (Liu et al., 2020a). The polysaccharide species extracted from Angelica are listed in Table 1.
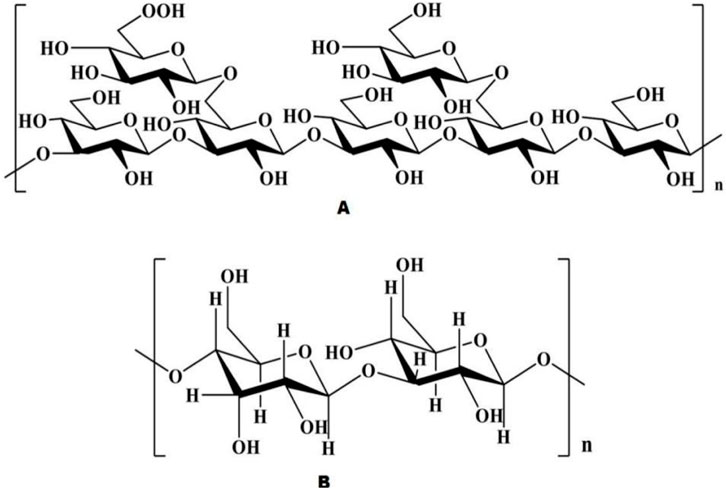
Figure 3. Chemical structure of ASP [Natural Product Communications. 2021; 16(3)].
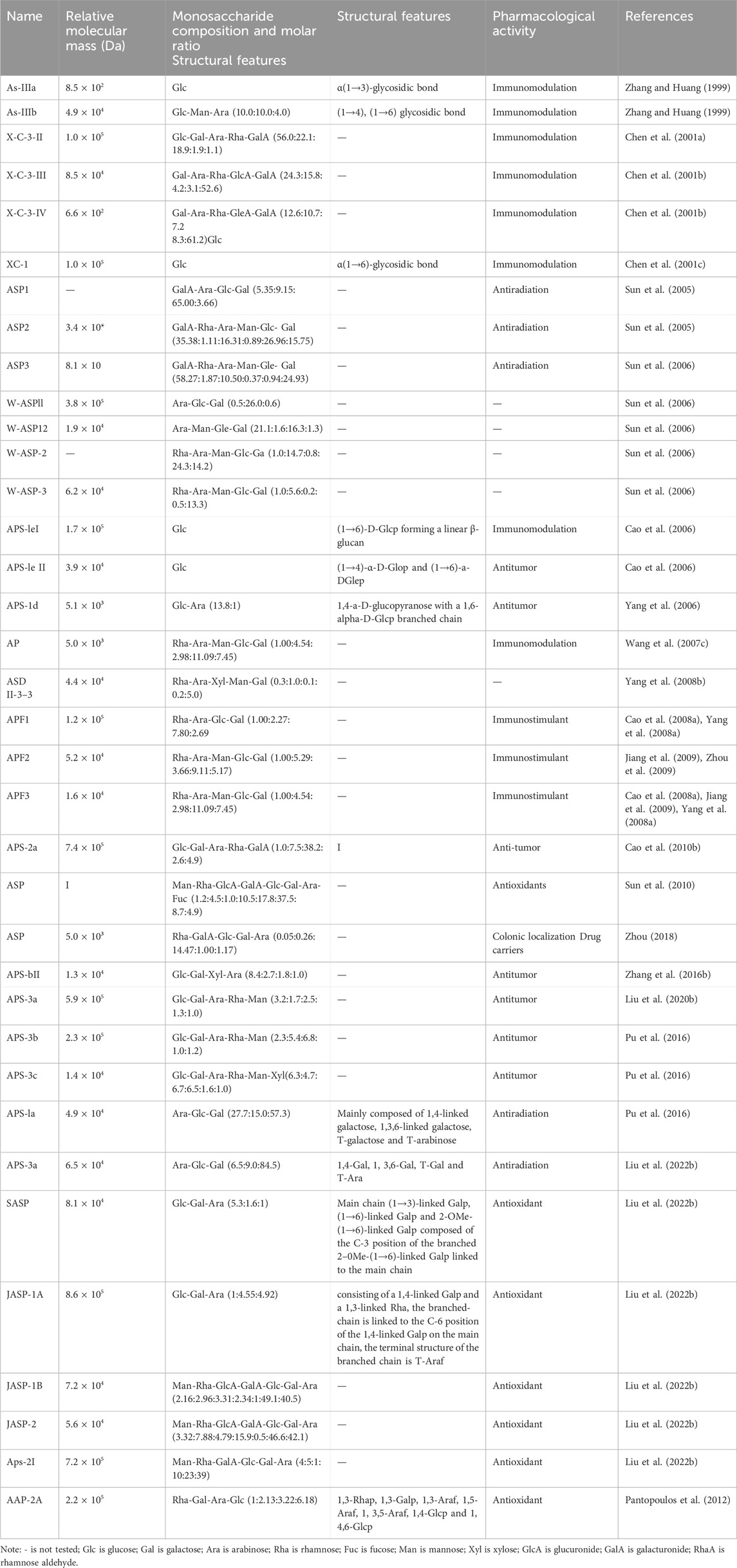
Table 1. Chemical structure and composition of natural polysaccharides from Angelica sinensis.
2.4 Pharmacological effects of ASPThe medicinal use of Angelica sinensis was first reported by Shennong Ben Cao Jing, where it was considered to have the effect of nourishing the five viscera and regenerating muscles and later by researchers as a sacred medicine for blood (Pantopoulos et al., 2012). Angelica sinensis has a wide range of clinical applications since ancient times. Several researchers have conducted numerous studies on its components and pharmacological effects. ASP is one of the main active components of Angelica sinensis, and its extraction, purification, and mechanism of action have been studied in recent years. However, few in-depth studies have been conducted on its pharmacological effects. In this paper, the pharmacological effects of ASP were investigated, and it was concluded that the pharmacological effects of ASP primarily include improving anemia and antitumour effects and enhancing immune function, antioxidation, hepatoprotection, anti-inflammation, anti-fibrosis, hypoglycemia, antiradiation and antiviral properties.
2.4.1 Improving anemiaASP can alleviate anemia symptoms by increasing the levels of hemoglobin, red blood cells, erythropoietin, and iron-regulating hormones in the liver. Ferroregulin is a peptide hormone synthesized and secreted by the liver and is involved in iron regulation. Ferroregulin downregulates membrane iron transport proteins, inhibits the release of serum iron, and negatively regulates iron homeostasis in the body (Zhang and Wang, 2018).
Anemia caused by chronic disease (ACD) is secondary to chronic infection, inflammation, and tumors. In a study, ASP (0.5, 1 g/kg) was administered intraperitoneally to rats with ACD and was found to alleviate anemia by interrupting the IL-6/STAT3 (interleukin-6/signal transducer and activator of transcription 3), and BMP/SMAD (bone morphogenetic protein/SMAD) pathways to inhibit inflammatory iron-regulated proteins. In an animal experimental study, researchers constructed ASP-modified iron oxide nanoparticles (IONPs) and demonstrated the therapeutic effects of IONPs-ASP on iron deficiency anemia (IDA), which was associated with IONPs supplementation and APS-stimulated hematopoietic cell generation (Ma et al., 2024). Additionally, ASP promoted erythropoiesis through the IkB kinase-IkBα pathway (Wang et al., 2017b). Wang et al. (2018) showed that ASP (0.5, 1 g/kg) alleviates the inhibition of erythropoietin by increasing the protein expression of hypoxia-inducible factor-2α (HIF-2α) and decreasing the expression of inflammatory cytokines in rats with chronic renal anemia to achieve an anti-anemic effect.
In addition to chronic anemia, iron deficiency anemia (IDA) is also common. Relevant animal experiments suggested that after the treatment of ASP (0.3, 0.6, and 1.2 g/kg) for blood loss rats, it was found that the expression of Smad 1/5/8, Jak and ERK were inhibited, and then the expression of hepcidin was inhibited, so as to alleviate the symptoms of blood loss in rats (Zhang et al., 2014; Zhang et al., 2012b). A study examining the expression of hepatic-related proteins in rats with blood loss after ASP (1 g/kg) administration via gavage revealed that A inhibited the expression of hepatic (C/EBPα) (CCAAT/enhancer binding protein α), STAT3/5 and SM (Bi et al., 2021). The expression of AD4 stimulates the secretion of erythropoietin and alleviates IDA symptoms (Liu et al., 2012; Wang et al., 2011b). In a study conducted by Wang et al. (2017a), who injected ASP (4, 6 mg/kg) intraperitoneally into blood-lost mice, it was found that ASP increased the number of bone marrow stromal cells and increased the expression of intercellular adhesion molecule-1 (ICAM-1), thereby enhancing the proliferation ability of hematopoietic stem cells.
Additionally, bone marrow suppression caused by radiotherapy and chemotherapeutic agents can cause anemia. ASP (10 mg/kg) was administered to mice after X-ray radiation and was found to inhibit cellular senescence through the phosphatidylinositol-3-hydroxyl kinase/protein kinase B (PI3K/Akt) pathway, promote the repair of platelets, blood cells, and progenitor cells, and alleviate anemia (Liu et al., 2010). He et al. (2012) reported that ASP (2, 8 mg/kg) decreased the percentage of apoptotic peripheral red blood cells, white blood cells, and bone marrow cavity blood cells in irradiated mice. These findings suggest that ASP can protect against radiation injury by altering microRNA expression in blood and restoring the bone marrow hematopoietic system in radiation-irradiated mice (Li and Xu, 2017). ASP (50, 200 mg/kg) also promoted the recovery of peripheral blood leukocyte counts and the transformation of splenic lymphocytes (Sun et al., 2009), enhancing radiation tolerance in mice. Radiation-exposed mice injected with ASP (0.2 mL/piece, once a day) had significantly reduced hematocrit and cellular damage caused by radiation (Hong et al., 2002). Through preclinical and cellular studies, researchers have shown that the polysaccharide components of traditional Chinese medicine have protective effects on the hematopoietic system. Ding Xuelan’s team administered ASP (100 μg/g, 200 μg/g, and 400 μg/g) by gavage to mice with cyclophosphamide-induced myelosuppression. The levels of red blood cells, white blood cell platelets, immunoglobulin G (IgG), and immunoglobulin M (IgM) were significantly increased, and the enhanced immunomodulation may act with cytokines on various lineages of hematopoietic progenitor cells, hematopoietic stem cells, and lymphocytes to promote the recovery of the hematopoietic system (Ding et al., 2016).
Lee et al. (2012) fractionated ASP (6,000 g/mL) with a DEAE-Sepharose CL-6B column and obtained four fractions (F1, F2, F3, and F4), of which the F2 fraction was found to have the highest hematopoietic activity by magnetically activated cell sorting. F2 accounted for 19% of the ASP and 0.53% of the protein content. The monosaccharide components of F2 were arabinose (51.82%), fructose (1.65%), galactose (29.96%), glucose (4.78%), and galacturonic acid (14.80%). It stimulates the secretion of granulocyte macrophage-colony stimulating factor (GM-CSF) and IL-3 by human peripheral monocytes and protects the hematopoietic function of CD34+ cells with strong hematopoietic activity.
2.4.2 AntitumorTumors are formed by the proliferation of local cells in response to various carcinogenic factors. Some studies have shown that iron overload increases the incidence of cancer (Basak and Kanwar, 2022). Tumors can be treated by several methods, such as increasing the body’s immunity, inducing tumor cell differentiation, promoting tumor cell apoptosis, and reducing iron content (Dienstmann and Tabernero, 2017). The antitumor activities of ASP and the underlying mechanisms are summarized in Table 2.
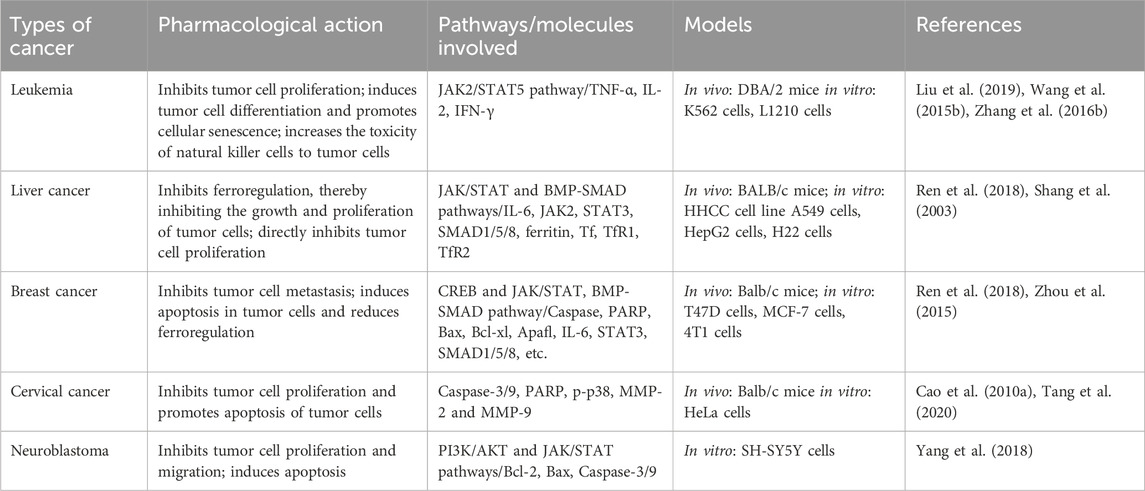
Table 2. Antitumor effects and mechanisms of ASP.
Leukemia is a malignant hematological tumor caused by the diffuse malignant growth of a certain type of immature leukocyte in the bone marrow that replaces normal bone marrow tissue and diffuses into the bloodstream and lymphatic system. Currently, the conventional treatments for leukemia include chemotherapy, radiotherapy, targeted therapy, bone marrow transplantation, and supportive therapy. Liu et al. (2019) reported that leukemic mice treated with different doses of ASP (12.5 μg/mL, 25 μg/mL, 50 μg/mL, 100 μg/mL) exhibited prolonged survival to varying degrees, and the leucocyte, lymphocyte, tumor necrosis factor-α (TNF-α), interleukin 2 (IL-2), and interferon-gamma (IFN-γ) levels increased significantly. The findings of this study suggest that ASP may suppress leukemia by enhancing specific and nonspecific immunomodulatory functions. In addition, one study revealed that ASP (12.5 μg/mL, 100 μg/mL) promotes ASP-induced differentiation of leukemia cells by inducing JAK2/STAT5 tyrosine phosphorylation and activating erythropoietin (EPO) (Wang et al., 2015b). In vitro experiments have shown that (Zhang et al., 2016b) ASP (100, 200, 300, 400, and 500 mg/L) can inhibit the proliferation of leukemic cells, promote the proliferation of splenocytes, and enhance macrophage and phagocytic activity and the cytotoxicity of natural killer cells.
Liver cancer is a malignant tumor with a high mortality rate. An in vitro study of H22 cells incubated with ASP showed that low doses of ASP promoted the proliferation of splenic lymphocytes, inhibited tumor growth, and significantly inhibited tumor growth by reducing iron concentrations in the liver, spleen, and tumor cells (Cheng et al., 2016). ASP (30, 100 and 300 mg/kg) can inhibit the invasion and metastasis of hepatocellular carcinoma cells in vitro (Shang et al., 2003). Several studies have shown that the expression levels of IL-6, STAT3, JAK2, SMAD1/5/8, ferritin, the transcription factor TfR1 (transferrin receptor 1), and TfR2 (transferrin receptor 2) are reduced in the liver tissues of mice with hepatocellular carcinoma after the administration of ASP (100 mg/kg) (Ren et al., 2018).
Breast cancer occurs frequently in the female population, is a common malignancy, and has multiple complex mechanisms. A study in which breast cancer was induced in nude mice by injecting breast cancer cells followed by intraperitoneal administration of ASP (0.2 mg/kg) showed that the cAMP-responsive element-binding protein cyclic adenosine effector element binding protein (CREB) (Zhou et al., 2015), poly ADP ribose polymerase (PARP), Caspase-3, Caspase-9, myeloid leukemia gene 1 (Mcl-1), Bcl-2, Bcl-xL and apoptosis-activating factor 1 (Apaf1) were upregulated, resulting in increased tumor cell apoptosis. Another study showed that ASP (100 mg/kg) inhibits tumor growth by decreasing the expression of ferro regulatory elements (Ren et al., 2018). Furthermore, by culturing breast cancer cells in vitro, ASP (100, 200, 300, 400, and 500 mg/L) was found to exhibit antitumor activity by directly inhibiting cancer cell proliferation (Zhang et al., 2016b).
Cervical cancer is a common gynecological malignancy, and its incidence has recently been reported in younger populations. In a study performed on cervical cancer-induced nude mice, after ASP treatment, the mitochondrial potential was reduced, and caspase-9, caspase-3, and PARP expression was greatly increased, indicating that ASP (3, 30 or 300 mg/mL) inhibits tumor proliferation in cervical cancer by activating mitochondrial apoptosis (Cao et al., 2010a). In addition, a dose-dependent ASP (100, 200, and 400 mg/L) study showed inhibition of the growth and migration ability of HeLa cervical cancer cells (Tang et al., 2020), a reduction in the scratch closure rate and number of invasive cells, and downregulation of p-p38, MMP-2, and MMP-9 protein expression. This inhibitory effect may be achieved in conjunction with the influence of MMP-2 and MMP-9 expression by modulating p38 signaling pathway activity.
Neuroblastoma occurs frequently in children and infants and has a high recurrence rate and poor prognosis. In vitro culture of neuroblastoma cells treated with ASP (100, 200, 300, 400 or 500 ug/mL) revealed that ASP downregulated NA-H19 expression in neuroblastoma cells and that miR-675 upregulated CD44 expression and may prevent the formation of neuroblastoma tumor cells by inhibiting the miR-675-mediated PI3K/AKT and JAK/STAT pathways (Yang et al., 2018).
2.4.3 AntioxidantsFree radicals are highly biologically active and oxidizing compounds produced by metabolic reactions in the body using oxygen. Under normal circumstances, the body maintains a dynamic balance between oxidation and antioxidation; however, when free radicals or the antioxidant capacity of the body is unbalanced, the body can damage cell structure, protein, and nucleic acid, thus accelerating aging and even inducing various diseases. Recent research has shown that ASP has a strong scavenging effect on free radicals in the body, regulating phenol oxidase (PO), superoxide dismutase (SOD), and glutathione peroxidase (GSH-PX) activities, reducing malondialdehyde (MDA) and alleviating the oxidative stress response of the body (Cao et al., 2018). ASP can protect the body from oxidative stress by improving the antioxidant capacity of body tissues; it is also important for enhancing beauty and delaying aging. Studies have shown that ASP can reduce damage to cellular oxidative reactions caused by H2O2, improve cell viability, and attenuate apoptosis and ROS production. In the H2O2-induced cardiomyocyte H9c2 oxidative stress model, APS (6.25, 12.5, 25, 50, and 100 mg/mL) exerts protection against H9c2 by reducing endoplasmic reticulum (ER) stress and oxidative stress, alleviating H2O2-induced cytotoxicity and apoptosis through activation of the ATF6 pathway (Niu et al., 2018). The antioxidant effects of ASP are shown in Table 3.
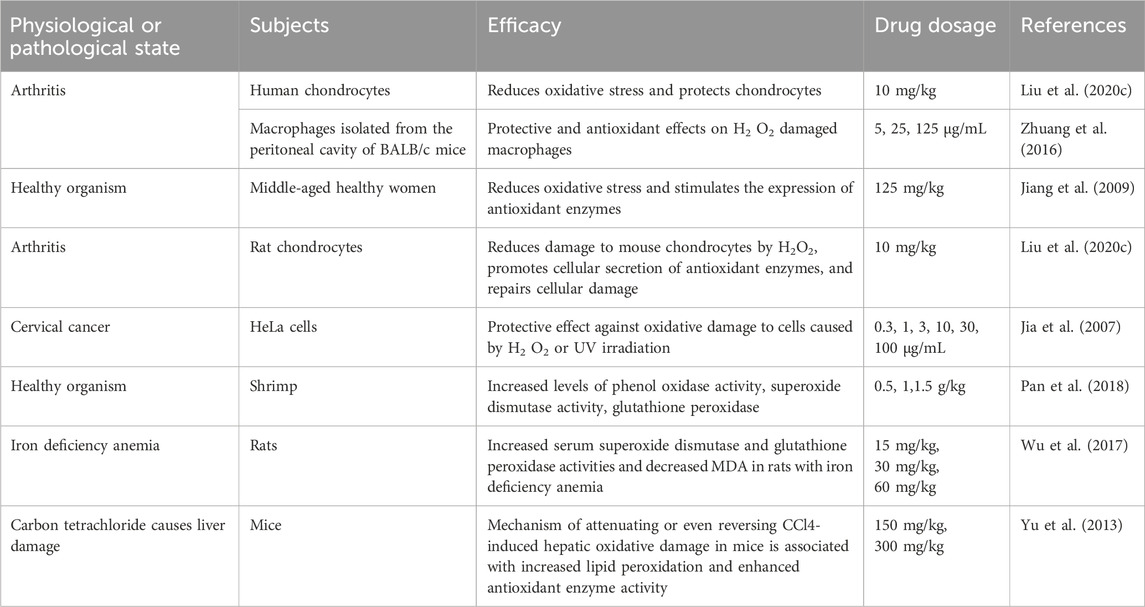
Table 3. Antioxidant effect of ASP.
2.4.4 ImmunomodulationThe immune system, which is composed of various components, protects the body by acting as an effective barrier against pathogenic invasion. ASP improves nonspecific immunity by enhancing immune cell activity. South American white shrimp were fed ASP (0.5, 1,1.5 g/kg) for 12 weeks and showed enhanced nonspecific immunity, increased survival, and increased resistance to Vibrio lysis (Pan et al., 2018). In vitro culture of mouse peritoneal macrophages revealed that ASP (20, 25, 50, 100, and 200 μg/mL) promoted macrophage proliferation, enhanced macrophage activity by modulating lysozyme activity, and increased the expression of H2O2, NO, TLR4 (toll-like receptor family 4), and ICAM-1 (Chen et al., 2010). ASP (10, 30, and 100 μg/mL) can enhance nonspecific immunity in addition to specific immunity. In a study conducted on murine leukemia virus (Yang et al., 2006), the percentage of CD4+ cells and the CD4+/CD8+ ratio in peripheral blood cells were found to be significantly increased by ASP at 3–30 mg/kg. It has also been demonstrated that ASP (3, 10, and 30 mg/kg) has immunomodulatory activity and is able to promote the proliferation of T cells (Yang et al., 2012), increase the production of IL-2 and IFN-γ, and decrease the production of IL-4 to regulate the expression of Th1- and Th2-related cytokines. Lu et al. (2017a) administered ASP (254.2 mg/kg) by gavage to rats exposed to whole-body X-ray radiation and observed a significant increase in the serum levels of IL-4 and IFN-γ, indicating that ASP has immunoprotective effects on X-ray radiation. A study conducted on ulcerative colitis-induced mice revealed that treatment with ASP (0.5 g/kg, 1 g/kg) reduced inflammation by decreasing the cytokine IL-10 (Pan et al., 2015). Ding et al. (2016) studied the effects of different doses of ASP (100, 200, and 400 ug/g) on myelosuppression in mice and reported that ASP increased the levels of IgG, IgM, and T-cell subsets, enhancing both humoral and cellular immunity.
2.4.5 Hepatoprotective effectThe liver is a crucial organ of the body and is involved in physiological functions, such as synthesis, metabolism, excretion, detoxification, and immunity. The incidence of liver injury, which seriously threatens human health, is increasing annually. It has been reported (Horvatits et al., 2019) that factors such as alcohol consumption, drug abuse, and viral infection can damage hepatocytes, leading to body imbalance and impaired energy regulation, which causes liver injury (Figure 4). ASP has shown protective effects against various types of liver injuries, and the mechanisms involved are reviewed below.

Figure 4. Various factors that can cause liver damage.
Alcoholic liver injury is a disease caused by long-term alcohol consumption, the incidence of which is increasing annually, and it is the second most common liver disease after viral hepatitis. Alcohol-induced liver injury is characterized by an unclear structure of the liver lobules, disorganized hepatocyte cords, hepatocytes filled with lipid droplets in the form of vacuoles, darker staining of nuclear consolidation, and the disappearance of most hepatic blood sinuses (Wang et al., 2016a). ASP (4 mg/kg) increases the activity of antioxidant enzymes, scavenges oxygen free radicals, and reduces the chain reaction of membrane lipid peroxidation, which subsequently restores the biofilm structure of hepatocytes in alcohol-induced liver injury (Jia et al., 2015). ASP (1.5 mg/kg, 6 mg/kg) alleviates viral and autoimmune hepatitis, reduces cytokine expression and inflammatory responses in experimental mice, and it may inhibit hepatocyte apoptosis by attenuating the c-Jun amino-terminal kinase (JNK)-mediated mitochondrial apoptotic pathway and inhibiting Caspase-8 expression (Wang et al., 2016c).
In addition to alcoholic liver injury and viral hepatitis, liver injury caused by drugs and cadmium poisoning is also common, with acetaminophen (APAP) being the most common cause of liver injury (Han et al., 2010). Long-term or excessive use of APAP is associated with acute liver failure and injury. Cao et al. (2018) investigated the reduced toxicity of different concentrations of ASP (3, 6, and 12 mg/kg) intravenously administered for 10 days in APAP-induced liver injury rats, and APAP-induced histological changes were significantly reversed with vacuolization and necrosis of the cytoplasm. In addition, the expression of caspase-3 and Bax decreased, the expression of Bcl-2 increased, and hepatocyte apoptosis was inhibited. Liu et al. (2018) showed that ASP (20 mg/kg) had a significant protective effect on liver injury in cadmium-treated rats. After treatment with ASP, the thymus, spleen index, lymphocyte transformation capacity, NK cell killing capacity, cytokine IL-2 content, and Bcl-2 protein expression were significantly increased in cadmium-treated rats, while AST, ALT, LDH activity, TGF-β1, and cadmium levels were increased. This mechanism may involve the enhancement of autoimmunity, the regulation of enzyme activity, and the expression of apoptosis-related proteins.
Furthermore, chemotherapeutic drugs used to treat tumors are associated with apoptosis and necrosis of liver cells. Fu and Ma (2018) reported that ASP (25, 50, and 100 mg/kg) could improve liver function by increasing the number of leukocytes, erythrocytes, and platelets, which decreases the level of Bax and increases the expression of Bcl-2. This results in alleviating liver injury induced by cisplatin in H22 ascites-derived tumors in mice.
Hyperglycemia and hyperlipidemia can cause liver damage. Kaiping Wang et al. (2016d) induced hyperglycemia and hyperlipidemia in mice using a high-fat diet and low-dose streptozotocin (STZ) and observed that the rats developed significant liver damage. During the experiment, the mice were administered different doses of ASP (100, 200 and 400 mg/kg) along with STZ injection. ASP has been found to reduce liver damage by stimulating insulin secretion, facilitating hepatic glycogen synthesis, releasing adipokines, and reducing hepatic fat accumulation. In addition, ASP promoted the expression of the antiapoptotic protein Bcl-2 and reduced the expression of the proapoptotic protein Bax, which inhibited the senescence of hepatocytes and reduced liver injury in mice.
2.4.6 Anti-inflammatory effectsExperiments have confirmed that ASP has therapeutic effects on several inflammatory conditions, such as osteoarthritis and colitis. Chondrocyte apoptosis is important in the development of osteoarthritis (OA). Xu et al. (2021) reported that ASP (50 ug/mL, 200 ug/mL) had a protective effect against sodium nitroprusside (SNP)-induced chondrocyte apoptosis, suggesting that ASP is a potential alternative for OA treatment. Xu et al. (2022) performed a study on an in vitro model of IL-1β injury and observed that ASP (10, 50, and 100 ug/mL) effectively reduced IL-1β damage to chondrocytes and increased their activity. Additionally, ASP reduced the expression levels of β-catenin, Wnt4a, GSK-3β, MMP-13, and ADAMTS4, inhibited IL-1β-induced cartilage degradation, and alleviated patient symptoms. ASP has been found to inhibit oxidative stress damage and inflammatory responses in osteoarthritic chondrocytes through the Wnt/β-catenin signaling pathway. Preclinical studies have shown that it (0.5 g/kg, 1 g/kg) significantly reduces the production of IL-6, TNF-α, and other proinflammatory factors and has a significant therapeutic effect on arthritic rats (Li et al., 2020).
Cheng et al. (2020) reported that ASP (200 mg/kg) reduced myeloperoxidase activity in colonic tissues, modulated the expression of proinflammatory cytokines and related proteins, and improved dextran sodium sulfate-induced colitis in mice. It has also been reported that ASP (200 mg/kg) significantly reduces the serum expression levels of IL-6, TNF-α, SDF-1, plasma TF, D-D, and FIB in rats prone to embolism during pregnancy (Ma et al., 2022), indicating that APS has anti-inflammatory and anticoagulant effects. In vitro studies have shown that ASP (80 ug/mL, 100 ug/mL) can reduce the inflammatory response to protect neuronal cells (Zhou et al., 2019). Zhou et al. (2018) analyzed the effects of ASP (0.009, 0.018, and 0.036 g/mL) on TLR4/MyD88/NF-κB pathway inhibition in rats with diabetic peripheral neuropathy (DPN) and reported that APS significantly decreased the levels of IL-6, myelin basic protein (MBP), TNF-α, CRP, NF-κB, MyD88, and TLR4 and significantly increased CAT mRNA expression in rats, reduced the levels of inflammatory factors, and decreased damage to the inflammatory response.
2.4.7 Anti-fibrosisWang et al. (2010) observed the effects of ASP (50, 100, and 200 g/kg) on pulmonary function and the lung coefficient in rats with pulmonary fibrosis induced by pulmonary fibrosis and reported that 0.3 s of expiratory volume as a percentage of forced expiratory volume (FEV0.3/FVC) and calm end-expiratory function residual capacity (FRC) increased to different extents, and it was shown that ASP could significantly improve pulmonary function, increase body weight, and decrease the lung coefficient in rat models of pulmonary fibrosis. Qian et al. (2020) reported that ASP (20 mg/kg) inhibited the development of fibrosis in rat lung and alveolar type II epithelial cells (RLE-6TN) through the DANCR/AUF1/FOXO3 regulatory axis, which facilitates the treatment of idiopathic pulmonary fibrosis (IPF). In addition, ASP (12.5, 25, and 50ug/mL) was found to inhibit pulmonary fibrosis, and its effects may be related to the downregulation of α-smooth muscle actin (α-SMA) and CTGF expression and the regulation of the balance between MMP-9 and TIMP-1 (Luo et al., 2017). Song et al. (2021) reported that ASP (80, 160, and 320 mg/kg) decreased the LV end-diastolic diameter, LV end-systolic diameter, LV end-diastolic volume, and LV end-systolic volume; decreased the expression levels of TGF-β1, α-SMA, type I collagen (CoI), fibronectin, vimentin, Bax, cleaved caspase-9, and cleaved caspase-3 in the LV; increased SOD and GSH-Px activities; decreased MDA, H2 O2, and ROS levels; and ultimately inhibited myocardial apoptosis and oxidative stress to prevent hypertensive myocardial fibrosis in HHD rats by increasing the ejection fraction and fractional shortening. Another study showed that ASP (200 mg/kg) improved chronic liver fibrosis by inhibiting HSC activation through the IL-22/STAT3 pathway (Wang et al., 2020), reducing serum alanine aminotransferase by approximately 50%, and inhibiting hepatic stellate cell (HSC) activation in mice with liver fibrosis.
2.4.8 Lowering blood sugarNon-starch polysaccharides lower blood glucose levels through multiple pathways. It was found that after 4 weeks of ASP4 (100, 200, 400, and 600 mg/kg) treatment in STZ-induced diabetic mice (Wang et al., 2015a), fasting blood glucose (FBG) decreased, dyslipidemia improved, and elevated serum total cholesterol (TC) and triglyceride (TG) levels decreased. Zhang et al. (2019) reported a protective effect of ASP on the islets of T2DM mice, and the possible mechanism was related to the promotion of insulin secretion and the inhibition of apoptosis by blocking both internal and external pathways of pancreatic β-cells. In another study, Chen (2010) reported that after oral administration of ASP (2 or 100 mg/kg) for 21 days, alloxan-induced diabetic rats exhibited significantly decreased blood glucose levels and improved plasma insulin levels, which may be associated with the repair and regeneration of damaged pancreatic β-cells. Several studies have shown that ASP (80, 160 and 320 mg/kg) reduces blood glucose levels and attenuates insulin resistance by modulating relevant metabolic enzymes and activating the PI3K/Akt pathway in high-fat diet-fed mice (Wang et al., 2016b), suggesting that ASP has therapeutic potential for treating hypoglycemia.
ASP-1I (12.5, 25, and 50 mg/kg) has been shown to enhance glucose absorption while simultaneously inhibiting the activation of p-IRS-1, p-IRS-2, p-JNK, and p-P38 pathways in insulin-resistant HepG2 cells. Furthermore, intraperitoneal administration of ASP-1I significantly ameliorates insulin resistance and suppresses the RAGE-JNK/P38-IRS pathway in the liver of diabetic rats, demonstrating a mild yet effective therapeutic effect (Liu et al., 2022a). Additionally, oral administration of ASP (40 mg/L) can improve bone health by reducing blood glucose levels and enhancing insulin sensitivity, thereby facilitating bone tissue repair in a rat model of type 2 diabetes (Liao et al., 2019).
2.4.9 Radiation resistanceCurrently, ionizing radiation has become the fourth major hazard to humans after air, water, and noise pollution. Polysaccharides are more suitable alternatives to medicinal radiation protection agents because they are less toxic and non-accumulative. ASP (63.5, 127, and 254 mg/kg) is used to protect against radiation-induced liver damage because of its ability to increase the resistance of the liver to radiation and to scavenge free radicals (Wang et al., 2017c). ASP (63.6, 127.1, and 254.2 mg/kg) regulates the body’s oxygen radical balance through activation of the transcription factor Nrf2, thereby counteracting radiation-induced oxidative damage (Lu et al., 2017b; Zhang et al., 2017). ASP (63.5, 127, and 254 mg/kg) has also shown efficacy against radiation-induced bone marrow and spleen damage in SD rats, probably by reducing radiation-induced damage to hematopoietic and immune cells in the bone marrow and spleen (Lu et al., 2017a; Xu et al., 2017). Zhang et al. (2020) showed that medium (127.1 mg/kg) and high (254.2 mg/kg) doses of ASP had significant effects on ionizing radiation-induced intestinal barrier damage in SD rats. ASP (100, 200, and 400 mg/kg) can regulate the ratio of regulatory cells (Tregs)/helper T cells 17 (Th17) in 60Co-γ-irradiated mice, improve the stability of mitochondrial membranes, and regulate abnormal levels of ROS and mitochondria-related apoptotic proteins, thus achieving antiradiation effects (Chen et al., 2022).
2.5 Other pharmacological effectsStudies have shown that ASP can lower blood lipids, improve diabetic nephropathy, and improve antiviral properties.
ASP (400, 600 mg/kg) significantly reduced the homeostasis model assessment-insulin resistance index (HOMA-IR) and body weight and was also effective in reducing serum TC and triglyceride concentrations and restoring pancreatic/liver or adipose tissue in BALB/c mouse models of prediabetes and STZ-induced diabetes (Wang et al., 2015a).
A major complication in long-term diabetic patients is diabetic nephropathy (DN). Intraperitoneal injection of the ASP branch Acanthopanax (AG) for 8 weeks in diabetic rats resulted in significant improvements in renal function, increased creatinine clearance, a significant reduction in blood urea nitrogen, and the expansion of glomeruli in patients with proteinuria. AG (20, 50, and 100 mg/kg) inhibited the RAGE/NF-KB (receptor for advanced glycosylation end products/nuclear factor-KB) signaling pathway by inhibiting the over-proliferation of glomerular mesangial cells (GMCs) via the RAGE/NF-KB signaling pathway and attenuating inflammatory mediators (Sui et al., 2019).
ASP (1.5 g/kg) was found to promote the maturation of dendritic cells (DC) in hepatitis B virus (HBV) transgenic mice, upregulate the expression of the surface costimulatory molecule CD86, improve the ability of DC to proliferate lymphocytes and secrete IL-12 and r-IFN, and may play a critical role in the antiviral immunity of HBV transgenic mice (Li et al., 2005). APS has shown a synergistic effect with dithiothreitol, suggesting that APS can be used in combination with antiviral drugs for the treatment of AIDS. ASP (10, 30 mg/kg) causes anti-cellular oxidative damage, which may be the mechanism associated with anti-AIDS activity (Jia, 2005). ASP have shown efficacy against human cytomegalovirus (HCMV)-infected cytomegalic lineage cells in vitro by inhibiting HCMV-induced apoptosis in a dose-dependent manner (Zhang et al., 2009). ASP enhance the proliferation of chicken embryonic fibroblasts and prevents infection by Newcastle disease virus (Hu et al., 2004). The chlorosulfate-pyridine-modified Angelica sulfated polysaccharides (0.244, 0.488, 0.977, 1.953, and 3.907 ug/mL) significantly improved resistance to Newcastle disease virus infection in chicken embryo fibroblasts (Wang et al., 2011a). An in vitro study demonstrated that ASP (1,399.531 ug/mL) inhibited chicken infectious bursal virus-infected cells in a quantitative and temporal manner (Hu et al., 2003).
Angelica sinensis polysaccharide, as a traditional Chinese medicine component, has shown significant antiviral potential in pharmacology, which has been widely recognized in the scientific community. However, any medicinal ingredient has its two sides, angelica polysaccharide is no exception. Studies have shown that angelica polysaccharide can effectively promote the proliferation of fibroblasts, which has a positive effect on the repair of damaged tissues and wound healing. Fibroblasts are the main cell type that constitutes connective tissue, and their proliferation is essential for maintaining the integrity and elasticity of skin and tissue. However, in some cases, excessive proliferation of fibroblasts can lead to undesirable side effects, such as excessive scar tissue formation during healing, and may even cause tissue sclerosis, affecting organ function. Therefore, when considering the use of ASP for treatment, we must carefully weigh its potential benefits against possible risks to ensure that unnecessary side effects are avoided while promoting health.
2.6 Effect of different extraction positions of Angelica sinensis on ASP activityAngelica sinensis, a traditional Chinese herbal medicine, shows unique differences in pharma cological effects of polysaccharide components contained in different parts. The content of ASP in Angelica sinensis is as high as 15% (Cao, 2019). As one of the main components of Angelica sinensis for nourishing blood and regulating menstruation (Ouyang et al., 2005), it can improve the hematopoietic function of the body by increasing the number of white blood cells, red blood cells and hemoglobin in peripheral blood. The study of Wu Guoxia et al.believed that in different medicinal parts of Angelica sinensis, the amount of ASP contained in Angelica sinensis was significantly higher than that in Angelica sinensis head and other parts (Wu et al., 2018a). Song et al. also confirmed the view that the content of ASP in Angelica sinensis was higher by investigating different cultivation methods of Angelica sinensis (Song et al., 2008). By comparing the content of ASP in the whole Angelica sinensis and the head of Angelica sinensis (Long and Maobao, 2008), think that the content of ASP in the whole angelica processed in the same origin is higher than that in the head of Angelica sinensis; considering that the whole Angelica sinensis and Angelica sinensis can be clustered into one class in the process of fingerprint cluster analysis (Wu et al., 2018b), we can still think that the polysaccharide content in Angelica sinensis is higher than that in Angelica sinensis head and Angelica sinensis tail, and is close to that in Angelica sinensis.
Some scholars have studied the antioxidant activity of polysaccharides from different medicinal parts of Angelica sinensis (Zou et al., 2022). Four kinds of polysaccharides, ASP-H-AP, ASP-B-AP, ASP-T-AP and ASP-Hb-AP, were obtained from different medicinal parts of Angelica sinensis, and their antioxidant activities were studied. It was found that the four polysaccharide components could reduce the oxidative stress of IPEC-J2 cells by up-regulating the expression of related genes and proteins of antioxidant enzymes. Among them, ASP-Hb-AP had better antioxidant effect, while ASP-T-AP had relatively poor antioxidant effect.
ASP in Angelica sinensis can induce the synthesis and secretion of hematopoietic regulatory factors by macrophages by enhancing the expression of GM-CSF protein and mRNA in bone marrow stromal cells, spleen cells and thymocytes, and accelerate the proliferation and differentiation of myeloid multi-directional hematopoietic progenitor cells, late erythroid progenitor cells and granulocyte mononuclear hematopoietic progenitor cells. Finally, it can restore the number of peripheral blood cells in blood deficiency animals, reconstruct the long-term hematopoietic ability of hematopoietic failure animals (Xu et al., 2020), improve the immune adhesion ability and hematopoietic ability of red blood cells after radiation injury, and improve anemia. In addition, it also has the effects of promoting blood circulation, hemostasis, anti-radiation, anti-oxidation, anti-tumor, anti-inflammation and improving immunity.
On the other hand, the pharmacological effects of polysaccharides in the head and tail of Angelica sinensis have not been reported in domestic and foreign studies, and further research and exploration by the research group are needed.
2.7 Summary and outlookIn summary, ASP is the main active component of the traditional Chinese medicine Angelica sinensis, which has multiple components, multiple target characteristics, and very broad pharmacological activities. It also plays an important role in the treatment of several diseases. In this review, we describe the ten major pharmacological activities of ASP reported in several studies, including anemia, antitumour, antioxidant, immunomodulatory, hepatoprotective, anti-inflammatory, hypoglycemic, antiradiation, antifibrosis, and antiviral activities. However, preclinical studies on ASP have primarily focused on crude compounds without further purification and isolation, which necessitates further investigation to determine the specific composition of ASP. Studies have shown that ASP modification helps amplify their pharmacological effects (Hou et al., 2021). Therefore, studying the effects of different modifying agents on the pharmacological activities of ASP is promising. ASP has been reported to play a crucial role in the treatment of diabetes mellitus and its complications, such as diabetic nephropathy and peripheral neuropathy. The incidence of diabetes mellitus is increasing annually worldwide, and patients require long-term medication. In such cases, the use of ASP as a natural plant extract with fewer side effects and a high safety profile is a potential alternative for the long-term treatment of diabetes mellitus. ASP has shown efficacy against tumors and organ fibrosis; however, its specific molecular mechanisms of action and drug targets remain undetermined. Therefore, in-depth studies are required to identify the underlying mechanisms and potential drug targets involved.
As research on ASP has advanced, the use of ASPs in clinical, food, healthcare, and cosmetic applications has also gradually increased. Iron ASP have been used for the treatment of iron deficiency anemia (Wang et al., 2007b), sulfated ASP have been used as antitumour agents (Wu et al., 2012; 2013), and Angelica hepatica capsules have been used to lower blood sugar (Wang et al., 2007a; Zhang et al., 1998). In addition, Angelica sinensis and Astragalus iron particles and Angelica Sinensis and Astragalus capsule have been developed for iron deficiency anemia and prevention of radiation exposure, as shown in Table 4. However, more research and development are require
留言 (0)