Paget’s disease of the breast (PD) is a rare condition characterized by unilateral skin changes in the nipple-areolar complex (NAC) and often associated with underlying carcinoma (BC) (1). An accurate and timely approach, including clinical examination and imaging techniques integration, is crucial when PD is suspected, although a definitive diagnosis requires histological confirmation through a skin punch biopsy (1). PD patients frequently present with unilateral changes in NAC, such as itching, erythema, eczematous-erosion lesions, ulceration, nipple retraction, and serous or bloody discharge (2); however, obvious symptoms may not be shown, and diagnosis is made through pathological evaluation of the NAC during mastectomy (1–3). Conventional imaging techniques, like Digital Mammography (DM) and Breast Ultrasonography (US), can miss underlying malignancies in up to 65% of cases (4). Magnetic Breast Resonance Imaging (MRI) is an essential tool in detecting clinically occult cancer; it also plays a key role in preoperative planning, guiding decisions between conservative and demolition surgical treatments (4–6). However, MRI’s limitations, such as false-negative, costs and patient contraindications (e.g., claustrophobia or non-MRI-compatible devices), necessitate exploring alternative techniques (1, 7).
Contrast-enhanced Mammography (CEM) offers comparable performance to MRI in terms of sensitivity and has shown utility in PD evaluation due to its ability to highlight neo angiogenesis and greater patient acceptability (8). Despite limited studies, CEM holds promise as a complementary or alternative tool to MRI in the evaluation of PD. Here, we discuss two clinical cases to illustrate the potential of CEM in staging and managing PD.
Clinical casesThe first case (Figure 1) involves a 46-year-old woman referred to our Breast Imagin Division for her annual follow-up examination. Her personal and family history of BC was negative, and a preliminary clinical assessment of the breasts revealed no alterations of NAC, palpable mass, or axillary adenopathy. Previous annual mammograms were unremarkable. DM revealed an area of fine pleomorphic microcalcifications in the left breast, with a segmental distribution extending from the upper-outer quadrant to the outer equatorial region, over approximately 6 cm, corresponding to ectasia ductal structures with multiple contextual echogenic spots on US. A US-guided biopsy diagnosed a high-grade in situ ductal carcinoma (DIN3). Subsequently, the patient underwent a staging CEM examination, refusing MRI due to claustrophobia. In addition to the known area, CEM showed contrast-enhancement of the ipsilateral NAC. A skin punch biopsy confirmed the PD diagnosis. Therefore, the patient was referred for left total mastectomy with reconstruction rather than a nipple-sparing procedure.
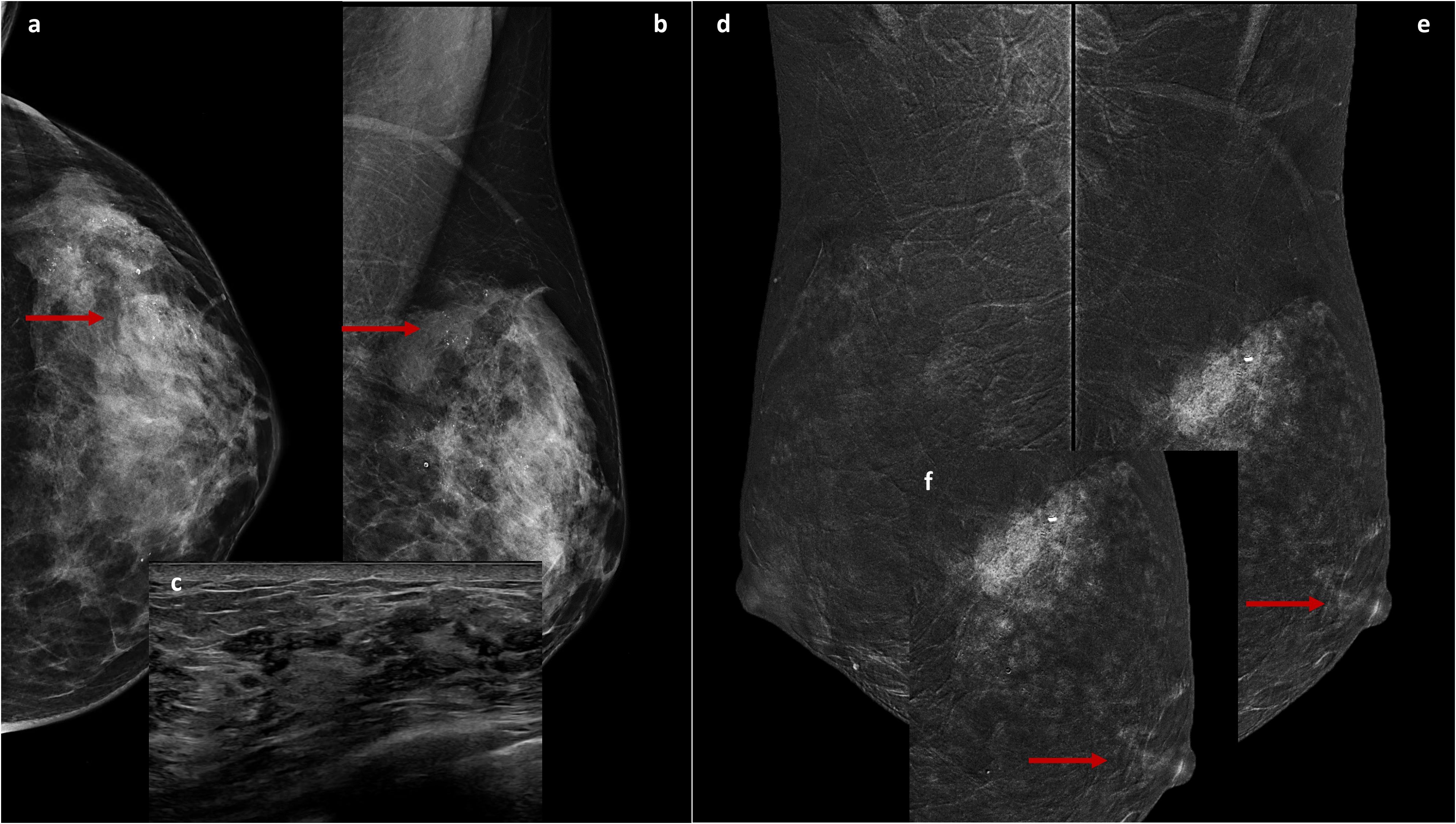
Figure 1. Cranio-caudal (A) and Medio-lateral-oblique (B) DM views of the left breast show suspicious fine and pleomorphic microcalcifications from the upper-outer quadrant to the outer equatorial region (arrows) corresponding to ectasia ductal structures with multiple contextual hyperechogenic spots on US (C). The recombined medio-lateral-oblique CEM images (D, E) show, in addition to non-mass-like enhancement in the upper quadrants of the left breast (with a post-biopsy clip inside, E), a contrastographic impregnation of the ipsilateral NAC (arrows), highlighted in the focused magnification (F).
The second case (Figures 2, 3) concerns a 57-year-old woman who was referred to our institute due to persistent pain, swelling and eczema of the left nipple, unresponsive to several weeks of antibiotic therapy. The patient was postmenopausal for five years and had not undergone hormone replacement therapy. Clinical examination confirmed marked erythematous eczematous changes in the left NAC without palpable breast masses or axillary adenopathies. DM and US performed at an external facility and did not identify any suspicious findings. However, a NAC punch biopsy performed elsewhere confirmed the PD diagnosis. Due to the discrepancy between the clinical examination and conventional imaging, a breast MRI was performed at the same external center, showing doubtful mild enhancement in the outer quadrants of the left breast. Upon reassessment of the initial DM, some amorphous calcifications were identified in the same region. Consequently, to achieve a morphofunctional correlation between calcifications and the observed enhancement to select a potential biopsy target, a CEM was performed, which confirmed a later inhomogeneous non-mass-like enhancement of approximately 8 cm in the outer quadrants of the left breast, with ipsilateral NAC contrast-enhancement associated. A subsequent CEM-guided biopsy verified a low-grade in situ ductal carcinoma (DIN1c) diagnosis. The patient was referred for a left total mastectomy with reconstruction instead of a central resection.
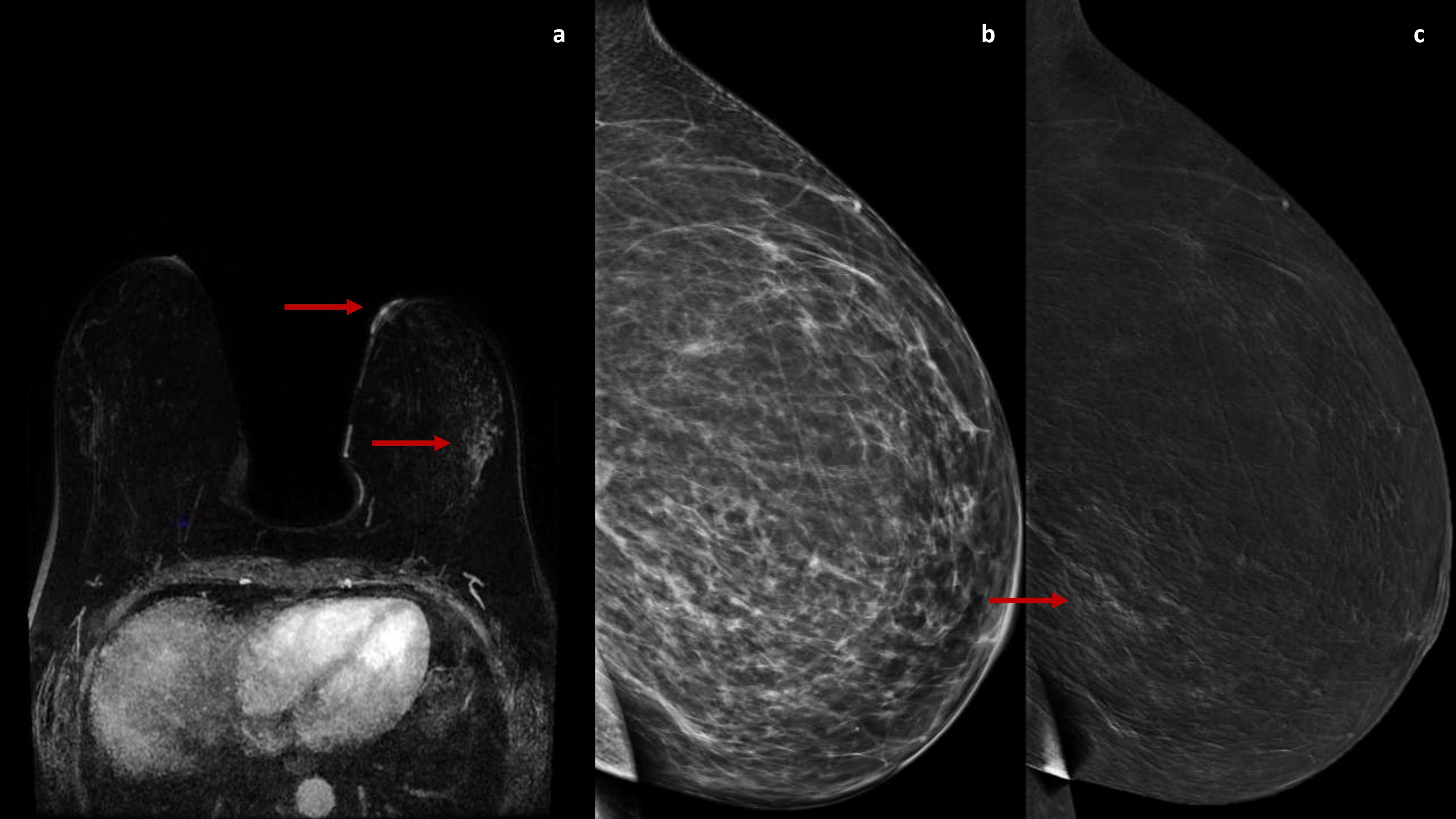
Figure 2. A DCE-MRI subtraction image (A) shows an enhancement of the left nipple with a non-mass-like enhancement in the outer quadrants (arrow). The 2D and recombined medio-lateral CEM images (B, C) confirm a later inhomogeneous non-mass-like enhancement in the outer quadrants of the left breast (arrow).
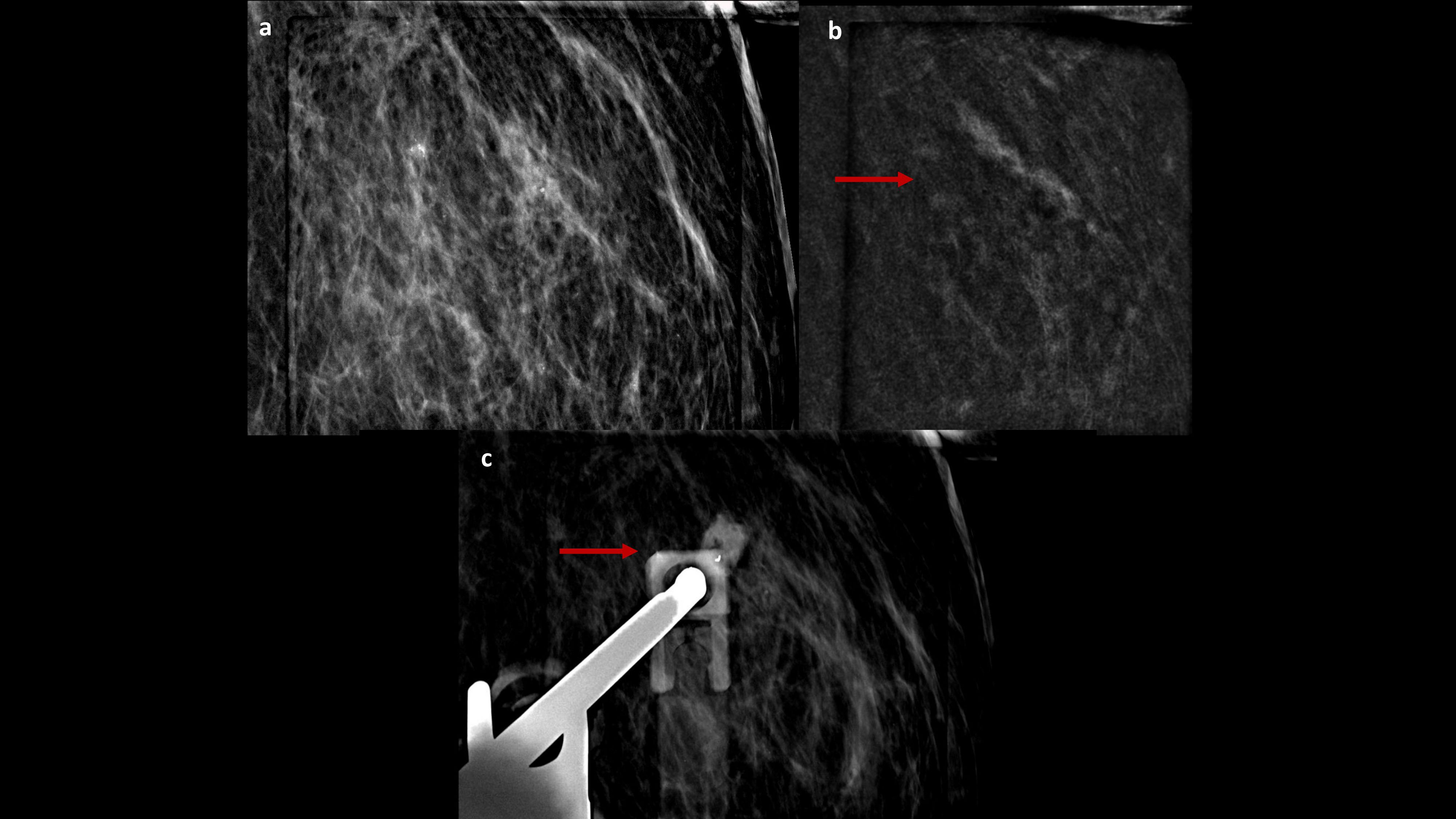
Figure 3. CEM-biopsy: 2D magnification and recombined focus (A, B) of the enhancement target in the outer quadrant of the left breast (arrow), where a vacuum-assisted biopsy was performed, followed by the clip placement (C, arrow).
CEM protocolCEM was performed using a full-field DM system (Pristina™ mammography system, GE Healthcare, Chalfont St. Giles, United Kingdom). Before breast compression, patients received an automated intravenous single injection of an iodinated contrast agent (Iopromide, 370 mg/ml, 1.5 ml/kg, Ultravist ®). Image acquisition started two minutes post-injection, capturing a series of bilateral cranio-caudal (CC) and medio-lateral-oblique (MLO) views starting from the suspicious breast.
Discussion and conclusionsPD is a rare condition, accounting for 1-3% of all breast malignancies (1). After diagnosis by NAC punch-biopsy, planning appropriate treatment for PD remains challenging for physicians due to the high rate (67-100% of cases) of associated underlying malignancy, often undetectable on conventional imaging techniques (1, 3). Lesions without a discernible mass typically correlate with in situ disease confined to the ductal system (DCIS), although they may also suggest the absence of underlying breast malignancy (2). MRI’s high sensitivity in PD detection is well-documented, particularly in assessing the retroareolar region and clinically occult malignancies with negative findings on DM and US, like non-mass enhancement (9). Additionally, MRI is critical for preoperative assessment of the overall extent of disease in patients eligible for breast-conserving therapy (4, 10). However, some of its limitations should be considered. False negatives may arise in low-grade or less aggressive disease forms (6, 7), and false positives can complicate diagnostic specificity (11, 12). Several studies demonstrated that sensitivity for DCIS is variable; some, especially those with a lower pathological grade (G1), can be missed (13–15). Furthermore, cost, availability, and patient-related issues (e.g., claustrophobia, incompatible devices) restrict its routine use.
In this context, CEM could emerge as a valuable alternative or complementary imaging tool in locoregional BC staging and diagnostic problem-solving when other imaging techniques yield inconclusive results. Studies demonstrate a high accuracy of CEM in measuring the main lesion (16–18) and identifying the multifocality and multicentricity of lesions, suggesting how its use in pre-surgical planning may offer significant benefits and surgical plan modification rates similar to MRI (19, 20). A recent Australian prospective investigation compared CEM to MRI for BC staging in 59 women with 68 sites of malignancy, demonstrating statistically equivalent sensitivities of 99% and 97%, respectively (21). CEM can also be evaluated as a tool to characterize additional breast findings that would otherwise be considered indeterminate. Nida et al. assessed the use of CEM as a second look modality to identify correlates of suspicious or indeterminate MRI findings, showing a higher detection fraction of CEM (76/109, 70%) compared to the US (50/109, 46%) (P < 0.001) (22).
To date, few studies have discussed the role of CEM in PD evaluation. Fakhry et al. evaluated the added value of incorporating CEM into the diagnostic workup of PD, demonstrating a higher sensitivity of 97.5% and a similar specificity of 54.2% compared to US-DM and a better performance in the assessment of disease extent, as it was able to detect multifocality, multicentricity, and diffuse abnormalities (8).
The clinical cases we presented support the value of CEM in diagnosing PD. In both cases, CEM led to better preoperative delineation of the disease extension and ultimately changed the surgical strategy. Given its ability to assess lesions’ neo angiogenesis, CEM could be an alternative method to MRI in assessing PD patients, especially when MRI is contraindicated or inconclusive.
Despite its promise, the findings of this presentation must be viewed within the context of limitations. It is based on only two cases from a single center, limiting the generalizability of its conclusions to broader and more diverse populations. However, the absence of quantitative data and statistical comparisons reduces the robustness of the observations.
Future research should address these gaps by conducting larger, multicentric, prospective studies with robust statistical methodologies.
PD of the breast represents a diagnostic and therapeutic challenge, particularly given its frequent association with underlying malignancy, often undetected by conventional imaging techniques. While MRI has historically been the gold standard for detecting clinically occult cancer, aiding in preoperative evaluation, its limitations necessitate the exploration of alternative imaging modalities.
Our cases highlight the potential utility of CEM, demonstrating its effectiveness in preoperative disease delineation and surgical planning. CEM offers several advantages, including comparable sensitivity, greater patient tolerance, and lower costs.
However, these findings must be interpreted cautiously. Future research should focus on multicentric studies with quantitative methodologies to validate CEM’s role and compare it comprehensively with MRI. Addressing these gaps can better establish the full potential of CEM as a diagnostic and planning tool in PD management. Nevertheless, the evidence thus far suggests that CEM could significantly improve PD’s diagnostic workup and treatment planning, mainly when MRI is not feasible.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementWritten informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributionsLuM: Writing – original draft, Writing – review & editing. LN: Writing – original draft, Writing – review & editing. AB: Writing – original draft, Writing – review & editing. FP: Writing – original draft, Writing – review & editing. FM: Writing – original draft, Writing – review & editing. GM: Writing – original draft, Writing – review & editing. LoM: Writing – original draft, Writing – review & editing. AS: Writing – original draft, Writing – review & editing. EC: Writing – original draft, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Mariano L, Nicosia L, Pupo D, Olivieri AM, Scolari S, Pesapane F, et al. A pictorial exploration of mammary paget disease: insights and perspectives. Cancers (Basel). (2023) 15:5276. doi: 10.3390/cancers15215276
PubMed Abstract | Crossref Full Text | Google Scholar
2. Ashikari R, Park K, Huvos AG, Urban JA. Paget’s disease of the breast. Cancer. (1970) 26:680–5. doi: 10.1002/1097-0142(197009)26:3<680::AID-CNCR2820260329>3.0.CO;2-P
Crossref Full Text | Google Scholar
3. Mendez-Fernandez MA, Henly WS, Geis RC, Schoen FJ, Hausner RJ. Paget’s disease of the breast after subcutaneous mastectomy and reconstruction with a silicone prosthesis. Plast Reconstr Surg. (1980) 5:683–5. doi: 10.1097/00006534-198005000-00026
PubMed Abstract | Crossref Full Text | Google Scholar
4. Morrogh M, Morris EA, Liberman L, Van Zee K, Cody HS 3rd, King TA. MRI identifies otherwise occult disease in select patients with Paget disease of the nipple. J Am Coll Surg. (2008) 206:316–21. doi: 10.1016/j.jamcollsurg.2007.07.046
PubMed Abstract | Crossref Full Text | Google Scholar
5. Echevarria JJ, Lopez-Ruiz JA, Martin D, Imaz I, Martin M. Usefulness of MRI in detecting occult breast cancer associated with Paget’s disease of the nipple-areolar complex. Br J Radiol. (2004) 77:1036–9. doi: 10.1259/bjr/94607773
PubMed Abstract | Crossref Full Text | Google Scholar
6. Kim HS, Seok JH, Cha ES, Kang BJ, Kim HH, Seo YJ. Significance of nipple enhancement of Paget’s disease in contrast enhanced breast MRI. Arch Gynecol Obstet. (2010) 282:157–62. doi: 10.1007/s00404-009-1244-4
PubMed Abstract | Crossref Full Text | Google Scholar
7. Frei KA, Bonel HM, Pelte MF, Hylton NM, Kinkel K. Paget disease of the breast: findings at magnetic resonance imaging and histopathologic correlation. Invest Radiol. (2005) 40:363–7. doi: 10.1097/01.rli.0000163742.40401.4e
PubMed Abstract | Crossref Full Text | Google Scholar
8. Fakhry S, Abdel Rahman RW, Shaalan HS, Hassan MHI, Tealab SH, Sayed SB. The added role of contrast-enhanced spectral mammography in the evaluation of pathological nipple discharge. Egypt J Radiol Nucl Med. (2022) 53:87. doi: 10.1186/s43055-022-00766-4
Crossref Full Text | Google Scholar
11. Jansen SA, Shimauchi A, Zak L, Fan X, Karczmar GS, Newstead GM. The diverse pathology and kinetics of mass, nonmass, and focus enhancement on MR imaging of the breast. J Magn Reson Imaging. (2011) 33:1382–9. doi: 10.1002/jmri.22567
PubMed Abstract | Crossref Full Text | Google Scholar
12. Siziopikou KP, Jokich P, Cobleigh M. Pathologic findings in MRI-guided needle core biopsies of the breast in patients with newly diagnosed breast cancer. Int J Breast Cancer. (2011) 2011:613285. doi: 10.4061/2011/613285
PubMed Abstract | Crossref Full Text | Google Scholar
13. Neubauer H, Li M, Kuehne-Heid R, Schneider A, Kaiser WA. High grade and non-high grade ductal carcinoma in situ on dynamic MR mammography: characteristic findings for signal increase and morphological pattern of enhancement. Br J Radiol. (2003) 76:3–12. doi: 10.1259/bjr/14883856
PubMed Abstract | Crossref Full Text | Google Scholar
14. Facius M, Renz DM, Neubauer H, Böttcher J, Gajda M, Camara O, et al. Characteristics of ductal carcinoma in situ in magnetic resonance imaging. Clin Imaging. (2007) 31:394–400. doi: 10.1016/j.clinimag.2007.04.030
PubMed Abstract | Crossref Full Text | Google Scholar
15. Kuhl CK, Schrading S, Bieling HB, Wardelmann E, Leutner CC, Koenig R, et al. MRI for diagnosis of pure ductal carcinoma in situ: a prospective observational study. Lancet. (2007) 370:485–92. doi: 10.1016/S0140-6736(07)61232-X
PubMed Abstract | Crossref Full Text | Google Scholar
16. Lobbes MBI, Lalji UC, Nelemans PJ, Houben I, Smidt ML, Heuts E, et al. The quality of tumor size assessment by contrast-enhanced spectral mammography and the benefit of additional breast MRI. J Cancer. (2015) 6:144–50. doi: 10.7150/jca.10705
PubMed Abstract | Crossref Full Text | Google Scholar
17. Jochelson MS, Dershaw DD, Sung JS, Heerdt AS, Thornton C, Moskowitz CS, et al. Bilateral contrast-enhanced dual-energy digital mammography: feasibility and comparison with conventional digital mammography and MR imaging in women with known breast carcinoma. Radiology. (2013) 266:743–51. doi: 10.1148/radiol.12121084
PubMed Abstract | Crossref Full Text | Google Scholar
18. Travieso-Aja MDM, Naranjo-Santana P, Fernández-Ruiz C, Severino-Rondón W, Maldonado-Saluzzi D, Rodríguez Rodríguez M, et al. Factors affecting the precision of lesion sizing with contrast-enhanced spectral mammography, Clin. Radiol. (2018) 73:296–303. doi: 10.1016/j.crad.2017.10.017
PubMed Abstract | Crossref Full Text | Google Scholar
19. Åhsberg K, Gardfjell A, Nimeus E, Rasmussen R, Behmer C, Zackrisson S, et al. Added value of contrast-enhanced mammography (CEM) in staging of Malignant breast lesions-a feasibility study. World J Surg Oncol. (2020) 18:100. doi: 10.1186/s12957-020-01865-0
PubMed Abstract | Crossref Full Text | Google Scholar
20. Kim EY, Youn I, Lee KH, Yun J-S, Park YL, Park CH, et al. Diagnostic value of contrast-enhanced digital mammography versus contrast-enhanced magnetic resonance imaging for the preoperative evaluation of breast cancer. J Breast Cancer. (2018) 21:453–62. doi: 10.1002/jmri.21147
PubMed Abstract | Crossref Full Text | Google Scholar
21. Taylor DB, Burrows S, Saunders CM, Parizel PM, Ives A. Contrast-enhanced mammography (CEM) versus MRI for breast cancer staging: detection of additional Malignant lesions not seen on conventional imaging. Eur Radiol Exp. (2023) 7:8. doi: 10.1186/s41747-022-00318-5
PubMed Abstract | Crossref Full Text | Google Scholar
22. Nida BA, Rooney TB, Miller MM. Utility of MRI-directed contrast-enhanced mammography for biopsy planning for suspicious MRI-detected breast lesions. AJR Am J Roentgenol. (2023) 220:202–11. doi: 10.2214/AJR.22.28055
留言 (0)